Transcription Factor NF-Y Inhibits Cell Growth and Decreases SOX2 Expression in Human Embryonal Carcinoma Cell Line NT2/D1
M. Mojsin*, V. Topalovic, J. Marjanovic Vicentic, and M. Stevanovic
Institute of Molecular Genetics and Genetic Engineering, University of Belgrade, Vojvode Stepe 444a, 11010 Belgrade, Serbia; fax: (+) 381-11-397-5808; E-mail: mojsin@imgge.bg.ac.rs; vladankatopalovic@imgge.bg.ac.rs; jelenamarjanovic@imgge.bg.ac.rs; milenastevanovic@imgge.bg.ac.rs* To whom correspondence should be addressed.
Received August 1, 2014; Revision received September 22, 2014
Transcription factor NF-Y belongs to the embryonic stem cell transcription factor circuitry due to its role in the regulation of cell proliferation. We investigated the role of NF-Y in pluripotency maintenance using NT2/D1 cells as one of the best-characterized human embryonal carcinoma cell line. We investigated the efficiency of protein transduction and analyzed the effects of forced expression of short isoform of NF-Y A-subunit (NF-YAs) on NT2/D1 cell growth and expression of SOX2. We found that protein transduction is an efficient method for NF-Y overexpression in NT2/D1 cells. Next, we analyzed the effect of NF-YAs overexpression on NT2/D1 cell viability and detected significant reduction in cell growth. The negative effect of NF-YAs overexpression on NT2/D1 cell pluripotency maintenance was confirmed by the decrease in the level of the pluripotency marker SOX2. Finally, we checked the p53 status and determined that the NF-Y-induced inhibition of NT2/D1 cell growth is p53-independent.
KEY WORDS: NF-Y transcription factor, NT2/D1 cell line, cell growth, SOX2, p53DOI: 10.1134/S0006297915020066
The NT2/D1 cell line is one of the best-characterized human embryonal carcinoma cell lines that is widely used in studies of pluripotency maintenance, cell fate determination and differentiation [1, 2]. These cells resemble human embryonal stem cells in gene expression profiles and DNA methylation status [3]. In addition, NT2/D1 cells show neuronal, mesodermal and ectodermal lineage potential [1, 4]. Since work with human embryonic stem cells raises complex ethical and legal issues, embryonal carcinoma cells represent an adequate in vitro model system for the study of human embryonic development [5].
NF-Y (nuclear transcription factor Y) has a dual role as both an activator and a repressor of transcription [6-8]. NF-Y regulates activity of target genes through CCAAT box, a widespread control element mapping to proximal promoters, tissue-specific enhancers, and selected subclasses of human endogenous retrovirus (HERV) long terminal repeats (LTR) [7, 9]. It is heterotrimer protein complex that comprises three subunits (NF-YA, NF-YB and NF-YC) [10]. NF-YA is considered the limiting and regulatory subunit of the trimer, since it is required for complex assembly and sequence-specific DNA binding [10]. There are several isoforms of NF-YA subunit resulting from differential splicing [11]. Tissue-specific alternative splicing produces two major isoforms, NF-YA short (NF-YAs) and NF-YA long (NF-YAl), differing in 28 amino acids in the Q-rich transcriptional activation domain [11]. Recently, it was shown that the short isoform of NF-YA (NF-YAs) is active in mouse embryonic stem cells (mESC), where it promotes proliferation and stemness and prevents differentiation of these cells [12].
Expression of transcription factor SOX2 is one of the hallmarks of embryonal stem cells and induced pluripotent stem cells [13-16]. Its expression is tightly regulated during development, since precise regulation of core transcription factors is essential not only for ESCs pluripotency maintenance but also for restraining their differentiation potential [17]. We have previously shown that NF-Y transcription factor regulates expression of several human SOX genes (SOX2, SOX3, SOX14, and SOX18) in NT2/D1 cells [18-22]. This transcriptional activation function of NF-Y is mediated, at least in part, by direct binding to CCAAT boxes within promoters of target genes and by making complex interplay with other factors involved in transcriptional regulation of human SOX genes [18-24]. To test the role of NF-Y in pluripotency maintenance of human embryonal carcinoma cells, we studied stemness features of NT2/D1 cells in NF-Y overexpression conditions. We used a strategy previously described for human hematopoietic progenitor cells [25] and for mESC [12]. To overcome problems with transient and stable transfections procedures for NF-YA overexpression, a GST-TAT-NF-YAs protein transduction procedure was used, where the TAT epitope enables GST-TAT-NF-YAs fusion protein rapid pass through biological membranes [12, 25].
In this study, we investigated the efficiency of the protein transduction procedure for the overexpression of NF-Y protein in NT2/D1 cells and analyzed the effects of forced expression of short isoform of the NF-YA subunit (NF-YAs) on some of the basic stemness features of human NT2/D1 cells: cell growth and expression of SOX2 pluripotency marker. We have shown that protein transduction is an efficient method for NF-Y overexpression in NT2/D1 cells. Next, we analyzed the effect of NF-YAs overexpression on NT2/D1 cell viability and detected significant reduction in the cell growth. Negative effect of forced NF-YAs expression on NT2/D1 cell pluripotency maintenance was confirmed by the decrease in the level of pluripotency marker SOX2. Finally, we checked the p53 status and detected no increase in p53 protein level after NF-Y protein transduction. However, protein transduction itself led to a significant increase in p53 level, suggesting that NF-Y-induced inhibition of NT2/D1 cell growth is p53-independent.
MATERIALS AND METHODS
Cell culture and protein transduction. NT2/D1 cells, kindly provided by Prof. P. W. Andrews (University of Sheffield, UK), were maintained in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 0.45% (w/v) glucose, 2 mM L-glutamine, and penicillin/streptomycin (all from Invitrogen, USA), at 37°C in 10% CO2 as previously described [2, 26]. For protein transduction treatments, cells were seeded at concentration 0.3·106 cells per well in 6-well plates.
Fusion proteins GST-TAT-NF-YAs and GST-TAT were a kind gift of Prof. Roberto Mantovani (University of Milan, Italy). Protein transduction of NT2/D1 cells was conducted as described previously [12]: cells were treated with 50 nM of the fusion proteins in complete medium for 48, 72 or 96 h with medium change every 24 h. After treatment, cells were counted manually using trypan blue dye exclusion staining.
Western blot. Whole cell lysates were prepared from either control or fusion protein-treated NT2/D1 cells using NP-40 buffer (20 mM Tris-HCl, pH 8.0, 5 mM EDTA, 150 mM NaCl, 1% Nonidet P-40, 10% glycerol and protease inhibitor cocktail) (Roche Diagnostics GmbH, Switzerland). Western blots were performed using anti-NF-YA Mab1a antibodies (a kind gift of Prof. Roberto Mantovani), anti-SOX2 antibodies (R&D Systems, USA), anti-p53 DO1 antibodies (Gene Spin, Italy), anti-α-tubulin DM1A antibodies (Calbiochem, Germany), and anti-GAPDH AM20337PU-S antibodies (Acris Antibodies Inc., Germany).
RESULTS AND DISCUSSION
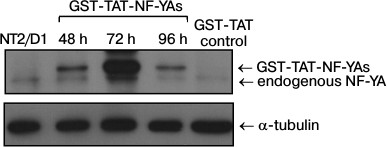
GST-TAT-NF-YAs protein transduction efficiency in NT2/D1 cells. To evaluate the efficiency of the protein transduction method in NT2/D1 cells, we employed a protocol previously used for transduction of GST-TAT-NF-YAs into mouse embryonic stem cells (mESC) [12]. We treated NT2/D1 cells with GST-TAT-NF-YAs and GST-TAT (as negative control) at concentration of 50 nM for different time intervals (48, 72 and 96 h) [12]. After treatment, whole cell lysates were prepared and Western blot analyses were used to assess the amount of intracellular recombinant fusion protein (Fig. 1). Our results showed that GST-TAT-NF-YAs was translocated efficiently in NT2/D1 cells after 48 h of treatment (Fig. 1). A 72 h treatment led to a tremendous increase in the recombinant protein in whole cell lysates (Fig. 1). It is interesting that prolonged treatment (96 h) did not increase further the amount of intracellular GST-TAT-NF-YAs. In contrast to results obtained with mESC, 96 h of transduction decreased the level of fusion protein to the amount seen after 48 h treatment (Fig. 1). These data indicate that protein transduction is an effective method for rapid delivery of GST-TAT-NF-YAs protein in NT2/D1 cells with the maximum increase in concentration of fusion protein achieved after 72-h treatment.
Fig. 1. Western blot analyses of levels of NF-YA proteins after treatments for 48, 72 and 96 h with GST-TAT-NF-YAs at concentration of 50 nM. NT2/D1, untreated cells; GST-TAT control, NT2/D1 cells transduced with 50 nM of control GST-TAT fusion protein for 72 h. α-Tubulin was used as loading control.
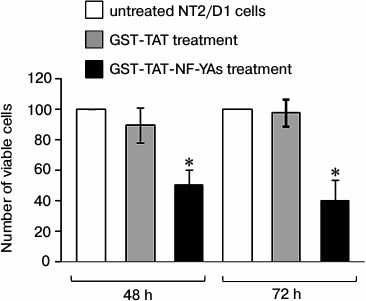
Effects of GST-TAT-NF-YAs fusion protein transduction on viability of NT2/D1 cells. It has been shown previously that the short isoform of NF-YA (NF-YAs) stimulates growth of mESCs and human hematopoietic progenitors [12, 25]. To check the potential effect of GST-TAT-NF-YAs on the viability and proliferation of NT2/D1 cells, we treated cells for 48 and 72 h as described above. Since treatment for 96 h resulted in a reduction of intracellular fusion protein, compared to 72 h treatment, we excluded 96 h transduction from further experiments. The cell viability was measured using trypan blue exclusion, and the resulting numbers of viable cells are presented in Fig. 2. After GST-TAT-NF-YAs protein transduction, growth rates of NT2/D1 cells were significantly reduced: 50% after 48 h and 60% after 72 h treatment. Under the same experimental conditions, control protein GST-TAT did not show the growth inhibition effect. These results are in concordance with the observations of Gurtner et al. that unrestricted NF-Y activity induced E2F1- and wtp53-dependent apoptosis in mouse embryonic fibroblasts and human cells [27]. In contrast, forced expression of NF-Y promotes proliferation and stemness in mESC and human hematopoietic progenitors [12, 25].
Fig. 2. Effect of GST-TAT-NF-YAs on cell growth. NT2/D1 cells were grown in medium containing 50 nM GST-TAT-NF-YAs or GST-TAT (as negative control) for 48 and 72 h. Cells were counted manually using trypan blue exclusion resulting in number of viable cells. Data are presented as percentages of untreated NT2/D1 cells. Values are presented as the means ± S.E.M. of at least three independent experiments, p < 0.05.
To explain this phenomenon and to elucidate the mechanisms involved in NF-Y-induced growth inhibition of NT2/D1 cells, we checked the status of SOX2 and p53 proteins after GST-TAT-NF-YAs transduction.
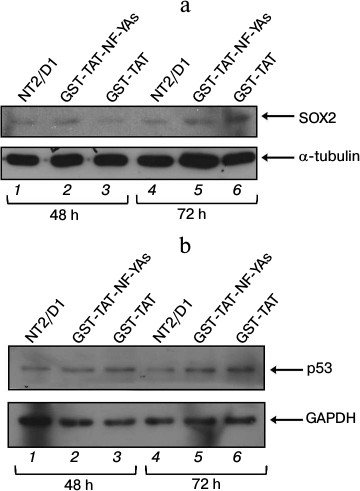
Effects of GST-TAT-NF-YAs transduction on expression of SOX2 and p53 proteins in NT2/D1 cells. Growing evidence suggests the role of NF-Y in the regulation of cell cycle progression in mouse and human embryonic stem cells [12, 28]. In mESC, NF-YAs has an important role in pluripotency maintenance by mechanisms that involve direct activation of stemness genes, including sox2 [12]. In our previous studies, we analyzed the expression of SOX2 protein in NT2/D1 cells transiently transfected with the short isoform of the NF-YA subunit, and detected only mild increase in the level of SOX2 protein [21]. Transient transfection allows only short-term protein overexpression studies due to the short half-life of NF-YA upon transfection. In this study, we took advantage of high concentration of GST-TAT-NF-YAs in NT2/D1 cells upon transduction to investigate the effect of prolonged overexpression of NF-YA (48 and 72 h) on the expression of SOX2. A 48 h treatment only slightly elevated the level of SOX2 in NT2/D1 cells (Fig. 3a, compare lanes 2 and 3), which correlates with the results obtained in our previous studies [21]. In contrast, 72 h treatment led to a marked decrease in SOX2 expression (Fig. 3a, compare lanes 5 and 6). This decline in SOX2 protein level correlates with the high level of intracellular GST-TAT-NF-YAs in NT2/D1 cells (Fig. 1). These data might suggest that deregulation of SOX2, one of the master regulators of pluripotency, is one of the mechanisms involved in NF-YA-induced inhibition of cell growth of NT2/D1 cells. It has been previously shown that elevated expression of SOX2 interferes with transcriptional activity of genes involved in cell cycle arrest and apoptosis in human embryonal stem cells and human embryonal carcinoma cells [29].
Fig. 3. Western blot analysis of SOX2 (a) and p53 (b) proteins expression in NT2/D1 cells after 48 and 72 h treatments with GST-TAT-NF-YAs or with GST-TAT as negative control. GAPDH and α-tubulin were used as loading controls. NT2/D1, untreated cells.
As mentioned above, p53-dependent apoptosis has been described previously as an additional mechanism of NF-YA antiproliferative effect [27]. Since NT2/D1 cell line is wt p53, we checked the level of p53 protein after GST-TAT-NF-YAs transduction (Fig. 3b). Western blot analysis showed that NF-YAs overexpression had no significant effect on p53 level during 48 and 72 h treatments (Fig. 3b, compare lanes 2 and 3 and lanes 5 and 6, respectively). Precisely, GST-TAT-NF-YAs transduction increased the level of p53 protein, but the same effect was observed in transductions with control GST-TAT protein at both time points (Fig. 3b, lanes 2 and 3 versus lane 1 for 48 h; lanes 5 and 6 versus lane 4 for 72 h). These data suggest that the increase in p53 level is not the result of NF-Y overexpression, but it is rather the effect of GST-TAT fusion protein or the procedure of protein transduction itself. At the same time, transduction with control GST-TAT protein did not affect viability and cell growth of NT2/D1 cells (Fig. 2). These results suggest that in NT2/D1 cells growth inhibition after NF-Y overexpression is either p53-independent, or it requires increased expression of both NF-YA and p53 proteins. It has been shown that NF-Y and p53 could function as partners in regulation of cell proliferation (for review, see [30]). Typically, p53 regulates transcription of target genes through binding to the consensus DNA sequence, p53RE (p53-responsive element) [31-37]. It has been shown that in the genes lacking p53RE, p53 can associate with NF-Y on CCAAT box elements and regulate transcription of important proapoptotic genes [38].
In conclusion, our results suggest that in human embryonal carcinoma cells NT2/D1, GST-TAT-NF-YAs transduction inhibits proliferation through a mechanism dependent on downregulation of stemness factor SOX2. Our data are in contradiction with results obtained on mESC and human hematopoietic progenitors [12, 25]. These discrepancies probably reflect cellular and developmental specificity of NF-Y action. This specificity is established through multiple mechanisms. NF-Y directly binds CCAAT box and regulates activity of target genes [7]. Another important mechanism involves recruitment of different cofactors and direct interactions of NF-Y with other transcription factors [39]. Also, histone-like properties of NF-Y enable its dual function in transcriptional regulation, as an activator or as a repressor [6].
In addition, posttranslational modifications of NF-Y add another level of complexity to NF-Y action by controlling the amount of NF-Y in the cell [27]. It was suggested previously that the total amount of NF-Y could define cell choice between proliferation, cell cycle arrest, or cell death [27]. NF-Y plays one of the central roles in regulation of cell cycle progression genes, such as cyclin A, cyclin B1, cyclin B2, cdc25A, cdc25C, cdk1, and E2F1 [40-47]. Precise activation of these genes determines the cell fate decision: cell division or cell death [48]. Activation of these genes is also highly dependent on cell type, genetic background and cellular environment [48].
Since role of NF-Y in the apoptotic cascade is not fully understood, the mechanisms underlying NF-Y induction of NT2/D1 cell growth inhibition and SOX2 protein decrease need to be further investigated.
We acknowledge Prof. Paul Andrews for kind gift of NT2/D1 cells. We thank Prof. Roberto Mantovani and GeneSpin (Milano, Italy) for generously providing anti-p53 antibody. We also thank Prof. Roberto Mantovani for his valuable input.
This work was supported by the Ministry of Education, Science, and Technological Development, Republic of Serbia (Grant No. 173051).
REFERENCES
1.Andrews, P. W. (2002) From teratocarcinomas to
embryonic stem cells, Philos. Trans. R. Soc. Lond. B. Biol.
Sci., 357, 405-417.
2.Andrews, P. W., Damjanov, I., Simon, D., Banting,
G. S., Carlin, C., Dracopoli, N. C., and Fogh, J. (1984) Pluripotent
embryonal carcinoma clones derived from the human teratocarcinoma cell
line Tera-2. Differentiation in vivo and in vitro,
Lab. Invest., 50, 147-162.
3.Bocker, M. T., Tuorto, F., Raddatz, G., Musch, T.,
Yang, F. C., Xu, M., Lyko, F., and Breiling, A. (2012) Hydroxylation of
5-methylcytosine by TET2 maintains the active state of the mammalian
HOXA cluster, Nat. Commun., 3, 818.
4.Pal, R., and Ravindran, G. (2006) Assessment of
pluripotency and multilineage differentiation potential of NTERA-2
cells as a model for studying human embryonic stem cells, Cell
Prolif., 39, 585-598.
5.Przyborski, S. A., Christie, V. B., Hayman, M. W.,
Stewart, R., and Horrocks, G. M. (2004) Human embryonal carcinoma stem
cells: models of embryonic development in humans, Stem Cells
Devel., 13, 400-408.
6.Ceribelli, M., Dolfini, D., Merico, D., Gatta, R.,
Vigano, A. M., Pavesi, G., and Mantovani, R. (2008) The histone-like
NF-Y is a bifunctional transcription factor, Mol. Cell Biol.,
28, 2047-2058.
7.Dolfini, D., Zambelli, F., Pavesi, G., and
Mantovani, R. (2009) A perspective of promoter architecture from the
CCAAT box, Cell Cycle, 8, 4127-4137.
8.Peng, Y., and Jahroudi, N. (2002) The NFY
transcription factor functions as a repressor and activator of the von
Willebrand factor promoter, Blood, 99, 2408-2417.
9.Fleming, J. D., Pavesi, G., Benatti, P., Imbriano,
C., Mantovani, R., and Struhl, K. (2013) NF-Y coassociates with FOS at
promoters, enhancers, repetitive elements, and inactive chromatin
regions, and is stereo-positioned with growth-controlling transcription
factors, Genome Res., 23, 1195-1209.
10.Romier, C., Cocchiarella, F., Mantovani, R., and
Moras, D. (2003) The NF-YB/NF-YC structure gives insight into DNA
binding and transcription regulation by CCAAT factor NF-Y, J. Biol.
Chem., 278, 1336-1345.
11.Li, X. Y., Hooft van Huijsduijnen, R., Mantovani,
R., Benoist, C., and Mathis, D. (1992) Intron-exon organization of the
NF-Y genes. Tissue-specific splicing modifies an activation domain,
J. Biol. Chem., 267, 8984-8990.
12.Dolfini, D., Minuzzo, M., Pavesi, G., and
Mantovani, R. (2012) The short isoform of NF-YA belongs to the
embryonic stem cell transcription factor circuitry, Stem Cells,
30, 2450-2459.
13.Sekido, R., and Lovell-Badge, R. (2009) Sex
determination and SRY: down to a wink and a nudge? Trends
Genet., 25, 19-29.
14.Takahashi, K., Tanabe, K., Ohnuki, M., Narita,
M., Ichisaka, T., Tomoda, K., and Yamanaka, S. (2007) Induction of
pluripotent stem cells from adult human fibroblasts by defined factors,
Cell, 131, 861-872.
15.Takahashi, K., and Yamanaka, S. (2006) Induction
of pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors, Cell, 126, 663-676.
16.Wegner, M. (2010) All purpose Sox: the many roles
of Sox proteins in gene expression, Int. J. Biochem. Cell Biol.,
42, 381-390.
17.Rizzino, A. (2013) Concise review: the Sox2-Oct4
connection: critical players in a much larger interdependent network
integrated at multiple levels, Stem Cells, 31,
1033-1039.
18.Djurovic, J., and Stevanovic, M. (2004)
Structural and functional characterization of the human SOX14 promoter,
Biochim. Biophys. Acta, 1680, 53-59.
19.Kovacevic Grujicic, N., Mojsin, M., Krstic, A.,
and Stevanovic, M. (2005) Functional characterization of the human SOX3
promoter: identification of transcription factors implicated in basal
promoter activity, Gene, 344, 287-297.
20.Krstic, A., Mojsin, M., and Stevanovic, M. (2007)
Regulation of SOX3 gene expression is driven by multiple NF-Y binding
elements, Arch. Biochem. Biophys., 467, 163-173.
21.Milivojevic, M., Nikcevic, G.,
Kovacevic-Grujicic, N., Krstic, A., Mojsin, M., Drakulic, D., and
Stevanovic, M. (2010) Involvement of ubiquitous and TALE transcription
factors, as well as liganded RXRA in the regulation of human SOX2 gene
expression in NT2/D1 embryonal carcinoma cell line, Arch. Biol. Sci.
(Belgrade), 62, 199-210.
22.Petrovic, I., Kovacevic-Grujicic, N., and
Stevanovic, M. (2009) ZBP-89 and Sp3 down-regulate while NF-Y
up-regulates SOX18 promoter activity in HeLa cells, Mol. Biol.
Rep., 36, 993-1000.
23.Kovacevic Grujicic, N., Yokoyama, K., and
Stevanovic, M. (2008) Trans-activation of the human SOX3 promoter by
MAZ in NT2/D1 cells, Arch. Biol. Sci., 60, 379-387.
24.Petrovic, I., Kovacevic-Grujicic, N., Popovic,
J., Krstic, A., Milivojevic, M., and Stevanovic, M. (2011) Members of
the CREB/ATF and AP1 family of transcription factors are involved in
the regulation of SOX18 gene expression, Arch. Biol. Sci.,
63, 517-525.
25.Domashenko, A. D., Danet-Desnoyers, G., Aron, A.,
Carroll, M. P., and Emerson, S. G. (2010) TAT-mediated transduction of
NF-Ya peptide induces the ex vivo proliferation and engraftment
potential of human hematopoietic progenitor cells, Blood,
116, 2676-2683.
26.Andrews, P. W. (1998) Teratocarcinomas and human
embryology: pluripotent human EC cell lines. Review article,
APMIS, 106, 158-167; discussion 167-158.
27.Gurtner, A., Fuschi, P., Martelli, F., Manni, I.,
Artuso, S., Simonte, G., Ambrosino, V., Antonini, A., Folgiero, V.,
Falcioni, R., Sacchi, A., and Piaggio, G. (2010) Transcription factor
NF-Y induces apoptosis in cells expressing wild-type p53 through E2F1
upregulation and p53 activation, Cancer Res., 70,
9711-9720.
28.Grskovic, M., Chaivorapol, C., Gaspar-Maia, A.,
Li, H., and Ramalho-Santos, M. (2007) Systematic identification of
cis-regulatory sequences active in mouse and human embryonic stem
cells, PLoS Genet., 3, e145.
29.Greber, B., Lehrach, H., and Adjaye, J. (2007)
Silencing of core transcription factors in human EC cells highlights
the importance of autocrine FGF signaling for self-renewal, BMC
Devel. Biol., 7, 46.
30.Imbriano, C., Gnesutta, N., and Mantovani, R.
(2012) The NF-Y/p53 liaison: well beyond repression, Biochim.
Biophys. Acta, 1825, 131-139.
31.Bourdon, J. C., Deguin-Chambon, V., Lelong, J.
C., Dessen, P., May, P., Debuire, B., and May, E. (1997) Further
characterization of the p53 responsive element – identification
of new candidate genes for trans-activation by p53, Oncogene,
14, 85-94.
32.El-Deiry, W. S. (1998) Regulation of p53
downstream genes, Semin. Cancer Biol., 8, 345-357.
33.El-Deiry, W. S., Kern, S. E., Pietenpol, J. A.,
Kinzler, K. W., and Vogelstein, B. (1992) Definition of a consensus
binding site for p53, Nat. Genet., 1, 45-49.
34.Funk, W. D., Pak, D. T., Karas, R. H., Wright, W.
E., and Shay, J. W. (1992) A transcriptionally active DNA-binding site
for human p53 protein complexes, Mol. Cell Biol., 12,
2866-2871.
35.Ko, L. J., and Prives, C. (1996) p53: puzzle and
paradigm, Genes Devel., 10, 1054-1072.
36.Menendez, D., Inga, A., and Resnick, M. A. (2009)
The expanding universe of p53 targets, Nat. Rev. Cancer,
9, 724-737.
37.Willis, A. C., and Chen, X. (2002) The promise
and obstacle of p53 as a cancer therapeutic agent, Curr. Mol.
Med., 2, 329-345.
38.Imbriano, C., Gurtner, A., Cocchiarella, F., Di
Agostino, S., Basile, V., Gostissa, M., Dobbelstein, M., Del Sal, G.,
Piaggio, G., and Mantovani, R. (2005) Direct p53 transcriptional
repression: in vivo analysis of CCAAT-containing G2/M promoters,
Mol. Cell Biol., 25, 3737-3751.
39.Su, M., Bansal, A. K., Mantovani, R., and Sodek,
J. (2005) Recruitment of nuclear factor Y to the inverted CCAAT element
(ICE) by c-Jun and E1A stimulates basal transcription of the bone
sialoprotein gene in osteosarcoma cells, J. Biol. Chem.,
280, 38365-38375.
40.Bolognese, F., Wasner, M., Dohna, C. L., Gurtner,
A., Ronchi, A., Muller, H., Manni, I., Mossner, J., Piaggio, G.,
Mantovani, R., and Engeland, K. (1999) The cyclin B2 promoter depends
on NF-Y, a trimer whose CCAAT-binding activity is cell-cycle regulated,
Oncogene, 18, 1845-1853.
41.Di Agostino, S., Strano, S., Emiliozzi, V.,
Zerbini, V., Mottolese, M., Sacchi, A., Blandino, G., and Piaggio, G.
(2006) Gain of function of mutant p53: the mutant p53/NF-Y protein
complex reveals an aberrant transcriptional mechanism of cell cycle
regulation, Cancer Cell, 10, 191-202.
42.Farina, A., Manni, I., Fontemaggi, G., Tiainen,
M., Cenciarelli, C., Bellorini, M., Mantovani, R., Sacchi, A., and
Piaggio, G. (1999) Down-regulation of cyclin B1 gene transcription in
terminally differentiated skeletal muscle cells is associated with loss
of functional CCAAT-binding NF-Y complex, Oncogene, 18,
2818-2827.
43.Gurtner, A., Fuschi, P., Magi, F., Colussi, C.,
Gaetano, C., Dobbelstein, M., Sacchi, A., and Piaggio, G. (2008) NF-Y
dependent epigenetic modifications discriminate between proliferating
and postmitotic tissue, PLoS One, 3, e2047.
44.Gurtner, A., Manni, I., Fuschi, P., Mantovani,
R., Guadagni, F., Sacchi, A., and Piaggio, G. (2003) Requirement for
down-regulation of the CCAAT-binding activity of the NF-Y transcription
factor during skeletal muscle differentiation, Mol. Biol. Cell,
14, 2706-2715.
45.Korner, K., Jerome, V., Schmidt, T., and Muller,
R. (2001) Cell cycle regulation of the murine cdc25B promoter:
essential role for nuclear factor-Y and a proximal repressor element,
J. Biol. Chem., 276, 9662-9669.
46.Linhart, C., Elkon, R., Shiloh, Y., and Shamir,
R. (2005) Deciphering transcriptional regulatory elements that encode
specific cell cycle phasing by comparative genomics analysis, Cell
Cycle, 4, 1788-1797.
47.Zwicker, J., Lucibello, F. C., Wolfraim, L. A.,
Gross, C., Truss, M., Engeland, K., and Muller, R. (1995) Cell cycle
regulation of the cyclin A, cdc25C and cdc2 genes is based on a common
mechanism of transcriptional repression, EMBO J., 14,
4514-4522.
48.Vermeulen, K., Berneman, Z. N., and Van
Bockstaele, D. R. (2003) Cell cycle and apoptosis, Cell Prolif.,
36, 165-175.