REVIEW: Warburg Effect Revisited: Embodiment of Classical Biochemistry and Organic Chemistry. Current State and Prospects
Leonid G. Menchikov1, Alexander A. Shestov2, and Anatoliy V. Popov3,a*
1N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 119991 Moscow, Russian Federation2University of Pennsylvania, Department of Pathology and Laboratory Medicine, 3400 Civic Center Blvd, Perelman Center for Advanced Medicine, Philadelphia, PA 19104, USA
3University of Pennsylvania, Department of Radiology, 3620 Hamilton Walk Anatomy Chemistry Building, Rm317, Philadelphia, PA 19104, USA
* To whom correspondence should be addressed.
Received August 16, 2022; Revised September 23, 2022; Accepted October 2, 2022
The Nobel Prize Winner (1931) Dr. Otto H. Warburg had established that the primary energy source of the cancer cell is aerobic glycolysis (the Warburg effect). He also postulated the hypothesis about “the prime cause of cancer”, which is a matter of debate nowadays. Contrary to the hypothesis, his discovery was recognized entirely. However, the discovery had almost vanished in the heat of battle about the hypothesis. The prime cause of cancer is essential for the prevention and diagnosis, yet the effects that influence tumor growth are more important for cancer treatment. Due to the Warburg effect, a large amount of data has been accumulated on biochemical changes in the cell and the organism as a whole. Due to the Warburg effect, the recovery of normal biochemistry and oxygen respiration and the restoration of the work of mitochondria of cancer cells can inhibit tumor growth and lead to remission. Here, we review the current knowledge on the inhibition of abnormal glycolysis, neutralization of its consequences, and normalization of biochemical parameters, as well as recovery of oxygen respiration of a cancer cell and mitochondrial function from the point of view of classical biochemistry and organic chemistry.
KEY WORDS: the Warburg effect, oncology, glycolysis, biochemistry, oxygen respiration, mitochondriaDOI: 10.1134/S0006297923140018
Abbreviations: LDH, lactate dehydrogenase; pHe, extracellular pH; pHi, intracellular pH.
INTRODUCTION
A hundred years ago, Otto Heinrich Warburg (Fig. 1), the Nobel Prize winner of 1931, found [1-7] that the main energy source in cancer cells is “aerobic glycolysis”, i.e., glycolysis in the presence of oxygen (the Warburg effect), that was in a sharp contrast to the Pasteur effect when the glycolysis rate abruptly decreases in the presence of oxygen. According to the opinion of Warburg, the key to the cancer treatment was in inhibition of glycolysis, recovery of oxygen respiration of cancer cells, and the normalization of biochemistry. Unfortunately, in 1920s the science did not know how to do this.
Fig. 1. Otto Heinrich Warburg. Portrait by Elena Polkh. Published with permission from L. G. Menchikov.
Based on his discoveries, O. Warburg also formulated the hypothesis that “the prime cause of cancer is the replacement of the respiration of oxygen (sugar oxidation) in normal body cells by a fermentation of sugar” [8]. According to the Warburg’s hypothesis, an irreversible damage of the respiratory function of mitochondria leads to tumor transformation of the cell and activation of glycolysis. During many years, the Warburg’s theory about the prime cause of cancer induced discussions of the scientists. Genetic theories of carcinogenesis became generally accepted [9-11], whereas the discovery of Warburg was believed to be a consequence, but not the prime cause of cancer, especially considering that the Warburg effect was not observed in some cancer cells. However, the Warburg hypothesis up to now attracts attention of the researchers, and the number of new scientific confirmations and interpretations is increasing [12-20].
Contrary to the hypothesis, the Warburg discovery has been accepted completely. Thus, the positron-emission tomography with fluorodeoxyglucose (18-FDG PET) developed in the University of Pennsylvania (USA) [21] and widely used in clinical diagnostics of cancer is based just on his discovery.
Nevertheless, the Warburg discovery was nearly forgotten among the battles about his hypothesis, and for a long time the studies on cancer metabolism progressed slowly, whereas the main attention of researchers was directed to the increased activity of oncogenes and also to inactivation of the genes onco-suppressors. Thus, in the visionary article “The Hallmarks of Cancer” issued in 2000 “the necessary and sufficient” functional features of a tumor cell were determined, and no place among them was found for the Warburg effect [22]. Later association between oncogenes and the Warburg effect was proven [19]. In the revised version of “The Hallmarks of Cancer” article issued in 2011, the metabolic reprogramming of the cancer cell energetics was added to the characteristic hallmarks of tumor growth [23]. During the last decade, the general hallmarks of cancer were revised not once and up to now it is discussed what should be included into this list, but the changes associated with the energetic metabolism reprogramming (the Warburg effect) are steadily involved into the list of undisputable hallmarks of a cancer cell [24-35].
Aerobic glycolysis providing tumor cells with energy (presented as ATP molecules) is the most specific feature of metabolism of the majority of malignant tumors, especially of rapidly growing ones. However, aerobic glycolysis represents only one part of changes in the metabolism, because the malignant tumor cells have also to enhance the rate of some other metabolic conversions, in particular, of those responsible for synthesis of amino acids, lipids, and nucleotides required for growth and proliferation of tumor cells [23].
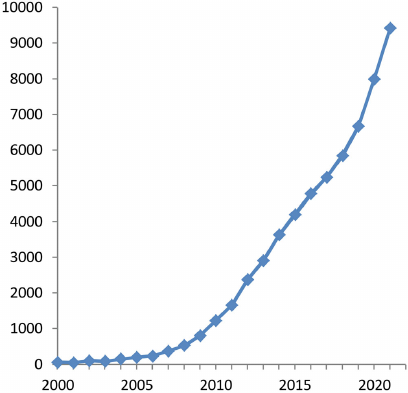
The acknowledgment that the Warburg effect is a specific hallmark of the tumor growth has led to the sharp increase in the interest of researchers in studying cancer metabolism [36, 37]. As a result, last decade became an epoch of the Warburg effect renaissance: growth of the number of publications became exponential (Fig. 2). Thus, Warburg’s work was ahead of its time by almost 90 years!
Fig. 2. Number (per year) of publications devoted to “The Warburg effect” (compiled by authors of this review based on the data of Google Academy).
The prime cause of cancer is important for the prevention and diagnosis, and yet the effects that influence on tumor growth are more important for cancer treatment. As the Warburg effect consequence, inhibition of glycolysis and other pathways of energy provision for the cancer cell, recovery of normal biochemistry and oxygen respiration could arrest the tumor growth and lead to remission [38]. In any case, normalization of biochemical parameters could increase efficiency of the traditional treatment.
However, the current works are associated only with investigations of mechanisms of changes in metabolic reactions in tumor cells. Moreover, the existing reviews consider the Warburg effect as a part of metabolic reprogramming, which is the result of interaction between hyperexpression of the hypoxia-induced transcription factors HIF-1, activation of oncogenes (cMyc, Ras), loss of tumor suppressor functions (the mutant p53), activated or deactivated signaling pathways, tumor microenvironment components, and interactions of HIF-1 with epigenetic mechanisms. Contrary to the traditional concept, we will consider for the first time the Warburg effect from the viewpoint of organic chemistry and classic biochemistry. We will discuss also (alongside with the main changes in the energetic metabolism in tumor cells) the available data and pathways of normalization of the key biochemical parameters, in particular, inhibition of the abnormal glycolysis and neutralization of its effect, as well as activation of oxygen respiration of a malignant tumor.
THE WARBURG PARADIGM IN THE THERAPY OF CANCER
Otto Warburg thought that to treat cancer it is necessary, first of all, to deprive the tumor cell of energy. This will result in starvation of the tumor that could arrest its growth and invasiveness.
Recovery of the normal biochemistry of the tumor and of the body as a whole is also an important component of the successful treatment of cancer. Regardless of the true causes of carcinogenesis, a number of significant changes in biochemical parameters are observed in the body of an oncological patient. Moreover, these biochemical changes are different from purely genetic changes, they are observed not only at the level of malignant tumor cell, but significant changes occur also at the whole-body level. In particular, lactic acidosis and increased activity of lactate dehydrogenase (LDH) are caused by the Warburg effect, i.e., by the changes in the energy metabolism [39]. And regardless of the prime cause of cancer, normalization of biochemical parameters in addition to the traditional therapeutic approaches could essentially improve the situation. Moreover, the treatment approaches corresponding to the Warburg theory do not contradict the traditional molecular genetic hypotheses, but supplement them!
At present, the prime cause of many diseases is unknown. Nevertheless, they are successfully treated saving and prolonging lives of the patients. When certain physiological (or biochemical) parameters change in humans along with manifestation of various symptoms, modern medicine does everything to return them to normal values. If the body temperature is increased, aspirin is given, for heartburn – antacids, for anemia – iron preparations, for diabetes – insulin, etc. Moreover, a diet is prescribed to normalize even insignificant deviations of blood parameters (e.g., bilirubin, glucose, etc.) from normal values. At the same time, knife remains a major weapon of the modern oncology (as well as radiation and cytostatics), whereas until recently nobody has dealt with normalizing biochemical parameters in oncological diseases (i.e., everybody treats the disease, but not the patient). If you bring biochemical parameters back to normal, the tumor will be deprived of the vitally important components (first of all, nutrition, growth mechanisms, protection against the immune system, etc.), and this could result in a synergism: efficiency of the traditional treatment will increase, survival will become higher, metastases will reduce. On the contrary, under conditions of tissue hypoxia, its malignant degeneration occurs [40, 41], and an increased content of lactic acid in the extracellular space modulates metabolism and functions of the adjacent cells in the tumor microenvironment, initiates angiogenesis and intensive growth of the tumor [42, 43].
Recently, the metabolic concept for treatment of oncological diseases has been actively developed [18, 44-47]. However, the proposed strategies usually are directed to only one metabolic pathway. The main specific feature of cancer is that the cells of malignant tumor use a number of alternative pathways to obtain energy and nutrients required for growth and proliferation. If necessary, the tumor cells quickly switch between these pathways. This is exactly what limits efficiency of numerous attempts to affect malignant tumor by targeting only one of the metabolic pathways, and this what differs this disease from many other diseases, when only one metabolic pathway is disturbed. Therefore, in the case of malignant tumors it is necessary to identify simultaneously all the pathways responsible for the key biochemical parameters, but not a single parameter, even the most characteristic. When speaking about the cancer bioenergetics, which O. Warburg had in mind, it is necessary to block all the pathways associated with energy production specific for a malignant tumor.
To apply the metabolic approach from the Warburg’s position, attention should be paid to biochemical changes in the tumor cell and in the body as a whole, which are caused by the changes in bioenergetics of the cell.
BIOENERGETICS OF CANCER CELLS
Aerobic glycolysis is the main distinctive feature of bioenergetics (generation of ATP) of the majority of cancer cells demonstrating the Warburg effect.
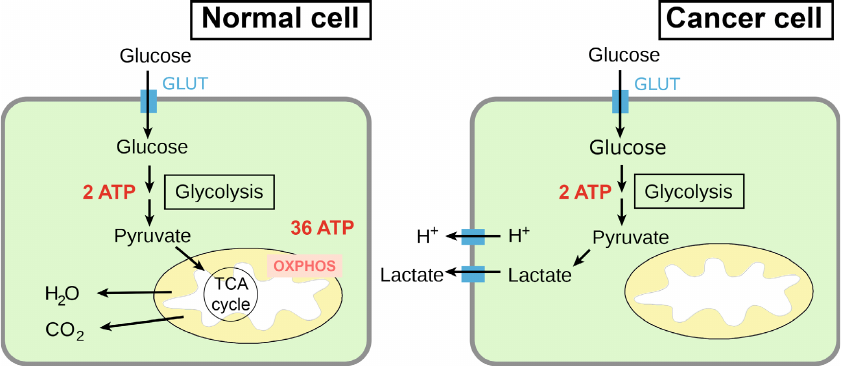
High level of aerobic glycolysis and defects of oxidative phosphorylation are the most characteristic changes in the energy metabolism of cancer cells [48, 49]. Glucose is the main source of energy for both normal and cancer cells. Glucose enters the cell by means of glucose transporter protein (GLUT) and in the cytosol is subjected to glycolysis to pyruvate (pyruvic acid) with production of two molecules of ATP (Fig. 3).
Fig. 3. The main pathways of energy production from glucose in normal and cancer cells. Designations: OXPHOS, oxidative phosphorylation; GLUT, glucose transporter; MCT, monocarboxylate transporter.
In a normal cell, pyruvate is transferred into mitochondria, where it is completely oxidized to CO2 and H2O in the tricarboxylic acid cycle (the Krebs cycle) and subjected to oxidative phosphorylation [50]. As a result, the maximal (theoretic) yield of ATP is 38 molecules per one glucose molecule, but in practice, the yield of ATP is 29-32 molecules per one molecule of glucose [51]. At the same time, in the cytosol of a tumor cell pyruvate is converted to lactate (lactic acid), and only two ATP molecules are produced per one molecule of glucose [15, 52].
Normal cells use processes of tissue respiration and oxidative phosphorylation to obtain up to 90% of all ATP and process of anaerobic glycolysis – to obtain 10% of ATP. The tumor cells exhibiting the Warburg effect use glycolysis to obtain 50-60% of ATP [52, 53]. This means that the majority of cancer cells are capable of both tissue respiration and oxidative phosphorylation simultaneously; however, the rates of these processes in them are significantly lower than in normal cells. An increased glycolysis in tumor cells could be caused not only by the deficiency of energy because of disrupted oxidative phosphorylation (OxPhos) complexes, but could be also a result of accelerated cell proliferation and high need of the malignant tumor cells in the intermediate products of glycolysis and Krebs cycle [54].
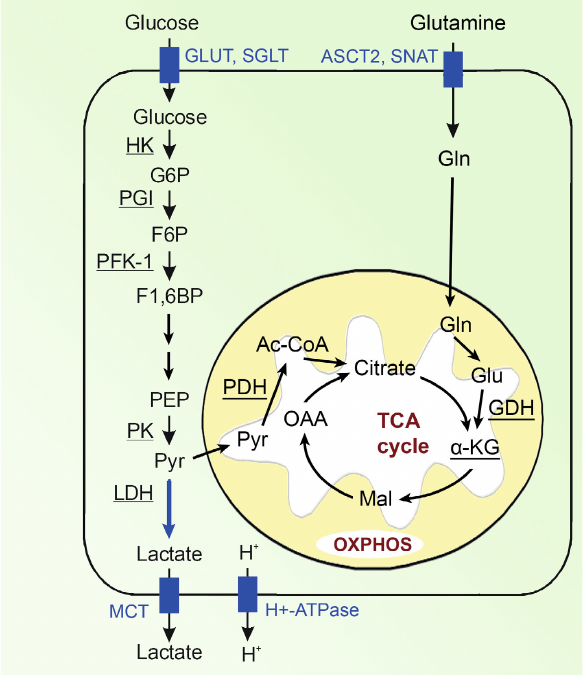
Let us consider the main pathways of energy generation in a cancer cell (Fig. 4).
Fig. 4. Bioenergetics of the cell. Designations: G6P, glucose 6-phosphate; F6P, fructose 6-phosphate; F1,6BP, fructose 1,6-bisphosphate; DHAP, dihydroxyacetone phosphate; GADP, glyceraldehyde 3-phosphate; 1,3BPG, 1,3-bisphosphoglycerate; 3-PG, 3-phosphoglycerate; 2-PG, 2-phosphoglycerate; PEP, phosphoenolpyruvate; Pyr, pyruvate; Gln, glutamine; Glu, glutamate; GLUT, glucose transporter; MCT, monocarboxylic acid transporter; OXPHOS, oxidative phosphorylation. Enzymes: HK, hexokinase; PGI, phosphoglucose isomerase; PFK-1, phosphofructokinase; ALDO, fructose bisphosphate aldolase; TPI, triosephosphate isomerase; GAPDH, glyceraldehyde phosphate dehydrogenase; PGK, phosphoglycerate kinase; PGM, phosphoglycerate mutase; ENO, enolase; PK, pyruvate kinase; LDH, lactate dehydrogenase; PDH, pyruvate dehydrogenase; GDH, glutamate dehydrogenase.
Glucose enters the cell mainly via glucose transporters GLUT. To obtain energy, cancer cells mainly use glycolysis in which from one glucose molecule only two ATP molecules are produced (i.e., approximately 15 times less than in the process of glucose oxidation) and, therefore, cancer cells require significantly more glucose than normal cells. The increased uptake of glucose by the malignant tumor cells is associated with hyperexpression of glucose transporters, in particular, of GLUT 1, 3, and 12 [55]. Under conditions of hypoxia, the roles of GLUT1 and GLUT3 significantly increase in the cells, as well as the roles of other proteins responsible for transporting glucose [56, 57]. Sodium-glucose cotransporters SGLT1 and SGLT2 also play a certain role in the glucose delivery into the cell, especially at the early stages of tumor development [58].
Upon entrance into the cell, glucose is phosphorylated by hexokinase-2 (HK2) to glucose-6-phosphate and then isomerized to fructose-6-phosphate. These products of the first two stages of glucose conversions could be later involved also into the pentose phosphate pathway, which is not associated with energy production, but facilitates anabolism of cancer cells [59-63]. The next important stage of glycolysis is conversion of fructose-6-phosphate to fructose-1,6-biphosphate under the action of phosphofructokinase-1 (PFK-1) that is the rate-limiting stage of glycolysis [64-66]. The last stage of glycolysis resulting in formation of pyruvate and ATP from phosphoenolpyruvate under the action of pyruvate kinase (PK) is also very important [67]. In tumor cells, the low-activity isoform of pyruvate kinase (PKM-2) predominates, and this leads to accumulation of the glycolysis intermediate products, which support also biosynthetic requirements of cancer cells [68-71].
Pyruvate, the final product of glycolysis, is located in the intersection of two pathways. Later, it can either be converted into lactate in the cytosol or be transferred into the mitochondria, where it can be oxidized to acetyl-CoA and then enter tricarboxylic acid cycle.
Pyruvate is converted to lactate under the action of LDH, and in tumor cells its A-isoform (LDH-A) predominates [72]. The final product of aerobic glycolysis, lactic acid, is actively eliminated from the malignant tumor cells by monocarboxylate transporters (first of all, by MCT-4), H+-lactate cotransporter, and proton pump (H+-ATPase) [72]. Intensive transport of lactic acid from cancer cell results in a noticeable increase in the intracellular pH of the tumor cells as compared to the normal cells, whereas extracellular pH of the tumor microenvironment, by contrast, becomes significantly lower, i.e., more acidic than in the healthy tissues [73-76]. Intracellular pH value is 6.99-7.05 in the normal and 7.12-7.7 in the tumor cells, whereas extracellular pH level is 7.35-7.45 in the normal tissues and 6.2-6.9 in the tumor microenvironment [75, 77]. On the one hand, increase in the intracellular pH value leads to the increase of activities of glycolytic enzymes. Thus, activity of the rate-limiting enzyme of glycolysis, phosphofructokinase (PFK-1), increases 100 times with increase of pH in the tumor cell by 0.2 units [75, 76, 78]. On the other hand, decrease in the extracellular space pH leads to the development of acidosis, which is one of the main characteristics of tumor tissues and promotes more aggressive behavior and metastasizing of the tumor [74]. Lactic acid in the intercellular space also plays an important role in the tumor emergence, its progression and survival, suppression of anti-cancer immune response; it also plays a regulatory role in various aspects of the energy metabolism and signaling, etc. [43, 79-83].
Another pathway of pyruvate conversion includes its transfer into mitochondria by the mitochondrial transporters MPC1 and MPC2 [84, 85] and then under the action of pyruvate dehydrogenase (PDH) pyruvate is converted into acetyl-CoA [86], which later is involved into tricarboxylic acid cycle and production of ATP through oxidative phosphorylation [87-89]. In cancer cells, some intermediates of tricarboxylic acid cycle are also used as starting compounds for synthesis of amino acids, lipids, and nucleotides, which are necessary for the tumor cell growth as a result of cataplerosis.
Another energy source in tumor cells is glutamine, which can partially compensate glycolysis, especially under conditions of glucose deficit or in the case of large amounts of inactive pyruvate kinase PKM-2 [90-94]. During glutaminolysis, glutamine is transported into the cell and converted into glutamate and then under the action of glutamate dehydrogenase (GDH) converted into α-ketoglutarate (α-KG) [95], which is involved into tricarboxylic acid cycle as its intermediate for generation of ATP (Fig. 4). In some cancer types more than 60% of the total energy in mitochondria are produced via glutaminolysis [96], which comprises more than 25% of the total energy of the cell. Note that glucose is the main source for generation of energy and survival of the cancer cell [97, 98].
Changes in glucose and glutamine metabolism of cancer cells are closely interrelated. When glucose level in cancer cell decreases, uptake of glutamine lowers, and when glutamine level in the cell increases, uptake of glucose increases [99, 100]. In the case of glucose deficit in the nutrient medium, conversion of glutamate to α-ketoglutarate is activated, which is next used in the tricarboxylic acid cycle for generating energy [100]. This is a pathway for providing a glucose-independent tumor growth [94, 101-103].
In addition to glucose and glutamine, a malignant tumor cells have also other energy sources. In particular, tumor cells can use lactic acid from blood and intercellular space to synthesize ATP [104-106]. Concentrations of lactate and pyruvate inside the tumor can significantly increase due to the tumor-associated fibroblasts. The activated fibroblasts in the tumor are able to produce these metabolites and release them into the intercellular space using the monocarboxylate transporter MCT4. The adjacent cancer cells uptake the released metabolites using the MCT1 transporter. Transfer of catabolites allows cancer cells to increase production of ATP and enhance proliferation concurrently with reducing the cell death. The transfer of catabolites from the tumor stromal cells to the cancer cells was named “Reverse Warburg Effect”. It is assumed that the metabolic connection between the cells is induced due to the changes in mitochondria functioning and redox state of the cancer cells. Therapy of such type tumors must target connection between the activated stromal cells and the cancer cells. This can be achieved through using acetylcysteine, in particular, which increases production of glutathione and thus acts as an antioxidant capable of destructing metabolic connection between the tumor cells [107]. Pyruvate in blood could be another energy source for a cancer cell. Despite its low concentration, pyruvate combined with lactate is an important modulator of RedOx-potential of the tissue cells [108]. Branched amino acids also are considered as a source of energy [109, 110]. However, all these sources are minor.
Cancer cells are genetically, biochemically, and phenotypically heterogenous from tumor to tumor and depend on the pathways of provision with ATP [54]. This means that each tumor type has its own set of unique physico-chemical and biochemical parameters, which distinguish them from normal cells and tissues. It is necessary to take these parameters in consideration, when strategies are being developed to affect the tumor metabolism and growth without a radical change in functionality of the tissues and organs. Such approach would successfully supplement traditional chemotherapeutical approaches.
We have considered only bioenergetic features of the cancer cell metabolism. Alongside with these features, many characteristic metabolic disorders occur in tumor cells, and for some of them targeted therapeutic approaches are under active development [47-49, 111-115].
WARBURG’S PARADIGM IN ACTION
Lactic acid is not simply “a waste” of the cancer cell vital activity, but it is a powerful weapon used by the tumor in its struggle for survival. Therefore, neutralization of accumulated lactic acid, recovery of pH level of the intercellular space and of the body as a whole, as well as neutralization of the consequences of the lactate effects and disorders in its homeostasis in the cancer cell is one of the major strategies in the therapy of oncological diseases.
The second strategy is inhibition of the characteristic pathways of ATP production by cancer cells in order to deprive these cells of energy sources. And the third strategy is activation of mitochondrial respiration of the cells, i.e., of tricarboxylic acid cycle and oxidative phosphorylation.
Moreover, to successfully use these three strategies, it is necessary to know the exact values of those parameters, which will be targeted. Cancer cells are biochemically heterogenous, and each tumor type has definite values of biochemical parameters different from those of healthy tissues [54]. Thus, the extracellular pH (pHe) in the tumor microenvironment is significantly lower than in normal tissues, and these values are different in different types of tumors [73, 74]. Therefore, for the successful treatment, it is necessary to obtain both qualitative and quantitative picture of metabolic changes for each tumor type in comparison with normal tissues. It is necessary also to develop suitable approaches for quantitative control of these biochemical parameters, which would make it possible to monitor their normalization.
Lactic acid. Lactic acid is the main metabolite of glycolysis, which is characteristic for the Warburg effect. Lactic acid is one of the strongest monocarboxylic acids, in the cell it is dissociated and exists as a lactate anion.
An active release of lactic acid by tumor cells is a serious and multi-faceted danger [42, 116]. Extracellular space of the majority of tumors is acidic due to intensive release of lactic acid and poor perfusion. Extracellular acidity of the tumor (pHe) itself has a tendency to correlate with the cancer aggressiveness. High extracellular acidity is toxic for many cells. However, tumor cells adapt successfully to such condition. Moreover, acidity is a cause of the tumor drug resistance to purely alkaline chemotherapeutic preparations, which become insoluble and inactive under the effect of strong lactic acid [117]. Unlike high extracellular acidity, the intracellular pH (pHi) of tumor cells is markedly higher than the pHi of normal cells [73]. This activates glycolytic enzymes and pathways of synthesis of the products necessary for proliferation of cancer cell [75]. Therefore, it is necessary to synchronously increase intracellular acidity (to reduce pH) of the cancer cell and “to acidify” the cancer cell from inside. We think that there are at least three pathways for normalizing pH levels caused by lactic acid. First, chemical neutralization of the intercellular space of the tumor and the body as a whole with alkaline agents, as well as restoration of the Na/K-balance of the malignant tumor cells. Second, inhibition of the lactic acid transport from the cancer cells. Third, targeted acidification of cancer cells from the inside (reducing the pHi of the tumor cells).
At present, all these approaches are used separately. However, synergism could be achieved, when all these approaches are used synchronously.
Thus, to neutralize an excess of lactic acid and support normal extracellular pH, the most obvious approach is to use sodium bicarbonate (NaHCO3). By now, this hypothesis has obtained a theoretical basis and practical confirmation [118-127]. In particular, it has been shown that peroral administration of baking soda selectively increases pHe of the tumor and reduces formation of metastases [120]. When using baking soda, it is extremely important to control pHe, because uncontrolled alkalization is also deleterious; that is the reason why for a long time efficiency of the treatment with baking soda was considered dubious [128, 129]. It is known that the urine pH of a healthy human correlates with the acidity of consumed nutrients [130-132], whereas pH of urine of oncological patients is markedly decreased [133-135]. Therefore, it seems promising to develop simple methods to control pHe by measuring the urine pH. Moreover, for alkalization only basic sodium compounds should be used, and, hence, control of the Na/K-balance in blood is necessary. The point is that the Na+-bound lactate transporter SMCT1, although its activity in cancer cells is reduced, removes sodium ions from the cell and this make us to expect that the intracellular concentration of sodium in the tumor cells may be lower than in the healthy tissues, whereas, by contrast, potassium concentration in the tumor cells may be higher than in the normal cells, because of necessity of maintaining of the total Na/K balance to facilitate the cell functioning. Such Na/K-disbalance is known [136], and decrease in the Na/K ratio within the cells would be especially pronounced in the rapidly growing tumors with especially intensive glycolysis. To normalize the Na/K balance within the tumor cells, it seems promising alongside with soda to use salts of lithium which is the lightest alkaline metal with the smallest ionic radius. It seems reasonable to expect that due to increase of the alkaline metal concentrations, lithium would promote removal of potassium from the cell. However, the uptake of lithium by the cells is not regulated by the processes of selective membrane transport (contrary to sodium and potassium ions) and lithium ions at certain concentrations are toxic. Therefore, their delivery into the tumor cells must be targeted and lithium level in the body must be strictly controlled. There are few data, which confirm efficiency of lithium salts for the therapy of some malignant tumors [137-141]. Cesium (Cs) is another alkaline metal, which potentially could be used for reducing potassium concentration in the cancer cell. The hydrated ionic radius of cesium is identical to that of potassium [142], therefore, it could be assumed that Cs+ will be transported into the cell by potassium transporters, replace potassium, and decrease activities of the potassium-dependent glycolytic enzymes, in particular, of pyruvate kinase. However, by now there are only few data confirming this assumption. Thus, cesium is known to suppress aerobic glycolysis and proliferation of the HeLa cells [143]. At the same time, high doses of cesium are toxic and can cause severe hypopotassemia and hypomagnesemia [144, 145]. Therefore, similarly to the case with lithium compounds, targeted delivery of cesium into the tumor cells is needed, as well as a strict control of its level in the body. Finally, to normalize the Na/K-balance, preparations must be used for decreasing transport of sodium and increasing potassium transport from the tumor cells. Considering that sodium is eliminated from the cells as lactate, it is most reasonable to use inhibitors of lactate dehydrogenase, first of all LDH-A, and it has been realized in practice [146-148]. As to the transport of potassium, it is promising to use preparations targeting potassium channels, which are usually activated in various tumor [149-151]. It is known that the neoplastic transformation is associated with changes in the transport of potassium across the plasma and intracellular membranes, and that potassium channels can promote initiation of cancer, malignant progression, and resistance of the tumor cells to therapy [152-156].
Inhibition of lactic acid transport from cancer cells by monocarboxylic acid transporters (mainly by MCT4) and by proton pumps also could be a strategy to combat the increased extracellular acidity. Moreover, activity of the Na+-bound transporter of lactate SMCT1 is suppressed in tumor cells and, as a result, lactic acid is removed from the tumor cells instead of neutral sodium lactate causing acidification of the intercellular space [157, 158]. In the process, alongside with the increase of extracellular acidity (decrease in pHe) the intracellular acidity of the tumor cell lowers (increase in pHi). To inhibit acidification of the intercellular space, it seems most reasonable to use inhibitors of the proton pump [159, 160] and MCT4 transporters [161]. In this case, another outcome of such effect on these transporters alongside with the decrease extracellular acidity is the increase of intracellular acidity, i.e., acidification of cancer cells themselves, which could slow down proliferation and promote apoptosis. However, in this case simple increase in the lactic acid concentration in cancer cells can lead not to the increase in intracellular acidity, but to reprogramming of metabolism and involvement of lactate into other conversions. Therefore, it is necessary to use simultaneously additional selective preparations for acidification of cancer cells from inside [162-165].
Presence of lactic acid is a serious problem for the vitally important minerals and microelements, especially those which are components of the active centers of enzymes and proteins playing an important role in the living cell. It is known that the microelement composition changes virtually in any disease, even in burns and contusions. Such changes in the levels of microelements (decrease in their concentrations) in blood and tissues of oncological patients are well known [166-186]. One of the causes of active removal of microelements from the body is their uptake (elimination) by lactic acid (especially from the intercellular space and blood under conditions of acidosis). The lactic acid molecule is very compact and is able to penetrate into almost all active protein cavities, pulling out key microelements from them (or binding microelements and affecting the protein function). The destructing action of lactic acid is enhanced by putative participation of its hydroxyl group in formation of additional complexes with different metal ions that further increases the strength of binding these metals with lactic acid and makes it an ideal “killer” of microelements. Different microelements will be captured and eliminated with different rates, and as a result their ratio will change in both the cells and the body as a whole. Taking into account chemical features of lactic acid, it may be expected that the bivalent chemical elements of the second group (Mg, Ca, Sr, Ba) and of the 12th group (Zn, Cd, Hg) of the Mendeleev Periodic Table, which form weak chemical bonds with proteins, will be captured most easily. Thus, the greatest danger for the cells is binding of magnesium and, especially, of calcium ions as lactates and their removal from the body. Such disturbances in the concentrations of magnesium and calcium ions are well known, they exacerbate the course of disease [187]. Among the elements of the 12th group, it should be expected that the intracellular concentration of zinc in the tumor could be markedly reduced due to active glycolysis, and this has been really observed [136, 188]. Chemical elements of the 11th group of the Mendeleyev Periodic Table (Cu, Ag, Au) can form complexes with both hydroxyl and amino groups (e.g., within proteins), therefore, their capture by lactic acid will be more difficult, and it may be expected that under equal conditions they will be removed from the tumor cells significantly slower than the elements of the 2nd and 12th groups. In particular, that is why the observed Cu/Zn ratio is significantly higher in tumor cells than in normal tissue cells (which is usually used to assess transformations of cancer cells) because zinc is much more easily bound by lactic acid than copper [136].
The produced lactic acid salts of microelements are actively eliminated from the body, mainly with urine, and as a result, concentrations of these elements (first of all, of bivalent ones) are increased in the urine and decreased in the blood/serum [176, 189-194]. Therefore, concentrations of microelements in these biological fluids can serve as diagnostic markers. Modern biochemistry considers mainly the level of genes and proteins, which represent only one part in the long chain of biochemical reactions in the cell and body as a whole. However, the final result on the elementary level is provided by classic biochemical reactions. Here, the decisive significance for the whole chain belongs to the presence of key microelements in the active centers of proteins, and in the case of their absence, any manipulation on the genetic level will be useless. Capturing (or binding) by lactic acid of microelements from active centers of enzymes and proteins (for instance, of zinc from p53) affects their functioning and makes the course of disease more severe [195-198]. Therefore, restoration of the microelement composition is another necessary condition for normalizing biochemistry of the body as a whole. Indeed, delivery of microelements into the cells, especially those of the 12th group, is a difficult task, which, to the best of the authors’ knowledge, has not been solved up to now. Attempts to use common zinc salts at nontoxic concentrations for this purpose seem to be ineffective because zinc ions will be bound by lactic acid in the blood and intercellular space. Therefore, it is necessary to develop methods for targeted delivery of zinc into the tumor cells. At present, various zinc preparations are being tested, in particular, quercetin-zinc complex [199], zinc gluconate [200], as well as nanoparticles of zinc oxide [201-203].
Glycolysis and glutaminolysis. Cancer cells rapidly adapt and change the main pathways to obtain energy depending on conditions, for example, glutaminolysis is activated at the inhibition of glycolysis. Therefore, to successfully inhibit ATP generation in tumor cells, it is necessary to maximally arrest all specific pathways of energy provision, which are different from those of normal cells. It is important in every pathway to act both on the energy source entry into the cancer cells and on the whole chain of conversions and elimination of the “wastes” from the cell.
The main energy source for cancer cells is glucose, which is intensively captured by the tumor cells. Therefore, the first step should be inhibition of the glucose transport into the cell [57, 204]. When entering the cell, glucose is subjected to glycolysis. In cancer cells, the glycolysis rate is many times higher than in normal cells [3, 6]. Therefore, decrease in the glycolysis intensity [205] could lead to deprivation of the tumor of the main nutrition (i.e., energy). Indeed, inhibitors of glycolysis suppress growth of cancer cells and could be successfully used in the combination therapy of cancer. Currently, this trend is under the most active development. In the reviews [46, 206-213] glycolysis mechanisms and the associated metabolic processes in cancer cells are summarized, as well as putative approaches to inhibit each stage of glycolysis. At present, many compounds inhibiting different stages of glycolysis as well as their targets in tumor cells are known, and they are under intensive study as potential targets for antitumor drugs [47, 214-217].
In addition to activation of enzymes within the tumor cells, there are enzymes, which are released into the extracellular space, and their activities increase in other tissues and blood, in particular, this concerns such enzymes as LDH and glutamate dehydrogenase (GDH) [218, 219]. As a result, an increased release of lactate is observed in the whole body. Therefore, neutralization of the cancer-associated metabolites in the intracellular space (first of all, inhibition of the glycolytic enzymes) also is an important strategy.
Finally, since glutaminolysis is an alternative pathway of energy generation in cancer cells, additional interference with the glutamine metabolism also is considered as an approach to antitumor therapy [98, 209, 220, 221].
Activation of oxygen respiration. Contrary to inhibition of cancer cell glycolysis, mechanisms of which are studied sufficiently [206, 214], it is more difficult to normalize oxygen (aerobic) respiration of the cell. At present, intensive searches are being conducted for preparations, which would be able to efficiently affect just the respiration processes in a cancer cell [222].
In particular, the halogen-substituted organic acids have been proposed, primarily salts of dichloroacetic acid, for stimulating aerobic respiration of cancer cells [223, 224], as well as their mixtures with avermectins [225, 226]. Dichloroacetate is able to switch glucose metabolism from aerobic glycolysis to OxPhos by stimulating mitochondrial activity. As a result, this leads to the decrease in production of lactic acid, activation of the respiratory chain, change in the mitochondrial membrane potential, and release of proapoptotic mediators (cytochrome c and apoptosis-inducing factor, AIF) into the cytosol. However, dichloroacetates are artificial compounds, which are absent in living organisms, and, hence, their use is accompanied with some adverse effects.
Another class of compounds that normalize aerobic respiration of cells are sources of an ultramicroelement germanium [227]. Germanium has long been considered a therapeutic agent with anticancer, antitumor, antiviral, and anti-inflammatory effects [228]. It is known that germanium concentrations in the blood sera of oncological patients are significantly lower than in the healthy individuals [229, 230]. In malignant tumor tissues, germanium concentration also is significantly lower than in the adjacent healthy tissues [231]. Therefore, germanium preparations have long attracted attention of scientists. The well-known natural source of germanium, ginseng, stimulates oxygen respiration of cells [232] and is widely used in combination therapy of cancer [233-236]. A stimulatory effect of germanium compounds on oxidizing enzymes, e.g., aldehyde reductase has been shown [237]. An antitumor activity of germanium compounds (as inorganic compounds) was established as early as in 1928 and was not once confirmed later [238]. However, germanium compounds were introduced into medical practice only after its organic water-soluble compounds were obtained [38, 239]. Germanium bis-2-carboxyethyl sesquioxide (Ge-132, CEGS) became the best-known among such compounds. Biological activity of this particular compound was studied in detail and after that was used in the combination therapy of cancer [227-229, 239, 240]. Clinically confirmed cases of successful use of such compounds in cancer therapy are known, for example, complete remission of the lung cancer was achieved following treatment with germanium sesquioxide [241].
At present, anti-tumor activity and low toxicity of Ge-132 have been established [242-245]. Toxicity of germanium organic compound is lower than of the table salt, NaCl [246]: LD50 of Sesquioxide Ge-132 is >6300 mg/kg per os for mice, >10000 mg/kg per os for rats, and >1000 mg/kg in the case of intravenous administration for rats. Germatranol: LD50 for mice is 8400 mg/kg per os and 300 mg/kg in the case of intravenous administration. Thus, these compounds are completely safe. Discussion about the supposedly high toxicity of germanium compounds, which continued for a long time, was caused by a misprint [247, 248] in an article published in 1987, where erroneous toxicity values were presented for Ge-132. Unfortunately, this misprint was not immediately noticed, which led to the erroneous criticism and illogical political decisions (for example, the sale of Ge-132 sesquioxide produced in Japan was banned in the USA). As a result, the works demonstrating anticancer potential of germanium compounds were ignored. Finally, the role of this microelement in the living nature was denied in some reviews based on the erroneous data and published in very influential journals. The misprint was corrected only in 1988, but until recently, many authors cited only secondary references with the erroneous data about the allegedly high toxicity of germanium compounds. The story of this misprint and “difficult fate” of the microelement germanium is described in detail in the review [248]. Combination of the misprint and reliance on the secondary sources of information became a cause of neglecting a putative clinical use of this unique microelement compounds up to the present time.
Mitochondria are especially rich with minerals acting as important cofactors for physiology of mitochondria. Currently only fragmentary data on the activity of some minerals in mitochondria are available, whereas for other minerals there is no established mitochondrial function at all [249]. The studies of regularities and interrelations of minerals in mitochondrial metabolism processes are very promising for normalizing mitochondria functioning.
Quantitative metabolism and metabolomics of cancer processes. At present, there is no exact notion of the activity levels to which it is necessary to inhibit (or activate) one or another enzyme in the tumor cells because, during malignant transformation, the enzymes themselves are changed. In particular, their isoforms are activated and absent in normal cells (for example, pyruvate kinase PKM-2). Therefore, by trial and error, researchers and physicians have to act blindly to restore the entire chain of glucose transformations in tumor cells corresponding to those in normal cells. To successfully fight cancer, it is necessary to compile a qualitative and quantitative picture of metabolic changes for each tumor type compared with the normal tissue.
For this, it is necessary, firstly, to determine regulation patterns, activities (and other features) of all enzymes of glycolysis and glutaminolysis, as well as concentrations of metabolites at every stage for each tumor type in comparison with normal cells, i.e., to model all steps of glycolysis [206, 250].
Secondly, it is necessary to determine flow rates of glucose and its intermediates at each stage of glycolysis and for other pathways of its conversion (for example, the pentose phosphate pathway) in normal and cancer cells, i.e., quantitative data are necessary for all stages of glycolysis. The system biology of metabolism is studying these problems, and results of such works began to appear [108, 206, 250-253].
In particular, quantitative studies on the activities of the metabolic pathways have shown that glutaminolysis generates more than 50% of the energy produced in mitochondria [96, 251]. One glutamine molecule can generate 22.5 molecules of ATP [251, 252]. Thus, even a small contribution of glutaminolysis can give a significant amount of energy.
Although the Warburg effect has been known for nearly 100 years, its quantitative description as a We parameter has been proposed recently [250]:
We = Fldh/Fmpc,
where Fldh is the rate of lactate generation from pyruvate under the action of LDH, Fmpc is the rate of pyruvate transport into mitochondria by the MPC1 and MPC2 transporters.
According to this equation, for normal oxygenated tissues, such as the brain and heart, Warburg’s parameter is equal to or near zero (We = 0). For tumor cells, Warburg’s parameter can be a few tens [252]. Quantitative interpretation of Warburg’s effect is essential for monitoring therapeutic response, understanding cancer pathophysiology, and diagnosing tumors. Thus, in the cancer cells of human melanoma and non-Hodgkin’s lymphoma (lymphosarcoma), the value of We is within the range 8-21 and markedly decreases after the treatment with drugs [96, 251, 252].
CONCLUSION
At present, a great number of various preparations have been developed and undergo clinical trials, which target specific stages of the cancer cell glycolysis (the monograph [11] and reviews [47, 254, 255]). In some cases, such preparations demonstrate good results on their own.
Using Warburg’s principles and conventional therapeutic approaches would make it possible to increase their efficiency significantly. To obtain this, it is necessary to develop regimens for taking the known drugs to ensure the same rates and metabolic pathways of glucose and glutamine conversion in cancer cells as in normal ones. In addition, it is necessary to normalize oxygen respiration and the functioning of mitochondria in cancer cells. Finally, it is essential to restore the normal biochemistry of the body as a whole, in particular, to neutralize lactic acid and other metabolites of the malignant tumor to inhibit such enzymes as LDH and GDH. Moreover, it is crucial to act simultaneously in all these directions. It is the time to unite all these parts. As Otto Warburg wrote: “But how long prevention [of cancer] will be avoided depends on how long the prophets of agnosticism will succeed in inhibiting the application of scientific knowledge in the cancer field” [256].
Contributions. LM and AP produced the first draft of manuscript. AS was involved in the critical review of the drafts and adding Quantitative metabolism and metabolomics of tumor processes. All authors approved the final version of the manuscript.
Ethics declarations. The authors declare no conflicts of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
REFERENCES
1.Warburg, O. (1924) Über den Stoffwechsel der
Carcinomzelle [in German], Naturwissenschaften, 12,
1131-1137, doi: 10.1007/BF01504608.
2.Warburg, O. (1925) The metabolism of carcinoma
cells, J. Cancer Res., 9, 148-163, doi:
10.1158/jcr.1925.148.
3.Warburg, O., Wind, F., and Negelein, E. (1927) The
metabolism of tumors in the body, J. Gen. Physiol., 8,
519-530, doi: 10.1085/jgp.8.6.519.
4.Warburg, O. H. (1931) Nobel Prize in Medicine 1931.
The official website of the Nobel Foundation (URL: http://nobelprize.org/nobel_prizes/medicine/laureates/1931/warburg-bio.html).
5.Warburg, O. (1956) On the origin of cancer cells,
Science, 123, 309-314, doi:
10.1126/science.123.3191.309.
6.Warburg, O. (1956) On respiratory impairment in
cancer cells, Science, 124, 269-270, doi:
10.1126/science.124.3215.269.
7.Warburg, O. (2010) The classic: the chemical
constitution of respiration ferment, Clin. Orthop. Relat. Res.,
468, 2833-2839, doi: 10.1007/s11999-010-1534-y.
8.Warburg, O. H. (1969) The Prime Cause and
Prevention of Cancer, Wurzburg, Verlag K. Trilsch.
9.Bertram, J. S. (2000) The molecular biology of
cancer, Mol. Aspects Med., 21, 167-223, doi:
10.1016/S0098-2997(00)00007-8.
10.Croce, C. M. (2008) Oncogenes and cancer, New
Engl. J. Med., 358, 502-511, doi: 10.1056/NEJMra072367.
11.Pecorino, L. (2021) Molecular Biology of
Cancer: Mechanisms, Targets, and Therapeutics, Oxford University
Press, Oxford.
12.Garber, K. (2004) Energy boost: the warburg
effect returns in a new theory of cancer, J. Natl. Cancer Inst.,
96, 1805-1806, doi: 10.1093/jnci/96.24.1805.
13.Chance, B. (2005) Was Warburg right? Or was it
that simple? Cancer Biol. Ther., 4, 125-126, doi:
10.4161/cbt.4.1.1462.
14.Schulz, T. J., Thierbach, R., Voigt, A., Drewes,
G., Mietzner, B., Steinberg, P., Pfeiffer, A. F. H., and Ristow, M.
(2006) Induction of oxidative metabolism by mitochondrial frataxin
inhibits cancer growth. Otto Warburg revisited, J. Biol. Chem.,
281, 977-981, doi: 10.1074/jbc.M511064200.
15.Vazquez, A., Liu, J., Zhou, Y., and Oltvai, Z. N.
(2010) Catabolic efficiency of aerobic glycolysis: the Warburg effect
revisited, BMC Syst. Biol., 4, 1-9, doi:
10.1186/1752-0509-4-58.
16.Upadhyay, M., Samal, J., Kandpal, M., Singh, O.
V., and Vivekanandan, P. (2013) The Warburg effect: insights from the
past decade, Pharmacol. Ther., 137, 318-330, doi:
10.1016/j.pharmthera.2012.11.003.
17.Potter, M., Newport, E., and Morten, K. J. (2016)
The Warburg effect: 80 years on, Biochem. Soc. Trans.,
44, 1499-1505, doi: 10.1042/bst20160094.
18.DeBerardinis, R. J., and Chandel, N. S. (2020) We
need to talk about the Warburg effect, Nat. Metabol., 2,
127-129, doi: 10.1038/s42255-020-0172-2.
19.Vaupel, P., and Multhoff, G. (2021) Revisiting
the Warburg effect: historical dogma versus current understanding,
J. Physiol., 599, 1745-1757, doi: 10.1113/JP278810.
20.Gaál, Z. (2021) MicroRNAs and metabolism:
revisiting the Warburg effect with emphasis on epigenetic background
and clinical applications, Biomolecules, 11, 1531, doi:
10.3390/biom11101531.
21.Basu, S., Kwee, T. C., Surti, S., Akin, E. A.,
Yoo, D., and Alavi, A. (2011) Fundamentals of PET and PET/CT imaging,
Ann. N. Y. Acad. Sci., 1228, 1-18, doi:
10.1111/j.1749-6632.2011.06077.x.
22.Hanahan, D., and Weinberg, R. A. (2000) The
hallmarks of cancer (review), Cell, 100, 57-70, doi:
10.1016/S0092-8674(00)81683-9.
23.Hanahan, D., and Weinberg, R. A. (2011) Hallmarks
of cancer: the next generation, Cell, 144, 646-674, doi:
10.1016/j.cell.2011.02.013.
24.Sonnenschein, C., and Soto, A. M. (2013) The
aging of the 2000 and 2011 hallmarks of cancer reviews: a critique,
J. Biosci. (Bangalore), 38, 651-663, doi:
10.1007/s12038-013-9335-6.
25.Fouad, Y. A., and Aanei, C. (2017) Revisiting the
hallmarks of cancer, Am. J. Cancer Res., 7,
1016-1036.
26.Schwartz, L., Supuran, T. C., and Alfarouk, O. K.
(2017) The Warburg effect and the hallmarks of cancer, Anticancer
Agents Med. Chem., 17, 164-170, doi:
10.2174/1871520616666161031143301.
27.Caon, I., Bartolini, B., Parnigoni, A.,
Caravà, E., Moretto, P., Viola, M., Karousou, E., Vigetti, D.,
and Passi, A. (2020) Revisiting the hallmarks of cancer: the role of
hyaluronan, Semin. Cancer Biol., 62, 9-19, doi:
10.1016/j.semcancer.2019.07.007.
28.Paul, D. (2020) The systemic hallmarks of cancer,
J. Cancer Metastas. Treat., 6, 29, doi:
10.20517/2394-4722.2020.63.
29.Senga, S. S., and Grose, R. P. (2021) Hallmarks
of cancer – the new testament, Open Biol., 11,
200358, doi: 10.1098/rsob.200358.
30.Hanahan, D. (2022) Hallmarks of cancer: new
dimensions, Cancer Discov., 12, 31-46, doi:
10.1158/2159-8290.cd-21-1059.
31.Ravi, S., Alencar Jr., A. M., Arakelyan, J., Xu,
W., Stauber, R., Wang, C.-C. I., Papyan, R., Ghazaryan, N., and
Pereira, R. M. (2022) An update to hallmarks of cancer, Cureus,
14, e24803, doi: 10.7759/cureus.24803.
32.Swain, N., Hosalkar, R., Thakur, M., and Prabhu,
A. H. (2022) Hallmarks of Cancer: Its Concept and Critique, in
Microbes and Oral Squamous Cell Carcinoma: A Network Spanning
Infection and Inflammation (Routray, S., ed.) Springer Nature
Singapore, Singapore, pp. 55-68, doi: 10.1007/978-981-19-0592-6_4.
33.Zhong, Z., Yu, J., Virshup, D. M., and Madan, B.
(2020) Wnts and the hallmarks of cancer, Cancer Metastasis Rev.,
39, 625-645, doi: 10.1007/s10555-020-09887-6.
34.Blagosklonny, M. V. (2022) Hallmarks of cancer
and hallmarks of aging, Aging (Milano), 14, 4176-4187,
doi: 10.18632/aging.204082.
35.Chitale, D. A. (2022) Hallmarks of Cancer:
Molecular Underpinnings, in Cancer Metastasis Through the
Lymphovascular System (Leong, S. P., Nathanson, S. D., and Zager,
J. S., eds) Springer International Publishing, Cham, pp. 3-14, doi:
10.1007/978-3-030-93084-4_1.
36.Otto, A. M. (2016) Warburg effect(s) – a
biographical sketch of Otto Warburg and his impacts on tumor
metabolism, Cancer Metab., 4, 5, doi:
10.1186/s40170-016-0145-9.
37.Liberti, M. V., and Locasale, J. W. (2016) The
Warburg effect: how does it benefit cancer cells? Trends Biochem.
Sci., 41, 211-218, doi: 10.1016/j.tibs.2015.12.001.
38.Popov, A. V., and Menchikov, L. G. (2013) The
Warburg Effect Is a Guide to Multipurpose Cancer Therapy Including
Trace Element Delivery, in Drug Delivery Systems: Advanced
Technologies Potentially Applicable in Personalised Treatment
(Coelho, J., ed.) Springer, Dordrecht (Netherlands), pp. 255-270, doi:
10.1007/978-94-007-6010-3_9.
39.Weljie, A. M., and Jirik, F. R. (2011)
Hypoxia-induced metabolic shifts in cancer cells: moving beyond the
Warburg effect, Int. J. Biochem. Cell Biol., 43, 981-989,
doi: 10.1016/j.biocel.2010.08.009.
40.Ferreira, L. M. R., Hebrant, A., and Dumont, J.
E. (2012) Metabolic reprogramming of the tumor, Oncogene,
31, 3999-4011, doi: 10.1038/onc.2011.576.
41.Miranda-Galvis, M., and Teng, Y. (2020) Targeting
hypoxia-driven metabolic reprogramming to constrain tumor progression
and metastasis, Int. J. Mol. Sci., 21, 5487, doi:
10.3390/ijms21155487.
42.Dhup, S., Dadhich, R. K., Porporato, P. E., and
Sonveaux, P. (2012) Multiple biological activities of lactic acid in
cancer: influences on tumor growth, angiogenesis and metastasis,
Curr. Pharm. Des., 18, 1319-1330, doi:
10.2174/138161212799504902.
43.Gao, Y., Zhou, H., Liu, G., Wu, J., Yuan, Y., and
Shang, A. (2022) Tumor microenvironment: lactic acid promotes tumor
development, J. Immunol. Res., 2022, 3119375, doi:
10.1155/2022/3119375.
44.DeBerardinis, R. J., and Chandel, N. S. (2016)
Fundamentals of cancer metabolism, Sci. Adv., 2,
e1600200, doi: 10.1126/sciadv.1600200.
45.Martinez-Outschoorn, U. E., Peiris-Pagés,
M., Pestell, R. G., Sotgia, F., and Lisanti, M. P. (2017) Cancer
metabolism: a therapeutic perspective, Nat. Rev. Clin. Oncol.,
14, 11-31, doi: 10.1038/nrclinonc.2016.60.
46.Stine, Z. E., Schug, Z. T., Salvino, J. M., and
Dang, C. V. (2022) Targeting cancer metabolism in the era of precision
oncology, Nat. Rev. Drug Discov., 21, 141-162, doi:
10.1038/s41573-021-00339-6.
47.Counihan, J. L., Grossman, E. A., and Nomura, D.
K. (2018) Cancer metabolism: current understanding and therapies,
Chem. Rev., 118, 6893-6923, doi:
10.1021/acs.chemrev.7b00775.
48.Pavlova, N. N., and Thompson, C. B. (2016) The
emerging hallmarks of cancer metabolism, Cell Metab., 23,
27-47, doi: 10.1016/j.cmet.2015.12.006.
49.Pavlova, N. N., Zhu, J., and Thompson, C. B.
(2022) The hallmarks of cancer metabolism: still emerging, Cell
Metab., 34, 355-377, doi: 10.1016/j.cmet.2022.01.007.
50.Martínez-Reyes, I., and Chandel, N. S.
(2020) Mitochondrial TCA cycle metabolites control physiology and
disease, Nat. Commun., 11, 102, doi:
10.1038/s41467-019-13668-3.
51.Flurkey, W. H. (2010) Yield of ATP molecules per
glucose molecule, J. Chem. Educ., 87, 271-271, doi:
10.1021/ed800102g.
52.Zheng, J. (2012) Energy metabolism of cancer:
glycolysis versus oxidative phosphorylation (review), Oncol.
Lett., 4, 1151-1157, doi: 10.3892/ol.2012.928.
53.Pedersen, P. (2007) Warburg, me and Hexokinase 2:
multiple discoveries of key molecular events underlying one of
cancers’ most common phenotypes, the “Warburg
Effect”, i.e., elevated glycolysis in the presence of oxygen,
J. Bioenerg. Biomembr., 39, 211-222, doi:
10.1007/s10863-007-9094-x.
54.Moreno-Sánchez, R.,
Rodríguez-Enríquez, S., Saavedra, E.,
Marín-Hernández, A., and Gallardo-Pérez, J. C.
(2009) The bioenergetics of cancer: is glycolysis the main ATP supplier
in all tumor cells? BioFactors, 35, 209-225, doi:
10.1002/biof.31.
55.Macheda, M. L., Rogers, S., and Best, J. D.
(2005) Molecular and cellular regulation of glucose transporter (GLUT)
proteins in cancer, J. Cell. Physiol., 202, 654-662, doi:
10.1002/jcp.20166.
56.Luo, X.-M., Zhou, S.-H., and Fan, J. (2010)
Glucose transporter-1 as a new therapeutic target in laryngeal
carcinoma, J. Int. Med. Res., 38, 1885-1892, doi:
10.1177/147323001003800601.
57.Pliszka, M., and Szablewski, L. (2021) Glucose
transporters as a target for anticancer therapy, Cancers
(Basel), 13, 4184, doi: 10.3390/cancers13164184.
58.Koepsell, H. (2017) The Na+-D-glucose
cotransporters SGLT1 and SGLT2 are targets for the treatment of
diabetes and cancer, Pharmacol. Ther., 170, 148-165, doi:
10.1016/j.pharmthera.2016.10.017.
59.Horecker, B. L. (2002) The pentose phosphate
pathway, J. Biol. Chem., 277, 47965-47971, doi:
10.1074/jbc.X200007200.
60.Stincone, A., Prigione, A., Cramer, T., Wamelink,
M. M. C., Campbell, K., Cheung, E., Olin-Sandoval, V., Grüning,
N.-M., Krüger, A., Tauqeer Alam, M., Keller, M. A., Breitenbach,
M., Brindle, K. M., Rabinowitz, J. D., and Ralser, M. (2015) The return
of metabolism: biochemistry and physiology of the pentose phosphate
pathway, Biol. Rev., 90, 927-963, doi:
10.1111/brv.12140.
61.Patra, K. C., and Hay, N. (2014) The pentose
phosphate pathway and cancer, Trends Biochem. Sci., 39,
347-354, doi: 10.1016/j.tibs.2014.06.005.
62.Alfarouk, K. O., Ahmed, S. B. M., Elliott, R. L.,
Benoit, A., Alqahtani, S. S., Ibrahim, M. E., Bashir, A. H. H.,
Alhoufie, S. T. S., Elhassan, G. O., Wales, C. C., Schwartz, L. H.,
Ali, H. S., Ahmed, A., Forde, P. F., Devesa, J., Cardone, R. A., Fais,
S., Harguindey, S., and Reshkin, S. J. (2020) The pentose phosphate
pathway dynamics in cancer and its dependency on intracellular pH,
Metabolites, 10, 285, doi: 10.3390/metabo10070285.
63.Ghanem, N., El-Baba, C., Araji, K., El-Khoury,
R., Usta, J., and Darwiche, N. (2021) The pentose phosphate pathway in
cancer: regulation and therapeutic opportunities, Chemotherapy,
66, 179-191, doi: 10.1159/000519784.
64.Lang, L., Chemmalakuzhy, R., Shay, C., and Teng,
Y. (2019) PFKP Signaling at a Glance: An Emerging Mediator of Cancer
Cell Metabolism, in Reviews on Biomarker Studies of Metabolic and
Metabolism-Related Disorders (Guest, P. C., ed.) Springer
International Publishing, Cham, pp. 243-258, doi:
/10.1007/978-3-030-12668-1_13.
65.Shi, X., You, L., and Luo, R.-Y. (2019)
Glycolytic reprogramming in cancer cells: PKM2 dimer predominance
induced by pulsatile PFK-1 activity, Phys. Biol., 16,
066007, doi: 10.1088/1478-3975/ab3f5a.
66.Moreno-Sánchez, R.,
Marín-Hernández, A., Gallardo-Pérez, J. C.,
Quezada, H., Encalada, R., Rodríguez-Enríquez, S., and
Saavedra, E. (2012) Phosphofructokinase type 1 kinetics, isoform
expression, and gene polymorphisms in cancer cells, J. Cell.
Biochem., 113, 1692-1703, doi: 10.1002/jcb.24039.
67.Oliveira, G. L., Coelho, A. R., Marques, R., and
Oliveira, P. J. (2021) Cancer cell metabolism: Rewiring the
mitochondrial hub, Biochim. Biophys. Acta, 1867, 166016,
doi: 10.1016/j.bbadis.2020.166016.
68.Dayton, T. L., Jacks, T., and Vander Heiden, M.
G. (2016) PKM2, cancer metabolism, and the road ahead, EMBO
Rep., 17, 1721-1730, doi: 10.15252/embr.201643300.
69.Zahra, K., Dey, T., Ashish, Mishra, S. P., and
Pandey, U. (2020) Pyruvate kinase M2 and cancer: the role of PKM2 in
promoting tumorigenesis, Front. Oncol., 10, 159, doi:
10.3389/fonc.2020.00159.
70.Zhu, S., Guo, Y., Zhang, X., Liu, H., Yin, M.,
Chen, X., and Peng, C. (2021) Pyruvate kinase M2 (PKM2) in cancer and
cancer therapeutics, Cancer Lett., 503, 240-248, doi:
10.1016/j.canlet.2020.11.018.
71.Gao, J., Zhao, Y., Li, T., Gan, X., and Yu, H.
(2022) The role of PKM2 in the regulation of mitochondrial function:
focus on mitochondrial metabolism, oxidative stress, dynamic, and
apoptosis. PKM2 in mitochondrial function, Oxid. Med. Cell.
Longev., 2022, 7702681, doi: 10.1155/2022/7702681.
72.Wu, P., Zhou, Y., Guo, Y., Zhang, S.-L., and Tam,
K. Y. (2021) Recent developments of human monocarboxylate transporter
(hMCT) inhibitors as anticancer agents, Drug Discov. Today,
26, 836-844, doi: 10.1016/j.drudis.2021.01.003.
73.Hao, G., Xu, Z. P., and Li, L. (2018)
Manipulating extracellular tumour pH: an effective target for cancer
therapy, RSC Adv., 8, 22182-22192, doi:
10.1039/C8RA02095G.
74.Kato, Y., Ozawa, S., Miyamoto, C., Maehata, Y.,
Suzuki, A., Maeda, T., and Baba, Y. (2013) Acidic extracellular
microenvironment and cancer, Cancer Cell Int., 13, 89,
doi: 10.1186/1475-2867-13-89.
75.Kobliakov, V. A. (2022) The role of extra- and
intracellular pH values in regulation of the tumor process, Cell
Tissue Biol., 16, 114-120, doi:
10.1134/S1990519X22020079.
76.Alfarouk, K. O., Ahmed, S. B. M., Ahmed, A.,
Elliott, R. L., Ibrahim, M. E., Ali, H. S., Wales, C. C., Nourwali, I.,
Aljarbou, A. N., Bashir, A. H. H., Alhoufie, S. T. S., Alqahtani, S.
S., Cardone, R. A., Fais, S., Harguindey, S., and Reshkin, S. J. (2020)
The interplay of dysregulated pH and electrolyte imbalance in cancer,
Cancers (Basel), 12, 898, doi:
10.3390/cancers12040898.
77.Gao, W., Zhang, H., Chang, G., Xie, Z., Wang, H.,
Ma, L., Han, Z., Li, Q., and Pang, T. (2014) Decreased intracellular pH
induced by cariporide differentially contributes to human umbilical
cord-derived mesenchymal stem cells differentiation, Cell. Physiol.
Biochem., 33, 185-194, doi: 10.1159/000356661.
78.Alfarouk, K. O., Verduzco, D., Rauch, C.,
Muddathir, A. K., Adil, H. H. B., Elhassan, G. O., Ibrahim, M. E.,
David Polo Orozco, J., Cardone, R. A., Reshkin, S. J., and Harguindey,
S. (2014) Glycolysis, tumor metabolism, cancer growth and
dissemination. A new pH-based etiopathogenic perspective and
therapeutic approach to an old cancer question, Oncoscience,
1, 777-802, doi: 10.18632/oncoscience.109.
79.Choi, S. Y. C., Collins, C. C., Gout, P. W., and
Wang, Y. (2013) Cancer-generated lactic acid: a regulatory,
immunosuppressive metabolite? J. Pathol., 230, 350-355,
doi: 10.1002/path.4218.
80.Mendes, C., and Serpa, J. (2020) Revisiting
lactate dynamics in cancer – a metabolic expertise or an
alternative attempt to survive? J. Mol. Med., 98,
1397-1414, doi: 10.1007/s00109-020-01965-0.
81.Zhang, D., Tang, Z., Huang, H., Zhou, G., Cui,
C., Weng, Y., Liu, W., Kim, S., Lee, S., Perez-Neut, M., Ding, J.,
Czyz, D., Hu, R., Ye, Z., He, M., Zheng, Y. G., Shuman, H. A., Dai, L.,
Ren, B., Roeder, R. G., et al. (2019) Metabolic regulation of gene
expression by histone lactylation, Nature, 574, 575-580,
doi: 10.1038/s41586-019-1678-1.
82.Jiang, J., Huang, D., Jiang, Y., Hou, J., Tian,
M., Li, J., Sun, L., Zhang, Y., Zhang, T., Li, Z., Li, Z., Tong, S.,
and Ma, Y. (2021) Lactate modulates cellular metabolism through histone
lactylation-mediated gene expression in non-small cell lung cancer,
Front. Oncol., 11, 647559, doi:
10.3389/fonc.2021.647559.
83.Certo, M., Llibre, A., Lee, W., and Mauro, C.
(2022) Understanding lactate sensing and signalling, Trends
Endocrinol. Metab., 33, 722-735, doi:
10.1016/j.tem.2022.07.004.
84.McCommis, K. S., and Finck, B. N. (2015)
Mitochondrial pyruvate transport: a historical perspective and future
research directions, Biochem. J., 466, 443-454, doi:
10.1042/bj20141171.
85.Ruiz-Iglesias, A., and Mañes, S. (2021)
The importance of mitochondrial pyruvate carrier in cancer cell
metabolism and tumorigenesis, Cancers (Basel), 13, 1488,
doi: 10.3390/cancers13071488.
86.Martin, W. F. (2020) Older than genes: the acetyl
CoA pathway and origins, Front. Microbiol., 11, 817, doi:
10.3389/fmicb.2020.00817.
87.Cantor, J. R., and Sabatini, D. M. (2012) Cancer
cell metabolism: one hallmark, many faces, Cancer Discov.,
2, 881-898, doi: 10.1158/2159-8290.cd-12-0345.
88.Alberghina, L., Gaglio, D., Gelfi, C., Moresco,
R., Mauri, G., Bertolazzi, P., Messa, C., Gilardi, M., Chiaradonna, F.,
and Vanoni, M. (2012) Cancer cell growth and survival as a system-level
property sustained by enhanced glycolysis and mitochondrial metabolic
remodeling, Front. Physiol., 3, 362, doi:
10.3389/fphys.2012.00362.
89.Chang, L., Fang, S., and Gu, W. (2020) The
molecular mechanism of metabolic remodeling in lung cancer, J.
Cancer, 11, 1403-1411, doi: 10.7150/jca.31406.
90.Le, A., Lane, A. N., Hamaker, M., Bose, S., Gouw,
A., Barbi, J., Tsukamoto, T., Rojas, C. J., Slusher, B. S., Zhang, H.,
Zimmerman, L. J., Liebler, D. C., Slebos, R. J. C., Lorkiewicz, P. K.,
Higashi, R. M., Fan, T. W. M., and Dang, C. V. (2012)
Glucose-independent glutamine metabolism via TCA Cycling for
proliferation and survival in B cells, Cell Metab., 15,
110-121, doi: 10.1016/j.cmet.2011.12.009.
91.Wise, D. R., and Thompson, C. B. (2010) Glutamine
addiction: a new therapeutic target in cancer, Trends Biochem.
Sci., 35, 427-433, doi: 10.1016/j.tibs.2010.05.003.
92.Wang, Z., Liu, F., Fan, N., Zhou, C., Li, D.,
Macvicar, T., Dong, Q., Bruns, C. J., and Zhao, Y. (2020) Targeting
glutaminolysis: new perspectives to understand cancer development and
novel strategies for potential target therapies, Front. Oncol.,
10, 589508, doi: 10.3389/fonc.2020.589508.
93.Halama, A., and Suhre, K. (2022) Advancing cancer
treatment by targeting glutamine metabolism – a roadmap,
Cancers (Basel), 14, 553, doi:
10.3390/cancers14030553.
94.Yang, L., Venneti, S., and Nagrath, D. (2017)
Glutaminolysis: a hallmark of cancer metabolism, Annu. Rev. Biomed.
Eng., 19, 163-194, doi:
10.1146/annurev-bioeng-071516-044546.
95.Yoo, H. C., Yu, Y. C., Sung, Y., and Han, J. M.
(2020) Glutamine reliance in cell metabolism, Exp. Mol. Med.,
52, 1496-1516, doi: 10.1038/s12276-020-00504-8.
96.Lee, S.-C., Shestov, A. A., Guo, L., Zhang, Q.,
Roman, J. C., Liu, X., Wang, H. Y., Pickup, S., Nath, K., Lu, P.,
Hofbauer, S., Mesaros, C., Wang, Y. L., Nelson, D. S., Schuster, S. J.,
Blair, I. A., Glickson, J. D., and Wasik, M. A. (2019) Metabolic
detection of Bruton’s tyrosine kinase inhibition in mantle cell
lymphoma cells, Mol. Cancer Res., 17, 1365-1377, doi:
10.1158/1541-7786.mcr-18-0256.
97.Sandulache, V. C., Ow, T. J., Pickering, C. R.,
Frederick, M. J., Zhou, G., Fokt, I., Davis-Malesevich, M., Priebe, W.,
and Myers, J. N. (2011) Glucose, not glutamine, is the dominant energy
source required for proliferation and survival of head and neck
squamous carcinoma cells, Cancer, 117, 2926-2938, doi:
10.1002/cncr.25868.
98.Seyfried, T. N., Arismendi-Morillo, G.,
Mukherjee, P., and Chinopoulos, C. (2020) On the origin of ATP
synthesis in cancer, iScience, 23, 101761, doi:
10.1016/j.isci.2020.101761.
99.Yoshida, G. J. (2020) Beyond the Warburg effect:
N-Myc contributes to metabolic reprogramming in cancer cells, Front.
Oncol., 10, 791, doi: 10.3389/fonc.2020.00791.
100.Sainero-Alcolado, L., Liaño-Pons, J.,
Ruiz-Pérez, M. V., and Arsenian-Henriksson, M. (2022) Targeting
mitochondrial metabolism for precision medicine in cancer, Cell
Death Differ., 29, 1304-1317, doi:
10.1038/s41418-022-01022-y.
101.Vincent, E. E., Sergushichev, A., Griss, T.,
Gingras, M.-C., Samborska, B., Ntimbane, T., Coelho, P. P., Blagih, J.,
Raissi, T. C., Choinière, L., Bridon, G., Loginicheva, E.,
Flynn, B. R., Thomas, E. C., Tavaré, J. M., Avizonis, D., Pause,
A., Elder, D. J. E., Artyomov, M. N., and Jones, R. G. (2015)
Mitochondrial phosphoenolpyruvate carboxykinase regulates metabolic
adaptation and enables glucose-independent tumor growth, Mol.
Cell, 60, 195-207, doi: 10.1016/j.molcel.2015.08.013.
102.Duraj, T., Carrión-Navarro, J.,
Seyfried, T. N., García-Romero, N., and Ayuso-Sacido, A. (2021)
Metabolic therapy and bioenergetic analysis: The missing piece of the
puzzle, Mol. Metab., 54, 101389, doi:
10.1016/j.molmet.2021.101389.
103.Li, C., Zhang, G., Zhao, L., Ma, Z., and Chen,
H. (2016) Metabolic reprogramming in cancer cells: glycolysis,
glutaminolysis, and Bcl-2 proteins as novel therapeutic targets for
cancer, World J. Surg. Oncol., 14, 15, doi:
10.1186/s12957-016-0769-9.
104.Rabinowitz, J. D., and Enerbäck, S. (2020)
Lactate: the ugly duckling of energy metabolism, Nat. Metabol.,
2, 566-571, doi: 10.1038/s42255-020-0243-4.
105.Hui, S., Ghergurovich, J. M., Morscher, R. J.,
Jang, C., Teng, X., Lu, W., Esparza, L. A., Reya, T., Le, Z., Yanxiang
Guo, J., White, E., and Rabinowitz, J. D. (2017) Glucose feeds the TCA
cycle via circulating lactate, Nature, 551, 115-118, doi:
10.1038/nature24057.
106.Faubert, B., Li, K. Y., Cai, L., Hensley, C.
T., Kim, J., Zacharias, L. G., Yang, C., Do, Q. N., Doucette, S.,
Burguete, D., Li, H., Huet, G., Yuan, Q., Wigal, T., Butt, Y., Ni, M.,
Torrealba, J., Oliver, D., Lenkinski, R. E., Malloy, C. R., et al.
(2017) Lactate metabolism in human lung tumors, Cell,
171, 358-371.e359, doi: 10.1016/j.cell.2017.09.019.
107.Wilde, L., Roche, M., Domingo-Vidal, M.,
Tanson, K., Philp, N., Curry, J., and Martinez-Outschoorn, U. (2017)
Metabolic coupling and the reverse Warburg effect in cancer:
implications for novel biomarker and anticancer agent development,
Semin. Oncol., 44, 198-203, doi:
10.1053/j.seminoncol.2017.10.004.
108.Shestov, A. A., Barker, B., Gu, Z., and
Locasale, J. W. (2013) Computational approaches for understanding
energy metabolism, WIREs Syst. Biol. Med., 5, 733-750,
doi: 10.1002/wsbm.1238.
109.Sivanand, S., and Vander Heiden, M. G. (2020)
Emerging roles for branched-chain amino acid metabolism in cancer,
Cancer Cell, 37, 147-156, doi:
10.1016/j.ccell.2019.12.011.
110.Lieu, E. L., Nguyen, T., Rhyne, S., and Kim, J.
(2020) Amino acids in cancer, Exp. Mol. Med., 52, 15-30,
doi: 10.1038/s12276-020-0375-3.
111.Broadfield, L. A., Pane, A. A., Talebi, A.,
Swinnen, J. V., and Fendt, S.-M. (2021) Lipid metabolism in cancer: new
perspectives and emerging mechanisms, Dev. Cell, 56,
1363-1393, doi: 10.1016/j.devcel.2021.04.013.
112.Wei, Z., Liu, X., Cheng, C., Yu, W., and Yi, P.
(2021) Metabolism of amino acids in cancer, Front. Cell Dev.
Biol., 8, 603837, doi: 10.3389/fcell.2020.603837.
113.Silyanova, E. A., Samet, A. V., Semenova, M.
N., and Semenov, V. V. (2021) Synthesis and antiproliferative
properties of 3,4-diarylpyrrole-2-carboxamides, Russ. Chem.
Bull., 70, 498-509, doi: 10.1007/s11172-021-3115-5.
114.Mühlethaler, T., Milanos, L.,
Martínez, J. A., Blum, T., Gioia, D., Roy, B., Prota, A.,
Cavalli, A., and Steinmetz, M. O. (2022) Rational design of a novel
tubulin inhibitor with a unique mechanism of action, Angew. Chem.
Int. Ed. Engl., 61, e202204052, doi:
10.1002/anie.202204052.
115.Silyanova, E. A., Ushkarov, V. I., Samet, A.
V., Maksimenko, A. S., Koblov, I. A., Kislyi, V. P., Semenova, M. N.,
and Semenov, V. V. (2022) A comparative evaluation of monomethoxy
substituted o-diarylazoles as antiproliferative microtubule
destabilizing agents, Mendeleev Commun., 32, 120-122,
doi: 10.1016/j.mencom.2022.01.039.
116.Pérez-Tomás, R., and
Pérez-Guillén, I. (2020) Lactate in the tumor
microenvironment: an essential molecule in cancer progression and
treatment, Cancers (Basel), 12, 3244, doi:
10.3390/cancers12113244.
117.McCarty, M. F., and Whitaker, J. (2010)
Manipulating tumor acidification as a cancer treatment strategy,
Altern. Med. Rev., 15, 264-272.
118.Forsythe, S. M., and Schmidt, G. A. (2000)
Sodium bicarbonate for the treatment of lactic acidosis, Chest,
117, 260-267, doi: 10.1378/chest.117.1.260.
119.Takigawa, S., Sugano, N., Ochiai, K., Arai, N.,
Ota, N., and Ito, K. (2008) Effects of sodium bicarbonate on butyric
acid-induced epithelial cell damage in vitro, J. Oral
Sci., 50, 413-417, doi: 10.2334/josnusd.50.413.
120.Robey, I. F., Baggett, B. K., Kirkpatrick, N.
D., Roe, D. J., Dosescu, J., Sloane, B. F., Hashim, A. I., Morse, D.
L., Raghunand, N., Gatenby, R. A., and Gillies, R. J. (2009)
Bicarbonate increases tumor pH and inhibits spontaneous metastases,
Cancer Res., 69, 2260-2268, doi:
10.1158/0008-5472.can-07-5575.
121.Estrella, V., Chen, T., Lloyd, M., Wojtkowiak,
J., Cornnell, H. H., Ibrahim-Hashim, A., Bailey, K., Balagurunathan,
Y., Rothberg, J. M., Sloane, B. F., Johnson, J., Gatenby, R. A., and
Gillies, R. J. (2013) Acidity generated by the tumor microenvironment
drives local invasion, Cancer Res., 73, 1524-1535, doi:
10.1158/0008-5472.can-12-2796.
122.Pilon-Thomas, S., Kodumudi, K. N., El-Kenawi,
A. E., Russell, S., Weber, A. M., Luddy, K., Damaghi, M., Wojtkowiak,
J. W., Mulé, J. J., Ibrahim-Hashim, A., and Gillies, R. J.
(2016) Neutralization of tumor acidity improves antitumor responses to
immunotherapy, Cancer Res., 76, 1381-1390, doi:
10.1158/0008-5472.can-15-1743.
123.Yang, M., Zhong, X., and Yuan, Y. (2020) Does
baking soda function as a magic bullet for patients with cancer? A mini
review, Integr. Cancer Ther., 19, 1534735420922579, doi:
10.1177/1534735420922579.
124.Wang, Y., Zhou, X., Wang, W., Wu, Y., Qian, Z.,
and Peng, Q. (2021) Sodium bicarbonate, an inorganic salt and a
potential active agent for cancer therapy, Chin. Chem. Lett.,
32, 3687-3695, doi: 10.1016/j.cclet.2021.06.032.
125.Gillies, R. J. (2022) Cancer heterogeneity and
metastasis: life at the edge, Clin. Exp. Metastasis, 39,
15-19, doi: 10.1007/s10585-021-10101-2.
126.Korang, S. K., Safi, S., Feinberg, J., Nielsen,
E. E., Gluud, C., and Jakobsen, J. C. (2021) Bicarbonate for acute
acidosis, Cochrane Database System. Rev., 3, 21,
doi: 10.1002/14651858.CD014371.
127.Ying, C., Jin, C., Zeng, S., Chao, M., and Hu,
X. (2022) Alkalization of cellular pH leads to cancer cell death by
disrupting autophagy and mitochondrial function, Oncogene,
41, 3886-3897, doi: 10.1038/s41388-022-02396-6.
128.Cuhaci, B., Lee, J., and Ahmed, Z. (2000)
Sodium bicarbonate controversy in lactic acidosis, Chest,
118, 882-884, doi: 10.1378/chest.118.3.882.
129.Velissaris, D., Karamouzos, V., Ktenopoulos,
N., Pierrakos, C., and Karanikolas, M. (2015) The use of sodium
bicarbonate in the treatment of acidosis in sepsis: a literature update
on a long term debate, Crit. Care Res. Pract., 2015,
605830, doi: 10.1155/2015/605830.
130.Berardi, J., Logan, A., and Rao, A. (2008)
Plant based dietary supplement increases urinary pH, J. Int. Soc.
Sports Nutr., 5, 1-8, doi: 10.1186/1550-2783-5-20.
131.Konig, D., Muser, K., Dickhuth, H. H., Berg,
A., and Deibert, P. (2009) Effect of a supplement rich in alkaline
minerals on acid-base balance in humans, Nutrition J., 8,
23, doi: 10.1186/1475-2891-8-23.
132.Welch, A. A., Mulligan, A., Bingham, S. A., and
Khaw, K.-T. (2008) Urine pH is an indicator of dietary acid –
base load, fruit and vegetables and meat intakes: results from the
European Prospective Investigation into Cancer and Nutrition
(EPIC)-Norfolk population study, Br. J. Nutr., 99,
1335-1343, doi: 10.1017/S0007114507862350.
133.Wright, M. E., Michaud, D. S., Pietinen, P.,
Taylor, P. R., Virtamo, J., and Albanes, D. (2005) Estimated urine pH
and bladder cancer risk in a cohort of male smokers (Finland),
Cancer Causes Control, 16, 1117-1123, doi:
10.1007/s10552-005-0348-9.
134.Maeda, T., Kikuchi, E., Matsumoto, K.,
Miyajima, A., and Oya, M. (2011) Urinary pH is highly associated with
tumor recurrence during intravesical mitomycin C therapy for nonmuscle
invasive bladder tumor, J. Urol., 185, 802-806, doi:
10.1016/j.juro.2010.10.081.
135.Ceylan, C., Doluoglu, O. G., and Yahşi,
S. (2020) A different perspective: can urine pH be important in the
diagnosis of prostate cancer? Urologia J., 87, 19-22,
doi: 10.1177/0391560319865724.
136.Juloski, J. T., Rakic, A., Ćuk, V. V.,
Ćuk, V. M., Stefanović, S., Nikolić, D.,
Janković, S., Trbovich, A. M., and De Luka, S. R. (2020)
Colorectal cancer and trace elements alteration, J. Trace Elem. Med.
Biol., 59, 126451, doi: 10.1016/j.jtemb.2020.126451.
137.Maeng, Y.-S., Lee, R., Lee, B., Choi, S.-I.,
and Kim, E. K. (2016) Lithium inhibits tumor lymphangiogenesis and
metastasis through the inhibition of TGFBIp expression in cancer cells,
Sci. Rep., 6, 20739, doi: 10.1038/srep20739.
138.Sun, A., Shanmugam, I., Song, J., Terranova, P.
F., Thrasher, J. B., and Li, B. (2007) Lithium suppresses cell
proliferation by interrupting E2F–DNA interaction and
subsequently reducing S – phase gene expression in prostate
cancer, Prostate, 67, 976-988, doi:
10.1002/pros.20586.
139.Ozerdem, A., Ceylan, D., and Targıtay, B.
(2018) The relationship between lithium and cancer proliferation: a
case-based review of the literature, Curr. Drug Metab.,
19, 653-662, doi: 10.2174/1389200219666180430095933.
140.Ge, W., and Jakobsson, E. (2019) Systems
biology understanding of the effects of lithium on cancer, Front.
Oncol., 9, 296, doi: 10.3389/fonc.2019.00296.
141.Taskaeva, I., Gogaeva, I., Shatruk, A., and
Bgatova, N. (2022) Lithium enhances autophagy and cell death in skin
melanoma: an ultrastructural and immunohistochemical study, Microsc.
Microanal., 28, 1703-1711, doi:
10.1017/S1431927622000745.
142.Israelachvili, J. N. (2011) 4 –
Interactions Involving Polar Molecules. in Intermolecular and
Surface Forces (Third Edition) (Israelachvili, J. N., ed.) Academic
Press, Boston, pp. 71-90, doi: 10.1016/B978-0-12-391927-4.10004-0.
143.Kobayashi, D., Nishimura, N., and Hazama, A.
(2021) Cesium treatment depresses glycolysis pathway in HeLa cell,
Cell. Physiol. Biochem., 55, 477-488, doi:
10.33594/000000399.
144.Melnikov, P., and Zanoni, L. (2010) Clinical
effects of cesium intake, Biol. Trace Elem. Res., 135,
1-9, doi: 10.1007/s12011-009-8486-7.
145.Horn, S., Naidus, E., Alper, S. L., and
Danziger, J. (2015) Cesium-associated hypokalemia successfully treated
with amiloride, Clin. Kidney J., 8, 335-338, doi:
10.1093/ckj/sfv017.
146.Woodford, M. R., Chen, V. Z., Backe, S. J.,
Bratslavsky, G., and Mollapour, M. (2020) Structural and functional
regulation of lactate dehydrogenase-A in cancer, Future Med.
Chem., 12, 439-455, doi: 10.4155/fmc-2019-0287.
147.Feng, Y., Xiong, Y., Qiao, T., Li, X., Jia, L.,
and Han, Y. (2018) Lactate dehydrogenase A: a key player in
carcinogenesis and potential target in cancer therapy, Cancer
Med., 7, 6124-6136, doi: 10.1002/cam4.1820.
148.Kozal, K., Jóźwiak, P., and
Krześlak, A. (2021) Contemporary perspectives on the Warburg
effect inhibition in cancer therapy, Cancer Control, 28,
10732748211041243, doi: 10.1177/10732748211041243.
149.Kasper, G., Weiser, A. A., Rump, A., Sparbier,
K., Dahl, E., Hartmann, A., Wild, P., Schwidetzky, U.,
Castaños-Vélez, E., and Lehmann, K. (2005) Expression
levels of the putative zinc transporter LIV-1 are associated with a
better outcome of breast cancer patients, Int. J. Cancer,
117, 961-973, doi: 10.1002/ijc.21235.
150.Serrano-Novillo, C., Capera, J.,
Colomer-Molera, M., Condom, E., Ferreres, J. C., and Felipe, A. (2019)
Implication of voltage-gated potassium channels in neoplastic cell
proliferation, Cancers (Basel), 11, 287, doi:
10.3390/cancers11030287.
151.Wrzosek, A., Augustynek, B., Żochowska,
M., and Szewczyk, A. (2020) Mitochondrial potassium channels as
druggable targets, Biomolecules, 10, 1200, doi:
10.3390/biom10081200.
152.Liu, J., Qu, C., Han, C., Chen, M.-M., An,
L.-J., and Zou, W. (2019) Potassium channels and their role in glioma:
a mini review, Mol. Membr. Biol., 35, 76-85, doi:
10.1080/09687688.2020.1729428.
153.Ganser, K., Klumpp, L., Bischof, H., Lukowski,
R., Eckert, F., and Huber, S. M. (2021) Potassium Channels in Cancer,
in Pharmacology of Potassium Channels (Gamper, N., and Wang, K.,
eds) Springer International Publishing, Cham, pp. 253-275, doi:
10.1007/164_2021_465.
154.Wrzosek, A., Gałecka, S.,
Żochowska, M., Olszewska, A., and Kulawiak, B. (2022) Alternative
targets for modulators of mitochondrial potassium channels,
Molecules, 27, 299, doi: 10.3390/molecules27010299.
155.Zúñiga, L., Cayo, A.,
González, W., Vilos, C., and Zúñiga, R. (2022)
Potassium channels as a target for cancer therapy: current
perspectives, Onco Targets Ther., 15, 783-797, doi:
10.2147/OTT.S326614.
156.Banderali, U., Leanza, L., Eskandari, N., and
Gentile, S. (2022) Potassium and chloride ion channels in cancer: a
novel paradigm for cancer therapeutics, in Targets of Cancer
Diagnosis and Treatment: Ion Transport in Tumor Biology (Stock, C.,
and Pardo, L. A., eds.) Springer International Publishing, Cham, Pp.
135-155, doi: 10.1007/112_2021_62.
157.Ganapathy, V., Thangaraju, M., Gopal, E.,
Martin, P. M., Itagaki, S., Miyauchi, S., and Prasad, P. D. (2008)
Sodium-coupled monocarboxylate transporters in normal tissues and in
cancer, AAPS J., 10, 193-199, doi:
10.1208/s12248-008-9022-y.
158.Ganapathy, V., Thangaraju, M., and Prasad, P.
D. (2009) Nutrient transporters in cancer: relevance to Warburg
hypothesis and beyond, Pharmacol. Ther., 121, 29-40, doi:
10.1016/j.pharmthera.2008.09.005.
159.De Milito, A., and Fais, S. (2005) Tumor
acidity, chemoresistance and proton pump inhibitors, Future
Oncol., 1, 779-786, doi: 10.2217/14796694.1.6.779.
160.De Milito, A., Lucia Marino, M., and Fais, S.
(2012) A rationale for the use of proton pump inhibitors as
antineoplastic agents, Curr. Pharm. Des., 18, 1395-1406,
doi: 10.2174/138161212799504911.
161.Payen, V. L., Mina, E., Van Hée, V. F.,
Porporato, P. E., and Sonveaux, P. (2020) Monocarboxylate transporters
in cancer, Mol. Metabol., 33, 48-66, doi:
10.1016/j.molmet.2019.07.006.
162.Nath, K., Nelson, D. S., Heitjan, D. F.,
Leeper, D. B., Zhou, R., and Glickson, J. D. (2015) Lonidamine induces
intracellular tumor acidification and ATP depletion in breast, prostate
and ovarian cancer xenografts and potentiates response to doxorubicin,
NMR Biomed., 28, 281-290, doi: 10.1002/nbm.3240.
163.Guo, L., Shestov, A. A., Worth, A. J., Nath,
K., Nelson, D. S., Leeper, D. B., Glickson, J. D., and Blair, I. A.
(2016) Inhibition of mitochondrial complex II by the anticancer agent
lonidamine, J. Biol. Chem., 291, 42-57, doi:
10.1074/jbc.M115.697516.
164.Nath, K., Nelson, D. S., Roman, J., Putt, M.
E., Lee, S.-C., Leeper, D. B., and Glickson, J. D. (2017) Effect of
lonidamine on systemic therapy of DB-1 human melanoma xenografts with
temozolomide, Anticancer Res., 37, 3413-3421, doi:
10.21873/anticanres.11708.
165.Nath, K., Roman, J., Nelson, D. S., Guo, L.,
Lee, S.-C., Orlovskiy, S., Muriuki, K., Heitjan, D. F., Pickup, S.,
Leeper, D. B., Blair, I. A., Putt, M. E., and Glickson, J. D. (2018)
Effect of differences in metabolic activity of melanoma models on
response to lonidamine plus doxorubicin, Sci. Rep., 8,
14654, doi: 10.1038/s41598-018-33019-4.
166.Malavolta, M., and Mocchegiani, E. (2018)
Trace Elements and Minerals in Health and Longevity, Springer
International Publishing, Cham, Switzerland, doi:
10.1007/978-3-030-03742-0.
167.Gregoriadis, G. C., Apostolidis, N. S.,
Romanos, A. N., and Paradellis, T. P. (1983) A comparative study of
trace elements in normal and cancerous colorectal tissues,
Cancer, 52, 508-519, doi:
10.1002/1097-0142(19830801)52:3<508::aid-cncr2820520322>3.0.co;2-8.
168.Drake, E. N., 2nd, and Sky-Peck, H. H. (1989)
Discriminant analysis of trace element distribution in normal and
malignant human tissues, Cancer Res., 49, 4210-4215.
169.Zaichick, V. Y., Tsyb, A. F., and Vtyurin, B.
M. (1995) Trace elements and thyroid cancer, Analyst,
120, 817-821, doi: 10.1039/an9952000817.
170.Zaichick, V., and Zaichick, S. (2018)
Associations between age and 50 trace element contents and
relationships in intact thyroid of males, Aging Clin. Exp. Res.,
30, 1059-1070, doi: 10.1007/s40520-018-0906-0.
171.Popescu, E., and Stanescu, A. M. A. (2019)
Trace elements and cancer, Mod. Med., 26, 169-175, doi:
10.31689/rmm.2019.26.4.169.
172.Bottoni, P., and Scatena, R. (2019)
Mitochondrial Metabolism in Cancer. A Tangled Topic. Which Role for
Proteomics? in Mitochondria in Health and in Sickness
(Urbani, A., and Babu, M., eds), Springer Singapore, Singapore, pp.
1-16, doi: 10.1007/978-981-13-8367-0_1.
173.Cilliers, K., Muller, C. J. F., and Page, B. J.
(2020) Trace element concentration changes in brain tumors: a review,
Anat. Rec., 303, 1293-1299, doi: 10.1002/ar.24254.
174.Lossow, K., Schwarz, M., and Kipp, A. P. (2021)
Are trace element concentrations suitable biomarkers for the diagnosis
of cancer? Redox Biol., 42, 101900, doi:
10.1016/j.redox.2021.101900.
175.Rodríguez-Tomàs, E., Baiges-Gaya,
G., Castañé, H., Arenas, M., Camps, J., and Joven, J.
(2021) Trace elements under the spotlight: A powerful nutritional tool
in cancer, J. Trace Elem. Med Biol., 68, 126858, doi:
10.1016/j.jtemb.2021.126858.
176.Gardner, D. S., Allen, J. C., Goodson, D.,
Harvey, D., Sharman, A., Skinner, H., Szafranek, A., Young, J. S.,
Bailey, E. H., and Devonald, M. A. J. (2022) Urinary trace elements are
biomarkers for early detection of acute kidney injury, Kidney Int.
Rep., 7, 1524-1538, doi: 10.1016/j.ekir.2022.04.085.
177.Beenken, A. (2022) Trace metaluria as a
biomarker of acute kidney injury, Kidney Int. Rep., 7,
1461-1462, doi: 10.1016/j.ekir.2022.05.028.
178.Fukuda, H., Ebara, M., Yamada, H., Arimoto, M.,
Okabe, S., Obu, M., Yoshikawa, M., Sugiura, N., and Saisho, H. (2004)
Trace elements and cancer, Japan Med. Assoc. J., 47,
391-395.
179.Wiernsperger, N., and Rapin, J. (2010) Trace
elements in glucometabolic disorders: an update, Diabetol. Metab.
Syndr., 2, 70, doi: 10.1186/1758-5996-2-70.
180.Kurtarkar, P., Aggarwal, S., Ahmed, I.,
Khadtare, S., and Digholkar, R. (2015) Effect of additives to sodium
hypochlorite on pulp tissue dissolution and physico-mechanical effects
on root canal dentin: a systematic review, J. Dent. Res. Rev.,
7, 75-85, doi: 10.4103/jdrr.jdrr_4_20.
181.Samavarchi Tehrani, S., Mahmoodzadeh Hosseini,
H., Yousefi, T., Abolghasemi, M., Qujeq, D., Maniati, M., and Amani, J.
(2019) The crosstalk between trace elements with DNA damage response,
repair, and oxidative stress in cancer, J. Cell. Biochem.,
120, 1080-1105, doi: 10.1002/jcb.27617.
182.Yang, Y.-W., Dai, C.-M., Chen, X.-H., and Feng,
J.-F. (2021) The relationship between serum trace elements and
oxidative stress of patients with different types of cancer, Oxid.
Med. Cell. Longev., 2021, 4846951, doi:
10.1155/2021/4846951.
183.Zaichick, S., Zaichick, V., Nosenko, S., and
Moskvina, I. (2012) Mass fractions of 52 Trace elements and zinc/trace
element content ratios in intact human prostates investigated by
inductively coupled plasma mass spectrometry, Biol. Trace Elem.
Res., 149, 171-183, doi: 10.1007/s12011-012-9427-4.
184.Zaichick, V., and Zaichick, S. (2016) Prostatic
tissue levels of 43 trace elements in patients with prostate
adenocarcinoma, Cancer Clin. Oncol., 5, 79-94, doi:
10.5539/cco.v5n1p79.
185.Zaichick, V., and Zaichick, S. (2018) Twenty
chemical element contents in normal and cancerous thyroid, Int. J.
Hematol. Blo. Dis., 3, 1-13, doi:
10.15226/ijhbd/3/2/00121.
186.Venturelli, S., Leischner, C., Helling, T.,
Renner, O., Burkard, M., and Marongiu, L. (2022) Minerals and cancer:
overview of the possible diagnostic value, Cancers (Basel),
14, 1256, doi: 10.3390/cancers14051256.
187.Rosner, M. H., and DeMauro Renaghan, A. (2021)
Disorders of divalent ions (magnesium, calcium, and phosphorous) in
patients with cancer, Adv. Chronic Kidney Dis., 28,
447-459.e441, doi: 10.1053/j.ackd.2021.09.005.
188.Murakami, M., and Hirano, T. (2008)
Intracellular zinc homeostasis and zinc signaling, Cancer Sci.,
99, 1515-1522, doi: 10.1111/j.1349-7006.2008.00854.x.
189.Schwartz, M. K. (1975) Role of Trace Elements
in Cancer, Cancer Res., 35, 3481-3487.
190.Tan, C., Chen, H., and Xia, C. (2009) Early
prediction of lung cancer based on the combination of trace element
analysis in urine and an Adaboost algorithm, J. Pharm. Biomed.
Anal., 49, 746-752, doi: 10.1016/j.jpba.2008.12.010.
191.Bornhorst, J., Kipp, A. P., Haase, H., Meyer,
S., and Schwerdtle, T. (2018) The crux of inept biomarkers for risks
and benefits of trace elements, TrAC, Trends Anal. Chem.,
104, 183-190, doi: 10.1016/j.trac.2017.11.007.
192.Lin, C.-N., Wang, L.-H., and Shen, K.-H. (2009)
Determining urinary trace elements (Cu, Zn, Pb, As, and Se) in patients
with bladder cancer, J. Clin. Lab. Anal., 23, 192-195,
doi: 10.1002/jcla.20318.
193.Saikawa, H., Nagashima, H., Cho, K., Chiba, R.,
Sera, K., Shigeeda, W., Tomoyasu, M., Deguchi, H., Takahashi, F.,
Saito, H., Sugai, T., and Maemondo, M. (2021) Relationship between
trace element in tumor and prognosis in lung cancer patients,
Medicina, 57, 209, doi: 10.3390/medicina57030209.
194.Burton, C., and Ma, Y. (2019) Current trends in
cancer biomarker discovery using urinary metabolomics: achievements and
new challenges, Curr. Med. Chem., 26, 5-28, doi:
10.2174/0929867324666170914102236.
195.Orlova, M. A., and Orlov, A. P. (2011) Role of
zinc in an organism and its influence on processes leading to
apoptosis, J. Adv. Med. Med. Res., 1, 239-305, doi:
10.9734/BJMMR/2011/488.
196.Franklin, R. B., and Costello, L. C. (2009) The
important role of the apoptotic effects of zinc in the development of
cancers, J. Cell. Biochem., 106, 750-757, doi:
10.1002/jcb.22049.
197.Story, M. J. (2021) Zinc, ω-3
polyunsaturated fatty acids and vitamin D: an essential combination for
prevention and treatment of cancers, Biochimie, 181,
100-122, doi: 10.1016/j.biochi.2020.11.019.
198.Zhang, Y., Tian, Y., Zhang, H., Xu, B., and
Chen, H. (2021) Potential pathways of zinc deficiency-promoted
tumorigenesis, Biomed. Pharmacother., 133, 110983, doi:
10.1016/j.biopha.2020.110983.
199.Lee, Y.-H., and Tuyet, P.-T. (2019) Synthesis
and biological evaluation of quercetin–zinc (II) complex for
anti-cancer and anti-metastasis of human bladder cancer cells, In
Vitro Cell. Dev. Biol. Anim., 55, 395-404, doi:
10.1007/s11626-019-00363-2.
200.Valenzano, M. C., Rybakovsky, E., Chen, V.,
Leroy, K., Lander, J., Richardson, E., Yalamanchili, S., McShane, S.,
Mathew, A., Mayilvaganan, B., Connor, L., Urbas, R., Huntington, W.,
Corcoran, A., Trembeth, S., McDonnell, E., Wong, P., Newman, G.,
Mercogliano, G., Zitin, M., et al. (2021) Zinc gluconate induces
potentially cancer chemopreventive activity in Barrett’s
esophagus: a phase 1 pilot study, Dig. Dis. Sci., 66,
1195-1211, doi: 10.1007/s10620-020-06319-x.
201.Racca, L., Limongi, T., Vighetto, V., Dumontel,
B., Ancona, A., Canta, M., Canavese, G., Garino, N., and Cauda, V.
(2020) Zinc oxide nanocrystals and high-energy shock waves: a new
synergy for the treatment of cancer cells, Front. Bioeng.
Biotechnol., 8, 577, doi: 10.3389/fbioe.2020.00577.
202.Anjum, S., Hashim, M., Malik, S. A., Khan, M.,
Lorenzo, J. M., Abbasi, B. H., and Hano, C. (2021) Recent advances in
zinc oxide nanoparticles (ZnO NPs) for cancer diagnosis, target drug
delivery, and treatment, Cancers (Basel), 13, 4570, doi:
10.3390/cancers13184570.
203.Hamrayev, H., Shameli, K., and Yusefi, M.
(2021) Preparation of zinc oxide nanoparticles and its cancer treatment
effects: a review paper, J. Adv. Res. Micro Nano Engineering,
2, 1-11, doi: 10.37934/jrnn.1.1.6274.
204.Shriwas, P., Roberts, D., Li, Y., Wang, L.,
Qian, Y., Bergmeier, S., Hines, J., Adhicary, S., Nielsen, C., and
Chen, X. (2021) A small-molecule pan-class I glucose transporter
inhibitor reduces cancer cell proliferation in vitro and tumor growth
in vivo by targeting glucose-based metabolism, Cancer Metab.,
9, 14, doi: 10.1186/s40170-021-00248-7.
205.Kim, J.-w., and Dang, C. V. (2006)
Cancer’s molecular sweet tooth and the Warburg effect, Cancer
Res., 66, 8927-8930, doi: 10.1158/0008-5472.
206.Marín-Hernández, A.,
Gallardo-Pérez, J. C., Rodríguez-Enríquez, S.,
Encalada, R., Moreno-Sánchez, R., and Saavedra, E. (2011)
Modeling cancer glycolysis, Biochim. Biophys. Acta, 1807,
755-767, doi: 10.1016/j.bbabio.2010.11.006.
207.Cairns, R. A., Harris, I. S., and Mak, T. W.
(2011) Regulation of cancer cell metabolism, Nat. Rev. Cancer,
11, 85-95, doi: 10.1038/nrc2981.
208.Porporato, P. E., Dadhich, R. K., Dhup, S.,
Copetti, T., and Sonveaux, P. (2011) Anticancer targets in the
glycolytic metabolism of tumors: a comprehensive review, Front.
Pharmacol., 2, 49, doi: 10.3389/fphar.2011.00049.
209.Akins, S. N., Nielson, C. T., and Le, V. H.
(2018) Inhibition of glycolysis and glutaminolysis: an emerging drug
discovery approach to combat cancer, Curr. Top. Med. Chem.,
18, 494-504, doi: 10.2174/1568026618666180523111351.
210.Abdel-Wahab, A. F., Mahmoud, W., and Al-Harizy,
R. M. (2019) Targeting glucose metabolism to suppress cancer
progression: prospective of anti-glycolytic cancer therapy,
Pharmacol. Res., 150, 104511, doi:
10.1016/j.phrs.2019.104511.
211.Icard, P., Loi, M., Wu, Z., Ginguay, A.,
Lincet, H., Robin, E., Coquerel, A., Berzan, D., Fournel, L., and
Alifano, M. (2021) Metabolic strategies for inhibiting cancer
development, Adv. Nutr., 12, 1461-1480, doi:
10.1093/advances/nmaa174.
212.Gyamfi, J., Kim, J., and Choi, J. (2022) Cancer
as a metabolic disorder, Int. J. Mol. Sci., 23, 1155,
doi: 10.3390/ijms23031155.
213.Dadgar, T., Ebrahimi, N., Gholipour, A. R.,
Akbari, M., Khani, L., Ahmadi, A., and Hamblin, M. R. (2022) Targeting
the metabolism of cancer stem cells by energy disruptor molecules,
Crit. Rev. Oncol. Hematol., 169, 103545, doi:
10.1016/j.critrevonc.2021.103545.
214.Pelicano, H., Martin, D. S., Xu, R. H., and
Huang, P. (2006) Glycolysis inhibition for anticancer treatment,
Oncogene, 25, 4633-4646, doi: 10.1038/sj.onc.1209597.
215.Kroemer, G., and Pouyssegur, J. (2008) Tumor
cell metabolism: cancer/s Achilles’ heel, Cancer Cell,
13, 472-482, doi: 10.1016/j.ccr.2008.05.005.
216.Akram, M. (2013) Mini-review on glycolysis and
cancer, J. Cancer Educ., 28, 454-457, doi:
10.1007/s13187-013-0486-9.
217.Sun, X., Peng, Y., Zhao, J., Xie, Z., Lei, X.,
and Tang, G. (2021) Discovery and development of tumor glycolysis
rate-limiting enzyme inhibitors, Bioorg. Chem., 112,
104891, doi: 10.1016/j.bioorg.2021.104891.
218.Mehdi, M., Menon, M. K. C., Seyoum, N., Bekele,
M., Tigeneh, W., and Seifu, D. (2018) Blood and tissue enzymatic
activities of GDH and LDH, index of glutathione, and oxidative stress
among breast cancer patients attending referral hospitals of Addis
Ababa, Ethiopia: hospital-based comparative cross-sectional study,
Oxid. Med. Cell. Longev., 2018, 6039453, doi:
10.1155/2018/6039453.
219.Moreno-Sánchez, R.,
Marín-Hernández, Á., Gallardo-Pérez, J. C.,
Pacheco-Velázquez, S. C., Robledo-Cadena, D. X., Padilla-Flores,
J. A., Saavedra, E., and Rodríguez-Enríquez, S. (2020)
Physiological role of glutamate dehydrogenase in cancer cells,
Front. Oncol., 10, 429, doi: 10.3389/fonc.2020.00429.
220.Sharma, S., Agnihotri, N., and Kumar, S. (2022)
Targeting fuel pocket of cancer cell metabolism: a focus on
glutaminolysis, Biochem. Pharmacol., 198, 114943, doi:
10.1016/j.bcp.2022.114943.
221.Vettore, L., Westbrook, R. L., and Tennant, D.
A. (2020) New aspects of amino acid metabolism in cancer, Br. J.
Cancer, 122, 150-156, doi: 10.1038/s41416-019-0620-5.
222.Bedi, M., Ray, M., and Ghosh, A. (2022) Active
mitochondrial respiration in cancer: a target for the drug, Mol.
Cell. Biochem., 477, 345-361, doi:
10.1007/s11010-021-04281-4.
223.Turke, A., Song, Y., Costa, C., Cook, R.,
Arteaga, C., and Asara, J. (2012) Dichloroacetate reverses the Warburg
effect, inhibiting growth and sensitizing breast cancer cells towards
apoptosis, Cancer Res., 72, 3228-3228, doi:
10.1158/0008-5472.CAN-11-3747.
224.Tataranni, T., and Piccoli, C. (2019)
Dichloroacetate (DCA) and cancer: an overview towards clinical
applications, Oxid. Med. Cell. Longev., 2019, 8201079,
doi: 10.1155/2019/8201079.
225.Ishiguro, T., Ishiguro, R. H., Ishiguro, M.,
Toki, A., and Terunuma, H. (2022) Synergistic anti-tumor effect of
dichloroacetate and ivermectin, Cureus, 14, e21884, doi:
10.7759/cureus.21884.
226.Menchikov, L. G., Dzhafarov, M. K., and
Zavarzin, I. V. (2022) Recent advances in avermectins chemistry,
Russ. Chem. Rev., 91, RCR5051, doi: 10.1070/RCR5051.
227.Awais, M., Aizaz, A., Nazneen, A., Bhatti, Q.
u. A., Akhtar, M., Wadood, A., and Atiq Ur Rehman, M. (2022) A review
on the recent advancements on therapeutic effects of ions in the
physiological environments, Prosthesis, 4, 263-316, doi:
10.3390/prosthesis4020026.
228.Cho, J. M., Chae, J., Jeong, S. R., Moon, M.
J., Shin, D. Y., and Lee, J. H. (2020) Immune activation of
Bio-Germanium in a randomized, double-blind, placebo-controlled
clinical trial with 130 human subjects: therapeutic opportunities from
new insights, PLoS One, 15, e0240358, doi:
10.1371/journal.pone.0240358.
229.Lukevics, E., Gar, T. K., Ignatovich, L. M.,
and Mironov, V. F. (1990) Biological Activity of Germanium
Compounds [in Russian], Zinatne, Riga.
230.Kamil, Z. H., and Ewadh, M. J. (2016)
Determination of serum trace elements and haematological parameters in
lymphoma patients receiving chemotherapy, J. Babylon Univ. Pure
Appl. Sci., 24, 2489-2500.
231.Cheng, X., Zhou, Y.-C., Zhou, B., Huang, Y.-C.,
Wang, G.-Z., and Zhou, G.-B. (2019) Systematic analysis of
concentrations of 52 elements in tumor and counterpart normal tissues
of patients with non-small cell lung cancer, Cancer Med.,
8, 7720-7727, doi: 10.1002/cam4.2629.
232.Chen, X.-C., Zhu, Y.-G., Zhu, L.-A., Huang, C.,
Chen, Y., Chen, L.-M., Fang, F., Zhou, Y.-C., and Zhao, C.-H. (2003)
Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by
suppressing oxidative stress, Eur. J. Pharmacol., 473,
1-7, doi: 10.1016/S0014-2999(03)01945-9.
233.Ahuja, A., Kim, J. H., Kim, J.-H., Yi, Y.-S.,
and Cho, J. Y. (2018) Functional role of ginseng-derived compounds in
cancer, J. Ginseng Res., 42, 248-254, doi:
10.1016/j.jgr.2017.04.009.
234.Najafi, T. F., Bahri, N., Tohidinik, H. R.,
Feyz, S., Bloki, F., Savarkar, S., and Jahanfar, S. (2021) Treatment of
cancer-related fatigue with ginseng: A systematic review and
meta-analysis, J. Herb. Med., 28, 100440, doi:
10.1016/j.hermed.2021.100440.
235.Sadeghian, M., Rahmani, S., Zendehdel, M.,
Hosseini, S. A., and Zare Javid, A. (2021) Ginseng and cancer-related
fatigue: a systematic review of clinical trials, Nutr. Cancer,
73, 1270-1281, doi: 10.1080/01635581.2020.1795691.
236.Hong, H., Baatar, D., and Hwang, S. G. (2021)
Anticancer activities of ginsenosides, the main active components of
ginseng, Evid. Based Complement. Alternat. Med., 2021,
8858006, doi: 10.1155/2021/8858006.
237.Unakar, N. J., Tsui, J., and Johnson, M. (1997)
Effect of pretreatment of germanium-132 on
Na+-K+-ATPase and galactose cataracts, Curr.
Eye Res., 16, 832-837, doi: 10.1076/ceyr.16.8.832.8980.
238.Ward, S. G., and Taylor, R. C. (1988) Antitumor
Activity of the Main-Group Metallic Elements: Aluminum, Gallium,
Indium, Thallium, Germanium, Antimony, and Bismuth, in Metal-Based
Antitumor Drugs (Gielen, M., ed.) Freund Publ. House Ltd., Tel
Aviv, Israel, pp. 1-54.
239.Menchikov, L. G., and Ignatenko, M. A. (2012)
Biological activity of organogermanium compounds (a review), Pharm.
Chem. J., 46, 635-638, doi: 10.1007/s11094-013-0860-2.
240.Asai, K. (1980) The Miracle Cure: Organic
Germanium, Japan Publications, New York.
241.Mainwaring, M. G., Poor, C., Zander, D. S., and
Harman, E. (2000) Complete remission of pulmonary spindle cell
carcinoma after treatment with oral germanium sesquioxide,
Chest, 117, 591-593, doi: 10.1378/chest.117.2.591.
242.Chase, T. A., Cupp, M. J., and Tracy, T. S.
(2003) Germanium. in Dietary Supplements: Toxicology and
Clinical Pharmacology (Cupp, M. J., and Tracy, T. S., eds) Humana
Press, Totowa, NJ, pp. 197-207, doi: 10.1385/1-59259-303-8:197.
243.Nakamura, T., Shimada, Y., Takeda, T., Sato,
K., Akiba, M., and Fukaya, H. (2015) Organogermanium compound, Ge-132,
forms complexes with adrenaline, ATP and other physiological cis-diol
compounds, Fut. Med. Chem., 7, 1233-1246, doi:
10.4155/fmc.15.62.
244.Kim, E., Hwang, S.-U., Yoon, J. D., Jeung,
E.-B., Lee, E., Kim, D. Y., and Hyun, S.-H. (2017)
Carboxyethylgermanium sesquioxide (Ge-132) treatment during in
vitro culture protects fertilized porcine embryos against oxidative
stress induced apoptosis, J. Reprod. Dev., 63, 581-590,
doi: 10.1262/jrd.2017-020.
245.Wada, T., Hanyu, T., Nozaki, K., Kataoka, K.,
Kawatani, T., Asahi, T., and Sawamura, N. (2018) Antioxidant activity
of Ge-132, a synthetic organic germanium, on cultured mammalian cells,
Biol. Pharm. Bull., 41, 749-753, doi:
10.1248/bpb.b17-00949.
246.Gerber, G. B., and Leonard, A. (1997)
Mutagenicity, carcinogenicity and teratogenicity of germanium
compounds, Mutat. Res. Rev. Mutat. Res., 387, 141-146,
doi: 10.1016/S1383-5742(97)00034-3.
247.Kaplan, B. J., Parish, W. W., Andrus, G. M.,
Simpson, J. S. A., and Field, C. J. (2004) Germane facts about
germanium sesquioxide: I. Chemistry and anticancer properties, J.
Altern. Complement. Med., 10, 337-344, doi:
10.1089/107555304323062329.
248.Kaplan, B. J., Andrus, G. M., and Parish, W. W.
(2004) Germane facts about germanium sesquioxide: II. Scientific error
and misrepresentation, J. Altern. Complement. Med., 10,
345-348, doi: 10.1089/107555304323062338.
249.Killilea, D. W., and Killilea, A. N. (2022)
Mineral requirements for mitochondrial function: a connection to redox
balance and cellular differentiation, Free Radic. Biol. Med.,
182, 182-191, doi: 10.1016/j.freeradbiomed.2022.02.022.
250.Shestov, A. A., Liu, X., Ser, Z., Cluntun, A.
A., Hung, Y. P., Huang, L., Kim, D., Le, A., Yellen, G., Albeck, J. G.,
and Locasale, J. W. (2014) Quantitative determinants of aerobic
glycolysis identify flux through the enzyme GAPDH as a limiting step,
eLife, 3, e03342, doi: 10.7554/eLife.03342.
251.Shestov, A. A., Lee, S.-C., Nath, K., Guo, L.,
Nelson, D. S., Roman, J. C., Leeper, D. B., Wasik, M. A., Blair, I. A.,
and Glickson, J. D. (2016) 13C MRS and LC–MS flux analysis of
tumor intermediary metabolism, Front. Oncol., 6, 135,
doi: 10.3389/fonc.2016.00135.
252.Shestov, A. A., Mancuso, A., Lee, S.-C., Guo,
L., Nelson, D. S., Roman, J. C., Henry, P.-G., Leeper, D. B., Blair, I.
A., and Glickson, J. D. (2016) Bonded cumomer analysis of human
melanoma metabolism monitored by 13C NMR spectroscopy of perfused tumor
cells, J. Biol. Chem., 291, 5157-5171, doi:
10.1074/jbc.M115.701862.
253.Shestov, A. A., Nath, K., Nelson, D. S., Wasik,
M. A., and Glickson, J. D. (2022) Bonded cumomer analysis of tumor
metabolism based on 13C magnetic resonance spectroscopy, NMR
Biomed., e4716, doi: 10.1002/nbm.4716.
254.Zhou, Y., Guo, Y., and Tam, K. Y. (2022)
Targeting glucose metabolism to develop anticancer treatments and
therapeutic patents, Expert Opin. Ther. Pat., 32,
441-453, doi: 10.1080/13543776.2022.2027912.
255.Kubik, J., Humeniuk, E., Adamczuk, G.,
Madej-Czerwonka, B., and Korga-Plewko, A. (2022) Targeting energy
metabolism in cancer treatment, Int. J. Mol. Sci., 23,
5572, doi: 10.3390/ijms23105572.
256.Warburg, O. (1969) Otto Warburg On The Prime
Cause & Prevention of Cancer: Respiration of Oxygen in Normal Body
Cells vs. Fermentation of Sugar in Cancer Cells (The Second Revised
Edition), Konrad Triltsch, Würzburg.