REVIEW: Ribosome as a Translocase and Helicase
Chen Bao1,a and Dmitri N. Ermolenko1,b,*
1Department of Biochemistry & Biophysics, School of Medicine and Dentistry and Center for RNA Biology, University of Rochester, Rochester, NY, USA* To whom correspondence should be addressed.
Received May 10, 2021; Revised May 21, 2021; Accepted May 21, 2021
During protein synthesis, ribosome moves along mRNA to decode one codon after the other. Ribosome translocation is induced by a universally conserved protein, elongation factor G (EF-G) in bacteria and elongation factor 2 (EF-2) in eukaryotes. EF-G-induced translocation results in unwinding of the intramolecular secondary structures of mRNA by three base pairs at a time that renders the translating ribosome a processive helicase. Professor Alexander Sergeevich Spirin has made numerous seminal contributions to understanding the molecular mechanism of translocation. Here, we review Spirin’s insights into the ribosomal translocation and recent advances in the field that stemmed from Spirin’s pioneering work. We also discuss key remaining challenges in studies of translocase and helicase activities of the ribosome.
KEY WORDS: ribosome, translocation, helicase, power stroke, Brownian ratchetDOI: 10.1134/S0006297921080095
Abbreviations: EF-G, elongation factor G.
INTRODUCTION
During the elongation phase of protein synthesis, the ribosome decodes sequences of codons by binding of the tRNA molecules charged with amino acids. Both small (30S in bacteria) and large (50S in bacteria) subunits contain three tRNA binding sites: the A (aminoacyl), the P (peptidyl) and the E (exit) sites. At the beginning of elongation cycle, the newly arrived aminoacyl-tRNA binds to the A site of the ribosome. Following the peptidyl transfer reaction, the resulting peptidyl- and deacylated tRNAs together with associated mRNA codons are translocated from the A and P to P and E sites, respectively (Fig. 1, a-f). The process is catalyzed by a universally conserved protein factor EF-G (EF-2 in eukaryotes). The molecular mechanism of translocation has fascinated scientists since the inception of the proteins synthesis field and remained one of the main areas of research in the laboratory of Alexander Spirin for over 50 years [1-6]. Spirin’s laboratory has discovered a number of important facets of the translocation mechanism [7-11]. Spirin’s locking–unlocking [2, 6] and Brownian ratchet [4, 12, 13] models of ribosomal translocation were highly influential and provided framework for investigations of translocation for decades. Below we review key contributions of the Alexander Spirin’s laboratory and recent progress in investigations of ribosomal translocation that stemmed from the Spirin’s ideas. More comprehensive reviews of the current state of understanding of the translocation mechanism can be found elsewhere [14-16].
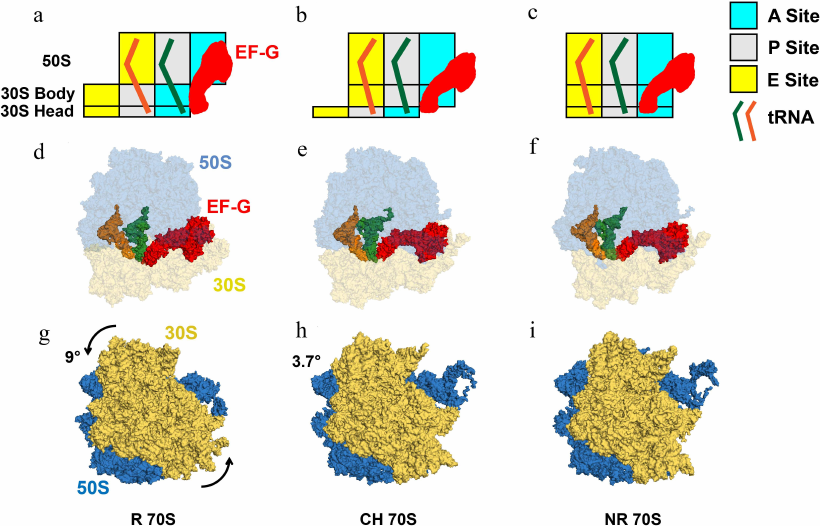
Fig. 1. tRNA movements and conformational rearrangements of the ribosome in EF-G–ribosome complexes. a-c) Box diagrams showing positions of peptidyl- (green) and deacylated (orange) tRNAs relative to the A (cyan), P (grey), and E (yellow) sites on the 50S subunit and 30S head and body. d-f) Structural view from the subunit interface of the 70S ribosomes bound with tRNAs and EF-G, in which 50S and 30S are shown in blue and yellow with transparency, tRNAs in solid orange and green, EF-G in solid red. g-i) Intersubunit rotation accompanying translocation viewed from the solvent side of the 30S. The 50S and 30S are displayed in blue and yellow, and the counter-clockwise rotation of 30S relative to 50S is indicated by the arrows. Degrees of intersubunit rotation (from [17]) measured relative to the structure of non-rotated ribosome (PDBID 4V51). a, d, g) The rotated (R) pre-translocation ribosome is bound with EF-G and tRNAs in A/P and P/E hybrid states (PDBID 4V7D). b, e, h) The partially rotated ribosome containing tRNAs in ap/P and pe/E tRNAs chimeric (CH) states (PDBID 4W29). c, f, i) The post-translocation EF-G-bound ribosome in nonrotated (NR) conformation (PDBID 4V5F), which contains tRNAs bound in classical P/P and E/E state.
ROLES OF EF-G AND tRNAs IN RIBOSOME TRANSLOCATION ALONG
mRNA
Relative to the uncatalyzed reaction, the universally conserved GTPase, EF-G, accelerates the rate of ribosome translocation by ~four orders of magnitude [18-20]. GTP hydrolysis by EF-G is activated by the interaction of the G domain of this protein with the sarcin–ricin loop (SRL) of the 23S rRNA [21, 22]. Works of Kaziro’s and Spirin’s laboratories established the role of GTP hydrolysis in translocation [9, 23-25]. They demonstrated that EF-G induces efficient translocation in the GTP-bound form. In the presence of GDP or in the absence of nucleotides, EF-G does not promote translocation. Replacing GTP with non-hydrolysable analogues preserves the ability of EF-G to induce translocation that was measured by the increase in puromycin reactivity of peptidyl-tRNA or by the release of deacylated tRNA from the ribosome. However, the non-hydrolysable analogues of GTP trap EF-G on the ribosome. These results suggest that GTP hydrolysis is not required for translocation but it is essential for EF-G release. Consistent with the Spirin–Kaziro experiments, more recent kinetic studies have shown that the non-hydrolysable analogues of GTP do not alter the translocation pathway [26, 27]. Furthermore, replacing GTP with non-hydrolysable analogues only moderately affects the rate of a single round of translocation, reducing it by 2-50 folds depending on experimental conditions [19, 26, 28, 29].
Another fundamental aspect of the translocation mechanism was discovered by Spirin and his colleagues in the experiments demonstrating that tRNAs can translocate through the ribosome in the absence of mRNA [10, 11]. These observations suggest that the movement of mRNA is driven by the translocation of the associated anti-codon stem-loops (ASLs) of A- and P-site tRNAs. More recent studies indicate that mRNA translocation requires the presence of ASL in the A site and full-length tRNA in the P site of the pre-translocation ribosome [30]. Consistent with the idea of tRNA-driven translocation of mRNA, single-molecule measurements showed that mRNA translocates three nucleotides at a time without detectable sub-steps [31]. Since tRNAs interact with both 30S and 50S subunits (Fig. 1, a-f), the tRNA-driven mechanism of translocation indicates that both ribosomal subunits are involved in this process.
STRUCTURAL REARRANGEMENTS OF THE RIBOSOME: THE
LOCKING–UNLOCKING HYPOTHESIS
In 1968-1969, Spirin proposed the locking–unlocking model of translocation based on the subunit organization of ribosome structure [1, 2, 6]. This model postulated that (i) tRNA translocation involves transition from the “locked” to the “unlocked” ribosome conformation that facilitates tRNA diffusion through the ribosome; (ii) peptidyl-transferase reaction triggers formation of an intermediate of translocation in which tRNAs are shifted on the large subunit but not yet translocated on the small subunit; (iii) translocation involves movement of the ribosomal subunits relative to each other. Similar ideas were independently proposed by M. S. Bretscher [32]. As discussed below, many aspects of this model turned out to be prophetic. To this day, the rate limiting step of translocation is often referred to as “unlocking”.
Neutron scattering experiments performed by Spirin, Serdyuk, and May provided an early indirect evidence for intersubunit rearrangements accompanying translocation [33, 34]. Nevertheless, further verification of the key predictions of the locking–unlocking model took several decades and required developing new experimental approaches such as chemical probing of RNA structure, cryogenic electron microscopy (cryo-EM), and single-molecule Förster resonance energy transfer (smFRET) microscopy [35].
Twenty years after the introduction of the locking–unlocking model, Danesh Moazed and Harry Noller used chemical probing for mapping of the tRNA binding sites to demonstrate that the reaction of transpeptidation triggers spontaneous translocation of the acceptors stems of the resulting peptidyl- and deacylated tRNAs from the A and P to P and E sites of the large subunit, respectively, while tRNA ASLs remain in the original A and P sites of the small subunit [36]. Hence, intermediate A/P and P/E hybrid states of tRNA binding were formed (Fig. 1, a and d). Completion of translocation of tRNAs on the small subunit was shown to require EF-G and GTP (Fig. 1, a-f).
A decade later, another key prediction of the locking–unlocking model was corroborated by cryo-EM reconstruction of the EF-G–ribosome complex performed by Joachim Frank and Rajendra Agrawal [37]. These experiments demonstrated that EF-G binding induces rotation of the small 30S subunit relative to the large 50S subunit parallel to the plane of the intersubunit interface (Fig. 1, g-i; Fig. 2, a and b). The discoveries of the hybrid-state intermediate and intersubunit rotation were followed by numerous structural and single-molecule studies that provided unprecedented insights into the structural rearrangements of the ribosome, tRNAs, and EF-G accompanying translocation [38, 39].
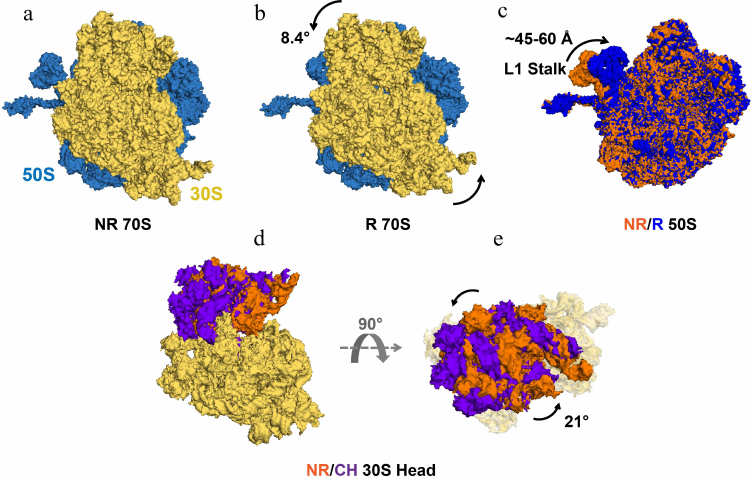
Fig. 2. Intersubunit rotation, L1 stalk movement and 30S head swivel observed in EF-G-free ribosomes. Structures of the 70S ribosome–tRNA complex (PDBID 4V9D) in the non-rotated (NR), classical state (a) and in the rotated (R), hybrid state (b). 50S and 30S ribosomal subunits are shown in blue and yellow, respectively. Both ribosome structures are viewed from the solvent side of the 30S subunit. The counter-clockwise rotation of 30S relative to 50S is indicated by the curved arrows. c) The L1 stalk in 50S subunits of NR (orange) and R (blue) ribosomes (PDBID 4V9D) superimposed by structural alignment of the 23S rRNA. d and e) The swiveling motion of the 30S head domain is shown by structural alignment of the body and platform domains (yellow) of the 16S rRNAs of NR (PDBID 4V51) and chimeric state (CH) (PDBID 4W29) ribosomes. The 30S is viewed from the solvent side of the 30S subunit (d) and from the “top” of the 30S head domain (e). The NR and CH 30S head domains are shown in orange and purple, respectively. Degrees of 30S head swivel in CH and intersubunit rotation in R (from [17]) are measured relative to the NR 70S (PDBID 4V51).
Cryo-EM and FRET experiments revealed that 6-10° intersubunit rotation is coupled with the movement of peptidyl- and deacylated tRNAs into A/P and P/E hybrid states (Fig. 2, a and b) [38, 40-43]. These data established equivalence of the nonrotated and rotated conformations with classical and hybrid states of tRNA binding, respectively. Cryo-EM and smFRET studies have also shown that formation of the rotated, hybrid state of the ribosome is accompanied by the inward movement of the mobile domain of the large ribosomal subunit named L1 stalk (Fig. 2c), which comprises ribosomal protein uL1 and helices 76, 77, and 78 of the 23S rRNA [35, 39]. Upon transition from the open to closed conformation, the extremity of the L1 stalk moves by as much as 60 Å and the L1 stalk becomes bound to the elbow of the P/E tRNA.
smFRET studies also demonstrated that in the absence of EF-G, the pretranslocation ribosome spontaneously fluctuates between the nonrotated, classical and the rotated, hybrid state conformations (Fig. 2, a and b) [38, 44-46]. Binding of EF-G•GTP transiently stabilizes the rotated, hybrid state conformation (Fig. 1, a, d, and g); translocation of mRNA and tRNA on the small subunit is coupled with the reverse transition into non-rotated, classical state conformation (Fig. 1, c, f, and i) [26, 40, 47].
In addition to intersubunit rearrangements and movement of the L1 stalk, translocation is accompanied by large structural changes within the small ribosomal subunit. The small subunit comprises three structural domains: head, body, and platform. Structural studies show that the 30S head rotates by up to 20° relative to the rest of the small subunit around the axis that is orthogonal to the axis of intersubunit rotation (Fig. 2, d and e) [48, 49]. In the EF-G-bound intermediate of translocation visualized by X-ray crystallography and cryo-EM, in which the 30S head is observed in a swiveled conformation, two tRNAs are translocated along the 50S subunit and the 30S platform/body but not yet translocated relative to the 30S head (Fig. 1, b, e, and h) [50, 51]. In these positions named ap/P and pe/E chimeric states, tRNAs are trapped midway between hybrid (A/P and P/E) and posttranslocation classical (P/P and E/E states) and likely represent a late intermediate of translocation.
The tip of domain IV of EF-G plays a critical role in translocation activity of EF-G and reading frame maintenance [19, 52-54]. When EF-G is bound in the rotated hybrid state conformation of the pretranslocation ribosome, domain IV of EF-G is positioned next to ASL of A/P tRNA (Fig. 1, a and d) [55]. Upon translocation, domain IV of EF-G docks into the A site of the small subunit vacated by the peptidyl-tRNA (Fig. 1, c and f) [38, 56]. Hence, upon reverse intersubunit rotation and 30S back-swivel, domain IV of EF-G displaces ASL of peptidyl-tRNA and prevents its backward movement.
Which of the aforementioned conformational rearrangements is the rate-limiting step that “unlocks” the ribosome (using Spirin’s terminology) and facilitates tRNA translocation is not entirely clear. Reaction of transpeptidation “unlocks” the ribosome in a sense that it enables spontaneous intersubunit rotation and fluctuations of tRNAs between the classical and hybrid states [44-46]. However, in the absence of EF-G, these fluctuations are unproductive and do not lead to tRNA/mRNA translocation on the small subunit [44-46]. Several lines of evidence suggest that the domain IV of EF-G destabilizes interactions of A-site tRNA with 16S rRNA [57-61]. Hence, the EF-G-induced changes in the A site may “unlock” the ribosome. Finally, another possible “unlocking” rearrangement is swiveling of the 30S head, which opens the path for tRNA movement from P to E site that is otherwise constricted [49]. Further studies are needed to establish complete sequence of the structural rearrangements accompanying translocation and identify the rate-limiting step in this process.
ENERGETICS OF TRANSLOCATION AND BROWNIAN RATCHET MODEL
Although translation is greatly accelerated by EF-G, the Spirin and Pestka laboratories demonstrated that translocation can occur spontaneously in the absence of protein factors [7, 8, 62]. It was found in the Spirin laboratory that spontaneous translocation is stimulated by modification of the universally conserved ribosomal protein (u)S12 of the 30S subunit by thiol-specific reagents, which were added to inactivate EF-G and thus rule out the presence of trace amounts of this protein [7, 63, 64]. More recent studies indicated that removal of the 30S proteins uS12 and uS13 enhanced the factor-free translocation possibly by weakening tRNA interactions with the 30S A and P sites, respectively [65]. Furthermore, it was reported that a single-round factor-free translocation could be induced by antibiotics sparsomycin, lincomycin, and chloramphenicol that bind to the 50S A site and thus destabilize the A-site tRNA binding [20, 66].
Based on the observations of factor-free translocation, Spirin postulated that translocation is an inherent property of the ribosome and that energy of the peptidyl-transfer reactions is sufficient to promote tRNA movement [3]. However, the slow rate of spontaneous translocation and the observation of reverse spontaneous translocation in some tRNA/mRNA contexts suggest that the reaction of transpeptidation is not the only energy source of translocation, which is also promoted by the energy stored in EF-G·GTP. Indeed, it was estimated that transpeptidation-driven translocation would require ~80% efficiency of the conversion of chemical energy into mechanical motion [67]. Such high efficiency is untypical for macromolecular motors [67].
Two alternative idealized models, the power stroke and the Brownian ratchet models, are employed to describe conversion of chemical energy into mechanical work by macromolecular motors [68, 69]. Chemical energy may be converted into elastic energy or conformational transition that drives large conformational change of the macromolecule, i.e., the power stroke. Alternatively, energy of chemical reaction may be used to bias random, thermally-driven motions of the macromolecule into unidirectional movement. Thus, chemical reaction plays a role that is similar to a pawl directing the movement of a wheel of mechanical ratchet. The chemical change either strictly precedes conformational change (the power stroke) or follows it (the Brownian ratchet) [68]. These two mechanisms can be distinguished by examining the load dependence of the molecular motor movement [68].
Several groups hypothesized that tRNA translocation is mediated by the power stroke of domain IV of EF-G driven by GTP hydrolysis [19, 70, 71]. This hypothesis is supported by kinetic data suggesting that GTP hydrolysis by EF-G precedes translocation [19]. However, the Spirin–Kaziro experiments and more recent kinetic measurements show that translocation occurs rapidly and efficiently in the absence of GTP hydrolysis, when GTP is replaced with non-hydrolysable analogues [9, 19, 23-26, 28, 29]. These data suggest that GTP hydrolysis by EF-G is not coupled with translocation and argue against the GTP hydrolysis-driven power stroke.
Based on the observations of spontaneous, factor-free translocation, Spirin reasoned that Brownian motions of tRNA are sufficient to explain translocation without invoking the power stroke by EF-G [3, 12, 13]. This hypothesis was further corroborated by the smFRET data demonstrating spontaneous intersubunit rotation and fluctuations of tRNA between the classical and hybrid states [44-46]. Spirin’s ideas were ultimately reinforced by the single-molecule optical tweezer measurements of ribosome translocation against applied force showing that EF-G-catalyzed translocation is also best described by the Brownian ratchet model [67].
In the Brownian ratchet mechanism of EF-G-catalyzed translocation, EF-G·GTP likely acts as a pawl of the ratchet that biases tRNA diffusion through the ribosome and couples translocation with the ribosome dynamics [14, 15]. The structure of EF-G trapped in the rotated pretranslocation ribosome (Fig. 1, a and d) reveals the basis for coupling of intersubunit rotation and tRNA/mRNA translocation [55]. Unproductive spontaneous fluctuations of the ribosome from the rotated into nonrotated conformation leads to the return of peptidyl-tRNA from the hybrid A/P into the classical A/A state. However, when EF-G is bound to the rotated pretranslocation ribosome, domain IV of EF-G creates steric hindrance for the return of peptidyl-tRNA from the hybrid A/P into classical A/A state [55]. Similarly, domain IV of EF-G sterically blocks the return of peptidyl-tRNA from the chimeric ap/P into the classical A/A state upon back-swivel of the 30S head [72]. Furthermore, upon translocation of peptidyl-tRNA from the A to P site of the small subunit, domain IV of EF-G docks into the 30S A site thus rendering the tRNA movement irreversible (Fig. 1, c and f).
RIBOSOME TRANSLOCATION IN REGULATION OF TRANSLATION: RIBOSOME AS
A HELICASE
While unprecedented molecular details of the translocation mechanism have recently emerged from the structural studies and single-molecule biophysical measurements, it remains less clear how the rate of translocation is modulated in live cells to regulate translation elongation. Eukaryotic translocase EF-2 was shown to be downregulated under stress conditions by phosphorylation [73-75]. Besides, EF-2 can be inactivated by ADP-ribosylation catalyzed by diphtheria toxin [76]. A number of antibiotics hamper cell growth by hindering translocation in bacteria [14]. Arguably the least understood and most fascinating aspect of the translocation regulation is modulation of the translocation rate by mRNA secondary structure.
Computational analyses suggest that most, if not all, mRNAs have the propensity to form extensive intramolecular secondary structures throughout the entire sequence including the Open Reading Frame (ORF) [77]. mRNA folding results in the formation of compact structures with short end-to-end distances [78]. In vivo transcriptome-wide RNA structure probing studies [79-84] show that mRNAs fold in live cells, at least to some degree, despite the presence of RNA helicases and other RNA binding proteins, which can disrupt RNA secondary structure. Consistent with the idea that mRNAs form extensive secondary structure in vivo, a number of structured mRNA elements were shown to regulate translation initiation, including bacterial riboswitches [85], frameshift-inducing hairpins and pseudoknots of eukaryotic viruses [86], Internal Ribosome Entry Sites (IRES) [87], Iron Response Elements (IRE) in the 5′-UTR of transcripts coding for proteins involved in iron metabolism [88], and Cap-Independent Translational Enhancers (CITEs) [89]. Furthermore, protein and miRNA binding to mRNA was found to be governed by the RNA structure, which can occlude sites [90-95] providing further evidence for the importance of mRNA secondary structure.
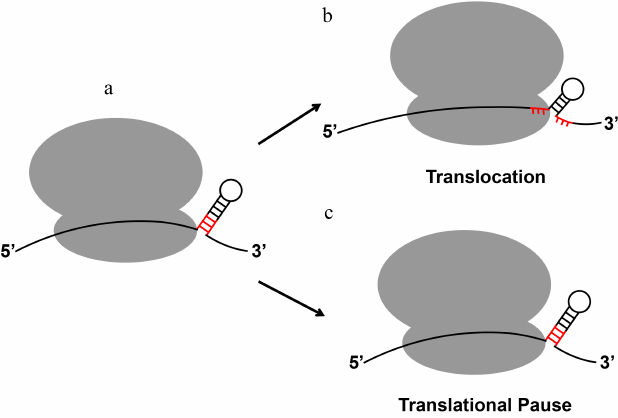
Biochemical and single-molecule experiments revealed that the translating ribosome is a very efficient helicase [96, 97], which unwinds three base pairs per translocation step (Fig. 3). The translating ribosome unfolds mRNA secondary structure because the narrow mRNA channel of the small ribosomal subunit can only accommodate a single-stranded mRNA [96, 98-101]. Consistent with the demonstrations of helicase activity of the ribosome, transcriptome-wide ribosome profiling analysis demonstrates that most of the secondary structure elements within the coding regions of mRNAs do not influence the rate of translation elongation [102]. Most structured mRNA elements, which regulate translation, reside either in the 5′- or 3′-UTRs.
Fig. 3. mRNA stem-loops can either be quickly unwound by the ribosome or induce ribosome pausing. When positioned at the mRNA entry channel, certain regulatory secondary structures of mRNA induce translational pause despite the helicase activity of the elongating ribosome. The three base pairs of the stem-loop adjacent to the mRNA entry channel that become unwound by a translocation step are shown in red.
Helicase activity of the ribosome likely plays a major role in the remodeling of mRNA structure and regulating mRNA interactions with RNA-binding proteins [103]. For example, mRNA translation in poly-ribosomes renders the mRNA ORF devoid of secondary structure due to the ribosome helicase activity [103-105]. The pioneer round of mRNA translation by the ribosome, which displaces exon junction protein complexes (EJCs) and other proteins deposited on mRNA in the nucleus [106], may enable mRNA folding into compact structures after termination of protein synthesis. Indeed, the single-molecule-resolution fluorescent in situ hybridization (smFISH) and proximity-ligation studies indicate lack of interactions between the distant segments of nuclear mRNAs bound with exon junction protein complexes (EJCs) [104, 107]. By contrast, the 5′- and 3′-ends of cytoplasmic mRNAs, which are not actively translated, are co-localized through the formation of intramolecular secondary structure [104, 105] that tend to bring mRNA ends in close proximity [78, 108].
Paradoxically, in spite of the ribosome helicase activity, certain RNA stem-loop structures can induce ribosome stalling that results in accumulation of truncated polypeptides [109] and No-Go mRNA decay (Fig. 3) [110]. Furthermore, the evolutionarily conserved mRNA stem-loops and pseudoknots trigger programmed translation pauses [111] and stimulate –1 programmed ribosomal frameshifting (PRF), which controls expression of a number of proteins in bacteria, viruses and eukaryotes [112]. In particular, –1 PRF regulates synthesis of DNA polymerase III in bacteria [113]; HIV cytokine receptor ccr5 in higher eukaryotes [114]; gag-pol proteins in retroviruses, including Human Immunodeficiency Virus (HIV) [115]; and C-terminally extended polyprotein in coronaviruses, including SARS-CoV-2, which caused the COVID-19 pandemic [116, 117].
The mechanism of ribosome pausing induced by mRNA secondary structure is not fully understood. A number of published single-molecule studies suggest that slow unwinding of a secondary structure, to which ribosome pausing is often attributed, is an unlikely explanation of the extent of translation inhibition induced by certain mRNA stem-loops [31, 118, 119]. Translocation through three GC base pairs is only 2 to 3-fold slower than translocation along a single-stranded codon [31, 118, 119] indicating that the stability of the three base pairs adjacent to the mRNA channel has a relatively moderate effect on translocation rate. By contrast, the frameshift-inducing stem-loops and pseudoknots were shown to produce extended ribosome pauses [120-127].
It appears that rather than creating a simple road block for the ribosome, mRNA stem-loops induce programmed ribosome pauses by making specific interactions with the ribosome. Recent studies suggest that the frameshift-inducing mRNA stem-loops can perturb translation elongation by docking into the 30S A site hindering tRNA binding [128]. Furthermore, when positioned at the entry of 30S mRNA channel, frameshift-inducing stem-loops and pseudoknots were shown to inhibit the rate of ribosomal translocation by more than one order of magnitude in a number of kinetic ensemble and single-molecule experiments [120, 121, 126-129]. It has been recently demonstrated that upon encountering the mRNA secondary structure the ribosome translocates through two alternative pathways (fast and slow) [31]. Interactions of the frameshift-inducing stem-loops and pseudoknots with the mRNA entry channel may increase the flux through the slow pathway and thus decrease the average rate of ribosome translocation [31].
Many of Spirin’s ingenious insights into the mechanism of ribosomal translocation were corroborated in the last few decades with advances brought by high-resolution structures of the ribosome and single-molecule biophysical experiments. Nevertheless, ribosomal translocation along mRNA remains one of the most fascinating steps of proteins synthesis. The complete “movie” reconstructing structural rearrangements of the ribosome, EF-G, and tRNA during translocation is yet to materialize. A more complete understanding of how ribosome translocation remodels mRNA secondary structure and modulates interactions of mRNA with many regulatory proteins is just beginning to emerge. Mechanisms of regulation of ribosome translocation by mRNA secondary structure await further investigation.
Acknowledgments. This article is dedicated to the memory of Prof. Spirin who was undergraduate research mentor to one of the authors (D. N. E.). D. N. E. is grateful to Alexander Sergeevich Spirin for his invaluable lessons of critical thinking and research rigor. The authors thank Gregory Ballard for his comments on the manuscript.
Funding. The research in the Ermolenko laboratory is supported by NIH grants R01GM099719 and R01GM132041.
Ethics declarations. The authors declare no conflicts of interest in financial or any other sphere. This article does not contain any studies involving human participants or animals performed by any of the authors.
REFERENCES
1.Spirin, A. S. (1968) How does the ribosome work? A
hypothesis based on the two subunit construction of the ribosome,
Curr. Mod. Biol., 2, 115-127, doi:
10.1016/0303-2647(68)90017-8.
2.Spirin, A. S. (1969) A model of the functioning
ribosome: locking and unlocking of the ribosome subparticles, Cold
Spring Harb. Symp. Quant. Biol., 34, 197-207, doi:
10.1101/sqb.1969.034.01.026.
3.Spirin, A. S. (1985) Ribosomal translocation: facts
and models, Prog. Nucleic Acid. Res. Mol. Biol., 32,
75-114, doi: 10.1016/s0079-6603(08)60346-3.
4.Spirin, A. S. (2009) The ribosome as a conveying
thermal ratchet machine, J. Biol. Chem., 284,
21103-21119, doi: 10.1074/jbc.X109.001552.
5.Finkelstein, A. V., Razin, S. V., and Spirin, A. S.
(2018) Intersubunit mobility of the ribosome, Mol. Biol.
(Mosk.), 52, 921-934, doi: 10.1134/S0026898418060083.
6.Spirin, A. S. (1968) On the mechanism of ribosome
function. The hypothesis of locking-unlocking of subparticles [in
Russsian], Dokl. Akad. Nauk SSSR, 179, 1467-1470.
7.Gavrilova, L. P., and Spirin, A. S. (1971)
Stimulation of “non-enzymic” translocation in ribosomes by
p-chloromercuribenzoate, FEBS Lett., 17, 324-326, doi:
10.1016/0014-5793(71)80177-1.
8.Gavrilova, L. P., and Spirin, A. S. (1974)
“Nonenzymatic” translation, Methods Enzymol.,
30, 452-462, doi: 10.1016/0076-6879(74)30045-6.
9.Belitsina, N. V., Glukhova, M. A., and Spirin, A.
S. (1975) Translocation in ribosomes by attachment-detachment of
elongation factor G without GTP cleavage: evidence from a column-bound
ribosome system, FEBS Lett., 54, 35-38, doi:
10.1016/0014-5793(75)81062-3.
10.Belitsina, N. V., Tnalina, G. Z., and Spirin, A.
S. (1981) Template-free ribosomal synthesis of polylysine from
lysyl-tRNA, FEBS Lett., 131, 289-292, doi:
10.1016/0014-5793(81)80387-0.
11.Belitsina, N. V., Tnalina, G. Z., and Spirin, A.
S. (1982) Template-free ribosomal synthesis of polypeptides from
aminoacyl-tRNAs, Biosystems, 15, 233-241, doi:
10.1016/0303-2647(82)90008-9.
12.Spirin, A. S. (2002) Ribosome as a molecular
machine, FEBS Lett., 514, 2-10, doi:
10.1016/s0014-5793(02)02309-8.
13.Spirin, A. S. (2004) The ribosome as an RNA-based
molecular machine, RNA Biol., 1, 3-9, doi:
10.4161/rna.1.1.889.
14.Ling, C., and Ermolenko, D. N. (2016) Structural
insights into ribosome translocation, Wiley Interdiscip. Rev.
RNA, 7, 620-636, doi: 10.1002/wrna.1354.
15.Noller, H. F., Lancaster, L., Zhou, J., and
Mohan, S. (2017) The ribosome moves: RNA mechanics and translocation,
Nat. Struct. Mol. Biol., 24, 1021-1027, doi:
10.1038/nsmb.3505.
16.Rodnina, M. V., Peske, F., Peng, B. Z.,
Belardinelli, R., and Wintermeyer, W. (2019) Converting GTP hydrolysis
into motion: versatile translational elongation factor G, Biol.
Chem., 401, 131-142, doi: 10.1515/hsz-2019-0313.
17.Mohan, S., Donohue, J. P., and Noller, H. F.
(2014) Molecular mechanics of 30S subunit head rotation, Proc. Natl.
Acad. Sci. USA, 111, 13325-13330, doi:
10.1073/pnas.1413731111.
18.Gavrilova, L. P., Kostiashkina, O. E.,
Koteliansky, V. E., Rutkevitch, N. M., and Spirin, A. S. (1976)
Factor-free (“non-enzymic”) and factor-dependent systems of
translation of polyuridylic acid by Escherichia coli
ribosomes, J. Mol. Biol., 101, 537-552, doi:
10.1016/0022-2836(76)90243-6.
19.Rodnina, M. V., Savelsbergh, A., Katunin, V. I.,
and Wintermeyer, W. (1997) Hydrolysis of GTP by elongation factor G
drives tRNA movement on the ribosome, Nature, 385, 37-41,
doi: 10.1038/385037a0.
20.Fredrick, K., and Noller, H. F. (2003) Catalysis
of ribosomal translocation by sparsomycin, Science, 300,
1159-1162, doi: 10.1126/science.1084571.
21.Parmeggiani, A., and Sander, G. (1981) Properties
and regulation of the GTPase activities of elongation factors Tu and G,
and of initiation factor 2, Mol. Cell. Biochem., 35,
129-158, doi: 10.1007/BF02357085.
22.Moazed, D., Robertson, J. M., and Noller, H. F.
(1988) Interaction of elongation factors EF-G and EF-Tu with a
conserved loop in 23S RNA, Nature, 334, 362-364, doi:
10.1038/334362a0.
23.Inoue-Yokosawa, N., Ishikawa, C., and Kaziro, Y.
(1974) The role of guanosine triphosphate in translocation reaction
catalyzed by elongation factor G, J. Biol. Chem., 249,
4321-4323, doi: 10.1016/S0021-9258(19)42519-2.
24.Belitsina, N. V., Glukhova, M. A., and Spirin, A.
S. (1976) Stepwise elongation factor G-promoted elongation of
polypeptides on the ribosome without GTP cleavage, J. Mol.
Biol., 108, 609-613, doi: 10.1016/s0022-2836(76)80140-4.
25.Belitsina, N. V., Glukhova, M. A., and Spirin, A.
S. (1979) Elongation factor G-promoted translocation and polypeptide
elongation in ribosomes without GTP cleavage: use of columns with
matrix-bound polyuridylic acid, Methods Enzymol., 60,
761-779, doi: 10.1016/s0076-6879(79)60070-8.
26.Ermolenko, D. N., and Noller, H. F. (2011) mRNA
translocation occurs during the second step of ribosomal intersubunit
rotation, Nat. Struct. Mol. Biol., 18, 457-462, doi:
10.1038/nsmb.2011.
27.Flis, J., Holm, M., Rundlet, E. J., Loerke, J.,
Hilal, T., et al. (2018) tRNA Translocation by the eukaryotic 80S
ribosome and the impact of GTP hydrolysis, Cell Rep., 25,
2676-2688.e7, doi: 10.1016/j.celrep.2018.11.040.
28.Pan, D., Kirillov, S. V., and Cooperman, B. S.
(2007) Kinetically competent intermediates in the translocation step of
protein synthesis, Mol. Cell, 25, 519-529, doi:
10.1016/j.molcel.2007.01.014.
29.Salsi, E., Farah, E., and Ermolenko, D. N. (2016)
EF-G activation by phosphate analogs, J. Mol. Biol., 428,
2248-2258, doi: 10.1016/j.jmb.2016.03.032.
30.Joseph, S., and Noller, H. F. (1998)
EF-G-catalyzed translocation of anticodon stem-loop analogs of transfer
RNA in the ribosome, EMBO J., 17, 3478-3483, doi:
10.1093/emboj/17.12.3478.
31.Desai, V. P., Frank, F., Lee, A., Righini, M.,
Lancaster, L., et al. (2019) Co-temporal force and fluorescence
measurements reveal a ribosomal gear shift mechanism of translation
regulation by structured mRNAs, Mol. Cell, 75,
1007-1019.e5, doi: 10.1016/j.molcel.2019.07.024.
32.Bretscher, M. S. (1968) Translocation in protein
synthesis: a hybrid structure model, Nature, 218,
675-677, doi: 10.1038/218675a0.
33.Spirin, A. S., Baranov, V. I., Polubesov, G. S.,
Serdyuk, I. N., and May, R. P. (1987) Translocation makes the ribosome
less compact, J. Mol. Biol., 194, 119-126, doi:
10.1016/0022-2836(87)90720-0.
34.Serdyuk, I., Baranov, V., Tsalkova, T.,
Gulyamova, D., Pavlov, M., et al. (1992) Structural dynamics of
translating ribosomes, Biochimie, 74, 299-306, doi:
10.1016/0300-9084(92)90107-p.
35.Frank, J., and Gonzalez, R. L., Jr. (2010)
Structure and dynamics of a processive Brownian motor: the translating
ribosome, Annu. Rev. Biochem., 79, 381-412, doi:
10.1146/annurev-biochem-060408-173330.
36.Moazed, D., and Noller, H. F. (1989) Intermediate
states in the movement of transfer RNA in the ribosome, Nature,
342, 142-148, doi: 10.1038/342142a0.
37.Frank, J., and Agrawal, R. K. (2000) A
ratchet-like inter-subunit reorganization of the ribosome during
translocation, Nature, 406, 318-322, doi:
10.1038/35018597.
38.Valle, M., Zavialov, A., Sengupta, J., Rawat, U.,
Ehrenberg, M., and Frank, J. (2003) Locking and unlocking of ribosomal
motions, Cell, 114, 123-134, doi:
10.1016/S0092-8674(03)00476-8.
39.Korostelev, A., Ermolenko, D. N., and Noller, H.
F. (2008) Structural dynamics of the ribosome, Curr. Opin. Chem.
Biol., 12, 674-683, doi: 10.1016/j.cbpa.2008.08.037.
40.Ermolenko, D. N., Majumdar, Z. K., Hickerson, R.
P., Spiegel, P. C., Clegg, R. M., and Noller, H. F. (2007) Observation
of intersubunit movement of the ribosome in solution using FRET, J.
Mol. Biol., 370, 530-540, doi:
10.1016/j.jmb.2007.04.042.
41.Ermolenko, D. N., Spiegel, P. C., Majumdar, Z.
K., Hickerson, R. P., Clegg, R. M., and Noller, H. F. (2007) The
antibiotic viomycin traps the ribosome in an intermediate state of
translocation, Nat. Struct. Mol. Biol., 14, 493-497, doi:
10.1038/nsmb1243.
42.Agirrezabala, X., Lei, J., Brunelle, J. L.,
Ortiz-Meoz, R. F., Green, R., and Frank, J. (2008) Visualization of the
hybrid state of tRNA binding promoted by spontaneous ratcheting of the
ribosome, Mol. Cell, 32, 190-197, doi:
10.1016/j.molcel.2008.10.001.
43.Julian, P., Konevega, A. L., Scheres, S. H.,
Lazaro, M., Gil, D., et al. (2008) Structure of ratcheted ribosomes
with tRNAs in hybrid states, Proc. Natl. Acad. Sci. USA,
105, 16924-16927, doi: 10.1073/pnas.0809587105.
44.Blanchard, S. C., Kim, H. D., Gonzalez, R. L.,
Jr., Puglisi, J. D., and Chu, S. (2004) tRNA dynamics on the ribosome
during translation, Proc. Natl. Acad. Sci. USA, 101,
12893-12898, doi: 10.1073/pnas.0403884101.
45.Cornish, P. V., Ermolenko, D. N., Noller, H. F.,
and Ha, T. (2008) Spontaneous intersubunit rotation in single
ribosomes, Mol. Cell, 30, 578-588, doi:
10.1016/j.molcel.2008.05.004.
46.Fei, J., Kosuri, P., MacDougall, D. D., and
Gonzalez, R. L., Jr. (2008) Coupling of ribosomal L1 stalk and tRNA
dynamics during translation elongation, Mol. Cell, 30,
348-359, doi: 10.1016/j.molcel.2008.03.012.
47.Marshall, R. A., Aitken, C. E., and Puglisi, J.
D. (2009) GTP hydrolysis by IF2 guides progression of the ribosome into
elongation, Mol. Cell, 35, 37-47, doi:
10.1016/j.molcel.2009.06.008.
48.Spahn, C. M., Gomez-Lorenzo, M. G., Grassucci, R.
A., Jorgensen, R., Andersen, G. R., et al. (2004) Domain movements of
elongation factor eEF2 and the eukaryotic 80S ribosome facilitate tRNA
translocation, EMBO J., 23, 1008-1019, doi:
10.1038/sj.emboj.7600102.
49.Schuwirth, B. S., Borovinskaya, M. A., Hau, C.
W., Zhang, W., Vila-Sanjurjo, A., et al. (2005) Structures of the
bacterial ribosome at 3.5 Å resolution, Science,
310, 827-834, doi: 10.1126/science.1117230.
50.Ramrath, D. J., Lancaster, L., Sprink, T.,
Mielke, T., Loerke, J., et al. (2013) Visualization of two transfer
RNAs trapped in transit during elongation factor G-mediated
translocation, Proc. Natl. Acad. Sci. USA, 110,
20964-20969, doi: 10.1073/pnas.1320387110.
51.Zhou, J., Lancaster, L., Donohue, J. P., and
Noller, H. F. (2014) How the ribosome hands the A-site tRNA to the P
site during EF-G-catalyzed translocation, Science, 345,
1188-1191, doi: 10.1126/science.1255030.
52.Martemyanov, K. A., and Gudkov, A. T. (1999)
Domain IV of elongation factor G from Thermus thermophilus is strictly
required for translocation, FEBS Lett., 452, 155-159,
doi: 10.1016/S0014-5793(99)00635-3.
53.Niblett, D., Nelson, C., Leung, C. S., Rexroad,
G., Cozy, J., et al. (2021) Mutations in domain IV of elongation factor
EF-G confer-1 frameshifting, RNA, 27, 40-53, doi:
10.1261/rna.077339.120.
54.Peng, B. Z., Bock, L. V., Belardinelli, R.,
Peske, F., Grubmuller, H., and Rodnina, M. V. (2019) Active role of
elongation factor G in maintaining the mRNA reading frame during
translation, Sci. Adv., 5, eaax8030, doi:
10.1126/sciadv.aax8030.
55.Brilot, A. F., Korostelev, A. A., Ermolenko, D.
N., and Grigorieff, N. (2013) Structure of the ribosome with elongation
factor G trapped in the pretranslocation state, Proc. Natl. Acad.
Sci. USA, 110, 20994-20999, doi:
10.1073/pnas.1311423110.
56.Salsi, E., Farah, E., Dann, J., and Ermolenko, D.
N. (2014) Following movement of domain IV of elongation factor G during
ribosomal translocation, Proc. Natl. Acad. Sci. USA, 111,
15060-15065, doi: 10.1073/pnas.1410873111.
57.Gao, Y. G., Selmer, M., Dunham, C. M.,
Weixlbaumer, A., Kelley, A. C., and Ramakrishnan, V. (2009) The
structure of the ribosome with elongation factor G trapped in the
posttranslocational state, Science, 326, 694-699, doi:
10.1126/science.1179709.
58.Khade, P. K., and Joseph, S. (2011) Messenger RNA
interactions in the decoding center control the rate of translocation,
Nat. Struct. Mol. Biol., 18, 1300-1302, doi:
10.1038/nsmb.2140.
59.Liu, G., Song, G., Zhang, D., Zhang, D., Li, Z.,
et al. (2014) EF-G catalyzes tRNA translocation by disrupting
interactions between decoding center and codon-anticodon duplex,
Nat. Struct. Mol. Biol., 21, 817-824, doi:
10.1038/nsmb.2869.
60.Abeyrathne, P. D., Koh, C. S., Grant, T.,
Grigorieff, N., and Korostelev, A. A. (2016) Ensemble cryo-EM uncovers
inchworm-like translocation of a viral IRES through the ribosome,
Elife, 5, e14874, doi: 10.7554/eLife.14874.
61.Taylor, D. J., Nilsson, J., Merrill, A. R.,
Andersen, G. R., Nissen, P., and Frank, J. (2007) Structures of
modified eEF2 80S ribosome complexes reveal the role of GTP hydrolysis
in translocation, EMBO J., 26, 2421-2431, doi:
10.1038/sj.emboj.7601677.
62.Pestka, S. (1968) Studies on the formation of
transfer ribonucleic acid-ribosome complexes. 3. The formation of
peptide bonds by ribosomes in the absence of supernatant enzymes, J.
Biol. Chem., 243, 2810-2820, doi:
10.1016/S0021-9258(18)93445-9.
63.Gavrilova, L. P., Koteliansky, V. E., and Spirin,
A. S. (1974) Ribosomal protein S12 and ‘non-enzymatic’
translocation, FEBS Lett., 45, 324-328, doi:
10.1016/0014-5793(74)80872-0.
64.Gavrilova, L. P., and Spirin, A. S. (1974)
Interaction of SH-reagents with the ribosomal 30 S subparticle and
‘non-enzymatic’ translocation, FEBS Lett.,
39, 13-16, doi: 10.1016/0014-5793(74)80005-0.
65.Cukras, A. R., Southworth, D. R., Brunelle, J.
L., Culver, G. M., and Green, R. (2003) Ribosomal proteins S12 and S13
function as control elements for translocation of the mRNA:tRNA
complex, Mol. Cell, 12, 321-328, doi:
10.1016/s1097-2765(03)00275-2.
66.Ermolenko, D. N., Cornish, P. V., Ha, T., and
Noller, H. F. (2013) Antibiotics that bind to the A site of the large
ribosomal subunit can induce mRNA translocation, RNA, 19,
158-166, doi: 10.1261/rna.035964.112.
67.Liu, T., Kaplan, A., Alexander, L., Yan, S., Wen,
J. D., et al. (2014) Direct measurement of the mechanical work during
translocation by the ribosome, Elife, 3, e03406, doi:
10.7554/eLife.03406.
68.Howard, J. (2006) Protein power strokes, Curr.
Biol., 16, R517-519, doi: 10.1016/j.cub.2006.06.045.
69.Hwang, W., and Karplus, M. (2019) Structural
basis for power stroke vs. Brownian ratchet mechanisms of motor
proteins, Proc. Natl. Acad. Sci. USA, 116, 19777-19785,
doi: 10.1073/pnas.1818589116.
70.Peske, F., Matassova, N. B., Savelsbergh, A.,
Rodnina, M. V., and Wintermeyer, W. (2000) Conformationally restricted
elongation factor G retains GTPase activity but is inactive in
translocation on the ribosome, Mol. Cell, 6, 501-505,
doi: 10.1016/S1097-2765(00)00049-6.
71.Chen, C., Cui, X., Beausang, J. F., Zhang, H.,
Farrell, I., et al. (2016) Elongation factor G initiates translocation
through a power stroke, Proc. Natl. Acad. Sci. USA, 113,
7515-7520, doi: 10.1073/pnas.1602668113.
72.Ratje, A. H., Loerke, J., Mikolajka, A., Brunner,
M., Hildebrand, P. W., et al. (2010) Head swivel on the ribosome
facilitates translocation by means of intra-subunit tRNA hybrid sites,
Nature, 468, 713-716, doi: 10.1038/nature09547.
73.Ryazanov, A. G., Natapov, P. G., Shestakova, E.
A., Severin, F. F., and Spirin, A. S. (1988) Phosphorylation of the
elongation factor 2: the fifth Ca2+/calmodulin-dependent
system of protein phosphorylation, Biochimie, 70,
619-626, doi: 10.1016/0300-9084(88)90245-3.
74.Ryazanov, A. G., Shestakova, E. A., and Natapov,
P. G. (1988) Phosphorylation of elongation factor 2 by EF-2 kinase
affects rate of translation, Nature, 334, 170-173, doi:
10.1038/334170a0.
75.Ryazanov, A. G., Rudkin, B. B., and Spirin, A. S.
(1991) Regulation of protein synthesis at the elongation stage. New
insights into the control of gene expression in eukaryotes, FEBS
Lett., 285, 170-175, doi: 10.1016/0014-5793(91)80798-8.
76.Davydova, E. K., and Ovchinnikov, L. P. (1990)
ADP-ribosylated elongation factor 2 (ADP-ribosyl-EF-2) is unable to
promote translocation within the ribosome, FEBS Lett.,
261, 350-352, doi: 10.1016/0014-5793(90)80589-B.
77.Ermolenko, D. N., and Mathews, D. H. (2021)
Making ends meet: new functions of mRNA secondary structure, Wiley
Interdiscip. Rev. RNA, 12, e1611, doi:
10.1002/wrna.1611.
78.Lai, W. C., Kayedkhordeh, M., Cornell, E. V.,
Farah, E., Bellaousov, S., et al. (2018) mRNAs and lncRNAs
intrinsically form secondary structures with short end-to-end
distances, Nat. Commun., 9, 4328, doi:
10.1038/s41467-018-06792-z.
79.Ding, Y., Tang, Y., Kwok, C. K., Zhang, Y.,
Bevilacqua, P. C., and Assmann, S. M. (2014) In vivo genome-wide
profiling of RNA secondary structure reveals novel regulatory features,
Nature, 505, 696-700, doi: 10.1038/nature12756.
80.Rouskin, S., Zubradt, M., Washietl, S., Kellis,
M., and Weissman, J. S. (2014) Genome-wide probing of RNA structure
reveals active unfolding of mRNA structures in vivo,
Nature, 505, 701-705, doi: 10.1038/nature12894.
81.Aw, J. G., Shen, Y., Wilm, A., Sun, M., Lim, X.
N., et al. (2016) In vivo mapping of eukaryotic RNA interactomes
reveals principles of higher-order organization and regulation, Mol.
Cell, 62, 603-617, doi: 10.1016/j.molcel.2016.04.028.
82.Lu, Z., Zhang, Q. C., Lee, B., Flynn, R. A.,
Smith, M. A., et al. (2016) RNA Duplex map in living cells reveals
higher-order transcriptome structure, Cell, 165,
1267-1279, doi: 10.1016/j.cell.2016.04.028.
83.Sharma, E., Sterne-Weiler, T., O’Hanlon,
D., and Blencowe, B. J. (2016) Global mapping of human RNA–RNA
interactions, Mol. Cell, 62, 618-626, doi:
10.1016/j.molcel.2016.04.030.
84.Ziv, O., Gabryelska, M. M., Lun, A. T. L.,
Gebert, L. F. R., Sheu-Gruttadauria, J., et al. (2018) COMRADES
determines in vivo RNA structures and interactions, Nat.
Methods, 15, 785-788, doi: 10.1038/s41592-018-0121-0.
85.Roth, A., and Breaker, R. R. (2009) The
structural and functional diversity of metabolite-binding riboswitches,
Annu. Rev. Biochem., 78, 305-334, doi:
10.1146/annurev.biochem.78.070507.135656.
86.Giedroc, D. P., and Cornish, P. V. (2009)
Frameshifting RNA pseudoknots: structure and mechanism, Virus
Res., 139, 193-208, doi: 10.1016/j.virusres.2008.06.008.
87.Mauger, D. M., Siegfried, N. A., and Weeks, K. M.
(2013) The genetic code as expressed through relationships between mRNA
structure and protein function, FEBS Lett., 587,
1180-1188, doi: 10.1016/j.febslet.2013.03.002.
88.Leipuviene, R., and Theil, E. C. (2007) The
family of iron responsive RNA structures regulated by changes in
cellular iron and oxygen, Cell. Mol. Life Sci., 64,
2945-2955, doi: 10.1007/s00018-007-7198-4.
89.Simon, A. E., and Miller, W. A. (2013) 3′
cap-independent translation enhancers of plant viruses, Annu. Rev.
Microbiol., 67, 21-42, doi:
10.1146/annurev-micro-092412-155609.
90.Shao, Y., Chan, C. Y., Maliyekkel, A., Lawrence,
C. E., Roninson, I. B., and Ding, Y. (2007) Effect of target secondary
structure on RNAi efficiency, RNA, 13, 1631-1640, doi:
10.1261/rna.546207.
91.Lu, Z. J., and Mathews, D. H. (2008) Efficient
siRNA selection using hybridization thermodynamics, Nucleic Acids
Res., 36, 640-647, doi: 10.1093/nar/gkm920.
92.Lu, Z. J., and Mathews, D. H. (2008) Fundamental
differences in the equilibrium considerations for siRNA and antisense
oligodeoxynucleotide design, Nucleic Acids Res., 36,
3738-3745, doi: 10.1093/nar/gkn266.
93.Tafer, H., Ameres, S. L., Obernosterer, G.,
Gebeshuber, C. A., Schroeder, R., et al. (2008) The impact of target
site accessibility on the design of effective siRNAs, Nat.
Biotechnol., 26, 578-583, doi: 10.1038/nbt1404.
94.Li, X., Quon, G., Lipshitz, H. D., and Morris, Q.
(2010) Predicting in vivo binding sites of RNA-binding proteins
using mRNA secondary structure, RNA, 16, 1096-1107, doi:
10.1261/rna.2017210.
95.Li, X., Kazan, H., Lipshitz, H. D., and Morris,
Q. D. (2014) Finding the target sites of RNA-binding proteins, Wiley
Interdiscip. Rev. RNA, 5, 111-130, doi:
10.1002/wrna.1201.
96.Takyar, S., Hickerson, R. P., and Noller, H. F.
(2005) mRNA helicase activity of the ribosome, Cell, 120,
49-58, doi: 10.1016/j.cell.2004.11.042.
97.Wen, J. D., Lancaster, L., Hodges, C., Zeri, A.
C., Yoshimura, S. H., et al. (2008) Following translation by single
ribosomes one codon at a time, Nature, 452, 598-603, doi:
10.1038/nature06716.
98.Yusupova, G. Z., Yusupov, M. M., Cate, J. H., and
Noller, H. F. (2001) The path of messenger RNA through the ribosome,
Cell, 106, 233-241, doi:
10.1016/s0092-8674(01)00435-4.
99.Balakin, A., Skripkin, E., Shatsky, I., and
Bogdanov, A. (1990) Transition of the mRNA sequence downstream from the
initiation codon into a single-stranded conformation is strongly
promoted by binding of the initiator tRNA, Biochim. Biophys.
Acta, 1050, 119-123, doi: 10.1016/0167-4781(90)90151-q.
100.De Smit, M. H., and van Duin, J. (1990)
Secondary structure of the ribosome binding site determines
translational efficiency: a quantitative analysis, Proc. Natl. Acad.
Sci. USA, 87, 7668-7672, doi: 10.1073/pnas.87.19.7668.
101.Poot, R. A., Tsareva, N. V., Boni, I. V., and
van Duin, J. (1997) RNA folding kinetics regulates translation of phage
MS2 maturation gene, Proc. Natl. Acad. Sci. USA, 94,
10110-10115, doi: 10.1073/pnas.94.19.10110.
102.Del Campo, C., Bartholomaus, A., Fedyunin, I.,
and Ignatova, Z. (2015) Secondary Structure across the bacterial
transcriptome reveals versatile roles in mRNA regulation and function,
PLoS Genet., 11, e1005613, doi:
10.1371/journal.pgen.1005613.
103.Khong, A., and Parker, R. (2020) The landscape
of eukaryotic mRNPs, RNA, 26, 229-239, doi:
10.1261/rna.073601.119.
104.Adivarahan, S., Livingston, N., Nicholson, B.,
Rahman, S., Wu, B., Rissland, O. S., and Zenklusen, D. (2018) Spatial
organization of single mRNPs at different stages of the gene expression
pathway, Mol. Cell, 72, 727-738.e5, doi:
10.1016/j.molcel.2018.10.010.
105.Khong, A., and Parker, R. (2018) mRNP
architecture in translating and stress conditions reveals an ordered
pathway of mRNP compaction, J. Cell Biol., 217,
4124-4140, doi: 10.1083/jcb.201806183.
106.Maquat, L. E., Tarn, W. Y., and Isken, O.
(2010) The pioneer round of translation: features and functions,
Cell, 142, 368-374, doi: 10.1016/j.cell.2010.07.022.
107.Metkar, M., Ozadam, H., Lajoie, B. R., Imakaev,
M., Mirny, L. A., et al. (2018) Higher-order organization principles of
pre-translational mRNPs, Mol. Cell, 72, 715-726.3, doi:
10.1016/j.molcel.2018.09.012.
108.Cetin, B., Song, G. J., and O’Leary, S.
E. (2020) Heterogeneous dynamics of protein–RNA interactions
across transcriptome-derived messenger RNA populations, J. Am. Chem.
Soc., 142, 21249-21253, doi: 10.1021/jacs.0c09841.
109.Yan, S., Wen, J. D., Bustamante, C., and
Tinoco, I., Jr. (2015) Ribosome excursions during mRNA translocation
mediate broad branching of frameshift pathways, Cell,
160, 870-881, doi: 10.1016/j.cell.2015.02.003.
110.Doma, M. K., and Parker, R. (2006)
Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation
elongation, Nature, 440, 561-564, doi:
10.1038/nature04530.
111.Young, J. C., and Andrews, D. W. (1996) The
signal recognition particle receptor alpha subunit assembles
co-translationally on the endoplasmic reticulum membrane during an
mRNA-encoded translation pause in vitro, EMBO J.,
15, 172-181.
112.Caliskan, N., Peske, F., and Rodnina, M. V.
(2015) Changed in translation: mRNA recoding by -1 programmed ribosomal
frameshifting, Trends Biochem. Sci., 40, 265-274, doi:
10.1016/j.tibs.2015.03.006.
113.Tsuchihashi, Z., and Kornberg, A. (1990)
Translational frameshifting generates the gamma subunit of DNA
polymerase III holoenzyme, Proc. Natl. Acad. Sci. USA,
87, 2516-2520, doi: 10.1073/pnas.87.7.2516.
114.Belew, A. T., Meskauskas, A., Musalgaonkar, S.,
Advani, V. M., Sulima, S. O., et al. (2014) Ribosomal frameshifting in
the CCR5 mRNA is regulated by miRNAs and the NMD pathway,
Nature, 512, 265-269, doi: 10.1038/nature13429.
115.Jacks, T., Power, M. D., Masiarz, F. R., Luciw,
P. A., Barr, P. J., and Varmus, H. E. (1988) Characterization of
ribosomal frameshifting in HIV-1 gag-pol expression, Nature,
331, 280-283, doi: 10.1038/331280a0.
116.Kelly, J. A., Olson, A. N., Neupane, K.,
Munshi, S., San Emeterio, J., et al. (2020) Structural and functional
conservation of the programmed -1 ribosomal frameshift signal of SARS
coronavirus 2 (SARS-CoV-2), J. Biol. Chem., 295,
10741-10748, doi: 10.1074/jbc.AC120.013449.
117.Kelly, J. A., Woodside, M. T., and Dinman, J.
D. (2021) Programmed -1 ribosomal frameshifting in coronaviruses: a
therapeutic target, Virology, 554, 75-82, doi:
10.1016/j.virol.2020.12.010.
118.Qu, X., Wen, J. D., Lancaster, L., Noller, H.
F., Bustamante, C., and Tinoco, I., Jr. (2011) The ribosome uses two
active mechanisms to unwind messenger RNA during translation,
Nature, 475, 118-121, doi: 10.1038/nature10126.
119.Chen, C., Zhang, H., Broitman, S. L., Reiche,
M., Farrell, I., et al. (2013) Dynamics of translation by single
ribosomes through mRNA secondary structures, Nat. Struct. Mol.
Biol., 20, 582-588, doi: 10.1038/nsmb.2544.
120.Chen, J., Petrov, A., Johansson, M., Tsai, A.,
O’Leary, S. E., and Puglisi, J. D. (2014) Dynamic pathways of -1
translational frameshifting, Nature, 512, 328-332, doi:
10.1038/nature13428.
121.Kim, H. K., Liu, F., Fei, J., Bustamante, C.,
Gonzalez, R. L., Jr., and Tinoco, I., Jr. (2014) A frameshifting
stimulatory stem loop destabilizes the hybrid state and impedes
ribosomal translocation, Proc. Natl. Acad. Sci. USA, 111,
5538-5543, doi: 10.1073/pnas.1403457111.
122.Tu, C., Tzeng, T. H., and Bruenn, J. A. (1992)
Ribosomal movement impeded at a pseudoknot required for frameshifting,
Proc. Natl. Acad. Sci. USA, 89, 8636-8640, doi:
10.1073/pnas.89.18.8636.
123.Somogyi, P., Jenner, A. J., Brierley, I., and
Inglis, S. C. (1993) Ribosomal pausing during translation of an RNA
pseudoknot, Mol. Cell. Biol., 13, 6931-6940, doi:
10.1128/mcb.13.11.6931-6940.1993.
124.Lopinski, J. D., Dinman, J. D., and Bruenn, J.
A. (2000) Kinetics of ribosomal pausing during programmed -1
translational frameshifting, Mol. Cell. Biol., 20,
1095-1103, doi: 10.1128/mcb.20.4.1095-1103.2000.
125.Kontos, H., Napthine, S., and Brierley, I.
(2001) Ribosomal pausing at a frameshifter RNA pseudoknot is sensitive
to reading phase but shows little correlation with frameshift
efficiency, Mol. Cell. Biol., 21, 8657-8670, doi:
10.1128/MCB.21.24.8657-8670.2001.
126.Caliskan, N., Katunin, V. I., Belardinelli, R.,
Peske, F., and Rodnina, M. V. (2014) Programmed -1 frameshifting by
kinetic partitioning during impeded translocation, Cell,
157, 1619-1631, doi: 10.1016/j.cell.2014.04.041.
127.Caliskan, N., Wohlgemuth, I., Korniy, N.,
Pearson, M., Peske, F., and Rodnina, M. V. (2017) Conditional switch
between frameshifting regimes upon translation of dnaX mRNA, Mol.
Cell, 66, 558-567 e554, doi:
10.1016/j.molcel.2017.04.023.
128.Bao, C., Loerch, S., Ling, C., Korostelev, A.
A., Grigorieff, N., and Ermolenko, D. N. (2020) mRNA stem-loops can
pause the ribosome by hindering A-site tRNA binding, Elife,
9, e55799, doi: 10.7554/eLife.55799.
129.Choi, J., O’Loughlin, S., Atkins, J. F.,
and Puglisi, J. D. (2020) The energy landscape of -1 ribosomal
frameshifting, Sci. Adv., 6, eaax6969, doi:
10.1126/sciadv.aax6969.