MINI-REVIEW: The Mechanisms of Electrogenic Reactions in Bacterial Photosynthetic Reaction Centers: Studies in Collaboration with Alexander Konstantinov
Olga P. Kaminskaya1 and Alexey Yu. Semenov2,3,a*
1Institute of Basic Biological Problems, Pushchino Scientific Center for Biological Research, Russian Academy of Sciences, 142290 Pushchino, Moscow Region, Russia2Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119992 Moscow, Russia
3Semenov Federal Research Center for Chemical Physics, Russian Academy of Sciences, 119991 Moscow, Russia
* To whom correspondence should be addressed.
Received October 15, 2020; Revised November 11, 2020; Accepted November 21, 2020
In this review, we discuss our studies conducted in 1985-1988 in collaboration with A. A. Konstantinov, one of the top scientists in the field of membrane bioenergetics. Studying fast kinetics of membrane potential generation in photosynthetic reaction centers (RCs) of purple bacteria in response to a laser flash has made it possible to examine in detail the mechanisms of electrogenic reactions at the donor and acceptor sides of RCs. Electrogenesis associated with the intraprotein electron transfer from the exogenous secondary donors, redox dyes, and soluble cytochrome (cyt) c to the photooxidized dimer of bacteriochlorophyll P870 was studied using proteoliposomes containing RCs from the non-sulfur purple bacterium Rhodospirillum rubrum. It was found that reduction of the secondary quinone electron acceptor QB accompanied by its protonation in the chromatophores from R. rubrum in response to every second light flash was electrogenic. Spectral characteristics and redox potentials of the four hemes in the tightly bound cyt c in the RC of Blastochloris viridis and electrogenic reactions associated with the electron transfer within the RC complex were identified. For the first time, relative amplitudes of the membrane potential generated in the course of individual electrogenic reactions were compared with the distances between the redox cofactors determined based on the three-dimensional structure of the Bl. viridis RC.
KEY WORDS: bacterial photosynthetic reaction centers, electron transfer, direct electrometric method, membrane potential, cytochrome c, quinoneDOI: 10.1134/S0006297921010016
Abbreviations: Δψ, transmembrane difference in electric potentials; cyt, cytochrome; DAD, 2,3,5,6-tetramethyl-p-phenylenediamine (diaminodurene); Em, midpoint redox potential; PMS, phenazine methosulfate; RC, reaction center; P870 (P960), bacteriochlorophyll dimer in RC; τ, characteristic reaction time; TMPD, N,N,N′,N′-tetramethyl-p-phenylenediamine; QA and QB, primary and secondary quinone electron acceptors, respectively.
BRIEF REVIEW OF THE PRECEDING STUDIES OF THE ELECTROGENIC
REACTIONS IN THE REACTION CENTERS OF PHOTOSYNTHETIC BACTERIA USING
DIRECT ELECTROMETRIC METHOD
In 1985-1988, the authors of this review had collaborated with Alexander Alexandrovich Konstantinov to investigate the nature and the mechanisms of electrogenic reactions in the chromatophores and photosynthetic reaction centers (RCs) isolated from the non-sulfur purple bacteria Rhodospirillum rubrum and Rhodobacter sphaeroides (previously classified as Rhodopseudomonas sphaeroides) and the sulfur purple bacterium Blastochloris viridis (previously classified as Rhodopseudomonas viridis) [1-6]. In 1974-1976, L. A. Drachev, A. D. Kaulen, and one of the authors of this review (A. S.) had developed a technique for direct measurement of electric activity of membrane proteins [7, 8]. It was established using this technique that continuous illumination of bacterial chromatophores associated with the artificial planar phospholipid membrane in the presence of redox mediators led to the formation of the transmembrane electric potential difference (Δψ) with positive charge inside the chromatophores [9, 10].
It was found that the photoinduced electron transfer in this system was limited to the reduction of the primary quinone acceptor QA, presumably due to the extraction of the secondary quinone QB and endogenous ubiquinone pool to the artificial membrane lipid phase [9-11]. Exogenous redox cofactors, electron donors and acceptors [ascorbate, N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD), diaminodurene (DAD), phenazine methosulfate (PMS), 2,6-dichlorophenol indophenol (DCPIP), methylene blue, and artificial quinones] maintained steady state photopotential, providing multiple re-reduction of the photooxidized primary RC donor (dimeric bacteriochlorophyll P870) and re-oxidation of the photoreduced QA [10].
The use of collodion film as a support for the planar phospholipid membrane allowed to record the kinetics of the Δψ generation in response to a laser flash with a ~200-ns time resolution [12]. It was demonstrated in the studies conducted by Drachev and coauthors [11-15] that illumination of chromatophores from the purple bacteria with a single laser flash caused Δψ formation that was too fast to resolve it with the used technique. The observed fast reaction step was due to the electron transfer between P870 (or P890 in the case of chromatophores from the sulfur purple bacteria Chromatium minutissimum and Ectothiorhodospira shaposhnikovii) and the primary quinone acceptor QA. Furthermore, an additional step in the Δψ generation was observed in the chromatophores from C. minutissimum and E. shaposhnikovii, whose RCs contain tightly bound four-heme cytochrome (cyt) c subunit. This step was associated with the reduction of the photooxidized P890 by cyt c. It was suggested that QA oxidation occurring either during electron transfer to an exogenous acceptor or during reduction of the secondary quinone acceptor QB could contribute to the Δψ generation.
The studies showed that Δψ generated in response to a single flash by chromatophores incorporated into the collodion phospholipid membrane dissipated with the heterogenous kinetics on a scale of tens of milliseconds to seconds [13, 14]. It was suggested that the fast component of the Δψ decay was due to the charge recombination between the photooxidized P870+ and reduced QA–. The slow component of the Δψ decay with the characteristic time τ ≥ 0.5 s reflected the process of passive discharge of Δψ through the artificial and the chromatophore membranes. It was found that the Δψ decay slowed down in the presence of reduced PMS.
ANALYSIS OF THE DECAY KINETICS OF THE PHOTOELECTRIC RESPONSE OF
CHROMATOPHORES FROM R. rubrum AND PROTEOLIPOSOMES WITH R.
rubrum RCs INCORPORATED INTO THE COLLODION PHOSPHOLIPID
MEMBRANE
The studies that were carried out in 1985-1988 in collaboration with A. A. Konstantinov [1-6] were devoted to the detailed investigation of the kinetics of formation and decay of the membrane potential generated by the photosynthetic RCs from purple bacteria in response to a laser flash. S. M. Dracheva and M. D. Mamedov have provided a large contribution to the experimental part of these studies.
It was proven using the double flash technique that the fast component of the laser flash induced Δψ decay with τ ~ 70 ms in R. rubrum chromatophores associated with the collodion membrane was due to the back electron transfer from QA– to P870+ [4]. It was demonstrated that the contribution of the slow component (τ ≥ 0.5 s) to the kinetics of Δψ decay increased in the presence of efficient electron donors and acceptors capable of reducing or oxidizing components of the ion-radical pair P870+QA– and providing formation of the long-lived states P870QA– and P870+QA. It was demonstrated that the relative amplitude of the slow component of the dark Δψ decay could be used to estimate the rates of P870+ reduction and QA– oxidation by exogenous redox cofactors. The redox mediators TMPD, DAD, PMS, and DCPIP were good reductants for P870+, and 1,4-benzoquinone, soluble analogues of ubiquinone Q-1 and Q-2, as well as ubiquinone 10 (Q-10) added to the phospholipid solutions in n-decane used for the impregnation of the collodion membrane, were efficient acceptors of electrons from QA– [4].
The fact that exogenous ubiquinone was capable of fast re-oxidation of QA–, thus preventing recombination of the ion-radical pair, indicated restoration of the QB function in the system of incorporated chromatophores. The QA– ® QB reaction was restored in 75% of RCs under saturating concentrations of ubiquinone in the collodion membrane [4]. The deceleration of the Δψ decay caused by addition of Q-10 was completely canceled by o-phenanthroline, an inhibitor of the QB site. These experiments corroborated the original suggestion that inhibition of the electron transfer from QA to QB in the chromatophores incorporated into the artificial phospholipid membrane was due to the extraction of ubiquinone bound at the QB site into the hydrophobic bulk of the artificial membrane [9, 10].
The use of membrane-permeable redox mediators as electron donors was required in the experiments with R. rubrum chromatophores, because the donor side of the RC protein complex was located in the vicinity of the chromatophore membrane inner surface. We demonstrated that proteoliposomes containing isolated R. rubrum RCs could serve as a good model for investigating the redox reaction of P870+ with non-permeable electron donors [3]. Illumination of such proteoliposomes with a laser flash resulted in the generation of the photopotential with the negative charge inside the vesicles indicated that P870 was located in the vicinity of the proteoliposome membrane outer surface, which is opposite to its location in R. rubrum chromatophores. Addition of non-permeable electron donors (hexammineruthenium and horse heart cyt c) resulted in a significant retardation of the decay of Δψ generated in response to the laser flash. This fact implied fast reduction of the photooxidized P870 by exogenous redox cofactors and emergence of the long-lived state P870 QA–.
ELECTROGENIC REACTIONS AT THE DONOR SIDE OF R. rubrum
RCs
Investigating electrogenesis associated with the electron transfer between the RC primary donor P870 and soluble cyt c is challenging in the case of R. rubrum chromatophores, because these closed vesicles lose significant part of endogenous cyt c2 during sonication and, moreover, the donor side of the RC is oriented inside the chromatophore, which makes it inaccessible to the exogenous cyt c. Hence, to investigate electrogenic reduction of P870+ by cytochromes c we used proteoliposomes containing isolated R. rubrum RCs.
Drachev et al. [3] measured the fast kinetics of the membrane potential generation in the course of electron transfer to P870 from the horse heart cyt c or from the isolated R. rubrum cyt c2. In the presence of ≥0.1 µM reduced cyt c, a kinetic component with the submillisecond to millisecond characteristic time associated with the electron donation from cyt c (step C) was observed in the kinetics of Δψ increase in addition to the fast time-unresolvable component A (τ < 0.2 µs) occurring due to the charge separation between P870 and QA. The relative contribution of this additional component of the Δψ increase to the total electrogenesis was found to be 22-24% in the presence of mitochondrial cyt c (at concentration > 5-10 µM) and ~16% in the presence of 7 µM cyt c2 from R. rubrum. Kinetic analysis of the step C indicated saturated second-order reaction between cyt c and P870+ with the maximum value of the reaction rate constant kv,max = 6·103 s–1 and Michaelis constant KM = 0.9 µM at low ionic strength. The rate of the increase of the step C slowed down with the increase in the ionic strength, which is in agreement with the notion on the ionic interactions of the RC protein with cyt c during formation of the bimolecular complex.
Understanding the fact that the electrogenic nature of the P870+ reduction is not specific for the soluble type c cytochromes as electron donors but also takes place in the case of reduced forms of redox mediators (TMPD, DAD, and PMS) has become an important step in the investigation of electrogenesis in RCs of photosynthetic bacteria. In the presence of high concentration of PMS and TMPD (>20 µM and >0.5 mM, respectively), an additional component in the Δψ increase was observed on the scale of hundreds of microseconds to milliseconds, which provided 15-18% contribution to the total electrogenesis in R. rubrum chromatophores [1]. The characteristic time τ of this additional Δψ component was ~250 µs at the PMS concentration of 50 µM and ~2 ms at the TMPD concentration of 4 mM. The electrogenic nature of P870+ reduction by low-molecular-weight electron donors indicated electric insulation of the special pair inside the RC protein globule. Based on the fact that the amplitude of the additional phase of Δψ increase provided by the electron transfer from the reduced TMPD or PMS was only slightly less than the amplitude of the step C observed in the presence of cyt c, it was concluded that the main contribution to electrogenesis associated with the reduction of P870+ was due to vectorial electron transfer inside the protein globule of the RC.
GENERATION OF Δψ BY R. rubrum CHROMATOPHORES
COUPLED TO ELECTRON TRANSFER AT THE ACCEPTOR SIDE OF RC
The possibility of reconstruction of QB function in chromatophores associated with the collodion membrane allowed to study electrogenesis in the process of QA oxidation by the secondary quinone QB [2]. It was demonstrated in our studies that the transfer of the first electron from QA to QB (QA–QB ® QAQB–) in R. rubrum chromatophores did not result in the Δψ increase. These data were in agreement with the earlier study of electrogenesis in Rba. sphaeroides RCs incorporated into the planar bilayer membrane [16] (later, a small pH-dependent electrogenic step was identified that occurred due to the protonation of an amino acid residue in the vicinity of QB during QB– formation in response to a single flash [17]). At the same time, we found that reduction of the semiquinone form of QB to ubiquinol was associated with the appearance of an additional Δψ component that comprised ~10% of the total response amplitude (step B) [2]. These data were the first evidence of electrogenesis coupled with the protonation of the secondary quinone in bacterial RCs.
The electrogenic step B associated with the QBH2 formation, had τ equal to 130 µs, 250 µs, and ~1 ms at pH 6.5, 7.5, and 9, respectively [2], which was close to the values measured for the electron transfer from QA to QB in the chromatophores from R. rubrum and Rba. sphaeroides RCs [18, 19] and the rate of antimycin-insensitive binding of H+ in Rba. sphaeroides chromatophores [20] (see also reviews [21, 22]). The fact that protonation of the QB during the ubiquinol formation in response to the flash is associated with the generation of Δψ indicates vectorial transfer of H+ from the external aqueous phase to the electroinsulated quinone QB located deep inside the RC protein globule.
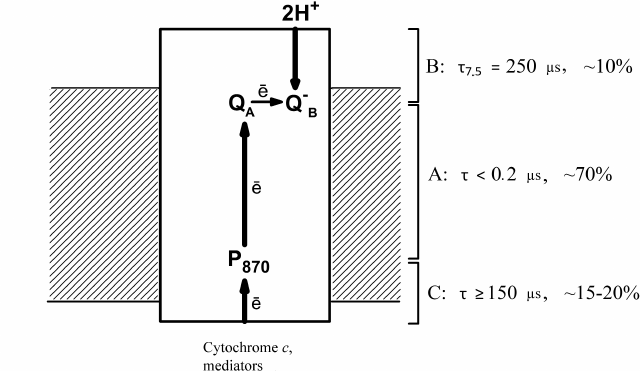
The data obtained by Drachev et al. [1-4] on the electrogenic reactions in R. rubrum RCs are summarized in Fig. 1 that shows three steps of electrogenesis on the chromatophore membrane coupled with the proton transfer in the RC complex. Step A is the primary charge separation; step B is protonation of the reduced QB during ubiquinol formation; and step C is reduction of the bacteriochlorophyll dimer by soluble electron donors.
Fig. 1. Electrogenic reactions in R. rubrum RCs. Electrogenic reduction of the photooxidized P870 by exogenous secondary electron donors is observed in response to each laser flash; electrogenic protonation of the secondary quinone acceptor QB occurs in response to every second flash. Characteristic times τ (for the component B – at pH 7.5) and relative contributions of the three steps to Δψ generation are shown.
ELECTRON TRANSFER AND ELECTROGENIC REACTIONS IN Bl.
viridis RCs
The studies by Dracheva et al. [5, 6] conducted in collaboration with A. A. Konstantinov were devoted to the electron transfer and electrogenic reactions in RCs isolated from the purple sulfur bacterium Bl. viridis. The interest in these particular RCs was due to two facts. Firstly, the three-dimensional crystal structure of this pigment-protein complex was elucidated with an atomic resolution [23, 24]. Secondly, in these RCs, the role of the secondary electron donor for the bacteriochlorophyll dimer plays the tightly-bound four-heme cyt c rather than to the water-soluble cyt c2 (as in the RCs from the non-sulfur bacteria R. rubrum and Rba. sphaeroides).
Prior to these studies, it had been assumed that the four-heme cyt c in the Bl. viridis RCs contained two equal high-potential and two equal low-potential hemes with the midpoint redox potentials Em of +340 and 0 mV and absorption maxima at 558 and 552-553 nm, respectively [25, 26]. We demonstrated that neither the high-potential nor the low-potential hemes are identical and that all four hemes exhibit different spectral and redox properties [5, 6], as they were found to have Em of +380, +310, +20, and –60 mV and absorption maxima at 559, 556, 552, and 554 nm, respectively. It had been shown previously that histidine residues serve as the axial ligands to the heme located second in a row from P960 in the Bl. viridis RC, which is typical for the low-potential heme [24, 27]. The measurements of the electron transfer kinetics in the Bl. viridis RCs using the time-resolved absorption spectrometry demonstrated that under physiological conditions (when hemes c559 and c556 are reduced), heme c559 (τ = 0.3 µs) served as a direct electron donor for the photooxidized P960, while heme c556 reduced heme c559 (τ = 2.5 µs) [5]. Hence, we suggested that the high-potential and the low-potential hemes were alternating in cyt c, and the high-potential heme c559 was the closest to P960, while the second high-potential heme c556 was the third in a row from P960. In this case, the redox centers must be arranged in the following order: c554 – c556 – c552 – c559 – P960 [6] (Fig. 2).
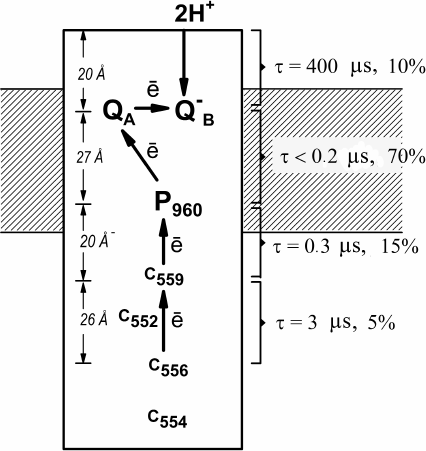
Fig. 2. Electrogenic reactions in Bl. viridis RCs. Relative contributions to the total electrogenesis (% Δψ) and characteristic times (τ) of individual electron transfer reactions are shown on the right; left panel, projections of the distances between the redox cofactors on the perpendicular to the membrane plane.
In order to investigate electrogenic reactions, proteoliposomes containing Bl. viridis RCs were incorporated into collodion phospholipid membrane. Analysis of photoelectric signals induced by laser flashes revealed that the kinetics of Δψ generation included three subsequent electrogenic reactions in response to each laser flash: A – time-unresolvable electron transfer from P960 to the primary quinone acceptor QA (τ < 0.2 µs); C1 – reduction of the photooxidized P960+ by the most high-potential heme c5592+ (τ = 0.3 µs); C2 – re-reduction of the oxidized c5593+ by the second high-potential heme c5562+ (τ = 2.5 µs); and component B observed in response to every second flash and associated with the protonation of the doubly-reduced secondary quinone acceptor QB with the formation of QBH2 (τ = 400 µs) [6]. Relative contributions of the kinetic steps C2, C1, A, and B to the total electrogenesis were 5, 15, 70, and 10%, respectively (Fig. 2). These results demonstrated that the relative contribution of electrogenic steps increases in the central part of the protein hydrophobic core and decreases at the protein globule periphery.
The first data of the X-ray diffraction analysis of Bl. viridis RCs available at the time of these studies [23, 24] allowed to reveal the correlation between the amplitudes of the steps of Δψ generation and the distances between the respective redox cofactors. It was found that the relative contribution of the charge transfer reactions to the total electrogenesis was defined not only by the distances between the redox cofactors, but also by the dielectric properties of the respective protein areas [6]. This observation is illustrated in Fig. 2 that shows projections of the distances between the cytochrome hemes c556 and c559, c559 and P960, P960, and QA, as well as between QB and the closest protein/water boundary, on the perpendicular to the membrane plane as determined based on the later publication [28]. The indicated distances were 27, 22, 29, and 22% of the distance between the heme c556 and the closest protein/water boundary at the acceptor side of the RC protein globule. It can be seen, for example, that the vectorial electron transfer from P960 to QA inside protein hydrophobic core corresponding to the central part of the membrane, contributes significantly more to electrogenesis than the electron transfer at the same distance between the hemes c556 and c559 located in the portion of the protein globule protruding from the membrane into the aqueous phase.
It should be emphasized in conclusion that the studies conducted in 1985-1988 in collaboration with A. A. Konstantinov and described in this review have revealed important mechanisms of electrogenic reactions in photosynthetic RCs from the purple bacteria R. rubrum, Rba. sphaeroides, and Bl. viridis. Many features of electrogenesis and electron transfer reactions in bacterial RCs were described for the first time. The kinetics of electrogenic electron transfer between the soluble cyt c and the bacterial RC complex was investigated with a high time resolution. It was demonstrated that electrogenesis associated with reduction of photooxidized P870 by cyt c and artificial redox dyes occurred due to the electron transfer inside the RC protein complex. In addition, the electrogenic nature of the protonation of the double-reduced secondary quinone acceptor QB was established. Spectral and redox characteristics of the four hemes in the tightly-bound cyt c were determined in the studies of Bl. viridis RCs. The rates of P960 reduction and electron transfer between the hemes were measured, as well as the steps of electrogenesis associated with the electron transport reactions in this pigment-protein complex were recorded. Comparison of the relative contributions of the individual electrogenic steps with the projections of distances between the redox cofactors on the perpendicular to the membrane plane led to the conclusion that the dielectric permittivity inside the RC protein is heterogenous and varies across the protein globule.
Acknowledgments. Alexander Konstantinov had played an invaluable role in the studies described in this review. He was one of the top scientists, a real professional, who valued the beauty of scientific experiments and had brilliant intuition. His broad scientific interests, clear identification of scientific problems, and thorough experiment planning, including selection of optimal experimental conditions, were the hallmarks of his work style. All this has made the results of experiments clear and effective. It was very interesting to work with Alexander Konstantinov. With his help and often with his direct supervision, I. A. Smirnova, S. M. Dracheva, O. P. Kaminskaya, D. L. Zaslavsky, and S. A. Siletsky have received their PhD degrees that involved investigations of electrogenesis mediated by the mitochondrial membrane proteins and reaction centers in photosynthetic bacteria using direct electrometric technique. His colleagues will always cherish the memory of Alexander Konstantinov.
Ethics declarations. The authors declare no conflict of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
REFERENCES
1.Drachev, L. A., Kaminskaya, O. P., Konstantinov, A.
A., Semenov, A. Yu., and Skulachev, V. P. (1985) Electrogenic reduction
of Rhodospirillum rubrum reaction centre bacteriochlorophyll
P870+ by redox dyes. Indication of intraprotein electron
transfer, FEBS Lett., 189, 45-49.
2.Kaminskaya, O. P., Drachev, L. A., Konstantinov, A.
A., Semenov, A. Yu., and Skulachev, V. P. (1986) Electrogenic reduction
of the secondary quinone acceptor in chromatophores of
Rhodospirillum rubrum. Rapid kinetic measurements, FEBS
Lett., 2, 224-228.
3.Drachev, L. A., Kaminskaya, O. P., Konstantinov, A.
A., Kotova, E. A., Mamedov, M. D., et al. (1986) The effect of
cytochrome c, hexaammineruthenium and ubiquinone-10 on the
kinetics of photoelectric responses of Rhodospirillum rubrum
reaction centers, Biochim. Biophys. Acta, 848,
137-146.
4.Drachev, L. A., Kaminskaya, O. P., Konstantinov, A.
A., Mamedov, M. D., Samuilov, V. D., et al. (1986) Effects of electron
donors and acceptors on the kinetics of the photoelectric responses in
Rhodospirillum rubrum and Rhodopseudomonas sphaeroides
chromatophores, Biochim. Biophys. Acta, 850, 1-9.
5.Dracheva, S. M., Drachev, L. A., Zaberezhnaya, S.
M., Konstantinov, A. A., Semenov, A. Yu., and Skulachev V. P. (1986)
Spectral, redox and kinetic characteristics of high-potential
cytochrome c hemes in Rhodopseudomonas viridis reaction centers,
FEBS Lett., 205, 41-46.
6.Dracheva, S. M., Drachev, L. A., Konstantinov, A.
A., Semenov, A. Yu., Skulachev, V. P., et al. (1988) Electrogenic steps
in the redox reactions catalysed by photosynthetic reaction centre
complex from Rhodopseudomonas viridis, Eur. J. Biochem.,
171, 253-264.
7.Drachev, L. A., Jasaitis, A. A., Kaulen, A. D.,
Kondrashin, A. A., Liberman, E. A., et al. (1974) Direct measurement of
electric current generation by cytochrome oxidase, H+-ATPase
and bacteriorhodopsin, Nature, 249, 321-324.
8.Drachev, L. A., Frolov, V. N., Kaulen, A. D.,
Liberman, E. A., Ostroumov, S. A., et al. (1976) Reconstitution of
biological molecular generators of electric current. Bacteriorhodopsin,
J. Biol. Chem., 251, 7059-7065.
9.Drachev, L. A., Frolov, V. N., Kaulen, A. D.,
Kondrashin, A. A., Samuilov, V. D., et al. (1976) Generation of
electric current by chromatophores of Rhodospirillum rubrum and
reconstitution of electrogenic function in subchromatophore
pigment-protein complexes, Biochim. Biophys. Acta, 440,
637-660.
10.Smirnova, I. A., Konstantinov, A. A., and
Skulachev, V. P. (1981) Role of cofactors in the formation of the
membrane potential by chromatophores of Rhodospirillum rubrum,
incorporated into a Teflon filter, Biochemistry (Moscow),
46, 925-934.
11.Drachev, L. A., Dracheva, S. M., Samuilov, V. D.,
Semenov, A. Yu., and Skulachev, V. P. (1984) Photoelectric effects in
bacterial chromatophores. Comparision of spectral and direct
electrometric methods, Biochim. Biophys. Acta, 767,
257-262.
12.Drachev, L. A., Semenov, A. Yu., and Skulachev,
V. P. (1979) Generation of electric potential difference by
chromatophores induced by laser flash, Dokl. Akad. Nauk SSSR,
245, 991-994.
13.Drachev, L. A., Semenov, A. Yu, Skulachev, V. P.,
Smirnova, I. A., Chamorovsky, S. K., et al. (1981) Fast stages of
photoelectric processes in biological membranes, Eur. J.
Biochem, 117, 483-489.
14.Semenov, A. Yu., Chamorovsky, S. K., Smirnova, I.
A., Drachev, L. A., Kononenko, A. A., et al. (1981) Kinetics of
formation of photo-induced electric potential difference by
chromatophores from photosynthesizing bacteria, Mol. Biol.
(Mosk), 15, 622-635.
15.Chamorovsky, S. K., Drachev, A. L., Karagulian,
A. K., Kononenko, A. A., Rubin, A. B., et al. (1985) Fast phases of the
generation of the transmembrane electric potential in chromatophores of
the photosynthetic bacterium Ectothiorhodospira shaposhnikovii,
Biochim. Biophys. Acta, 808, 201-208.
16.Packham, N. K., Dutton, P. L., and Mueller, P.
(1982) Photoelectric currents across planar bilayer membranes
containing bacterial reaction centers. Response under conditions of
single electron turnover, Biophys. J., 37, 465-473.
17.Drachev, L. A., Mamedov, M. D., Mulkidjanian, A.
Ya., Semenov, A. Yu., Shinkarev, V. P., and Verkhovsky, M. I. (1990)
Electrogenesis associated with proton transfer in the reaction center
protein of the purple bacterium, FEBS Lett., 259,
324-326.
18.Wraight, C. A. (1979) Electron acceptors of
bacterial photosynthetic reaction centers II. H+ binding
coupled to secondary electron transfer in the quinone acceptor complex,
Biochim. Biophys. Acta, 548, 309-327.
19.Vermeglio, A. (1982) Electron transfer between
primary and secondary electron acceptors in chromatophores and reaction
centers of photosynthetic bacteria, in: Function of Quinones in
Energy Conserving Systems (Trumpower, B. L., ed.) Academic Press,
New York, pp. l69-180.
20.Petty, K. M., and Dutton, P. L. (1976) Properties
of the flash-induced proton binding encountered in membranes of
Rhodopseudomonas sphaeroides: a functional pK on the
ubisemiquinone? Arch. Biochem. Biophys., 172,
335-345.
21.Sebban, P., Maroti, P., and Hanson, D. K. (1995)
Electron and proton transfer to the quinones in bacterial
photosynthetic reaction centers: insight from combined approaches of
molecular genetics and biophysics, Biochimie, 77,
677-694.
22.Okamura, M. Y., Paddock, M. L., Graige, M. S.,
and Feher, G. (2000) Proton and electron transfer in bacterial reaction
centers, Biochim. Biophys. Acta, 1458, 148-163.
23.Deisenhofer, J., Epp, O., Miki, K., Huber, R.,
and Michel, H. (1984) X-ray structure analysis of a membrane protein
complex: electron density map at 3 Å resolution and a model of
the chromophores of the photosynthetic reaction center from
Rhodopseudomonas viridis, J. Mol. Biol., 180,
385-398.
24.Deisenhofer, J., Epp, O., Miki, K., Huber, R.,
and Michel, H. (1985) Structure of the protein subunits in the
photosynthetic reaction centre of Rhodopseudomonas viridis at 3
Å resolution, Nature, 318, 618-624.
25.Clayton, R. K., and Clayton, B. J. (1978) Molar
extinction coefficients and other properties of an improved reaction
center preparation from Rhodopseudomonas viridis, Biochim.
Biophys. Acta, 501, 478-487.
26.Case, G. D., Parson, W. W., and Thornber, J. P.
(1970) Photooxidation of cytochromes in reaction center preparations
from Chromatium and Rhodopseudomonas viridis, Biochim.
Biophys. Acta, 223, 122-128.
27.Weyer, K. A., Lottspeich, F., Gruenenberg, H.,
Lang, F., Oesterhelt, D., and Michel, H. (1987) Amino acid sequence of
the cytochrome subunit of the photosynthetic reaction centre from the
purple bacterium Rhodopseudomonas viridis, EMBO J.,
6, 2197-2202.
28.Deisenhofer, J., Epp, O., Sinning, I., and
Michel, H. (1995) Crystallographic refinement at 2.3 Å resolution
and refined model of the photosynthetic reaction centre from
Rhodopseudomonas viridis, J. Mol. Biol., 246,
429-457.