Bioactive Amide of Prostaglandin E1 and Ethanolamine Plasmalogen Analog of Platelet-Activating Factor Inhibits Several Pathways of Human Platelet Aggregation
V. I. Kulikov* and G. I. Muzya
Research and Development Center for Medical Biotechnology, Ministry of Public Health of the Russian Federation, ul. Shchukinskaya 6, Moscow, 123182 Russia; fax: (095) 190-0100* To whom correspondence should be addressed.
Received September 15, 1999; Revision received November 9, 1999
The influence of an amide of prostaglandin E1 and ethanolamine plasmalogen platelet-activating factor analog 1-O-alk-1´-enyl-2-acetyl-sn-glycero-3-phospho-(N-11alpha,15alpha-dioxy-9-keto-13-prostenoyl)ethanolamine (PGE1-PPAF) on platelet-activating factor (PAF)-, ADP-, and thrombin-induced human platelet aggregation has been studied. It was found that PGE1-PPAF inhibits the PAF-, ADP-, and thrombin-induced platelet aggregation in platelet-rich plasma. 1-O-alk-1´-enyl-2-acetyl-sn-glycero-3-phosphoethanolamine inhibited PAF-induced aggregation up to 50% but had no influence on platelet aggregation induced by ADP or thrombin. The ethanolamine plasmalogen analog of PAF 1-O-alk-1´-enyl-2-acetyl-sn-glycero-3-phospho-(N-palmitoyl)ethanolamine, having a palmitoyl residue instead of PGE1, did not inhibit platelet aggregation induced by PAF, ADP, or thrombin. We propose that inhibition of human platelet aggregation by PGE1-PPAF is mediated by its action on platelet PAF-receptors and the adenylate cyclase system.
KEY WORDS: platelet-activating factor, amide of prostaglandin E1 and a PAF analog, prostaglandin E1, platelets
Abbreviations: PAF) platelet-activating factor (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine); PGE1) prostaglandin E1; PGE1-PPAF) 1-O-alk-1´-enyl-2-acetyl-sn-glycero-3-phospho-(N-11alpha,15alpha-dioxy-9-keto-13-prostenoyl)ethanolamine.
Ethanolamides of fatty acids are a new group of lipid bioregulators that
are considered as endogenous ligands of cannabinoid receptors of the
central nervous system and peripheral tissues [1].
One of the well-studied fatty acid ethanolamides is anandamide--the
ethanolamide of arachidonic acid [1]. Cell
cyclooxygenase-2 can synthesize the ethanolamide of prostaglandin
E2 from anandamide, and 12- or 15-lipoxygenase can
synthesize the 12- or 15-hydroxy derivatives of anandamide; these have
a different spectrum of biological activity than anandamide [2].
The hydrolysis of N-acylated phosphatidylethanolamines is one of the pathways of biosynthesis of fatty acid ethanolamides [1]. N-Acylphosphatidylethanolamines have been identified in a number of cells and tissues [3] and obtained by chemical synthesis [4]. Previously, the phospholipid derivatives of prostaglandins having prostaglandins joined by an amide bond with the ethanolamine residue of phosphatidylethanolamine were obtained [5]. These phospholipid derivatives of prostaglandins revealed a broad spectrum of biological activity, more stable against inactivation in plasma and tissues than prostaglandins, and are of interest as precursors of bioactive prostaglandin ethanolamides.
In this work, we studied the influence of prostaglandin E1 and an ethanolamine plasmalogen analog of PAF on human platelet aggregation induced by PAF, ADP, or thrombin. We chose this compound for our study for the following reasons. Under physiological conditions in the bloodstream, platelet activation is not induced by a single agent, but it is the result of the action of a combination of different proaggregatory agents. A great number of proaggregatory agents operate by three general mechanisms involving ADP, thromboxane A2, and PAF [6]. The selective or simultaneous action of these three general aggregation mechanisms give rise to the possibility of direct regulation of the functional activity of platelets. It was shown previously that a plasmalogenic analog of PAF (1-O-alk-1´-enyl-2-acetyl-sn-glycero-3-phosphocholine) binds with PAF-receptors, induces platelet desensitization to PAF, inhibits PAF-induced platelet desensitization to PAF, and inhibits PAF-induced platelet aggregation, but it has no effect on ADP- or thrombin-induced aggregation [7]. In contrast to PAF, the plasmalogenic analog of PAF increases [3H]PGE1 binding with human platelets [7].
It is known that PGE1 and prostacyclin activate platelet adenylate cyclase by a receptor-dependent route that increases platelet cAMP level and inhibits platelet aggregation, and this effect can be increase by phosphodiesterase inhibitors [8]. Since the plasmalogenic analog of PAF (1-O-alk-1´-enyl-2-acetyl-sn-glycero-3-phosphocholine) blocks PAF-receptors and the PAF-dependent aggregation pathway, and PGE1 inhibits platelet aggregation due to adenylate cyclase activation, we combine these two functions in the PGE1-PPAF compound that is aimed at simultaneous action on two platelet receptor systems and inhibition of platelet aggregation induced by different proaggregatory agents.
MATERIALS AND METHODS
PGE1 and thrombin from Sigma (USA) and ADP from Reanal (Hungary) were used is this study. PAF was obtained from beef heart choline plasmalogens by a classic method [9].
1-O-alk-1´-enyl-2-acetyl-sn-glycero-3-phosphoethanolamine (compound 1) was obtained by phospholipase D-catalyzed transesterification of 1-O-alk-1´-enyl-2-acetyl-sn-glycero-3-phosphocholine in the presence of ethanolamine as described previously [4].
1-O-alk-1´-enyl-2-acetyl-sn-glycero-3-phospho-(N-palmitoyl)ethanolamine (compound 2) was obtained by treatment of compound 1 with palmitic anhydride in benzene and purified by column chromatography on silica gel as described previously [4].
The amide of PGE1 and the ethanolamine plasmalogen analog of PAF of the chemical structure
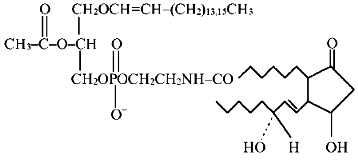
was synthesized by condensation of compound 1 (44 µmoles) with PGE1 (8.5 µmoles) and N,N-dicyclohexylcarbodiimide (29 µmoles) in the presence of chloroform (2 ml) and argon at room temperature for 20 h. After termination of the reaction, the mixture was purified by TLC using the solvent system chloroform-methanol (9:1 v/v).
The physicochemical characteristics of PGE1-PPAF are: Rf = 0.13 in CHCl3O-CH3OH-AcOH-H2O (130:12:4:2 v/v); IR-spectrum (numax, cm-1): 3450, 3050, 2825, 2860, 1740, 1650, 1550, 1470, 1240, 1065; UV-spectrum (lambdamax, nm (epsilon) in CH3OH): 275.6 (3..·104). The ratio PGE1/Pi (mole per g-atom) was 1.1 ± 0.1. The PGE1 content in the PGE1-PPAF was determined as described previously [10]. Lipid phosphorus was analyzed as described by Vaskovsky et al. [11].
Platelets were isolated from blood of healthy donors with 3.8% trisodium citrate solution as anticoagulant (blood/anticoagulant ratio 9:1). Platelet-rich plasma was obtained by centrifugation of blood samples at 200g for 15 min. PAF-, ADP-, or thrombin-induced platelet aggregation was measured turbidimetrically in platelet-rich plasma at 520 nm as described previously [12].
Results given in Tables 1 and 2 are the means of three parallel measurements ± the standard error of the means. Similar results were obtained in 4-5 independent experiments.
RESULTS AND DISCUSSION
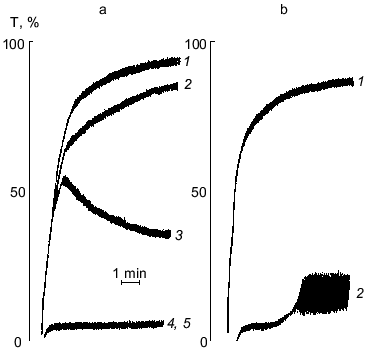
The results of the study on the influence of PGE1-PPAF on PAF-, ADP-, or thrombin-induced platelet aggregation are presented in Table 1. Pretreatment of platelets by PGE1-PPAF (10-6 M) results in a complete inhibition of PAF-, ADP-, or thrombin-induced platelet aggregation. To determine whether these effects depend on the PGE1 residue, we compare the influence of compound 2 having a palmitic acid residue instead of PGE1 and compound 1, an ethanolamine plasmalogen PAF analog having a free amino group, on platelet aggregation. The data in Table 1 show that compounds 1 and 2 inhibit PAF-induced aggregation to some extent but has no influence on ADP- or thrombin-induced aggregation. The data on the dependence of inhibition of platelet aggregation on the concentrations of PGE1-PPAF are presented in Table 2. The data show that PGE1-PPAF inhibits PAF-induced aggregation at concentrations as low as 10-9 M, and 100%-inhibition was observed at 10-7-10-6 M PGE1-PPAF. The curves of PAF-induced platelet aggregation are presented in the figure. The data in the figure show that PGE1-PPAF at concentration of 10-8 M significantly decreases the extent of platelet aggregation; this represents a reverse aggregation. At PGE1-PPAF concentrations of 10-7 and 10-6 M there is complete inhibition of the platelet aggregation, and the first wave of aggregation is not observed. PGE1-PPAF has a less inhibitory effect on the ADP-induced aggregation than on the PAF-induced aggregation, and 100%-inhibition is observed at 10-6 M PGE1-PPAF (Table 2). Inhibition of the thrombin-induced aggregation by PGE1-PPAF revealed some peculiarities (see the figure). There are no first waves of aggregation and formation of platelet aggregates in the presence of PGE1-PPAF, and after a definite time (3-4 min) a sharp uneven change in light transmission is observed, indicating the formation of a jelly-like clot in the medium. The data show that PGE1-PPAF has an inhibitory effect on PAF-, ADP-, and thrombin-induced platelet aggregation. What are the reasons for these effects?
Table 1. Influence of PGE1-PPAF,
1-O-alk-1´-enyl-2-acetyl-sn-glycero-3-phosphoethanolamine
(compound 1), and
1-O-alk-1´-enyl-2-acetyl-sn-glycero-3-phospho-(N-palmitoyl)ethanolamine
(compound 2) on the human platelet aggregation
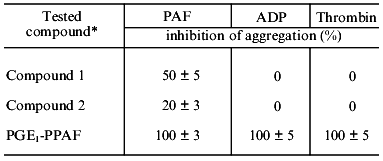
*The concentration of the tested compounds was 10-6 M;
the concentrations of the proaggregatory agents were: PAF,
10-6 M; ADP, 10-5 M; thrombin, 0.1 U/ml.
Table 2. Inhibition of PAF- and ADP-induced
aggregation of human platelets by PGE1-PPAF in platelet-rich
plasma
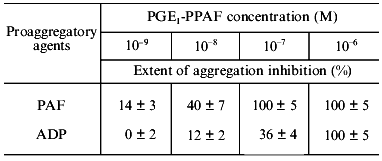
It is obvious that inhibition of PAF-induced platelet aggregation by PGE1-PPAF depends on the presence of the PGE1 residue since compound 1 lacking PGE1 and compound 2 having a palmitic acid residue instead of PGE1 are not able to provide 100%-inhibition of platelet aggregation. The inhibitory effect of PGE1-PPAF has clear concentration dependence. It was shown previously that PGE1-stimulated cAMP formation in platelets occurs in parallel with the increase in specific binding of PGE1 to platelets. The maximal cAMP formation in platelets was observed as the level of specific PGE1 binding reached 0.5-1 µM [13]. These data suggest that PGE1-PPAF can act on the platelets in at least two ways: 1) to block PAF-receptors and PAF-mediated platelet activation; 2) PGE1-PPAF binds with PGE1-receptors, resulting in adenylate cyclase activation and subsequent inhibition of platelet aggregation.Figure. Influence of PGE1-PPAF on PAF- or thrombin-induced platelet aggregation. a) PAF-induced aggregation: 1) platelet aggregation induced by 10-6 M PAF; 2-5)platelet aggregation after pretreatment (4-5 min) of platelets with PGE1-PPAF (concentrations of PGE1-PPAF in the medium: 10-9 M (2), 10-8 M (3), 10-7 M (4), or 10-6 M (5)). b) Thrombin-induced aggregation: 1) platelet aggregation induced by thrombin (0.1 U/ml); 2) platelet aggregation after pretreatment of platelets with PGE1-PPAF followed by addition of thrombin (0.1 U/ml). Ordinate, light transmission, T (%); abscissa, time (min).
ADP as well as the other proaggregatory agents induce platelet activation that involves platelet shape changes, exposure of fibrinogen receptors, aggregation, and inhibition of PGE1- or prostacyclin-stimulated adenylate cyclase [14]. It is believed that ADP-induced platelet activation and adenylate cyclase inhibition may be relatively independent events and mediated by different ADP-receptors [14]. However, changes in platelet shape and aggregation were observed at ADP concentration of 0.1-0.5 and 1-5 µM, respectively, whereas half-maximal inhibition of adenylate cyclase was observed at ADP concentration 3 µM [14]. According to our data, complete inhibition of ADP-induced aggregation is observed at 1 µM PGE1-PPAF, closely corresponding to the concentration range of ADP where inhibition of adenylate cyclase was observed. Since the synthesis of endogenous PAF is not followed by ADP-induced platelet aggregation, it may be assumed that the inhibition of ADP-induced platelet aggregation by PGE1-PPAF depends on adenylate cyclase activation and increasing platelet cAMP level.
It is known that thrombin at very low concentrations induces a reverse platelet aggregation, and at higher concentrations (0.1-1 U/ml) thrombin induces a classical two-phase aggregation followed by a reverse reaction in the course of the second phase [14]. Thrombin-induced platelet activation begins with its binding to high-affinity receptors and is followed by the inhibition of platelet adenylate cyclase [14]. In our study, we found the inhibition of the first and second phase of thrombin-induced aggregation by PGE1-PPAF and observe the formation of a jelly-like fibrin clot. The reason for the formation of a fibrin clot in the absence of aggregation is unclear, but this effect appears to be linked to stimulation of the release reaction in platelets under these conditions.
Are there some other mechanisms of inhibition of platelet aggregation by PGE1-PPAF? The formation of PGE1 ethanolamide from the PGE1-PPAF in the incubation medium is an alternative mechanism. Recently the inhibition of adrenaline- or arachidonic acid-induced platelet aggregation by arachidonic acid amides with dopamine, histamine, or serotonin was described [1]. It has been shown previously that amide PGE2 with phosphatidylethanolamine slowly hydrolyzed in human plasma, and for 10-min incubation no more than 2% of PGE2 release was observed [5]. For this reason, under our experimental conditions the possibility of PGE1-PPAF hydrolysis with free PGE1 release, where all inhibitory effect is observed by 2-5 min after addition of PGE1-PPAF to platelet-rich plasma, seems unlikely. The formation of PGE1 ethanolamide as the result of the action of membrane-bound phospholipase D on PGE1-PPAF appears possible. There is a thrombin-stimulated platelet phospholipase D, and its maximal activation is observed for 10 min after the action of thrombin on platelets [15].
The activity of ethanolamides of prostaglandins with respect to adenylate cyclase is unknown, whereas the ethanolamides of polyunsaturated fatly acids inhibit adenylate cyclase of various cells [1]. Thus, the data presented above show that inhibition of platelet aggregation by PGE1-PPAF could be accomplished by several mechanisms, including its action on the PGE1- and PAF-receptors as well as the formation of ethanolamide of PGE1. The data presented suggest the possibility of simultaneous action on the platelet receptor system (PAF-receptor)-mediated platelet activation and the receptor system (adenylate cyclase) that takes part in the inhibition of platelet activation.
REFERENCES
1.Bezuglov, V. V., Bobrov, M. Yu., and Archakov, A.
V. (1998) Biochemistry (Moscow), 63,22-27.
2.Ueda, N., Yamamoto, K., Yamamoto, S., Tokunaga, T.,
Shirakawa, E., Shinkai, H., Ogawa, M., Sato, T., Kudo, I., and Inoue,
K. (1995) Biochim. Biophys. Acta,1254,127-134.
3.Natarajan, V., Reddy, P. V., Schmid, P. C., and
Schmid, H. H. O. (1981) Biochim. Biophys.
Acta,664,445-448.
4.Kuznetsova, T. I., and Kulikov, V. I. (1992)
Biokhimiya,57,16-20.
5.Lekim, D., Stieck, A., Betzing, H., and Kunze, H.
(1978) Advances in Prostaglandin and Thromboxane
Research,3,193-198.
6.Vargaftig, B. B., Chignard, M., and Benveniste, J.
(1981) Biochem. Pharmacol.,30,263-271.
7.Kulikov, V. I., and Muzya, G. I. (1999)
Biochemistry (Moscow),64,631-635.
8.Harby, J. H. (1999) J. Biol.
Chem.,274,7599-7602.
9.Demopoulos, C. A., Pinckard, R. N., and Hanahan, D.
J. (1979) J. Biol. Chem.,254,9355-9358.
10.Bergelson, L. D., Dyatlovitskaya, E. V.,
Molotkovsky, Yu. G., Batrakov, S. G., Barsukov, L. I., and Prokazova,
N. V. (1981) Preparative Lipid Biochemistry [in Russian], Nauka,
Moscow, p. 88.
11.Vaskovsky, V. E., Kostetsky, E. Y., and Vasendin,
I. M. (1975) J. Chromatogr.,114,129-141.
12.Muzya, G. I., and Kulikov, V. I. (1996)
Biochemistry (Moscow),61,337-340.
13.Kahn, N. N., and Sinha, A. K. (1988) Biochim.
Biophys. Acta,972,45-53.
14.Siess, W. (1989) Physiol.
Rev.,69,58-178.
15.Rubin, R. (1988) Biochem. Biophys. Res.
Commun.,156,1090-1096.