REVIEW: Lel A. Drachev and the Direct Electrometric Method
Vasily V. Ptushenko1 and Alexey Y. Semenov1,a*
1Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119992 Moscow, Russia* To whom correspondence should be addressed.
Received June 23, 2023; Revised July 14, 2023; Accepted July 17, 2023
In the bioenergetics studies, the direct electrometric method played an important role. This method is based on measuring the electrical potential difference (Δψ) between two compartments of the experimental cell generated by some membrane proteins. These proteins are incorporated into closed lipid–protein membrane vesicles associated with an artificial lipid membrane that separates the compartments. The very existence of such proteins able to generate Δψ was one of the consequences of Peter Mitchell’s chemiosmotic concept. The discovery and investigation of their functioning contributed to the recognition of this concept and, eventually the well-deserved awarding of the Nobel Prize to P. Mitchell. Lel A. Drachev (1926-2022) was one of the main authors of the direct electrometrical method. With his participation, key studies were carried out on the electrogenesis of photosynthetic and respiratory membrane proteins, including bacteriorhodopsin, visual rhodopsin, photosynthetic bacterial reaction centers, cytochrome oxidase and others.
KEY WORDS: Mitchell’s chemiosmotic hypothesis, transmembrane electric potential difference (Δψ), methods of membrane potential measurement, molecular electric generators, proteoliposomes, chromatophores, bacteriorhodopsin, photosystem IDOI: 10.1134/S0006297923100012
Abbreviations: Δψ, transmembrane potential difference; ΔµH+, transmembrane electrochemical potential difference of hydrogen atoms; ALM, artificial lipid membrane; bR, bacteriorhodopsin; PSI and PSII, photosystems I and II; RC, bacterial reaction center.
INTRODUCTION
Lel Aleksandrovich Drachev started his work at the Interfaculty Research Laboratory of Molecular Biology and Bioorganic Chemistry (hereinafter, referred to as Interfaculty Laboratory; currently, Belozersky Institute of Physical-Chemical Biology of the Moscow State University) in 1973 as a Chair of the newly established Department of New Physical Methods. He was offered this position by Rem Viktorovich Khokhlov, Rector of the Moscow State University, who had been appointed shortly before that and who was Drachev’s course mate at the Physical Faculty of Moscow State University, through the intermediary of another course mate, well-known biophysicist Efim Arsentyevich Liberman. At that time, Liberman, who several years before had developed the method of membrane potential measurements with synthetic penetrating ions, closely collaborated with the newly appointed Director of the Interfaculty Laboratory, Vladimir Petrovich Skulachev and his co-workers.
MITCHELL’S HYPOTHESIS AND SEARCH FOR APPROPRIATE RESEARCH
METHODS
Direct electrometric method is based on the concept of transmembrane electric potential difference (Δψ) formed on the intracellular, the so-called coupling, membranes of mitochondria and chloroplasts and on the inner bacterial membranes. This concept has stemmed from the chemiosmotic theory of coupling between the respiratory and photosynthetic electron transfer and phosphorylation suggested by the future Nobel laureate P. Mitchell. The chemiosmotic theory considered the transmembrane difference of electrochemical potentials of hydrogen ions (ΔµH+) as an intermediate form of energy in the processes of oxidative and photosynthetic phosphorylation. Existence of ΔµH+ on the coupling membranes proved to be one of the experimentally verified statements of the Mitchell’s hypothesis, and the efforts of research teams headed by Skulachev and Liberman, who were among the first to accept the chemiosmotic theory, had been focused on proving it. Note that as it has become clear much later, Δψ is not always the main component of ΔµH+. In chloroplasts, FΔψ (where F is the Faraday constant) is much lower than the concentration component of ΔµH+ (–RTΔpH) [1, 2]. In the sodium bioenergetics, which was discovered later, the transmembrane electrochemical potential of sodium ions plays the role of the membrane form of energy, which allowed to generalize the Mitchell’s concepts [3]. Here, we will omit the discussion between Mitchell and R. Williams about the role of protons in membrane energization, as it has been already described in detail in the literature (e.g., see the article by Weber and Prebble [4]).
Selection of an appropriate research method was essential for Δψ evaluation, so development of quite different methods for measuring Δψ had started at that time almost simultaneously. Apparently, it was the discussion of the Mitchell’s hypothesis that has initiated the outburst of research activity in the field. In 1968, Junge and Witt [5] used electrochromic shift in the absorption spectrum of chlorophyll b (though they did not refer to it as such) to study photoinduced generation of the transmembrane potential and ion transport in photosynthesis in isolated spinach chloroplasts. A little later, Bulychev et al. [6-8] developed a microelectrode method for measuring Δψ that was used to study giant (up to 15-25 µm) chloroplasts of the higher plant Peperomia metallica, both isolated and in vivo. The method for measuring electric potential in a light gradient was proposed shortly afterwards [9, 10]. This method used macroscopic changes in Δψ (and consequently, macroelectrode techniques to record them) that originate due to the summation of dipole moments of individual vesicles in an optically dense suspension of chloroplast thylakoids. Here, we will not discuss these methods, which have been widely used later and provided a lot of valuable data on the light-dependent reactions of photosynthesis. Instead, let us get straight to their limitations, as this will be important in the context of further article. The method for recording transmembrane potential based on the measurement of electrochromic shifts of photosynthetic pigments (mainly carotenoids), is widely used in the studies of plant chloroplasts and chromatophores of purple bacteria; however, it proved to be inefficient for investigating cyanobacteria and isolated pigment–protein complexes in model systems. Also, this method provided a relatively low signal to noise ratio, a problem that was largely solved only 1.5 decades later in the works of Joliot and Joliot [11].
The methods based on the microelectrode technique have provided an important information on the light-dependent currents generated on the chloroplast membranes but could not be extended to smaller objects (including proteoliposomes and chromatophores of purple bacteria). Unavoidably high active resistance of microelectrodes also limited the time resolution of this method (about 1 ms). The light gradient method has allowed to record the kinetics of membrane potential generation within a time interval from 20 ps to 50 ns; however, in the case of slower processes, recording was limited by the dissipation of the formed electric potential gradient due to the ionic conductivity of the buffer solution in the suspension of membrane vesicles.
Further development of methods for measuring in a light gradient using multilayered electrically oriented fragments of protein–lipid membranes has followed a decade and a half later [12]. Switching to gels and decreasing ionic conductivity has allowed to raise the upper limit of the time window [13]. We should mention that the initial variants of the method described below, which was developed by Liberman and Skulachev, as well as of the direct electrometric method that has evolved from it, also has limitation with respect to many of the above-mentioned parameters.
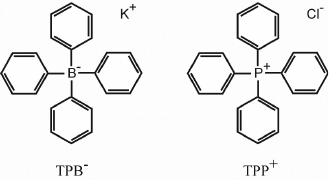
Liberman and Skulachev have solved this problem by coming up with an ingenious idea of synthetic penetrating ions. “Penetrating (through the membrane) ions” seemed as an oxymoron at that time, because the charge of an ion obviously makes it hydrophilic and creates an energy barrier to its immersion in the hydrophobic environment of the membrane. However, Liberman assumed that the barrier could be considerably reduced by distributing the charge over a large volume of the particle or surrounding it by relatively hydrophobic molecular groups. The search for such “lipophilic ions” in the collections of colleagues working in chemistry had been successful and helped to confirm the original idea: some of the found compounds, in particular, phenyl dicarbaundecaborane (PCB–) and tetraphenylboron (TPB–) anions and tetraphenylphosphonium (TPP+) cation turned out to be able to easily penetrate through the membrane (Fig. 1). At the same time, their distribution on the two sides of the membrane was determined, in accordance with the Nernst equation, by the electrochemical gradient ΔµH+: positively charged ions accumulated inside the mitochondria that generated Δψ on the inner membrane (with a negative sign inside in the case of mitochondria and positive sign in the case of submitochondrial particles) due to the process of oxidative phosphorylation.
Fig. 1. Lipophilic tetraphenylboron (TPB–) and tetraphenylphosphonium (TPP+) ions.
As a result, the concentration of these ions in the suspension decreased, which could be recorded potentiometrically [14]. This approach has demonstrated for the first time that the process of cellular respiration is accompanied by the Δψ generation on the membranes of mitochondria and submitochondrial particles, which could be suppressed by respiratory inhibitors or uncouplers of oxidation and phosphorylation [15].
SYNTHESIS OF TWO APPROACHES
In 1971, Oesterhelt and Stoeckenius [16] published an article in Nature New Biology, in which they described a rhodopsin-like protein from the purple membranes of Halobacterium halobium. At the initiative of Skulachev, a strain of H. halobium was brought to Moscow and the protein bacteriorhodopsin (bR) capable of light-dependent proton translocation across the membrane was isolated from the purple membranes. The studies of this membrane protein at the Department of Bioenergetics and then at the Department of New Physical Methods of the Interfaculty Laboratory were the first step on the path to the development of direct electrometric method.
In the summer of 1973, Drachev moved from the All-Union Institute of Scientific and Technical Information (VINITI) to the Interfaculty Laboratory. Together with the graduate student A. D. Kaulen, he performed direct measurements of the electric potential generated by bR embedded in a flat artificial lipid membrane (ALM) separating two sections of a Teflon cell. bR was incorporated in the membrane directly from a phospholipid solution in n-decane, to which it had been added in a solubilized form in the presence of detergent [17]. In contrast to the indirect measurements with the involvement of penetrating ions, this approach allowed direct measurements of Δψ generation by the protein. However, it was characterized by considerable variations in the values and even directions of photoinduced signal from one experiment to another. Later, it has become clear that these variations were associated with random orientation of bR in the membrane: approximately the same amounts of protein were incorporated in the membrane in the “plus” and “minus” orientations, and even minor variations in their ratio led to significant changes in the determined difference between the signals and fluctuations in the sign of Δψ.
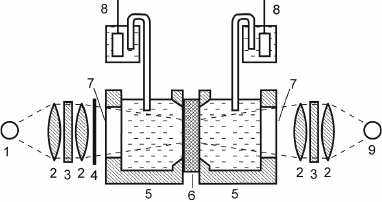
In the autumn of 1973, one of the authors of this article (A.Yu.S.), who had just graduated from the Department of Molecular Biology, Faculty of Biology, Moscow State University, became involved in this work to study bR-containing proteoliposomes, which had been introduced in the research practice shortly before by Kagawa and Racker, specialists in bioenergetics [18]. bR incorporated in proteoliposomes generated transmembrane potential in response to illumination [19]. The experimental system was a cell consisting of two sections filled with an electrolyte solution and separated with a flat ALM applied onto a hole in the partition between the sections. Silver chloride electrodes connected to an electrometric voltmeter were submerged into both sections of the cell to measure changes in the potential difference between the sections throughout the experiment (Fig. 2). Penetrating anions (PCB–) were added to both cells, and bR proteoliposomes were added to one of them. When the cell was illuminated, PCB– ions were absorbed by proteoliposomes due to the generation of transmembrane potential, resulting in the generation of potential difference between the sections. Although the amplitude of this potential difference was small (~0.01 V), it was sufficient for reliable measurement.
Fig. 2. Scheme of the experimental system: 1) continuous light source (halogen lamp); 2) lens; 3) optical filter; 4) shutter; 5) Teflon cuvette; 6) ALM formed on membrane porous filter; 7) glass window; 8) AgCl electrode; 9) pulse laser.
As we know from the history of science, discovery of a new phenomenon is often a matter of chance. Becquerel stored uranium salts together with photographic plates; Pasteur kept tartaric acid crystals for too long; Fleming failed to prevent Petri dishes from fungal contamination. In our case, ALMs were not stable enough during long-term experiments and periodically lost their resistance because of electrical or mechanical fluctuations. As a result, the photoelectric signal disappeared and a drop of phospholipid solution had to be injected into the hole in the Teflon cell partition to form the ALM again. Another factor contributing to the discovery of this effect, which had become the key element in the direct electrometric method development, was high concentration of ammonium ions in the medium. Since the electrochemical potential on the liposome membrane could contain both electrical and concentration (pH-dependent) components, to maximally improve the measured electric signal, it was decided to reduce the “competing” transmembrane pH gradient. For this purpose, ammonium salts were added to the medium at a significant concentration (0.1 M) as a pH buffer penetrating into proteoliposomes. Under these conditions, repeated formation of ALM was accompanied by the increase in the photoelectric signal with time, which was difficult to explain within the framework of existing ideas on the action mechanism of penetrating ions. The fact that this effect was observed at high salt concentrations suggested that it could be related to changes in the surface charges. When the singly-charged ions in the background electrolyte were replaced with lower concentrations (10-20 mM) of doubly-charged ions (Ca2+ and Mg2+), the signal increased much more rapidly. This suggested the shielding of fixed negative charges of phospholipids by cations of the background electrolyte, in which doubly-charged cations were much more efficient than singly-charged ones. The shielding of surface charges on the proteoliposome membranes and ALM should have accelerated proteoliposome aggregation and adsorption on the ALM separating the cell sections. This implied that the increase in the light-induced Δψ occurred due to the activity of bR in the membranes of proteoliposomes attached to the ALM. To verify this suggestion, Liberman proposed to perform the same experiment in the absence of penetrating ions. Indeed, even without the addition of PCB– to the medium, incubation of proteoliposome suspension for 30 min in the presence of 20 mM CaCl2 was accompanied by a gradual increase in the amplitude of photoinduced Δψ up to ~250 mV.
Unlike the method of ALM formation by mixing a bR suspension in detergent with a solution of phospholipids in n-decane, which had been originally used by Drachev and Kaulen, experiments with bR incorporated into liposome membranes showed a high degree of orientation asymmetry: more than 95% protein molecules were oriented with the “plus” directed inward (this was proven later using lanthanum ions inhibiting the activity of bR upon its binding in the “entrance” proton channel on the proton-donor side). As a result, light-induced Δψ always had the same sign and a relatively small dispersion of values that was mostly determined by the accuracy with which the total internal volume of proteoliposomes was controlled. Apparently, such asymmetry of protein incorporation was due to the asymmetry of the membrane that had a nonzero curvature in the case of closed spherical vesicles, while flat ALMs lacked the curvature that could provide the asymmetry of protein incorporation.
Therefore, both methods (based on synthetic penetrating ions and mixing bR with a solution of phospholipids) had their pros and cons. The discovery of proteoliposome adsorption on the ALM surface allowed to create a “hybrid” of these two methods by combining their advantages: a high degree of asymmetry of protein incorporation into proteoliposome membrane (and, therefore, higher signal value) typical of the indirect method of synthetic penetrating ions and direct measurement of protein-generated potential in the direct electrometric method. Further development of this hybrid method, that has received the name of direct electrometric method, or Drachev’s method, has been associated with the works of Drachev and his coworkers A. D. Kaulen and A. Yu. Semenov.
PROOF OF EQUIVALENT CIRCUIT AND SELECTION OF ARTIFICIAL
MEMBRANE
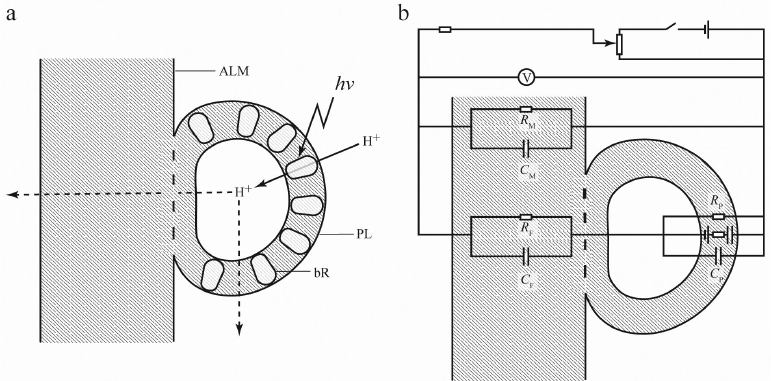
“Hybridization” of the two methodological approaches has provided new experimental opportunities but simultaneously posed new problems. Above, we mentioned association of proteoliposomes with flat ALM surface. But was this protein–lipid system equivalent to the one formed by direct protein incorporation in the ALM in the presence of detergent (except for the differences in the asymmetry of incorporation)? This is what correct quantitative interpretation of measurements depended on. The systems would be equivalent if after adsorption on a flat ALM, the membrane of a proteoliposome ruptured, proteoliposome phospholipids “spread” over the ALM, and membrane proteins were incorporated in the ALM. However, an alternative scenario is possible, when proteoliposome membranes do not undergo topological changes and proteoliposomes remain associated with the ALM surface as closed vesicles. In this case, proteoliposomes retain aqueous phase inside (Fig. 3a). At the same time, hypothetically, there might be also different variants of protein distribution, as proteins could be transferred from the proteoliposome membrane into the ALM or remain in the proteoliposome membrane. Later, a similar question was raised for the distribution of a pool of ubiquinone-10 molecules in the chromatophore membranes in the studies using chromatophores of photosynthetic bacteria (natural closed vesicles). However, if hydrophobic ubiquinone freely redistributed between the chromatophore membranes and ALM, no such exchange was observed for protein molecules. The proof that the aqueous phase (which is referred to as the “third water” in the literature) was retained inside the vesicles and all proteins were located on the vesicle external surface was based on the measurements of potentials generated in the presence of protonophore uncouplers of different types. For example, gramicidin A, which forms channels in the membrane, substantially reduced the potential generated by bR upon illumination, but had almost no effect on the total electrical conductivity of the membrane. At the same time, the “shuttle” protonophores (e.g., carbonyl cyanide-m-chlorophenylhydrazone) decreased both the light-dependent potential and the membrane conductivity [20]. Such difference could result only from the presence of two types of membranes in the system. Gramicidin formed channels in the bilayer membrane containing ΔμH+ generators (bR), but not in a relatively thick flat ALM that separated the compartments of the experimental system and determined conductivity of this system; although ALM remained permeable for the “shuttle” protonophores (Fig. 3b).
Fig. 3. Interaction between bR-containing proteoliposome (PL) and flat phospholipid ALM (a) and equivalent circuit diagram (b). Orientation of bR molecules in the PL membrane is asymmetric (~95% molecules transferred protons inside PL). According to the experimental data, bR was almost absent in the area of membrane fusion. V, electrometric voltmeter with high input resistance; CP, RP, CM, RM, CF, RF, electrical conductivity (C) and resistance (R) of PL membrane, ALM, and area of membrane fusion, respectively. The image is out of scale, as the ALM thickness exceeds the PL membrane thickness by several orders of magnitude.
Experiments with direct incorporation of protein molecules into a flat membrane conducted by Drachev and Kaulen, as well as experiments of Semenov with proteoliposomes, were performed using thick (about 100-µm) phospholipid membranes separating two compartments of a Teflon cell. Although proteins cannot not penetrate such membrane or transfer a proton across it, their incorporation into the membrane, nevertheless, transformed it in an analog of H+-selective electrode, which made possible the recording of an electric signal. However, such membrane had one significant drawback: because of a significant volume of lipids, it had low electrical capacity, resulting in a considerable increase in the electrical response time. The minimum characteristic time of Δψ generation that could be obtained in this system was about 10 ms, which was significantly higher than the characteristic times of main processes of charge transfer in bR, so experiments on the kinetics of this transfer required much thinner membrane. Unfortunately, a phospholipid bilayer (analog of natural membrane) could not be used in this case, because despite numerous attempts, the bilayer membrane (referred to as “black membrane” due to reduced optical transmission resulting from the interference of light reflected from the two surfaces) remained extremely unstable. The attempts to incorporate proteins into the “black membrane” failed as well, since addition of detergent-containing proteins to the electrolyte surrounding the membrane resulted in membrane rupture. The attempts to use flat bilayer membranes in the electrometric method had been abandoned, demanding the search for new approaches to the problem.
This search for other variants of flat ALM has been very long and difficult. It became obvious that such membrane had to be somehow “reinforced” to increase its strength, and various porous membrane filters soaked in a phospholipid solution have been tested as rigid base for the ALM. Although the filters themselves were rather thick (about 0.1 mm, which is similar to the thickness of thick phospholipid membrane), there was hope that the phospholipid films formed over the filter pores would be thinner. These studies have also shown that not only the filter material and pore size, but also the nature of the vesicle was important for the development of the experimental system. Thus, almost all tested types of filters could be used for the measurements with bR-containing proteoliposomes, while the results of studies of chromatophores were affected by the filter properties. Filters made of chemically inert materials (Fluoropore, Teflon) provided the maximal electric responses that were comparable with the potentials obtained with the phospholipid membrane (up to 0.25 V; [21, 22]) irrespectively of the pore diameter. Electrical responses recorded for chromatophores (but not proteoliposomes) with cellulose-based filters were lower (and even less for cellulose acetate–nitrate filters compared to pure cellulose nitrate filters). In the case of cellulose acetate-containing filters, the results also depended on the pore size, as larger pores ensured higher values of measured potentials [21]. However, irrespectively of the filter used, the same insurmountable time resolution limit remained – about 10 ms.
In addition to the use of available commercially filters, there were attempts to create suitable “frames” using contemporary “high technologies”. This brings to mind the visit of Drachev and his young colleagues to the Laboratory of Nuclear Reactions headed by G. N. Flerov at the Joint Institute for Nuclear Research in Dubna, where the scientists synthesized their own filters for different technical tasks by placing polymer films under a particle accelerator beam which turned them into “sieves”. The diameter and distribution density of pores could be varied by varying the beam parameters. However, none of such filters turned to be suitable for the membrane experiments, as a phospholipid membrane failed to settle on long narrow cylindrical pores and did not impregnate the filter.
Finally, the filters that were found by Drachev and his colleagues and proved to be the best for their studies, were nitrocellulose collodion films. These films were actively used as supports in electron microscopy and, therefore, were available. Nitrocellulose collodion films consisted of long cellulose filaments that form a small number of layers and had a comparatively small thickness (~200-300 nm). When impregnated with the phospholipid solution, they ensured sufficient membrane stability and, simultaneously, a fast electrical response (about 200 ns). Most of the following studies at the Interfaculty Laboratory were performed using collodion ALMs. The search for this key component of the measurement system took almost five years.
MAIN RESULTS OBTAINED BY DIRECT ELECTROMETRIC METHOD
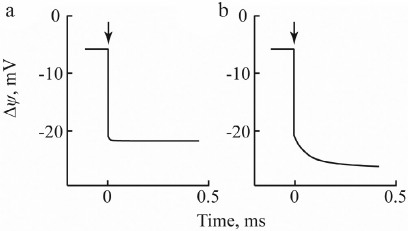
From 1974 to 1980, experiments using a combination of thick phospholipid membrane and closed vesicles (proteoliposomes containing various membrane proteins or chromatophores) have demonstrated generation of potential difference by bR, visual rhodopsin, mitochondrial cytochrome c oxidase, ATPase, and transhydrogenase, as well as by chromatophores of photosynthetic bacteria [23-27]. In 1979-1980, analogous measurements were performed using different membrane pore filters impregnated with a phospholipid solution in n-decane. A typical photoelectric response of the membrane filter/ bR-containing proteoliposome system to light switching on and off is shown in Fig. 4. In this case, illumination caused rapid generation of Δψ with a positive sign inside the proteoliposomes and amplitude up to ~200 mV. The photoelectric signal remained at approximately the same level for at least 10 min of light exposure and completely decayed when light was switched off. When the system was kept in the dark, the light-dependent potential with an amplitude of >150 mV was maintained for 72 h and then decayed simultaneously with the decrease in the ALM resistance.
Fig. 4. Photoelectric response of proteoliposomes containing bacterial photosynthetic RC without additions (a) and in the presence of 2 mM ascorbate and 2 µM natural electron donor cytochrome c (b).
In the previous section of this article, we mentioned that when using “thick” phospholipid ALMs and ALMs based on a membrane filter, low-capacity CM comparable with the input capacity of the operational amplifier (~5 pF) distorted the kinetics of Δψ generation in response to a laser flash. Therefore, rapid kinetics was studied using collodion phospholipid ALMs (~200 nm thick) with the CM value of ~5000 pF. This system characterized by the time resolution of 0.2 µs, was used to investigate the kinetics of laser-induced Δψ generation in proteoliposomes containing bR and bacterial RCs and chromatophores from photosynthetic bacteria [28-30].
As shown in Fig. 4a, in the system of collodion ALM/proteoliposomes with incorporated photosynthetic RC from Rhodobacter sphaeroides, laser flash in the absence of electron donor caused generation of Δψ with a negative sign inside with an amplitude of ~15-20 mV due to the formation of the ion–radical pair P870+QA– during electron transfer from the primary donor of R. sphaeroides RCs (bacteriochlorophyll dimer P870) to the acceptor (ubiquinone QA incorporated in the RC) [31]. Upon addition of 2 mM ascorbate and 2 µM cytochrome c2 (native electron donor), kinetics of the enhanced photoelectric response, apart from the quick phase, showed an additional slower component with the characteristic time τ = 250 µs (Fig. 4b). The observed increase in the Δψ generation was due to the electrogenic reduction of P870+ by cytochrome c2. Beside electrogenic reactions resulting from the reduction of oxidized primary donor P+ by different c-type cytochromes, the use of chromatophores from R. sphaeroides, Rhodospirillum rubrum, Ectothiorhodospira shaposhnikovii, Chromatium minutissimum, and Blastochloris viridis demonstrated the presence of one more electrogenic component associated with protonation of the secondary quinone acceptor QB from the external aqueous phase in response to even-numbered light flashes [32]. Chromatophores from R. sphaeroides were also used to study laser-induced electrogenic responses caused by electron and proton transfer in the cytochrome bc1 complex [33].
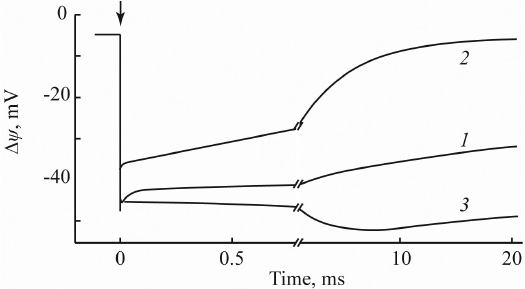
A similar approach was used to study electrogenic responses in proteoliposomes containing photosystem (PS) I and II complexes from cyanobacteria and higher plants [34, 35]. Figure 5 shows typical photoelectric responses of proteoliposomes containing PSI complexes from the cyanobacterium Synechocystis sp. PCC 6803.
Fig. 5. Photoelectric response of PSI-containing proteoliposomes: 1) without additions; 2) in the presence of sodium dithionite; 3) in the presence of 10 mM ascorbate and 0.4 mM plastocyanin (native PSI external donor).
In the absence of additions (curve 1), these preparations demonstrated a photoelectric response with the negative sign inside and amplitude of ~50 mV that decayed with τ = 100 ms. Addition of a strong reducer (100 mM sodium dithionate) slightly decreased the amplitude of Δψ and accelerated its decay to several milliseconds (curve 2). This effect was due to the reduction by dithionite of the terminal 4Fe4S clusters FA/FB; as a result, the decay of Δψ occurred from the preceding 4Fe4S cluster FX. At the same time, addition of 0.4 M plastocyanin caused an appearance of an additional electrogenic component with τ = 3 ms (curve 3) resulting from the electrogenic reduction of the photooxidized PSI primary electron donor (chlorophyll dimer P700+) by plastocyanin.
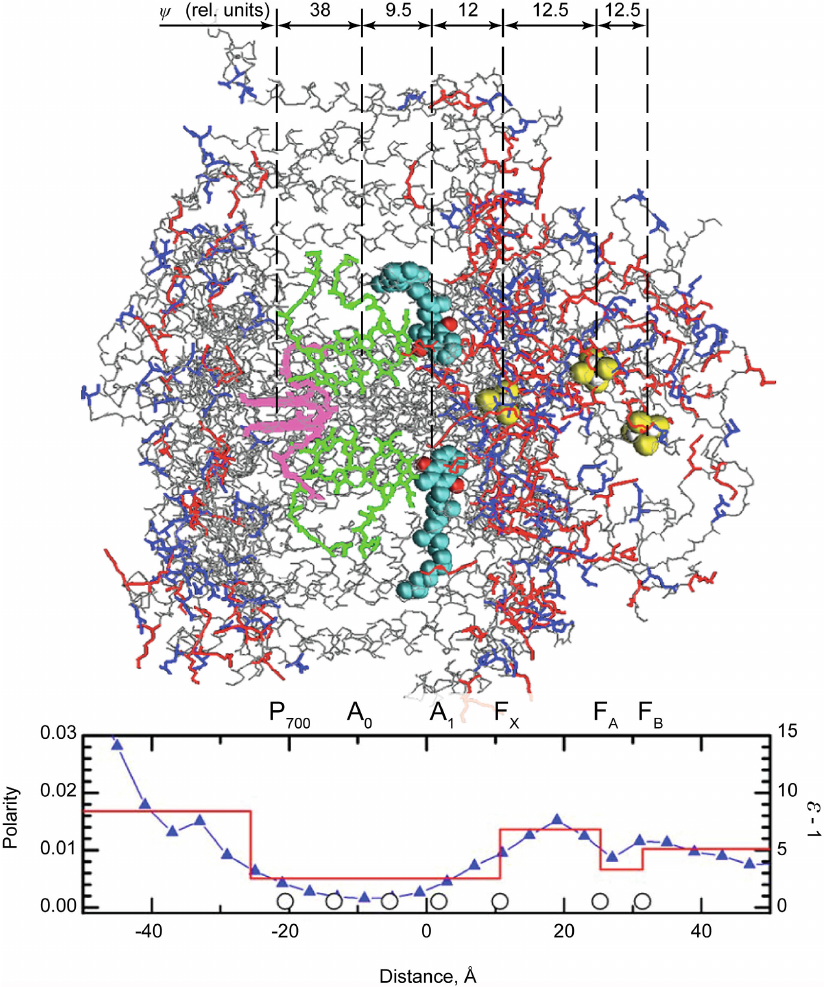
One of the important features of direct electrometric method is that can be used to evaluate the distribution of dielectric permittivity (ε) in hydrophobic membrane proteins containing redox cofactors. The transfer of a charge (electron or proton) from one group to another inside the protein causes a change in the potential difference between the two sides of the membrane that depends both on the distance over which the charge is transferred in the membrane (or more precisely a transfer component, which is normal to the membrane plain) and the ε value of the protein domain where this transfer takes place. The potential difference caused by the charge transfer is lower in a domain with a greater number of polar amino acid residues and, consequently, higher ε value, but is greater in the domains with hydrophobic residues (and low ε value). Evaluations of the distances between cofactors (based on X-ray structure) have allowed to employ the data of direct electrometric method to estimate the average ε value in protein regions between two cofactors. The use of this method made it possible to determine ε distribution in the bacterial photosynthetic RC from R. sphaeroides, PSI from cyanobacteria, and PSII from higher plants [36, 37]. Of course, these data were merely evaluative because the polarity of adjacent protein domains also affects the value of the measured Δψ. However, estimated ε values were in good agreement with the data on the distribution of polar and nonpolar residues in proteins. Figure 6 shows the distribution of PSI dielectric permittivity along the normal to the membrane plain estimated by direct electrometric method [38]. One can see that it is in agreement with the distribution of polar groups in the structure of protein complex. The proportion of atoms carrying a noticeable partial charge (side-chains of Asp and Glu marked in blue and those of Lys, Arg, and His marked in red) and causing the dielectric response of the protein atomic subsystem determined based on the X-ray structure analysis of the PSI three-dimensional structure is presented in the same graph. In the figure, redox cofactors (primary electron donor P700, primary and secondary acceptors A0 and A1 in the two branches of redox cofactors A and B, and 4Fe4S acceptors FX, FA, and FB) and the relative contributions of each electron transfer stage to Δψ are also shown.
Fig. 6. Three-dimensional structure of PSI (the donor side exposed to the lumen is on the left; the acceptor side exposed to the stroma is on the right) and distribution of the polarity and dielectric permittivity of protein complexes along the normal to the membrane plane. Polarity was calculated as a proportion of atoms carrying a partial charge (N, O, and S atoms of amino acid side chains). Circles on the x-axis indicate coordinates of redox cofactors. Electron transfer between the primary donor P700 (pink) and quinone acceptors A1 (turquoise and red spheres, carbon and oxygen atoms, respectively) can occur via two different branches: A and B (upper and lower, respectively, in the figure). Each branch contains a pair of chlorophyll molecules (green) between P700 and A1 that function as a single cofactor A0 due to the electronic coupling between two parallel porphyrin rings. In the structure of PSI proteins, only peptide backbone (gray) and side chains of aniono- and cationogenic amino acid residues (blue and red, respectively) are shown.
The data on distribution of ε in PS1 served as a basis for creating an algorithm for calculation of electrostatic interactions in protein complexes and, eventually, for determination of redox characteristics of electron carrier cofactor. The other contribution to the proposed algorithm was the ideas of L. I. Krishtalik on how to overcome the apparent contradiction between the paradigm of macroscopic electrostatics and calculations using X-ray analysis data on the 3D protein structure, in particular, partial atom charges. Krishtalik has shown that these data carry different kinds of information about various components of changes in the free energy of transfer of cofactor into the protein. Therefore, these components should be calculated with different approaches and by taking into account the properties of protein via using various dielectric constants [39, 40]. As a result, for the first time, redox potentials of all PSI cofactors, from the primary donor P700/P700+ to the iron–sulfur clusters FX, FA, and FB, were calculated within the framework of the same approach [38].
CONCLUSIONS
One of the consequences of the Mitchell’s chemiosmotic concept was discovery of Δψ generators as a class of membrane proteins. Existence of these proteins was demonstrated by the direct electrometric method, which served as one of the proofs of the chemiosmosis theory and contributed to the decision to award Mitchell with a well-deserved Nobel Prize in Chemistry.
Direct electrometric method and its variants were (and are) used not only in the Drachev’s laboratory and, later, in the Kaulen and Semenov’s laboratories, but also in some groups in other countries. In particular, this method was used in the studies of electrogenic reactions in bacterial RCs at the laboratory of G. Feher (La Jolla, USA) [41], electrogenic reactions in PSI at the laboratory of P. Brzezinski (Göteborg, Sweden) [42], kinetics of Δψ generation during transitions between the S states in PSII complexes at the laboratory of W. Junge (Osnabrück, Germany) [43], and electrogenic reactions during the functioning of terminal oxidases of mitochondria and bacteria at the laboratories of M. Wikström and M. Verkhovsky (Helsinki, Finland) (see review by Wikström and Verkhovsky [44]).
The development of direct electrometric method has been recognized as one of the main achievements (among approximately 40) of experimental studies in the field of oxidative and photosynthetic phosphorylation in the 20th century, along with the works by G. Embden and O. Meyerhof, V. Engelhardt, O. Warburg, V. Belitzer and E. Tsybakova, A. Lehninger, D. Arnon, B. Chance, P. Boyer, etc. [45].
We would like to emphasize the role of Drachev in the development and modification of direct electrometric method. Drachev was a radiophysicist, a highest-level specialist in electronics and laser technology. When he started to work at the Interfaculty Laboratory of the Moscow State University, he had a rather superficial understanding of proteins, as well as biochemistry and molecular biology in general. Liberman and Skulachev had played an essential role in attracting his interest to membrane proteins.
From 1973 to 1993, he had worked with younger biochemists Kaulen and Semenov, who carried out biochemical experiments. They isolated proteins, obtained proteoliposomes and chromatophores, varied the composition of proteoliposome phospholipids and ALM, and tested membrane filters as a support for ALM. They were also directly engaged in the measurement of Δψ produced by various membrane proteins in response to light or addition of respective substrates, performed pH and redox titration of photoelectric signals, and studied kinetics of Δψ generation in response to laser flashes. However, detailed elaboration of the method, including development of a system for recording membrane potential generation and corroboration of equivalent electric circuit would be impossible without painstaking and highly professional work of Drachev. Among other things, he was a top-level engineer, who identified and very effectively eliminated instrumentation problems.
Once his co-workers told him in some context: “...You are our scientific supervisor!” Drachev answered half-jokingly: “I am not a scientific supervisor, but a technical director”. Of course, Lel Aleksandrovich has significantly understated his role. In fact, all scientific findings were discussed by Drachev and his co-workers, and his opinion played an extremely important role in the data interpretation and planning of future experiments.
Contributions. A.Yu.S. formulated the concept of the article; A.Yu.S. and V.V.P. wrote the manuscript, discussed and edited the text.
Funding. This work was supported by the Russian Science Foundation (project no. 23-74-00025).
Ethics declarations. The authors declare no conflict of interests. This article does not contain description of studies with human participants or animals performed by any of the authors.
REFERENCES
1.Tikhonov, A. N. (2012) Energetic and regulatory
role of proton potential in chloroplasts, Biokhimiya, 77,
1155-1176, doi: 10.1134/S0006297912090027.
2.Johnson, M. P., and Ruban, A. V. (2014) Rethinking
the existence of a steady-state Δψ component of the proton
motive force across plant thylakoid membranes, Photosynth. Res.,
119, 233-242, doi: 10.1007/s11120-013-9817-2.
3.Skulachev, V. P. (1984) Sodium bioenergetics,
Trends Biochem. Sci., 9, 483-485, doi:
10.1016/0968-0004(84)90317-7.
4.Weber, B. H., and Prebble, J. N. (2006) An issue of
originality and priority: the correspondence and theories of oxidative
phosphorylation of Peter Mitchell and Robert JP Williams, 1961-1980,
J. History Biol., 39, 125-163, doi:
10.1007/s10739-005-3052-4.
5.Junge, W., and Witt, H. T. (1968) On the ion
transport system of photosynthesis – investigations on a
molecular level, Zeitschrift Für Naturforschung B,
23, 244-254, doi: 10.1515/znb-1968-0222.
6.Bulychev, A., Andrianov, V., Kurella, G., and
Litvin, F. (1971) Transmembrane potential of a cell and a chloroplast
of a higher terrestrial plant, Fiziol. Rast., 18,
248-256.
7.Bulychev, A., Andrianov, V., Kurella, G., and
Litvin, F. (1971) Transmembrane potential of a chloroplast and its
photoinduced changes, Dokl. Akad. Nauk SSSR, 197,
473-477.
8.Bulychev, A. A., Andrianov, V. K., Kurella, G. A.,
and Litvin, F. F. (1972) Micro-electrode measurements of the
transmembrane potential of chloroplasts and its photoinduced changes,
Nature, 236, 175-177, doi: 10.1038/236175a0.
9.Witt, H. T., and Zickler, A. (1974) Vectorial
electron flow across the thylakoid membrane. Further evidence by
kinetic measurements with an electrochromic and electrical method,
FEBS Lett., 39, 205-208, doi:
10.1016/0014-5793(74)80051-7.
10.Fowler, C. F., and Kok, B. (1974) Direct
observation of a light-induced electric field in chloroplasts,
Biochim. Biophys. Acta Bioenergetics, 357, 308-318, doi:
10.1016/0005-2728(74)90069-3.
11.Joliot, P., and Joliot, A. (1984) Electron
transfer between the two photosystems. I. Flash excitation under
oxidizing conditions, Biochim. Biophys. Acta Bioenergetics,
765, 210-218, doi: 10.1016/0005-2728(84)90015-X.
12.Deprez, J., Trissl, H. W., and Breton, J. (1986)
Excitation trapping and primary charge stabilization in
Rhodopseudomonas viridis cells, measured electrically with
picosecond resolution, Proc. Natl. Acad. Sci. USA, 83,
1699-1703, doi: 10.1073/pnas.83.6.1699.
13.Trissl, H.-W., Leibl, W., Deprez, J., Dobek, A.,
and Breton, J. (1987) Trapping and annihilation in the antenna system
of photosystem I, Biochim. Biophys. Acta Bioenergetics,
893, 320-332, doi: 10.1016/0005-2728(87)90053-3.
14.Liberman, E., Mokhova, E., Skulachev, V., and
Topaly, V. (1968) Effects of the uncouplers of oxidative
phosphorylation on bimolecular phospholipid membranes,
Biofizika, 13, 188-193membranes, Biofizika, 13,
188-193..
15.Liberman, E. A., Topaly, V. P., Tsofina, L. M.,
Jasaitis, A. A., and Skulachev, V. P. (1969) Mechanism of coupling of
oxidative phosphorylation and the membrane potential of mitochondria,
Nature, 222, 1076-1078, doi: 10.1038/2221076a0.
16.Oesterhelt, D., and Stoeckenius, W. (1971)
Rhodopsin-like protein from the purple membrane of Halobacterium
halobium, Nat. New Biol., 233, 149-152, doi:
10.1038/newbio233149a0.
17.Drachev, L. A., Kaulen, A. D., Ostroumov, S. A.,
and Skulachev, V. P. (1974) Electrogenesis by bacteriorhodopsin
incorporated in a planar phospholipid membrane, FEBS Lett.,
39, 43-45, doi: 10.1016/0014-5793(74)80012-8.
18.Kagawa, Y., and Racker, E. (1971) Partial
resolution of the enzymes catalyzing oxidative phosphorylation: XXV.
Reconstitution of vesicles catalyzing 32Pi-adenosine triphosphate
exchange, J. Biol. Chem., 246, 5477-5487, doi:
10.1016/S0021-9258(18)61930-1.
19.Kayushin, L. P., and Skulachev, V. P. (1974)
Bacteriorhodopsin as an electrogenic proton pump: Reconstitution of
bacteriorhodopsin proteoliposomes generating Δψ and
ΔpH, FEBS Lett., 39, 39-42, doi:
10.1016/0014-5793(74)80011-6.
20.Drachev, L. A., Frolov, V. N., Kaulen, A. D.,
Liberman, E. A., Ostroumov, S. A., Plakunova, V. G., Semenov, A. Y.,
and Skulachev, V. P. (1976) Reconstitution of biological molecular
generators of electric current. Bacteriorhodopsin, J. Biol.
Chem., 251, 7059-7065, doi:
10.1016/S0021-9258(17)32940-X.
21.Drachev, L., Kaulen, A., Samuilov, V., Severina,
I., Semenov, A, Skulachev, V. P., and Chekulaeva, L. N. (1979)
Incorporation of proteoliposomes and chromatophores into membranes
based on filters, Biofizika, 24, 1035-1042.
22.Drachev, L. A., Kaulen, A. D., Semenov, A. Y.,
Severina, I. I., and Skulachev, V. P. (1979) Lipid-impregnated filters
as a tool for studying the electric current-generating proteins,
Anal. Biochem., 96, 250-262, doi:
10.1016/0003-2697(79)90580-3.
23.Bol’shakov, V. I., Drachev, A. L.,
Kalamkarov, G. R., Kaulen, A. D., Ostrovsky, M. A., and Skulachev, V.
P. (1979) Common properties of bacterial and visual rhodopsins:
conversion of light energy into electric potential difference, Dokl.
Akad. Nauk SSSR, 249, 1462-1466.
24.Drachev, L. A., Kalamkarov, G. R., Kaulen, A. D.,
Ostrovsky, M. A., and Skulachev, V. P. (1981) Fast stages of
photoelectric processes in biological membranes: II. Visual rhodopsin,
Eur. J. Biochem., 117, 471-481, doi:
10.1111/j.1432-1033.1981.tb06362.x
25.Drachev, L. A., Jasaitis, A. A., Kaulen, A. D.,
Kondrashin, A. A., Liberman, E. A., Nemecek, I. B., Ostroumov, S. A.,
Semenov, A. Yu., and Skulachev, V. P. (1974) Direct measurement of
electric current generation by cytochrome oxidase, H+-ATPase
and bacteriorhodopsin, Nature, 249, 321-324, doi:
10.1038/249321a0.
26.Drachev, L. A., Kondrashin, A. A., Semenov, A.
Y., and Skulachev, V. P. (1980) Reconstitution of biological molecular
generators of electric current: transhydrogenase, Eur. J.
Biochem., 113, 213-217, doi:
10.1111/j.1432-1033.1980.tb06158.x.
27.Drachev, L. A., Frolov, V. N., Kaulen, A. D.,
Kondrashin, A. A., Samuilov, V. D., Semenov, A. Y., and Skulachev, V.
P. (1976) Generation of electric current by chromatophores of
Rhodospirillum rubrum and reconstitution of electrogenic
function in subchromatophore pigment-protein complexes, Biochim.
Biophys. Acta Bioenergetics, 440, 637-660, doi:
10.1016/0005-2728(76)90048-7.
28.Drachev, L. A., Kaulen, A. D., Khitrina, L., and
Skulachev, V. P. (1981) Fast stages of photoelectric processes in
biological membranes: I. Bacteriorhodopsin, Eur. J. Biochem.,
117, 461-470, doi: 10.1111/j.1432-1033.1981.tb06361.x.
29.Drachev, L. A., Semenov, A. Y., Skulachev, V. P.,
Smirnova, I. A., Chamorovsky, S. K., Kononenko, A. A., Rubin, A. B.,
and Uspenskaya, N. Ya. (1981) Fast stages of photoelectric processes in
biological membranes: III. Bacterial photosynthetic redox system,
Eur. J. Biochem., 117, 483-489, doi:
10.1111/j.1432-1033.1981.tb06363.x.
30.Chamorovsky, S. K., Drachev, A. L., Drachev, L.
A., Karagul’yan, A. K., Kononenko, A. A., Rubin, A. B., Semenov,
A. Yu., and Skulachev, V. P. (1985) Fast phases of the generation of
the transmembrane electric potential in chromatophores of the
photosynthetic bacterium Ectothiorhodospira
shaposhnikovii, Biochim. Biophys. Acta Bioenergetics,
808, 201-208, doi: 10.1016/0005-2728(85)90044-1.
31.Drachey, L. A., Kaminskaya, O. P., Konstantinov,
A. A., Kotova, E. A., Mamedov, M. D., Samuilov, V. D., Semenov, A. Y.,
and Skulachev, V. P. (1986) The effect of cytochrome c,
hexammineruthenium and ubiquinone-10 on the kinetics of photoelectric
responses of Rhodospirillum rubrum reaction centres, Biochim.
Biophys. Acta Bioenergetics, 848, 137-146, doi:
10.1016/0005-2728(86)90169-6.
32.Kaminskaya, O. P., Drachev, L. A., Konstantinov,
A. A., Semenov, A. Y., and Skulachev, V. P. (1986) Electrogenic
reduction of the secondary quinone acceptor in chromatophores of
Rhodospirillum rubrum: rapid kinetics measurements, FEBS
Lett., 202, 224-228, doi: 10.1016/0014-5793(86)80691-3.
33.Drachev, L. A., Kaurov, B. S., Mamedov, M. D.,
Mulkidjanian, A. Y., Semenov, A. Y., Shinkarev, V. P., Skulachev, V.
P., and Verkhovsky, M. I. (1989) Flash-induced electrogenic events in
the photosynthetic reaction center and bc1 complexes
of Rhodobacter sphaeroides chromatophores, Biochim. Biophys.
Acta Bioenergetics, 973, 189-197, doi:
10.1016/S0005-2728(89)80421-9.
34.Mamedov, M. D., Mamedova, A. A., Chamorovsky, S.
K., and Semenov, A. Y. (2001) Electrogenic reduction of the primary
electron donor P700 by plastocyanin in photosystem I complexes, FEBS
Lett., 500, 172-176, doi: 10.1016/S0014-5793(01)02615-1.
35.Mamedov, M. D., Gourovskaya, K. N., Vassiliev, I.
R., Golbeck, J. H., and Sememov, A. Y. (1998) Electrogenicity
accompanies photoreduction of the iron-sulfur clusters FA and FB in
photosystem I, FEBS Lett., 431, 219-223, doi:
10.1016/S0014-5793(98)00759-5.
36.Chamorovsky, K., Chamorovsky, S., and Semenov, A.
(2005) Dielectric and photoelectric properties of photosynthetic
reaction centers, Biokhimiya, 70, 315-322, doi:
10.1007/s10541-005-0109-0.
37.Semenov, A. Y., Mamedov, M. D., and Chamorovsky,
S. K. (2006) Electrogenic reactions associated with electron transfer
in photosystem I, Photosystem I: The Light-driven Plastocyanin:
Ferredoxin Oxidoreductase, Springer, p. 319-338, doi:
10.1007/978-1-4020-4256-0_21.
38.Ptushenko, V. V, Cherepanov, D. A., Krishtalik,
L. I., and Semenov, A. Y. (2008) Semi-continuum electrostatic
calculations of redox potentials in photosystem I, Photosynth.
Res., 97, 55-74, doi: 10.1007/s11120-008-9309-y.
39.Krishtalik, L. I. (1989) Dielectric constant in
calculations of the electrostatics of biopolymers, J. Theor.
Biol., 139, 143-154, doi: 10.1016/S0022-5193(89)80097-9.
40.Krishtalik, L. I., Kuznetsov, A. M., and Mertz,
E. L. (1997) Electrostatics of proteins: description in terms of two
dielectric constants simultaneously, Proteins Struct. Funct.
Bioinformatics, 28, 174-182, doi:
10.1002/(SICI)1097-0134(199706)28:2<174::AID-PROT6>3.0.CO;2-F.
41.Brzezinski, P., Okamura, M. Y., and Feher, G.
(1992) Structural changes following the formation of D+
QA– in bacterial reaction centers:
measurement of light-induced electrogenic events in RCs incorporated in
a phospholipid monolayer, The Photosynthetic Bacterial Reaction
Center II: Structure, Spectroscopy and Dynamics, pp. 321-330, doi:
10.1007/978-1-4615-3050-3_36.
42.Sigfridsson, K., Hansson, O., and Brzezinski, P.
(1995) Electrogenic light reactions in photosystem I: resolution of
electron-transfer rates between the iron-sulfur centers, Proc. Natl.
Acad. Sci. USA, 92, 3458-3462, doi:
10.1073/pnas.92.8.3458.
43.Haumann, M., Mulkidjanian, A., and Junge, W.
(1997) Electrogenicity of electron and proton transfer at the oxidizing
side of photosystem II, Biochemistry, 36, 9304-9315, doi:
10.1021/bi963114p.
44.Wikström, M., and Verkhovsky, M. I. (2007)
Mechanism and energetics of proton translocation by the respiratory
heme-copper oxidases, Biochim. Biophys. Acta Bioenergetics,
1767, 1200-1214, doi: 10.1016/j.bbabio.2007.06.008.
45.Beinert, H. (1992) Trails of inquiry and thought
leading toward today’s bioenergetics, Biochim. Biophys. Acta
Bioenergetics, 1101, 125-133, doi:
10.1016/S0005-2728(05)80002-7.