Phytofluene as a Highly Efficient UVA Photosensitizer of Singlet Oxygen Generation
A. A. Ashikhmin1,a, A. S. Benditkis2,b, A. A. Moskalenko1,c, and A. A. Krasnovsky, Jr.2,d*
1Institute of Basic Biological Problems, Federal Research Center “Pushchino Scientific Center for Biological Research, Russian Academy of Sciences, 142290 Pushchino, Moscow Region, Russia2Bach Institute of Biochemistry, Federal Research Center of Biotechnology, Russian Academy of Sciences, 119071 Moscow, Russia
* To whom correspondence should be addressed.
Received April 29, 2020; Revised May 26, 2020; Accepted June 4, 2020
Phytoene and phytofluene – uncolored C40 carotenoids with short chain of conjugated double bonds (3 and 5, respectively) – are known to be universal precursors in biosynthesis of colored carotenoids in photosynthesizing organisms. It is commonly recognized that C40 carotenoids are photoprotectors of cells and tissues. We have shown that phytofluene is an exception to this rule. By measuring photosensitized phosphorescence of singlet oxygen (1O2) we found out that phytofluene was very effective photosensitizer of 1O2 formation in aerated solutions under UVA irradiation (quantum yield of 85 ± 5%), whereas phytoene was almost inactive in this process. It was demonstrated that both carotenoids quench singlet oxygen in the dark. The obtained quenching rate constants [(4 ± 1) × 106 M–1·s–1 for phytoene and (2 ± 0.5) × 107 M–1·s–1 for phytofluene] were smaller than the rate constant of the diffusion-controlled reactions by 3-4 orders of magnitude. Thus, both carotenoids displayed rather weak protector properties. Moreover, phytofluene due to its high photosensitizing activity might be considered as a promoter of cell photodamage and a promising UVA photosensitizer for medical purposes.
KEY WORDS: phytofluene, phytoene, singlet oxygen, photosensitization, UVADOI: 10.1134/S0006297920070056
Abbreviations: CDB(s), conjugated double bond(s); 1O2, singlet oxygen; UV, ultra violet; ΦΔ, the quantum yield of 1O2 generation.
INTRODUCTION
Colored carotenoids with nine and more conjugated double bonds (CDBs) synthesized by green plants and photosynthetic bacteria are recognized as efficient protectors of living tissues against photooxidative stress. It has been suggested that protection is based on capabilities of carotenoids to efficiently deactivate the triplet states of chlorophylls and bacteriochlorophylls, quench singlet oxygen (1O2), and scavenge free radicals ([1, 2] and refs therein). On the contrary, visual pigment – retinal, which is polyene with short CDB chain (6 CDBs), is an efficient photosensitizer of 1O2 formation in non-polar environment ([2, 3] and refs therein). This process is thought to cause photodynamic damage of retina and various eye diseases ([4] and refs therein). The C40 carotenoids with short CDB chains (3-7 CDBs) (phytoene, phytofluene, ζ-carotene) are known to be formed in photosynthetic bacteria, green plants, and algae as universal precursors in biosynthesis of colored carotenes and xanthophylls that have a long chain of CDBs [1]. Based on these facts, it was suggested that polyenes with short CDB chains might cause photodynamic damage of photosynthetic tissues [5]. In addition, it was recently found that the membranes of purple bacteria photosensitize oxygenation of bacteriochlorophyll B-850 and 1O2 production under blue-green light, which is predominantly absorbed by carotenoids ([6-10] and refs therein). On the other hand, phytoene and phytofluene are present in commonly consumed foods and are among the predominant carotenoids in human plasma and tissues. Moreover, they were shown to react with free radicals and therefore, were used as nutrients providing protection of skin and other tissues against oxidative stress and UV light ([11, 12] and refs therein). Nevertheless, to the best of our knowledge, systematic analysis of 1O2 generation by C40 carotenoids – precursors in β-carotene biosynthesis – has never been carried out, although flash photolysis studies of the triplet states of certain compounds of this group have been reported [13, 14]. Recently, we observed in the preliminary test measurements that phytofluene was an exceptionally strong photosensitizer of 1O2 formation, which exceeds by this ability all-trans retinal. This observation stimulated us to detailed investigation of 1O2 generation by this carotenoid and its biosynthetic precursor phytoene using measurements of photosensitized phosphorescence of 1O2 [15]. The obtained results are represented below.
MATERIALS AND METHODS
All-trans phytoene and phytofluene were obtained from cells of two sulfur photosynthetic bacteria Ectothiorhodospira haloalkaliphila (ATCC 51935T strain) and Allochromatium vinosum (MSUT strain). According to earlier nomenclature, this bacterium was named Allochromatium minutissimum. The cell culture of E. haloalkaliphila was obtained from Professor J. F. Imhoff (Helmholtz Center for Ocean Research, Germany), and the A. vinosum culture was provided by the Department of Microbiology, Lomonosov Moscow State University). Bacterial cells were grown anaerobically in the presence of 53 and 71 µM of diphenylamine (DPA) (“Reachim”, Russia) under 90 W/m2 white light on the Pfennig’s and Larsen’s media. The collected cells were disrupted by sonication using a UZG13-0.1-22 ultrasound generator (“Ultrasound technology”, Russia). Intact cells and fragments of cell walls were removed by centrifugation in a K24 centrifuge (“Janetzki”, Germany) for 10 min at 4500 rpm and 10,000 rpm for E. haloalkaliphila and A. vinosum, respectively [16-18]. Carotenoids were extracted from bacterial membranes using a mixture of acetone, methanol and petroleum ether and followed by purification using chromatography techniques. More details on the carotenoid isolation procedure were presented in [18].
Singlet oxygen was detected using measurement of its own infrared phosphorescence at 1270 nm, which arose due to the energy transfer from the triplet state of the photosensitizer molecules to oxygen followed by population of the excited singlet (1Δg) state of oxygen molecules. Measurements were carried out using a laser/LED spectrometer recently assembled at the A. N. Bach Institute of Biochemistry, Russian Academy of Sciences [19]. The spectrometer allowed phosphorescence detection upon excitation by continuous LEDs with the emission maxima at 281, 350, and 371.5 nm (AQMEPAN, China) and 399.5 nm (Polironic, Russia), and pulsed LED with the emission maximum at 405 nm (Alkom Medica, Russia). LED radiation was focused onto a 5 mm spot on the surface of a quartz cuvette with the test solution. Intensity of the excitation light was controlled by a ThorLabs PM-100D power meter with a S120VC sensor head (ThornLabs, USA). Phosphorescence of 1O2 was recorded at a 90° angle by a cooled FEU-112 photomultiplier (Ekran Optical systems, Russia) with the S-1 spectral response through the cut-off filters that transmit IR light at λ ≥ 1000 nm and one of three interchangeable interference filters with transmission maxima at 1230, 1270, and 1310 nm and half-width of 10 nm. The steady-state phosphorescence intensity was measured with a digital millivoltmeter (Econix-Expert, Russia).
For kinetic measurements, samples were irradiated with pulses of blue LED at 405 nm. Pulse duration and pulse repetition rate varied from 1 to 10 µs, and from 5 to 100 Hz, respectively [19]. The photomultiplier signal passed through a preamplifier and was directed to a USB board operating in the time-resolved photon-counting mode (Parsec, Russia). The time constant of the recording system was about 10 ns. The counting board, which was launched by an additional pulse of the control unit synchronously with the LED pulse, divided the time interval between flashes into 512 or 1024 channels. Kinetic curves were obtained by accumulating photomultiplier pulses in each channel. The quantum yields of 1O2 generation by carotenoids were measured using an organic photosensitizer phenalenone (perinaphthenone, 1H_phenalen-1-one) (Merk, EU) as a reference, which had a wide absorption band with maximum at 350 nm and considerable absorbance at 405 nm. The quantum yield of 1O2 photogeneration by phenalenone is known to be close to 1 (0.95 ± 0.05) [20, 21]. The experiments were performed using specially purified hexafluorobenzene (C6F6) and perflorohexane as solvents (Piminvest, Russia).
RESULTS
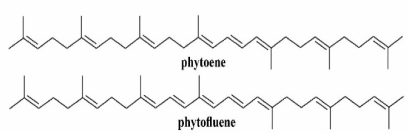
Figure 1 illustrates chemical structures of the carotenoids under study. It is seen that phytoene has 3 CDBs and phytofluene – 5 CDBs.
Fig. 1. Structural formulae of the studied carotenoids.
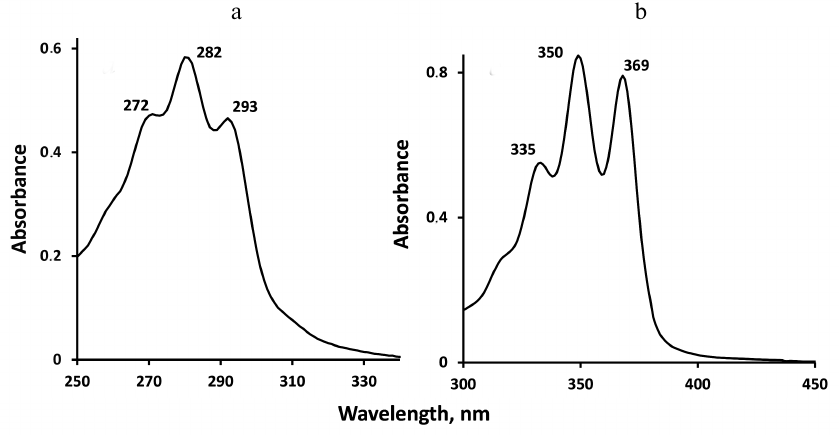
Absorption spectra of carotenoids are shown in Fig. 2. It is seen that the absorption maxima of phytoene are in the UV-B region between 250 and 310 nm, therefore for spectroscopic measurements phytoene was dissolved in perfluorohexane, which transmits light in this wavelength range. Absorption maxima of phytofluene are in the UV-A region between 320 and 400 nm. However, phytofluene is poorly soluble in perfluorohexane. Therefore, phytofluene was dissolved in hexafluorobenzene, which was transparent in the UVA region but did not transmit the UV-B light. That is why different solvents were used for spectroscopic analyses of the carotenoids. There were also other reasons of using hexafluorobenzene and perfluorohexane. Molecules of these solvents have no hydrogen atoms and, hence, weakly quench 1O2. Therefore, the lifetimes (τΔ) and intensities of 1O2 phosphorescence are several hundred times greater than in the organic solvents containing H-atoms, where the 1O2 lifetimes usually vary in the range 10-300 µs ([19] and refs therein). The use of less expensive hydrogen-free solvents containing chlorine (CCl4 and freon) appeared to be impossible for our studies because under irradiation in the chlorine-containing solvents rapid photodestruction of carotenoids occurred due to an efficient photochemical reaction. In fluoro-containing solvents such reaction was not observed. The following molar absorption coefficients (in M–1·cm–1) were used for calculation of the pigment concentrations: phytoene, 50,000 (at 282 nm); phytofluene, 73,000 (at 350 nm) [22], and phenalenone 97,000 (at 351 nm) [20, 21].
Fig. 2. Absorption spectra of phytoene in perfluorohexane (a) and phytofluene in hexafluorobenzene (b) (with absorbance of the solvents subtracted) in the 1 cm quartz cuvettes.
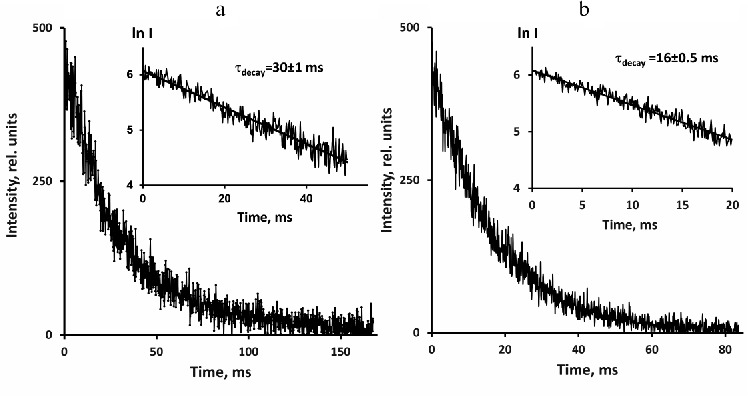
Decays of photosensitized 1O2 phosphorescence in perfluorohexane and hexafluorobenzene after excitation by short pulses of the violet LED (405 nm) are presented in Fig. 3. Phenalenone was employed as a photosensitizer. The decays were exponential with the lifetimes of 16 ms in hexafluorobenzene and 30 ms in perfluorohexane.
Fig. 3. Decays of 1O2 phosphorescence in solutions of phenalenone (~3 µM) in perfuorohexane (a) and hexafluorobenzene (b) after irradiation with 10 µs LED pulses (405 nm) in the presence of air. The decays were obtained using a pulse repetition rate of 5 and 10 Hz after 30- and 15-min signal accumulation. Average excitation power was 30 and 60 µW, respectively. The insets show semilogarithmic plots of the decay curves.
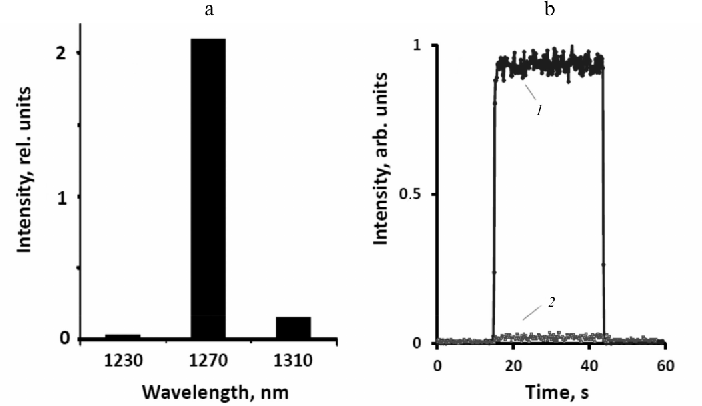
Both phytoene and phytofluene practically do not absorb light at the wavelength of pulsed LED (405 nm), therefore for excitation of these carotenoids cw LED radiation at 281 and 371 nm was employed. Under irradiation by these LEDs, phelanenone solutions emitted rather strong phosphorescence of 1O2 with a maximum at 1270 nm in both solvents.
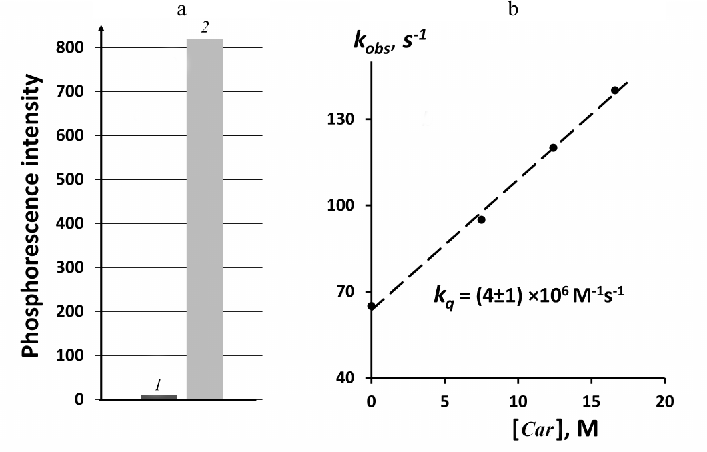
Figure 4 illustrates relative quantum yields of 1O2 phosphorescence in solutions of phytoene and phenalenone. Phytoene was studied in perfluorohexane under excitation at 281 nm (3.5 mW). Very weak luminescence was observed at 1270 nm under these conditions in phytoene solutions. Phenalenone absorbance at 281 nm was low, therefore phenalenone was irradiated by the LED at 371 nm to obtain reliable results. Absorbance of both compounds was adjusted to 0.13 at the excitation wavelengths that corresponded to the concentrations of 2.6 µM for phytoene and 1.7 µM for phenalenone. After normalization to the number of incident photons and subtraction of the background luminescence signal emitted by the solvents without photosensitizers, we established that under the applied conditions the intensity of the 1O2 phosphorescence in the phytoene solutions was ≈100 times lower than in the phenalenone solution.
It is known that phytoene and phytofluene are able to moderate quenching of 1O2, although accurate measurements of the rates of this process have not been reported [23]. Quenching activity of phenalenone is known to be very low ([19] and refs therein). In order to find out how quenching of 1O2 by phytoene affects the relative yield of 1O2 phosphorescence in phytoene solutions, we studied quantitatively the rate of 1O2 quenching by phytoene. This was performed based on measuring the decay rates of the phenalenone-photosensitized 1O2 phosphorescence upon addition of phytoene. Hexafluorobenzene was employed as a solvent, because both phytoene and phenalenone were reasonably soluble in hexafluorobenzene, whereas both carotenoids were poorly soluble in perfluorohexane. Figure 4b shows that quenching obeys the Stern–Volmer equation:
kobs = ko + kq[Car], (1)
where kobs is the observed rate constant of phosphorescence decay (in s–1) (kobs = 1/τΔ, where τΔ is the 1O2 lifetime) after LED pulses in solutions containing both photosensitizer and Car; k0 is the rate constant of the phosphorescence decay in phenalenone solutions without Car; kq is the bimolecular rate constant of 1O2 quenching by Car; [Car] is the molar concentration of the carotenoid.
It follows from Fig. 4b that kq = (4 ± 1) × 106 M–1·s–1 for phytoene. This value agrees with the data reported by Mathews–Roth et al. indicating that kq < 107 M–1·s–1 [23]. Hence, phytoene is a rather poor quencher of 1O2. It follows from the Stern–Volmer plot presented in Fig. 4b that phytoene at the concentration of 2.6 µM increases the decay rate of 1O2 phosphorescence in hexafluorobenzene by a factor of 1.16 (kq[Car]/ko) = 0.16). By rewriting Eq. (1) using the steady-state phosphorescence intensities, the following equation is obtained:
Io/Iq = 1 + (kq/ko)[Car], (2)
where Io and Iq are the steady-state intensities of phenalenone-photosensitized 1O2 phosphorescence in the absence and in the presence of a phytoene, respectively. It can be estimated from this equation that in hexafluorobenzene, quenching by phytoene causes 16% reduction of the steady-state intensity of 1O2 phosphorescence compared to the solution of phenalenone alone.
Taking into account that the 1O2 lifetime in perfluorohexane is two-fold longer (or ko is two-fold lower) than in hexafluorobenzene (Fig. 4), and kq are equal in both solvents, one can calculate using Eq. (2) that quenching of 1O2 by phytoene (2.6 µM) in perfluorohexane should reduce the phosphorescence intensity by a factor of 1.3 ((kq[Car]/ko) ≈ 0.3). Hence, 1O2 quenching by phytoene cannot explain low intensity of 1O2 phosphorescence in the phytoene solution (Fig. 4). It should be concluded that this effect is due to the very low quantum yield of 1O2 generation (ΦΔ) by phytoene. Based on these results, we can argue that the phytoene ΦΔ is less than 2%. This value agrees with the flash photolysis data of Bensansson et al., who reported that the yield of phytoene triplet state is very low, being less than 0.002 [14]. Studies of phytoene solutions are currently underway to generate more detailed data.
Fig. 4. Generation and quenching of 1O2 by phytoene. a) Relative intensities of photosensitized phosphorescence of 1O2 in solutions of phytoene (1) and phenalenone (2) in perfluorohexane with equal absorbance (0.13) at the excitation wavelengths (281 and 371 nm, respectively); b) quenching of phenalenone-photosensitized 1O2 phosphorescence in hexafluorobenzene by addition of phytoene, excitation wavelength was 405 nm.
Phytofluene was studied in hexafluorobenzene under excitation by both cw LED at 371 nm (2-3 mW) and pulsed LED at 405 nm. After irradiation of phytofluene solution by the cw LED a rather strong IR phosphorescence with the spectral maximum at 1270 nm was observed (Fig. 5). After addition of 50% acetone, which led to a sharp decrease in the 1O2 lifetime, the photosensitized phosphorescence weakened by approximately 500-fold (Fig. 5). Similar effect was observed in the phenalenone solutions in hexafluorobenzene [19]. The action spectrum of this phosphorescence evaluated using three LEDs with fixed wavelengths 350, 371, and 399 nm (not shown) corresponded to the absorption spectrum of phytofluene.
Fig. 5. Parameters of 1O2 phosphorescence photosensitized by phytofluene in hexafluorobenzene. a) Phosphorescence spectrum; b) intensity of phosphorescence before (1) and after (2) addition of 50% acetone. Phosphorescence was excited by cw LED at 371 nm (≈3.5 mW).
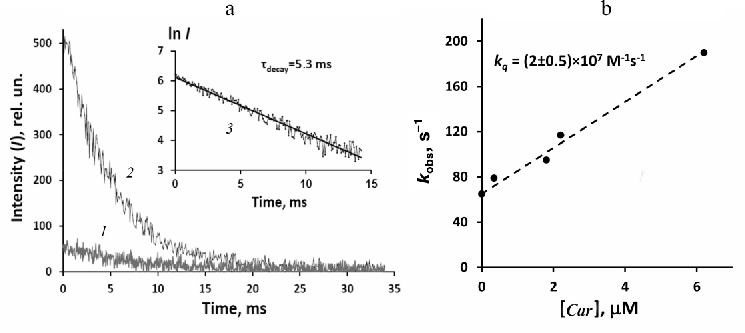
Due to low absorbance of phytofluene at the wavelength of our pulsed LED (405 nm), it was difficult to perform accurate time-resolved measurement of 1O2 phosphorescence. Nevertheless, weak signal of the 1O2 phosphorescence was detected under the pulsed irradiation of rather concentrated (6.2 µM) phytofluene solution with absorbance of ~0.01 at 405 nm (Fig. 6). The decay time was about 5 ms, being about three-fold shorter than in the dilute solution of phenalenone alone (Fig. 3). This fact indicated that phytofluene quenched 1O2 at the used concentration. In order to verify this observation, a solution was prepared, which contained phytofluene of the same concentration and phenalenone with absorbance of 0.1 at 405 nm. Strong phosphorescence at 1270 nm was observed in this solution. Its lifetime was found to coincide with the phosphorescence lifetime in the solution of pure phytofluene (Fig. 6). Moreover, the initial intensity of phosphorescence in phenalenone solution was about 10 times stronger than in the solution of phytofluene. Hence, relative phosphorescence intensities of both solutions were proportional to the values of their absorbance at the wavelength of excitation. This fact suggests that the quantum yields of 1O2 generation by phytofluene and phenalenone were approximately the same.
Further experiments were directed to more detailed investigation of phytofluene ability to generate and quench 1O2. Figure 6b shows the Stern–Volmer plot [Eq. (1)] for quenching of 1O2 phosphorescence photosensitized by phenalenone (2 µM) upon addition of phytofluene. It follows from this plot that the rate constant of 1O2 quenching by phytofluene is (2.0 ± 0.5) × 107 M–1·cm–1. This value agrees with the estimate of kq < 108 M–1·s–1 reported by Mathews–Roth et al. [23].
Fig. 6. Generation and quenching of 1O2 phosphorescence by phytofluene in hexafluorobenzene. a) Decays of phosphorescence after irradiation with LED pulses (10 µs, 405 nm) in solution of phytofluene (6.2 µM) (1) and in solution of phenalenone with added 6.2 µM phytofluene (2) (pulse repetition rate 30 Hz; averaging time – 15 min); semilogarithmic plot of the curve 2 (3). b) Stern–Volmer plot for phytofluene quenching of 1O2 phosphorescence photosensitized by phenalenone (2 µM). See additional explanations in the text.
To determine the quantum yield of 1O2 generation (ΦΔ) by phytofluene, the intensities of 1O2 phosphorescence were compared in the solutions of phytofluene and phenalenone in hexafluorobenzene of equal absorbance (0.15) at 371 nm. This absorbance corresponded to the concentrations of phytofluene and phenalenone of about 2 and 1.4 µM, respectively. Under these conditions, the 1O2 phosphorescence intensity in the phytofluene solution was about 60% of that in the phenalenone solution. Reduction of 1O2 phosphorescence in the phytofluene solution is partially due to the quenching of 1O2 by phytofluene (Fig. 6b). Using Eq. (2) and obtained kq for phytofluene, one can calculate that 2 µM phytofluene causes 1.5-fold reduction in the phosphorescence intensity compared to the solution of phenalenone alone. Taking into account this quenching and the value of ΦΔ for phenalenone equal to 0.95 [20, 21], we arrive to the following value of ΦΔ for phytofluene: ΦΔ = 0.95 × 0.6 × 1.5 = 0.85 with estimated error of ±5%. Table summarizes the data obtained.
Quantum yields of 1O2 generation and rate
constants of 1O2 quenching by carotenoids studied
in the present work
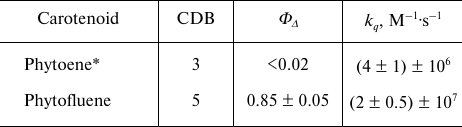
* ΦΔ for phytoene was obtained in
hexafluorohexane, other data – in hexafluorobenzene.
DISCUSSION
Our data demonstrate that phytoene and phytofluene, which are universal precursors in biosynthesis of colored carotenoids with the long CDB chains in plant and bacteria, differ greatly in their ability to photosensitize and quench 1O2. Phytoene was much less efficient in both these processes. Phytofluene is more active as a 1O2 quencher than phytoene but still the quenching activities of both carotenoids are much (by 2-3 orders of magnitude) lower than those of lycopene or β-carotene [2, 5, 15]. At the same time, phytofluene shows very high photosensitizing activity. To the best of our knowledge this property of phytofluene was observed in the present paper for the first time. The data suggest that upon photoexcitation, phytofluene efficiently populate rather long-lived triplet state, which is capable to efficiently transfer its energy to oxygen. We could not find any information on the quantum yield and lifetime of phytofluene triplets in the literature. However, energy of the triplet state (ET) can be estimated from the linear dependence between ET–1 and number of the CDBs reported by Bensasson et al. [14]. Using this linear function, we obtained that ET = 1.45 ev for phytofluene, hence it is higher than the energy of the 1Δg state of oxygen (1 ev). Therefore, generation of 1O2 by triplet phytofluene is energetically allowed.
In addition, it is known that phytofluene emits rather strong fluorescence at 500 nm with the quantum yield of 0.05 ± 0.01 at room temperature [24, 25]. This is unusual for C40 carotenoids, whose fluorescence yield is normally less than 10–3 [24, 25]. Hence, by photophysical properties phytofluene resembles more chlorophyll and porphyrins than C40 polyenes. The data suggest, that phytofluene should be an efficient promoter of photochemical processes. In our opinion this fact changes views on the biological roles of this compound. In particular, it may be involved in biological effects of UV-A light [26], photodamage of the photosynthetic apparatus and skin tissues. Moreover, this compound can be considered as a potential UV-A photosensitizer in cosmetology and medicine. However considering high photosensitizing activity of phytofluene, the use of this compound for protection of skin from ultraviolet radiation seems questionable.
Acknowledgements. The authors are grateful to companies “Polironik Ltd.” (Moscow) and “Alkom Medika Ltd.” (St. Petersburg) for technical assistance and to “Pimimvest” (Moscow) for purification of the solvents.
Funding. This study was financially supported in part by the Russian Foundation for Basic Research (projects Nos. 18-04-00684-a and 19-04-00331-a), and by the State Assignments of the Federal Research Centre for Biotechnology, Russian Academy of Sciences, Moscow, and by the Federal Research Centre “Pushchino Scientific Center for Biological Research”, Russian Academy of Sciences, Pushchino, Russia.
Ethics declarations. The authors declare that they have no conflicts of interest. This article does not contain any studies involving human participants or animals performed by any of the authors.
REFERENCES
1.Britton, G. (2008) Functions of Intact
Carotenoids., in Carotenoids. Natural Functions
(Britton, G., Liaaen-Jensen, S., and Pfanger, H. eds.) Birkhauser
Verlag, Switzerland, pp. 265-308.
2.Edge, R., and Truscott, T. G. (2018) Singlet oxygen
and free radical reactions of retinoids and carotenoids – a
review, Antioxidants, 7, 5-16.
3.Krasnovsky, A. A., Jr., and Kagan, V. E. (1979)
Photosensitization and quenching of singlet oxygen by pigments and
lipids of photoreceptor cells of the retina, FEBS Lett.,
108, 152-154.
4.Ostrovskii, M. A., and Fedorovich, I. B. (1994)
Retinal as sensitizer of photodamage of retinal-containing proteins in
the retina of the eye, Biophysics, 39, 13-25.
5.Krasnovskii, A. A., Jr. (1986) Singlet oxygen in
photosynthesizing organisms, Zhurn. Vsesoyuz. Khim. Obsch. im. D. I.
Mendeleeva, (Mendeleev Chemical Journal), 31, 562-567.
6.Makhneva, Z. K., Erokhin, Yu. E., and Moskalenko,
A. A. (2007) Carotenoid-photosensitized oxidation of
bacteriochlorophyll dimers in light-harvesting complexes B800-850 in
Allochromatium minutissimum cells, Dokl. Biochem.
Biophys., 416, 256-259.
7.Makhneva, Z. K., Bolshakov, M. A., Ashikhmin, A.
A., Erokhin, Y. E., and Moskalenko, A. A. (2009) Influence of blue
light on the structure stability of antenna complexes from
Allochromatium minutissimum with different content of
carotenoids, Biochemistry Suppl. Ser. A Membr. Cell Biol.,
3, 123-127.
8.Makhneva, Z. K., Ashikhmin, A. A., Bolshakov, M.
A., and Moskalenko, A. A. (2020) Carotenoids are probably involved in
singlet oxygen generation in the membranes of purple photosynthetic
bacteria under light irradiation, Microbiology, 89,
164-173.
9.Makhneva, Z. K., Ashikhmin, A. A., Bolshakov, M.
A., and Moskalenko, A. A. (2019) Bacteriochlorophyll interaction with
singlet oxygen in membranes of purple photosynthetic bacteria: does the
protective function of carotenoids exist? Dokl. Biochem.
Biophys., 486, 216-219.
10.Makhneva, Z. K., Ashikhmin, A. A., Bolshakov, M.
A., and Moskalenko, A. A. (2019) Quenchers protect BChl850 from action
of singlet oxygen in the membranes of a sulfur photosynthetic bacterium
Allochromatium vinosum Strain MSU, Microbiology,
88, 79-86.
11.Meléndez-Martínez, A. J., Stinco,
C. M., and Mapelli-Brahm, P. (2019) Skin carotenoids in public health
and nutricosmetics: the emerging roles and applications of the UV
radiation-absorbing colourless carotenoids phytoene and phytofluene,
Nutrients, 11, 1093.
12.Meléndez-Martínez, A. J.,
Mapelli-Brahm, P., and Stinco, C. M. (2018) The colourless carotenoids
phytoene and phytofluene: from dietary sources to their usefulness for
the functional foods and nutricosmetics industries, J. Food
Composit. Anal., 67, 91-103.
13.Mathis, P., and Kleo, J. (1973) The triplet state
of β-carotene and of analog polyenes of different length,
Photochem. Photobiol., 18, 343-346.
14.Bensasson, R., Land, E. J., and Maudinas, B.
(1976) Triplet states of carotenoids from photosynthetic bacteria
studied by nanosecond ultraviolet pulse irradiation, Photochem.
Photobiol., 23, 189-193.
15.Krasnovsky, A. A., Jr. (1979) Photoluminescence
of singlet oxygen in pigment solutions, Photochem. Photobiol.,
29, 29-36.
16.Moskalenko, A. A., and Makhneva, Z. K. (2012)
Light-harvesting complexes from purple sulfur bacteria
Allochromatium minutissimum assembled without
carotenoids, J. Photochem. Photobiol. B Biol., 108,
1-7.
17.Ashikhmin, A., Makhneva, Z., Bolshakov, M., and
Moskalenko, A. (2014) Distribution of colored carotenoids between
light-harvesting complexes in the process of recovering carotenoid
biosynthesis in Ectothiorhodospira haloalkaliphila cells, J.
Photochem. Photobiol. B Biol., 141, 59-66.
18.Ashikhmin, A., Makhneva, Z., Bolshakov, M., and
Moskalenko, A. (2017) Incorporation of spheroidene and spheroidenone
into light-harvesting complexes from purple sulfur bacteria, J.
Photochem. Photobiol. B Biol., 170, 99-107.
19.Krasnovsky, A. A. Jr., Benditkis, A. S., and
Kozlov, A. S. (2019) Kinetic measurements of singlet oxygen
phosphorescence in the solvents lacking hydrogen atoms using the method
of time resolved photon counting, Biochemistry (Moscow),
84, 153-163.
20.Oliveros, E., Suardi-Murasecco, P.,
Aminian-Saghafi, T., and Braun, A. M. (1991) 1H-Phenalen-1-one:
photophysical properties and singlet oxygen production, Helv. Chim.
Acta, 74, 79-90.
21.Schmidt, R., Tanelian, C., Dunsbach, R., and
Wolf, C. (1994) Phenalenone, a universal compound for the determination
of quantum yields of singlet oxygen O2
(1Δg) sensitization, J. Photochem.
Photobiol. A Chem., 79, 11-17.
22.Britton, G. (1995) UV/visible spectroscopy, in
Carotenoids (Britton, G., Liaaen-Jensen, S., and Pfander, H.,
eds.) vol. 1B, Birkhäuser Verlag, Basel.
23.Mathews-Roth, M. M., Wilson, T., Fujimori, E.,
and Krinsky, N. I. (1974) Carotenoid chromophore length and protection
against photosensitisation, Photochem. Photobiol., 19,
217-222.
24.Cogdell, R. J., Gillbro, T., Andersson, P. O.,
Liu, R. S. H., and Asato, A. E. (1994) Carotenoids as accessory
light-harvesting pigments, Pure Appl. Chem., 66,
1041-1046.
25.Andersson, P. O., Takaichi, S., Cogdell, R. J.,
and Gillbro, T. (2001) Photophysical characterization of natural
cis-carotenoids, Photochem. Photobiol., 74,
549-557.
26.Fraikin, G. Y., and Rubin, L. B. (1979) Some
physiological effects of near-ultraviolet light on microorganisms,
Photochem. Photobiol., 29, 185-187.