Immune Response and Protective Efficacy of Inactivated and Live Influenza Vaccines Against Homologous and Heterosubtypic Challenge
E. Y. Boravleva1, A. V. Lunitsin2, A. P. Kaplun3, N. V. Bykova3, I. V. Krasilnikov4, and A. S. Gambaryan1,a*
1Chumakov Federal Scientific Center for Research and Development of Immune and Biological Products, Russian Academy of Sciences, 108819 Moscow, Russia2FSBSI Federal Research Center for Virology and Microbiology, 601125 Volginsky, Vladimir Region, Russia
3Lomonosov Moscow University of Fine Chemical Technology, 119571 Moscow, Russia
4Saint Petersburg Institute of Vaccines and Sera, FMBA, 198320 St.-Petersburg, Russia
* To whom correspondence should be addressed.
Received February 19, 2020; Revised March 19, 2020; Accepted March 20, 2020
Inactivated (whole-virion, split, subunit, and adjuvanted) vaccines and live attenuated vaccine were tested in parallel to compare their immunogenicity and protective efficacy. Homologous and heterosubtypic protection against the challenge with influenza H5N1 and H1N1 viruses in a mouse model were studied. Single immunization with live or inactivated whole-virion H5N1 vaccine elicited a high level of serum antibodies and provided complete protection against the challenge with the lethal A/Chicken/Kurgan/3/05 (H5N1) virus, whereas application of a single dose of the split vaccine was much less effective. Adjuvants increased the antibody levels. Addition of the Iso-SANP adjuvant to the split vaccine led to a paradoxical outcome: it increased the antibody levels but reduced the protective effect of the vaccine. All tested adjuvants shifted the ratio between IgG1 and IgG2a antibodies. Immunization with any of the tested heterosubtypic live viruses provided partial protection against the H5N1 challenge and significantly reduced mouse mortality, while inactivated H1N1 vaccine offered no protection at all. More severe course of illness and earlier death were observed in mice after immunization with adjuvanted subunit vaccines followed by the challenge with the heterosubtypic virus compared to challenged unvaccinated animals.
KEY WORDS: influenza virus A, live and inactivated vaccine, adjuvantsDOI: 10.1134/S0006297920050041
Abbreviations: Ca, cold-adapted; CE, embryonated chicken egg; EID50, 50% infection dose in CE; HA, hemagglutinin; HPAIV, highly pathogenic avian influenza virus; IIV, inactivated influenza vaccine; LAIV, live attenuated influenza vaccines; LD50, 50% lethal dose; LPAIV, low pathogenic avian influenza virus; NA, neuraminidase; SANP, spherical amorphous nanoparticles consisting of birch bark triterpenoid mixture; TCID50, 50% infection dose in tissue culture; TIV, trivalent influenza vaccine; VAF, virus-containing allantoic fluid; VE, vaccine efficacy; WIV, whole-virion inactivated vaccine.
INTRODUCTION
Vaccination is a primary method for the prophylactics of influenza infection. Influenza vaccination had prevented an estimated 4.4 million illnesses, 2.3 million medical visits, 58,000 hospitalizations, and 3500 deaths during the US 2018-2019 influenza season [1]. Vaccination reduces the risk of influenza illness by ~50%, although its efficiency can significantly differ depending on the season, epidemiological situation, age group, virus type/subtype, and degree of antigenic match between the vaccine and circulating viruses [2]. Many different types of influenza vaccines are currently produced, including whole-virion inactivated vaccines (WIVs), split vaccines, and subunit vaccines contained hemagglutinin (HA) and neuraminidase (NA) antigens only. These vaccines can be supplemented with adjuvants. Another type of produced vaccines is live attenuated vaccines (LAIVs) [3].
Whole-virion inactivated vaccines. Numerous studies suggest that WIVs induce a more powerful immune response than split and subunit vaccines. WIVs provide influenza-specific induction of CD8+ T cells and ensure protection against lethal homosubtypic and heterosubtypic challenge in mice [4]. WIVs induce two waves of antibody (Ab) response: early T cell-independent burst of high-affinity memory B cells and late T cell-dependent response. High-affinity Abs with an improved neutralizing activity are synthesized during the early response [5]. Split vaccines elicit the late T cell-dependent response only.
WIVs provide a significant reduction in the peripheral blood leukocytes in mice, which is accompanied by a rapid induction and massive production of interferon (IFN)-α, whereas split and subunit vaccines prepared from the same influenza virus strain do not elicit such effect [6].
The limiting factor preventing wide application of WIVs is a development of inflammatory reactions at the site of vaccine administration. Nevertheless, monovalent WIVs might perform best in a pandemic, when vaccine production time is a key factor [7].
Split and subunit vaccines. Split and subunit vaccines are more tolerated, but exhibit low vaccine efficacy (VE) [8].
Adjuvanted vaccines. One way to improve immune response to a vaccine is to add adjuvants that facilitate antigen delivery to the antigen-presenting cells [9]. MF59, AS03, and aluminum hydroxide (the most common adjuvants), enhance immune response and contribute to the production of higher Ab levels [8].
Safety and efficacy data from the clinical trials and observational studies attest to the safety of MF59 and its ability to enhance the efficacy of influenza vaccines in children and elderly [10]. MF59 and AS03 successfully stimulate production of more effective antibodies even when used with a smaller dose of H5N1 vaccine [11]. In a randomized trial in healthy elderly volunteers, MF59-adjuvanted vaccine exhibited superior immunogenicity compared with a conventional subunit vaccine. However, vaccination-associated generalized myalgia was observed in 8.1% and 0.9% patients, respectively [12]. Relative efficacy, immunogenicity, and safety were the same for the MF59-adjuvanted subunit influenza vaccine (aIIV4) and US-licensed non-adjuvanted vaccine in the overall children population; but the efficacy of aIIV4 was considerably higher compared to the non-adjuvanted vaccine in the 6 to 23 months-old subgroup. The safety profiles were similar, for both vaccines; however, a higher number of adverse effects was reported for aIIV4 [13, 14].
Prime-boost-boost vaccination of ferrets with recombinant hemagglutinin (HA) mixed with Addavax (MF59-like adjuvant) elicited stronger Ab response with a broader cross-reactivity than the actual influenza infection; this cross-reactive response likely correlated with the increased content of the anti-stalk antibodies [15].
The effects of seasonal trivalent influenza vaccine (TIV) and TIV/AS03-adjuvanted vaccine in elderly people were compared in an observer-blind study. The relative content of CD4+ T cells specific to the vaccine strains was higher in the TIV/AS03 recipients than in TIV recipients [16].
The immunogenicity and safety of the aluminum hydroxide-adjuvanted, whole-virion, prepandemic A/H5N1 influenza vaccine (MG1109) were assessed in [17]. Administration of two doses of MG1109 was highly immunogenic and well tolerated in adults. The disadvantage of the aluminum-based adjuvant, especially when it used with a subunit vaccine, is a poor cellular immune response, since aluminum only induces the Th2-type response [18].
G3, a new saponin-based adjuvant formulated with a diterpenoid from steviol glycosides, induced anamnestic virus-specific CD8+ T cells, which ensured broader protection against antigenically different influenza viruses [19]. The ability of G3 to induce Th1 and Th2 cell responses in mice immunized with a split influenza vaccine was evaluated in comparison with Al(OH)3. It was found that G3 increased both IgG1 and IgG2a levels, whereas Al(OH)3 suppressed IgG2a production, thus indicating a strong ability of G3 to induce both cellular and humoral immune responses [20].
The CAF01 adjuvant, with induces both CD4+ T-cellular and Ab responses, was investigated in ferrets for the cross-protective efficacy with a heterologous split vaccine. CAF01-adjuvanted vaccine elicited protection after heterologous challenge despite the absence of specific antibodies. The immunity induced by TIV+CAF01 reduced virus shedding and systemic disease symptoms, but not local inflammation in the nasal cavity [21].
A combination glucopyranosyl lipid (TLR4 agonist) adjuvant formulated as a stable oil-in-water emulsion (GLA-SE), with a split influenza vaccine boosted the IgG2c/IgG1 ratio, increased hemagglutination inhibition titers, and promoted protection in aged mice [22].
The CpG adjuvant suppressed induction and expansion of antigen-specific Treg cells, thus attenuating the immunity against the influenza virus. CpG-adjuvanted peptide vaccines provided protection against heterosubtypic influenza viruses presumably by inhibiting Treg development and enhancing T cell immunity [23].
A combination of human pulmonary surfactant containing carboxy vinyl polymer as a viscosity improver (SF-10) and influenza HA vaccine (HAv) induced the HA-specific cytotoxic T lymphocytes and upregulated granzyme B expression in splenic CD8+ T cells exhibiting high cytotoxicity against HA-expressing target cells. The T cell-mediated cytotoxicity induced by HAv-SF-10 in the lungs during the early phase of influenza virus infection was higher than that induced by HAv alone [24].
Synthetic hemozoin (protein byproduct of malaria infection) was tested as an adjuvant for a WIV in a mouse model. Hemozoin improved the immunogenicity of inactivated influenza viruses and was proved to be a promising adjuvant for WIV [25].
Polyoxidonium and sovidon adjuvants are used in the Grippol and Sovigripp vaccines in Russia. Grippol accounts for ~60% of the Russian market for influenza vaccines, but no large-scale trials of Grippol and Sovigripp have been conducted so far [26].
Live vaccines. LAIVs are constructed by the reverse genetics methods or reassortment using the HA and NA genes from epidemic strains and six remaining genome segments from attenuated cold-adapted (Ca) master donor strains [A/Ann Arbor/6/60 (H2N2) and A/Leningrad/134/17/57 (H2N2) in the USA and Russia, respectively].
LIAV FluMist was approved for use in the United States in 2003. This vaccine is administered intranasally and offers an advantage of immunity stimulation at the site of infection in the upper respiratory tract. By mimicking natural infection, it has a potential to elicit a multifaceted immune response [27].
Numerous studies have proven the safety and efficacy of LAIVs, especially in young children [28-40]. LAIVs have a lower chance of inducing severe adverse side effects (Guillain-Barre syndrome and paralysis) than TIVs [41]. In children aged 6 months to 7 years, LAIVs are superior to placebo and TIVs [34, 36, 42].
The safety of LAIVs has been shown not only in the cohort, for which they are mostly recommended (2- to 7-year-old children), but also in 6-24-month-old children and children with mild forms of asthma or prior wheezing [43]. LAIVs also demonstrated a low risk of systemic allergic reactions in young people with egg allergy [44]. LAIVs are well tolerated in patients with well controlled asthma or recurrent wheeze LAIVs used in asthma were not associated with an increased risk of adverse respiratory effects that required medical attention [45, 46].
TIVs elicit higher geometric mean antibody titers than LAIVs, whereas LAIVs induce stronger T-cell response, especially against hypervariable HA regions [47]. The advantage of live vaccines is that they mainly induce development of virus-specific CD4+ and CD8+ T cells that provide a broader immune response; therefore, LAIVs are less sensitive to the antigenic match between the vaccine and circulating viruses [48]. It was found that LAIV elicited a significant increase in naive, memory, and transitional B cells on day 30 after vaccination, whereas TIV elicited an increased number of plasmablasts on day 7, which suggested that LAIV and TIV induced different B-cell responses in vaccinated children [49].
LAIV showed significant protection against mismatched influenza strains in 6 to 36-month-old children [50]. Induction of heterosubtypic cross-protection indicates the possibility of LAIV application as a universal influenza vaccine for the prime-boost vaccination in the case of a new pandemic [51].
Although LAIVs are highly efficacious in young children, they are less efficacious than TIVs in adults. A nation-wide cohort study in Finland during the 2015-2016 season revealed that TIV provided more efficient protection than LAIV [52]. During the 2004-2005 season, in which most circulating viruses were dissimilar to those included in the vaccine, the inactivated vaccine was efficacious in preventing laboratory-confirmed symptomatic influenza illnesses in healthy adults; LAIV also prevented influenza but with less efficiency [53, 54].
Although all types of vaccines have proven their safety and efficacy in research studies, the results of randomized controlled blind trials are sometimes not so impressive.
Analysis of the Cochrane Database of Systematic Reviews revealed that inactivated influenza vaccines reduce influenza in healthy adults from 2.3% (without vaccination) to 0.9%. Vaccinations also reduced influenza-like illness (ILI), but to the varied extent. No unequivocal evidence for the reduction in the number of hospitalizations and time off work has been obtained. Protection against influenza and ILI in mothers and newborns was less pronounced than in other populations. At the same time, vaccination was often associated with negative side effects [2].
Immunization with inactivated influenza vaccine (IIV) in children with pre-existing medical conditions did not reduce respiratory illness episodes during the influenza season [55].
Vaccination with TIV sometimes results in paradoxical effects. The efficacy of the vaccine against A(H3N2) was –3% during the 2014-2015 influenza season [56]. Previous season vaccination usually provides residual protection, yet sometimes decreases current vaccine efficacy [57]. During the 2015-2016 season, the vaccine efficacy in preventing A(H1N1) pdm09 infection was low, especially for persons vaccinated in the previous season [58]. The negative interference between previous and current vaccinations against A(H3N2) virus was reported by Gherasim et al. [59] and Rondy et al. [60].
The best effect of vaccination was observed during the 1968-1969 pandemic: the monovalent whole-virion vaccine matching the circulating virus had a VE of 66-93% [7].
Pandemics are extremely dangerous to human life and health. Efficient protection against new pandemics might be more important than protection during the periods between them. The optimal mitigation strategy was determined using the age-specific compartmental model. It was found that if the vaccine stockpile is limited at the start of the influenza pandemic, the vaccination campaign should start early in order to delay the arrival of a large wave of infections and to slow down its growth. The most efficient strategy would be to use seasonal LAIV and to target the group of 5- to 19-year-old [61].
In this study, we used a mouse model to test different types of influenza vaccines, such as WVI, split vaccine, and subunit IIV, vaccines with different adjuvants, and LAIV and to study homologous and heterosubtypic protection against the challenge with the H1N1 and H5N1 influenza viruses.
MATERIALS AND METHODS
Viruses used in this study are listed in Table 1.
Table 1. Influenza A viruses used in the
study
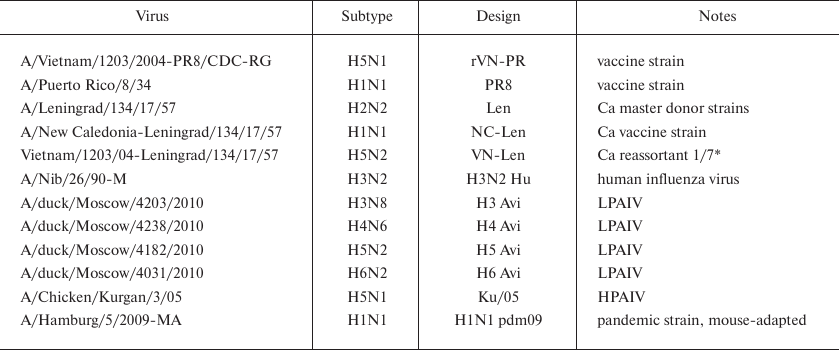
* HA H5 gene and 7 genome segments from the donor strain.
Low-pathogenic avian influenza viruses A/duck/Moscow/4203/2010 (H3N8), A/duck/Moscow/3735/2009 (H4N6), A/duck/Moscow/4182/2010 (H5N3), and A/duck/Moscow/4031/2010 (H6N2) were isolated from wild mallard feces as described previously [62, 63].
The vaccine strain A/Vietnam/1203/04-PR8/CDC-RG (rVN-PR) was a reassortant containing the NA and modified HA genes from A/Vietnam/1203/2004 (H5N1) and remaining genes from the vaccine strain A/Puerto Rico/8/34 (PR8). This vaccine was constructed at the Influenza Department of the Center for Disease Control and Prevention (CDC, Atlanta, USA) and was kindly provided by Dr. R. Donis. Cold-adapted (Ca) A/Leningrad/134/17/57 (H2N2) strain and A/New Caledonia-Leningrad/134/17/57 (NC-Len) reassortant were kindly provided by Dr. Rudenko (Research Institute of Experimental Medicine, RAMS, St.-Petersburg, Russia). Ca reassortant A/Vietnam-Leningrad/134/17/57 (VN-Len) was generated by classical reassortment using A/Leningrad⁄134⁄17⁄57 donor strain as previously described [64]. Human virus A/Nib/26/90M (H3N2) (not adapted to CE) was kindly provided by Dr. James Robertson (National Institute for Biological Standards and Control, United Kingdom). Pandemic strain A/Hamburg/5/2009 (H1N1) was kindly provided by Dr. Matrosovich (Institute of Virology, Philipps University, Marburg, Germany). Mouse-adapted A/Hamburg/5/2009-MA strain was obtained via seven consecutive passages in mouse lungs and differed from the original virus by the HA-Asp225Gly and HA-Lys123Asn substitutions [65].
Highly pathogenic avian influenza virus (HPAIV) A/Chicken/Kurgan/3/05 (Ku/05) was kindly provided by Dr. S. S. Yamnikova (Gamaleya National Research Center for Epidemiology and Microbiology, Moscow, Russia). All studies with Ku/05 were conducted in a BSL-3 containment facility at the Federal Research Center for Virology and Microbiology, 601125 Volginsky, Vladimir Region, Russia.
Propagation of viruses. Ten-day-old embryonated chicken eggs (CEs) were inoculated with 102 viral infection units (50% infection dose in CE, EID50) and incubated at 32°C (Ca strains) or 36°C (other viruses). Allantoic fluid was harvested 96 or 48 h post infection, respectively. The viral titer in the allantoic fluid (VAF) was determined with the HA assay [26]; VAF infectivity was assessed by titration in CEs and expressed in EID50.
Samples for immunization. WIVs, split vaccines with and without adjuvants, subunit vaccines with polyoxidonium, and live vaccines were used for immunization (Table 2). The GrippolR plus vaccine (Petrovax, Russia) contained viral surface antigens from the H1N1 A/California7/2009/, H3N2 A/Victoria 210/208 NYMC X-187, and V/Brisben/60/2008 viruses (5 μg of HA from each virus) and 0.5 mg of polyoxidonium in a single human dose. All vaccines were stored at the recommended temperatures.
Table 2. Vaccine formulations used in this
work
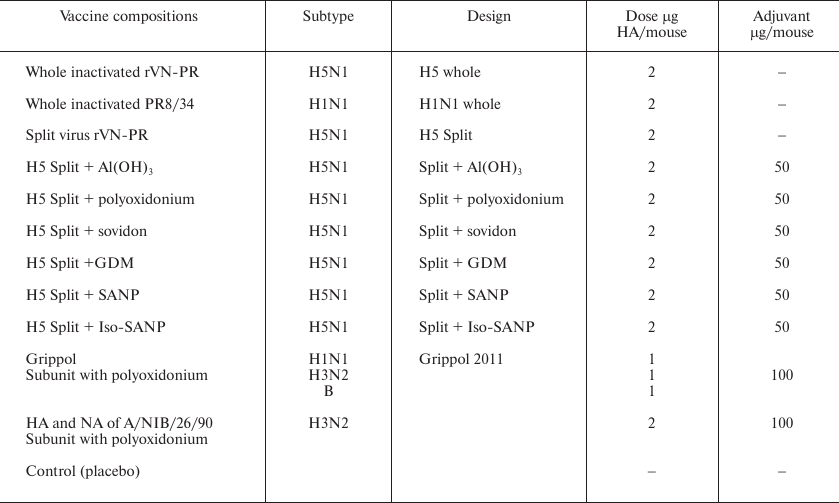
The adjuvants were aluminum hydroxide (Serva, German), copolymer of N-oxy-1,4-ethylene piperazine and (N-carboxy)-1,4-ethylene piperazine bromide (polyoxidonium), and copolymer of N-vinylpyrrolidone and 2-methyl-5-vinylpyridine (sovidon) (NPO Petrovax Farm, Russia). Positively charged nanoparticles composed of glycosphingolipid, miramistin, and dihydroquercetin (GMD), negatively charged spherical amorphous nanoparticles (SANP) containing birch bark triterpenoids, and SANP with addition of isopropyl palmitate (Iso-SANP) were produced as described in [66].
Immunization of mice with inactivated vaccines. Six weak-old BALB/c mice (Stolbovaya FSBSI, Russian FMBA) were inoculated intramuscularly with inactivated vaccines or placebo (PBS). The mice were weighed daily; serum samples for Ab titration were taken on days 11, 18, and 30 post immunization.
Infection of mice with live influenza viruses. Mice were anesthetized with ether and inoculated intranasally with 50 μl of VAF (infection dose, ~107 EID50 per mouse).
Blood samples for Ab assay. The tail tip was cut off; the blood was collected and mixed at a 1/2 ratio with PBS containing with 0.01 M sodium citrate and 25 IU/ml heparin. The samples were centrifuged; 10% suspension of kaolin was added to the supernatant (1/10 of the supernatant volume). The mixture was incubated for 5 h with periodical shaking. Then, the samples were centrifuged and stored frozen. The concentration of serum in the resulting preparation was 1/5 of the total volume.
Anti-influenza virus Abs to the in the mouse serum were identified by ELISA. VAF containing 64 hemagglutinating units of the virus of a corresponding subtype was added to the plate wells Nunc, MaxiSorp (Sigma-Aldrich, USA) sensitized with fetuin and incubated overnight at 4°C. Next, the plates were washed and blocked with 0.2% BSA solution in PBS for 1 h. The blocking solution was removed, and 100 μl of buffer (0.1% Tween-20, 0.2% BSA in PBS) containing different dilutions of the serum (staring from 1 : 20 dilution) was added to each well. The wells without the virus served as a negative control. After incubation for 4 h at 4°C, the plates were washed, and peroxidase-labeled antibodies against mouse IgG1 and IgG2a (Serotec, Germany) were added. The color reaction was performed with ortho-phenylenediamine [64].
Aerosol challenge was performed on day 30 post-immunization. All mice were infected using an in-house constructed apparatus for the whole-body aerosol exposure as described in [68]. The apparatus consisted of a transparent plastic chamber connected to a Musson-1 ultrasound inhaler (Rotor, Altai, Russia) that generated virus aerosol (particle size, 1-5 μm). The aerosol entered the chamber through an inlet in the upper lid and was pumped out through an outlet in the chamber bottom, which was connected via HEPA filter to a peristaltic pump operating at 0.5 liter per minute. The mice were exposed to the aerosol containing ~105-106 EID50 of the virus per liter for 10 min. The dosage of the A/Chicken/Kurgan/3/05 virus was ~103 EID50 per mouse (~100 LD50). The dosage of the mouse-adapted A/Hamburg/5/2009-MA virus was ~104 EID50 per mouse, which was lower than LD50. On day 4 post challenge, the lungs of 3 mice from each group were surgically removed, and viral titers were determined using MDCK cell cultures as described below. Mouse survival and body weight were monitored daily following the challenge.
Virus titer in the mouse lungs. Mouse lungs were homogenized with glass beads under sterile conditions; 1 ml of PBS with gentamicin (0.1 mg/ml) was added. The resulting suspension was centrifuged, and the supernatant was collected. MDCK cells grown in 96 well panels were washed, and 200 μl of fresh medium (Eagle-MEM with the addition of 0.1% L-glutamine 1 mg/ml gentamicin and 5 mg/ml BSA) was added to the wells. Serial dilutions of lung extracts were prepared by adding 50 μl of the lung extract to the well and then transferring 50 μl of the solution from well to well (8 wells altogether). After incubation for 16 h, 20 μl of glutaraldehyde solution was added to the wells to a final concentration of 0.02%; the plates were incubated 30 minutes; the medium was then discarded, and the wells were washed. Horseradish peroxidase-conjugated fetuin (50 μl) in PBS containing 0.01% Tween-20, 1 mg/ml BSA, and 1 μM NA inhibitor oseltamivir phosphate was added to each well. After incubation for 60 min at 4°C, the plates were washed and 3-amino-9-ethylcoarbazole solution with hydrogen peroxide was added to the wells for 30 minutes. Virus-infected cells (stained red) were counted using an inverted BIOLAM P-1 microscope (LOMO, Russia).
Statistical analysis. Statistical analysis was performed using the log-rank test.
RESULTS
Comparison of live, whole-virion, and split vaccines with and without adjuvants. The VE of experimental vaccines was estimated by challenging immunized mice with the influenza virus. Groups of mice were inoculated intranasally with live vaccines and intramuscularly with inactivated vaccines and placebo. The H5N1 WIV, split vaccine, and split vaccine with the adjuvants were compared with the live H5N1 vaccine. Aluminum hydroxide, polyoxidonium, and sovidon (standard adjuvants), as well as experimental preparations GMD, SANP, and Iso-SANP, were used as adjuvants.
Inactivated vaccines and cold-adapted reassortants VN-Len and NC-Len were well tolerated. The weight loss and the survival in these groups were almost the same as in the placebo group (data not shown).
WIV and live vaccine provided the maximum increase in the Ab content and almost 100% protection against subsequent challenge with highly pathogenic H5N1 virus. The split vaccine provided lesser increase in the Ab content and 50% protection. Addition of adjuvants to the split vaccine led to very different outcomes. Three adjuvants (aluminum hydroxide, polyoxidonium, and SANP) caused a rise in the Ab levels and increased vaccine protectivity. Sovidon insignificantly affected the Ab level of antibodies and slightly increased the survival of mice. GMD slightly affected the Ab level and reduced the survival of mice. Iso-SANP increased the Ab levels but dramatically reduced the protective effect of the split vaccine (p < 0.05). Even the efficient adjuvants (aluminum hydroxide, polyoxidonium, and SANP) failed to improve the quality of the split vaccine to the levels of the whole-virion vaccine and, especially, live vaccine (Table 3).
Table 3. Effect of adjuvants on the
immunogenicity and protectivity of H5N1 split vaccine
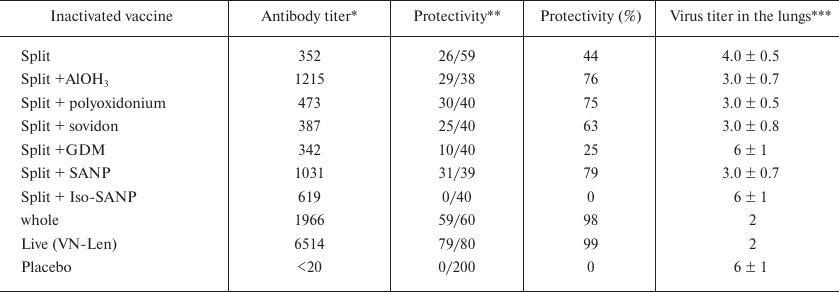
Notes: Data from several experiments.
* Anti-H5N1 Abs, geometrical mean of titer (ELISA).
** Survival of infected mice on day 14 post challenge.
*** Log of TCID50 value.
Some of the tested adjuvants that did not cause visible negative effects in mice after immunization and increased the Ab levels, but, nevertheless, reduced the survival of mice after the challenge. To better understand this phenomenon, we studied accumulation of IgG1 and IgG2a, which reflects the levels of the humoral and cellular immune responses, respectively.
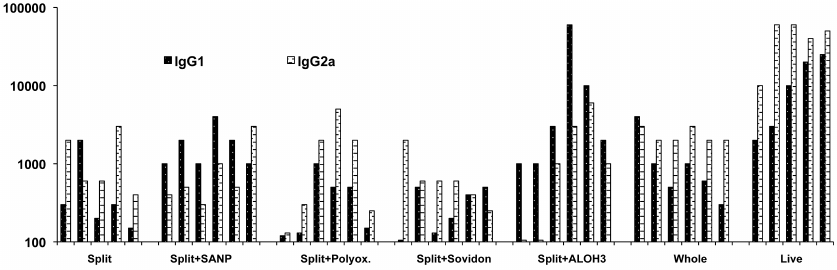
The levels of IgG1 and IgG2a in mice after vaccination with the split, split + adjuvants, whole-virion, and live vaccines are shown in Fig.1.
Fig. 1. Post vaccination levels of IgG1and IgG2a against H5N1 in the sera of mice vaccinated with split, split + adjuvants, whole-virion, and live H5 vaccines. Each pair of columns in the chart shows Ab levels in an individual mouse.
The tested vaccines differed in the ability to increase the Ab levels, the ratio of IgG1 to IgG2a, and the spread in the Ab levels between individual animals. The highest content of Abs was observed after vaccination with the live vaccine. WIV also induced high and steady Ab levels with a desired IgG1/IgG2a ratio. The split vaccine induced low but reliable increase in the content of both IgG1 and IgG2a in all mice. Aluminum hydroxide significantly increased the level of IgG1 antibodies (i.e., promoted humoral immune response), but the IgG2a levels in some mice dropped significantly. The difference in the IgG2a content in the mice of this group was more than 50 times. In other words, immunization with the split + Al(ОН)3 vaccine suppressed cell-mediated immunity in these mice. Polyoxidonium increased the level of IgG2a, but dramatically reduced the level of IgG1 in some mice, indicating a weakening of humoral response. Low levels of IgG1 were detected in some mice inoculated with the split + sovidon combination.
Both split and live vaccines increased the levels of IgG1 and IgG2a for up to 30 days after immunization (Table 4). AlОН3, polyoxidonium, and SANP, which improved the quality of the subunit vaccine, provided a higher increase in the IgG1 and IgG2a levels compared to the split vaccine without adjuvants; the high level of antibodies persisted for up to 30 days. At the same time, Iso-SANP increased the level of IgG2a up to day 11, but no further growth in the Ab content occurred, and by day 30, the Ab levels were lower than after vaccination with the split vaccine, which explained lower survival levels of mice vaccinated with the split + Iso-SANP vaccine vs. split vaccine only. Interestingly, mice in the split + Iso-SANP group died earlier than non-vaccinated mice with the zero level of anti-H5N1 Abs, which suggested serious impairments of the body defense systems.
Table 4. Post vaccination levels of IgG1and
IgG2a against H5N1 in the mouse sera and post-challenge survival of
mice
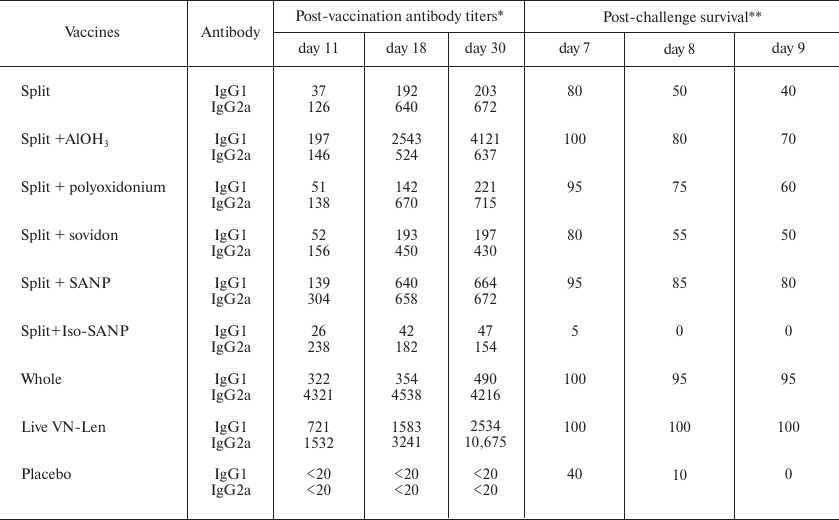
Notes: Each group contained 20 mice. The difference between the placebo
group and the split + Iso-SANP group is statistically significant
(p < 0.05, log-rank test).
* Anti-H5N1 Abs on days 11, 18, and 30 post vaccination, geometrical
mean of ELISA titer.
** % Survival on days 7, 8, and 9 post challenge with H5N1 virus.
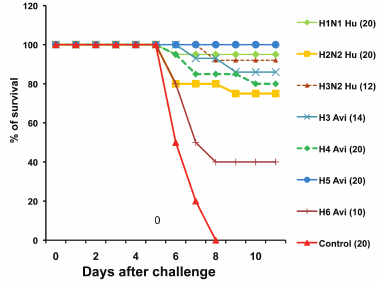
Effect of different types of vaccines after heterosubtypic immunization. To study the effects of nonspecific immunization, we conducted a series of experiments in which the mice were vaccinated with the influenza virus of one subtype and then challenged with the viruses of another subtype. We were primarily interested in how infection with the low-pathogenic influenza virus cross-protects against the following infection with a highly virulent virus. The mice were infected with the Ca H1N1 and H2N2 viruses, human A/Nib/26/90M (H3N2) virus, and wild duck LPAIVs with the H3, H4, H5, and H6 HAs. No signs of disease were observed after infection with these viruses. Twenty day later, the mice were challenged with HPAIV A/chicken/Kurgan/3/2005 (H5N1).
Figure 2 shows the survival of infected and control mice after the viral challenge. All control mice died by day 8, whereas 40-95% mice preliminarily infected with the nonspecific viruses survived. Therefore, asymptomatic infection with heterosubtypic influenza viruses protected against subsequent challenge with a lethal dose of highly pathogenic virus.
Fig. 2. Survival of control mice and mice preliminarily infected with heterosubtypic influenza viruses after the challenge with the A/chicken/Kurgan/3/2005 (H5N1) virus (results of typical experiment). Viruses used for infection were Ca vaccine strain A/New Caledonia-Leningrad/134/17/57 (H1N1 Hu), Ca A/Leningrad/134/17/57 virus (H2N2 Hu), and human and wild duck viruses (Table 1). The number of mice in a group is shown in parentheses.
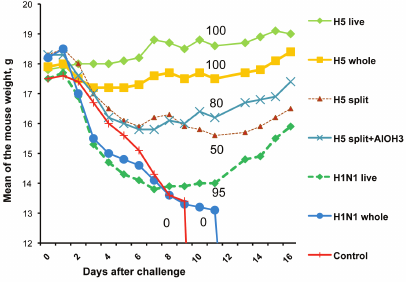
To compare live and inactivated vaccines for the ability to provide cross-protection, we immunized mice with H5 and H1 vaccines and then challenged them with HPAIV A/chicken/Kurgan/3/2005 (Fig.3).
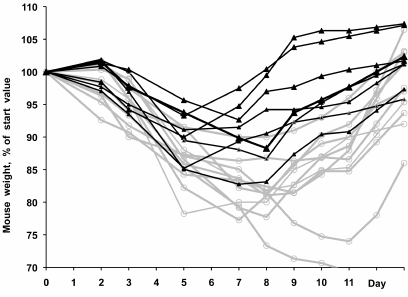
Fig. 3. Weight dynamics and survival of mice immunized with specific or heterosubtypic vaccines and challenged with HPAI H5N1 virus A/Chicken/Kurgan/3/05 (result of typical experiment). Vaccines used were split H5N1, whole-virion H5N1, live H5N2 (Ca strain VN-Len), whole-virion inactivated H1N1 (strain A/Puerto Rico/8/34), and live H1N1 Ca A/New Caledonia-Leningrad/134/17/57. Each experimental group contained 20 mice. The numbers on the curves indicate % survived mice.
Mice vaccinated with live attenuated H5N2 virus continued to gain weight after the challenge and have shown no signs of the disease. Some mice vaccinated with the inactivated whole H5N1 virus were depressed and lost appetite, which resulted in a slight weight drop on days 4-7. All mice in these two groups survived. In the group vaccinated with the H5N1 split vaccine, 50% mice died. Survived mice began to gain weight after day 10. All mice immunized with inactivated H1N1 virus PR8, as well as the control (nonvaccinated) mice, have died. Mice vaccinated with live Ca H1N1 vaccine A/New Caledonia-Leningrad/134/17/57 demonstrated rapid weight loss during the first post-challenge days. Starting day 8, they began to gain weight and recovered; 90-95% of these mice survived. These data demonstrate a fundamental difference between live and inactivated vaccines after heterosubtypic immunization. Inactivated vaccines have no apparent efficacy in this case, while LAIV conferred almost complete cross-protection.
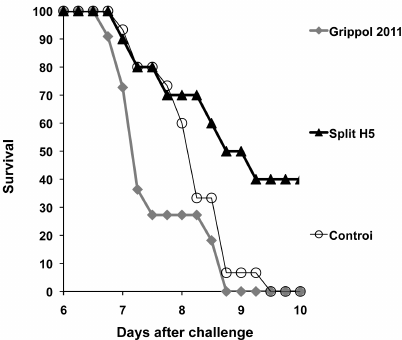
To examine the effect of the current standard vaccines on the disease outcome in the event of a new pandemic virus, we vaccinated mice with the GrippolR plus subunit seasonal vaccine containing surface antigens of the H1N1 (A/California 7/2009), H3N2 (A/Victoria/210/208 NYMC X-187), and B/Brisben/60/2008 viruses and 0.5 mg polyoxidonium in a single humans dose. Twenty days post vaccination, the mice were challenged with the H5N1 HP virus. Mice vaccinated with the H5N1 split vaccine and intact mice were used for comparison. Post-challenge survival curves are shown in Fig. 4.
Fig. 4. Survival of mice immunized with heterosubtypic subunit adjuvanted vaccine and challenged with A/chicken/Kurgan/3/2005 virus (H5N1) (results of typical experiment). The composition of the GrippolR plus and split H5 vaccines is described in Materials and Methods and in Table 2. The differences between the groups were statistically significant (p = 0.09; log-rank test).
In agreement with our previous findings, single vaccination with the virus-specific split vaccine provided 40% survival rate, while all control mice have died by day 9. All mice immunized with the non-specific Grippol vaccine have also died; moreover, they started to die one day before the control mice.
Similar results were obtained by immunization of mice with the surface antigens of the H3N2 A/NIB/26/90 virus with polyoxidonium (2 μg HA + 100 μg polyoxidonium per mouse) followed by the challenge with the mouse-adapted pandemic H1N1 virus. The virulence of the A/Hamburg/5/2009 (H1N1) virus was increased by 7 passages through the mouse lungs, resulting in the HA-Asp225Gly and HA-Lys123Asn substitutions. This virus caused a well manifested disease in the control mice, with the maximum weight loss by days 5-7 post infection, after which the mice started to gain weight and usually recovered by day 10.
Figure 5 shows individual weight curves for each of the vaccinated and control mice after the challenge. It is evident that the weight loss was more significant in the vaccinated mice; these mice also started to recover on average two days later than the control mice. It is possible that previous heterosubtypic vaccination depleted the immune system and slowed down the formation of protective Abs, resulting in the increased mortality rate and slower recovery.
Conclusions:
1) heterosubtypic vaccination with the live vaccine did not prevent disease development after subsequent infection with the H5N1 virus but promoted healing and almost completely prevented death in mice;
2) heterosubtypic vaccination with the WIV provided no protection against subsequent challenge with the H5N1 virus;
3) heterosubtypic subunit vaccine with polyoxidonium made mice more susceptible to subsequent infection with the H5N1 and H1N1 viruses.
Fig. 5. Weight dynamics in mice immunized with the H3N2 subunit vaccine with polyoxidonium (see Table 2 for the vaccine composition) and challenged with the pandemic A/Hamburg/5/2009-MA (H1N1) virus (results of typical experiment). Black and gray curves, unimmunized and immunized mice, respectively. The difference between the groups was statistically significant (р = 0.03, log-rank test).
DISCUSSION
The strategy for influenza vaccination continues to be a widely debated subject. Thus, it had been believed in the last century that only persons in risk groups should be vaccinated.
In 2003, World Health Assembly recommended to increase vaccine coverage against influenza in order to provide protection of at least 50% of elderly population by 2006 (http://www.who.int/immunization/sage/1_WHA56_19_).
In 2005, due to concerns about the H5N1 pandemic, the Second Joint WHO/European Commission recommended the review of policies on seasonal influenza vaccination with the purpose to increase seasonal vaccine usage, thereby promoting increase in the global production capacity of influenza vaccines [69].
In 2012, WHO Strategic Advisory Group of Experts recommended to assign pregnant women to the risk groups requiring seasonal immunization with inactivated influenza vaccine. Other risk groups were healthcare workers, children 6- to 59-month-old, elderly, and those with the high-risk health conditions (www.who.int/wer/2012/wer8721.pdf).
In 2017, the Center for Disease Control and Prevention recommended that “everyone 6 months of age and older, including pregnant women and people with certain health conditions, should get a flu vaccine every season, with rare exception” (https://www.cdc.gov/features/fluhighrisk/index.html).
Such recommendations may cause skepticism, given a moderate efficacy of vaccination in the interpandemic periods and the risk of adverse side effects [2]. Moreover, annual vaccination can lead to negative interference between the previous and current vaccinations [59, 60].
In some countries, schoolchildren are immunized against influenza annually [70]. However, this practice may be a matter of concern. Although annual vaccines are effective against seasonal influenza, they could make people more vulnerable to novel pandemics [71]. As noted by Bodewes et al. [72], “an age-dependent increase of the influenza virus-specific CD8+ T cell response was observed in unvaccinated healthy control children and was absent in vaccinated children. Influenza vaccination is effective against seasonal influenza but hampers the development of virus-specific CD8+ T cell responses”.
It has been shown that “influenza A virus-specific cytotoxic T lymphocytes recognize epitopes located in the relatively conserved proteins like the nucleoprotein and that they cross-react with various subtypes of influenza A viruses. This implies that these CD8+ T lymphocytes may contribute to protective heterosubtypic immunity induced by an accidental influenza A virus infections” [73].
Repeated vaccinations with inactivated subunit vaccines prevent natural immunization with circulating influenza viruses. Antibodies are produced only to the surface proteins; therefore, they do not provide any cross-protection against new strains. Such repeatedly vaccinated children are particularly vulnerable in the case of pandemic.
Inactivated vaccines are inefficient against heterosubtypic infection [73, 74]. Thus, the 2009-2010 seasonal inactivated vaccine did not provide protection against the pandemic virus [75]. At the same time, cross-protection with live influenza vaccines has been shown [73, 76-80].
In accordance with the above works, our study demonstrated that the immunity induced by a live virus not only prevents infection with closely related viruses, but also ensures partial protection against antigenically distant influenza viruses. Comparison of the ability of various types of vaccines to cross-protect showed that prior infection with any non-pathogenic influenza virus (Ca vaccine, low-pathogenic human virus, or virus of wild birds) provides protection against challenge with a heterosubtypic virus, while immunization with inactivated vaccines is inefficient in these cases.
In the pilot study with various adjuvants, we found that mice vaccinated with the split vaccine with Iso-SANP died before unvaccinated mice. Some of the adjuvants, which were safe for mice and elevated the Ab level post vaccination, negatively affected mouse survival after the viral challenge.
Despite this, the effectiveness of adjuvants is often be determined only by measuring the immune response, without checking the protective effect [81].
Despite that commonly adjuvants, such as polyoxidonium and aluminum hydroxide, enhance parameters of immune response on average, they alter the IgG1/IgG2 balance and increase the difference between Ab levels in the immunized animals, which results in the suppression of either cellular of humoral immunity in some of the animals. Such variations in the Ab levels and dramatic shift in the IgG1/IgG2 ratio cause concern, since they suggest that although adjuvants increase the vaccine efficacy, they may be harmful in specific cases.
Only few immune system failures can go undetected in human trials. This dictates an extreme caution when using adjuvanted vaccines. Although a vaccine can be found suitable for practical application, it may cause complications in individual patients during widespread immunization.
The strategy of influenza vaccination must take into account not only protection from the current epidemic strains, but also a possible risk of a new pandemic. In the latter case, persons vaccinated with live vaccines would be partially protected, while individuals immunized with adjuvanted vaccines will be even at a higher risk than unvaccinated individuals.
Funding. This work was supported by the Russian Foundation for Basic Research (projects Nos. 11-04-00517-a and 17-04-00148-a).
Acknowledgements. The authors thank Dr. R. Donis (Centers for Disease Control and Prevention, Atlanta, GA, USA) for providing the vaccine strain VNH5N1-PR8/CDC-RG; Dr. Rudenko (Institute of Experimental Medicine , St. Petersburg, Russia) for providing the A/Leningrad/134/17/57 (H2N2) and A/New Caledonia-Leningrad/134/17/57 (H1N1) strains; Dr. S. S. Yamnikova (Gamaleya National Research Center for Epidemiology and Microbiology, Moscow, Russia) for providing influenza H5N1 A/chicken/Kurgan/3/2005 virus; Dr. James Robertson (National Institute for Biological Standards and Control, United Kingdom) for providing A/Nib/26/90-M (H3N2) strain; and Dr. Matrosovich (Institute of Virology, Philipps University, Marburg, Germany) for providing A/Hamburg/5/2009 (H1N1) strain.
Author contributions. A. P. K., I. V. K., and A. S. G. conceived and designed the experiments; E. Y. B. and A. V. L. performed the experiments; E. Y. B. and A. S. G. wrote the manuscript.
Conflicts of interest. The authors declare no conflict of interest.
Ethics statement. All procedures performed with the animals were conducted according to the “International recommendations (code of ethics) for conducting biomedical research using animals” (http://www.msu.ru/bioetika/doc/recom.doc) and met the ethical standards of the institutions where the studies were carried out.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Chumakov Federal Scientific Center for Research and Development of Immune-and-Biological Products, Russian Academy of Sciences.
REFERENCES
1.Chung, J. R., Rolfes, M. A., Flannery, B., Prasad,
P., O’Halloran, A., Garg, S., Fry, A. M., Singleton, J. A.,
Patel, M., and Reed, C. (2020) Effects of influenza vaccination in the
United States during the 2018-2019 influenza season, Clin. Infect.
Dis., doi: 10.1093/cid/ciz1244, [Epub ahead of print].
2.Demicheli, V., Jefferson, T., Ferroni, E., Rivetti,
A., and Di Pietrantonj, C. (2018) Vaccines for preventing influenza in
healthy adults, Cochrane Database Syst. Rev., 2,
CD001269, doi: 10.1002/14651858.CD001269.pub6.
3.Grohskopf, L. A., Sokolow, L. Z., Broder, K. R.,
Walter, E. B., Bresee, J. S., Fry, A. M., and Jernigan, D. B. (2017)
Prevention and control of seasonal influenza with vaccines:
recommendations of the advisory committee on immunization practices
– United States, 2017–18 influenza season, MMWR Recomm.
Rep., 66, 1-20, doi: 10.15585/mmwr.rr6602a1.
4.Budimir, N., de Haan, A., Meijerhof, T., Gostick,
E., Price, D. A., Huckriede, A., and Wilschut, J. (2013) Heterosubtypic
cross-protection induced by whole inactivated influenza virus vaccine
in mice: influence of the route of vaccine administration, Influenza
Other Respir. Viruses, 7, 1202-1209,
doi: 10.1111/irv.12142.
5.Onodera, T., Hosono, A., Odagiri, T., Tashiro, M.,
Kaminogawa, S., Okuno, Y., Kurosaki, T., Ato, M., Kobayashi, K., and
Takahashi, Y. (2016) Whole-virion influenza vaccine recalls an early
burst of high-affinity memory B cell response through TLR signaling,
J. Immunol., 196, 4172-4184.
6.Ato, M., Takahashi, Y., Fujii, H., Hashimoto, S.,
Kaji, T., Itamura, S., Horiuchi, Y., Arakawa, Y., Tashiro, M., and
Takemori, T. (2013) Influenza A whole virion vaccine induces a rapid
reduction of peripheral blood leukocytes via
interferon-α-dependent apoptosis, Vaccine, 31,
2184-2190, doi: 10.1016/j.vaccine.2013.02.016.
7.Jefferson, T. O., Rivetti, D., Di Pietrantonj, C.,
Rivetti, A., and Demicheli, V. (2010) Vaccines for preventing influenza
in healthy adults, Cochrane Database Syst. Rev., 7,
CD001269, doi: 10.1002/14651858.CD001269.pub4.
8.Allwinn, R., and Doerr, H. W. (2011) Comparison of
seasonal influenza vaccines: composition and properties, Dtsch. Med.
Wochenschr., 136, 2315-2318,
doi: 10.1055/s-0031-1292046.
9.Even-Or, O., Samira, S., Ellis, R., Kedar, E., and
Barenholz, Y. (2013) Adjuvanted influenza vaccines, Expert Rev.
Vaccines, 9, 1095-1108,
doi: 10.1586/14760584.2013.825445.
10.Black, S. (2015) Safety and effectiveness of
MF-59 adjvanted influenza vaccines in children and adults,
Vaccine, 33, Suppl. 2:B3-5,
doi: 10.1016/j.vaccine.2014.11.062.
11.Guo, Q., Liu, Z., Gao, J., Zhou, J., Hu, W., Cun,
Y., Li, W., and Liao, G. (2016) Immunogenicity and safety of pandemic
influenza H5N1 vaccines in healthy adults through meta-analysis,
Cell. Physiol. Biochem., 40, 921-932.
12.Seo, Y. B., Choi, W. S., Lee, J., Song, J. Y.,
Cheong, H. J., and Kim, W. J. (2014) Comparison of the immunogenicity
and safety of the conventional subunit, MF59-adjuvanted, and
intradermal influenza vaccines in the elderly, Clin. Vaccine
Immunol., 21, 989-996, doi: 10.1128/CVI.00615-13.
13.Vesikari, T., Forstén, A., Arora, A.,
Tsai, T., and Clemens, R. (2015) Influenza vaccination in children
primed with MF59-adjuvanted or non-adjuvanted seasonal influenza
vaccine, Hum. Vaccin. Immunother., 11, 2102-2112,
doi: 10.1080/21645515.2015.1044167.
14.Vesikari, T., Kirstein, J., Devota Go, G., Leav,
B., Ruzycky, M. E., Isakov, L., de Bruijn, M., Oberye, J., and Heijnen,
E. (2018) Efficacy, immunogenicity, and safety evaluation of an
MF59-adjuvanted quadrivalent influenza virus vaccine compared with
non-adjuvanted influenza vaccine in children: a multicentre, randomised
controlled, observer-blinded, phase 3 trial, Lancet Respir.
Med., 6, 345-356.
15.Wang, J., Hilchey, S. P., DeDiego, M., Perry, S.,
Hyrien, O., Nogales, A., Garigen, J., Amanat, F., Huertas, N., Krammer,
F., Martinez-Sobrido, L., Topham, D. J., Treanor, J. J., Sangster, M.
Y., and Zand, M. S. (2018) Broad cross-reactive IgG responses elicited
by adjuvanted vaccination with recombinant influenza hemagglutinin
(rHA) in ferrets and mice, PLoS One, 13, e0193680,
doi: 10.1371/journal.pone.0193680.
16.Couch, R. B., Bayas, J. M., Caso, C., Mbawuike,
I. N., López, C. N., Claeys, C., El Idrissi, M., Hervé,
C., Laupèze, B., Oostvogels, L., and Moris, P. (2014) Superior
antigen-specific CD4+ T-cell response with
AS03-adjuvantation of a trivalent influenza vaccine in a randomised
trial of adults aged 65 and older, BMC Infect. Dis., 14,
425, doi: 10.1186/1471-2334-14-425.
17.Song, J. Y., Choi, M. J., Noh, J. Y., Choi, W.S.,
Cheong, H. J., Wie, S. H., Lee, J. S., Woo, G. J., Lee, S. H., and Kim,
W. J. (2017) Randomized, double-blind, multi-center, phase III clinical
trial to evaluate the immunogenicity and safety of MG1109 (egg-based
pre-pandemic influenza A/H5N1 vaccine) in healthy adults, Hum.
Vaccin. Immunother., 13, 1190-1197,
doi: 10.1080/21645515.2016.1263410.
18.Li, P., and Wang, F. (2015) Polysaccharides:
candidates of promising vaccine adjuvants, Drug Discov. Ther.,
9, 88-93, doi: 10.5582/ddt.2015.01025.
19.Van de Sandt, C. E., Kreijtz, J. H.,
Geelhoed-Mieras, M. M., Vogelzang-van Trierum, S. E., Nieuwkoop, N.
J.., van de Vijver, D. A., Fouchier, R. A., Osterhaus, A. D., Morein,
B., and Rimmelzwaan, G. F. (2014) Novel G3/DT adjuvant promotes the
induction of protective T cells responses after vaccination with a
seasonal trivalent inactivated split-virion influenza vaccine,
Vaccine, 32, 5614-5623,
doi: 10.1016/j.vaccine.2014.08.003.
20.Hjertner, B., Bengtsson, T., Morein, B., Paulie,
S., and Fossum, C. (2018) A novel adjuvant G3 induces both Th1 and Th2
related immune responses in mice after immunization with a trivalent
inactivated split-virion influenza vaccine, Vaccine, 36,
3340-3344, doi: 10.1016/j.vaccine.2018.04.054, [Epub ahead of
print].
21.Christensen, D., Christensen, J. P., Korsholm, K.
S., Isling, L. K., Erneholm, K., Allan, R Thomsen, A. R., and Andersen,
P. (2018) Seasonal influenza split vaccines confer partial
cross-protection against heterologous influenza virus in ferrets when
combined with the CAF01 adjuvant, Front. Immunol., 8,
1928, doi: 10.3389/fimmu.2017.01928.
22.Baldwin, S. L., Hsu, F.-C., Van Hoeven, N., Gage,
E., Granger, B., Guderian, J. A., Larsen, S. E., Lorenzo, E. C.,
Haynes, L., Reed, S. G., and Coler, R. N. (2018) Improved immune
responses in young and aged mice with adjuvanted vaccines against H1N1
influenza infection, Front. Immunol., 9, 295.
23.Lin, P. H., Wong, W. I., Wang, Y. L., Hsieh, M.
P., Lu, C. W., Liang, C. Y., Jui, S. H., Wu, F. Y., Chen, P. J., and
Yang, H. C. (2018) Vaccine-induced antigen-specific regulatory T cells
attenuate the antiviral immunity against acute influenza virus
infection, Mucosal Immunol.,
doi: 10.1038/s41385-018-0004-9.
24.Hyejin, K., Kimoto, T., Sakai, S., Takahashi, E.,
and Kido, H. (2018) Adjuvanting influenza hemagglutinin vaccine with a
human pulmonary surfactant-mimicking synthetic compound SF-10 induces
local and systemic cell-mediated immunity in mice, PLoS One,
13, e0191133, doi: 10.1371/journal.pone.0191133.
25.Uraki, R., Das, S. C., Hatta, M., Kiso, M.,
Iwatsuki-Horimoto, K., Ozawa, M., Coban, C., Ishii, K. J., and Kawaoka,
Y. (2014) Hemozoin as a novel adjuvant for inactivated whole
virioninfluenza vaccine, Vaccine, 32, 5295-5300,
doi: 10.1016/j.vaccine.2014.07.079.
26.El’shina, G. A., Gorbunov, M. A.,
Shervarli, V. I., Lonskaia, N. I., Pavlova, L. I., Khaitov, R. M.,
Nekrasov, A. V., Ivanova, A. S., Matrosovich, M. N., Puchkova, N. G.,
Belashev, V. P., and Malinovskiĭ, A. A. (1998) Evaluation of the
effectiveness of influenza trivalent polymer subunit vaccine
“Grippol”, J. Mikrobiol. Epidemiol. Immunobiol.
(Moscow), 3, 40-43.
27.Shannon, I., White, C. L., and Nayak, J. L.
(2019) Understanding immunity in children vaccinated with live
attenuated influenza vaccine, J. Pediatric. Infect. Dis. Soc.,
9, (Supplement_1), S10-S14, doi: 10.1093/jpids/piz083.
28.Subbarao, K. (1999) Influenza vaccines: present
and future, Adv. Virus Res., 54, 349-373.
29.Piedra, P. A., Gaglani, M. J., Kozinetz, C. A.,
Herschler, G. B., Fewlass, C., Harvey, D., Zimmerman, N., and Glezen,
W. P. (2007) Trivalent live attenuated intranasal influenza vaccine
administered during the 2003-2004 influenza type A (H3N2) outbreak
provided immediate, direct, and indirect protection in children,
Pediatrics, 120, e553-e564.
30.Carter, N. J., and Curran, M. P. (2011) Live
attenuated influenza vaccine (FluMist®; Fluenz™): a review of
its use in the prevention of seasonal influenza in children and adults,
Drugs, 71, 1591-1622.
31.Hoft, D. F., Babusis, E., Worku, S., Spencer, C.
T., Lottenbach, K., Truscott, S. M., Abate, G., Sakala, I. G., Edwards,
K. M., Creech, C. B., Gerber, M. A., Bernstein, D. I., Newman, F.,
Graham, I., Anderson, E. L., and Belshe, R. B. (2011) Live and
inactivated influenza vaccines induce similar humoral responses, but
only live vaccines induce diverse T-cell responses in young children,
J. Infect. Dis., 204, 845-853,
doi: 10.1093/infdis/jir436.
32.Chen, G. L., Lau, Y. F., Lamirande, E. W.,
McCall, A. W., and Subbarao, K. (2011) Seasonal influenza infection and
live vaccine prime for a response to the 2009 pandemic H1N1 vaccine,
Proc. Natl. Acad. Sci. USA, 108, 1140-1145.
33.Loving, C. L., Vincent, A. L., Pena, L., and
Perez, D. R. (2012) Heightened adaptive immune responses following
vaccination with a temperature-sensitive, live-attenuated influenza
virus compared to adjuvanted, whole-inactivated virus in pigs,
Vaccine, 30, 5830-5838,
doi: 10.1016/j.vaccine.2012.07.033.
34.Osterholm, M. T., Kelley, N. S., Sommer, A., and
Belongia, E. A. (2012) Efficacy and effectiveness of influenza
vaccines: a systematic review and meta-analysis, Lancet Infect.
Dis., 12, 36-44,
doi: 10.1016/S1473-3099(11)70295-X.
35.Moore, D. L., Canadian Paediatric Society, and
Infectious Diseases and Immunization Committee (2014) Vaccine
recommendations for children and youth for the 2014/2015 influenza
season, Paediatr. Child. Health, 19, 440-444.
36.Andersohn, F., Bornemann, R., Damm, O., Frank,
M., Mittendorf, T., and Theidel, U. (2014) Vaccination of children with
a live-attenuated, intranasal influenza vaccine – analysis and
evaluation through a Health Technology Assessment, GMS Health
Technol. Assess, 10, Doc03, doi: 10.3205/hta000119.
37.Helmeke, C., Gräfe, L., Irmscher, H.-M.,
Gottschalk, C., Karagiannis, I., and Oppermann, H. (2015) Effectiveness
of the 2012/13 trivalent live and inactivated influenza vaccines in
children and adolescents in Saxony-Anhalt, Germany: a test-negative
case-control study, PLoS One, 10, e0122910,
doi: 10.1371/journal.pone.0122910.
38.Schotsaert, M., and García-Sastre, A.
(2017) Inactivated influenza virus vaccines: the future of TIV and QIV,
Curr. Opin. Virol., 23, 102-106,
doi: 10.1016/j.coviro.2017.04.005.
39.Brooks, W. A., Zaman, K., Lewis, K. D., Ortiz, J.
R., Goswami, D., Feser, J., Sharmeen, A. T., Nahar, K., Rahman, M.,
Rahman, M. Z., Barin, B., Yunus, M., Fry, A. M., Bresee, J., Azim, T.,
and Neuzil, K. M. (2016) Efficacy of a Russian-backbone live attenuated
influenza vaccine among young children in Bangladesh: a randomised,
double-blind, placebo-controlled trial, Lancet Glob. Health,
4, e946-e954, doi: 10.1016/S2214-109X(16)30200-5.
40.Raburn, M. M., Yu, J., Kameo, S., Tanaka, M.,
Rito, K., Itoh, Y., and Dubovsky, F. (2018) The safety and efficacy of
quadrivalent live attenuated influenza vaccine in Japanese children
aged 2-18 years: results of two phase 3 studies, Influenza Other
Respir. Viruses, 12, 438-445,
doi: 10.1111/irv.12555.
41.Sarntivijai, S., Xiang, Z., Shedden, K. A.,
Markel, H., Omenn, G. S., Athey, B. D., and He, Y. (2012)
Ontology-based combinatorial comparative analysis of adverse events
associated with killed and live influenza vaccines, PLoS One,
7, e49941, doi: 10.1371/journal.pone.0049941.
42.Ambrose, C. S., Levin, M. J., and Belshe, R. B.
(2011) The relative efficacy of trivalent live attenuated and
inactivated influenza vaccines in children and adults, Influenza
Other Respir. Viruses, 5, 67-75.
43.Ambrose, C. S., Dubovsky, F., Yi, T., Belshe, R.
B., and Ashkenazi, S. (2012) The safety and efficacy of live attenuated
influenza vaccine in young children with asthma or prior wheezing,
Eur. J. Clin. Microbiol. Infect. Dis., 31, 2549-2557.
44.Turner, P. J., Southern, J., Andrews, N. J.,
Miller, E., Erlewyn-Lajeunesse, M., and SNIFFLE study investigators
(2015) Collaborators (12) safety of live attenuated influenza vaccine
in atopic children with egg allergy, J. Allergy. Clin. Immunol.,
136, 376-381, doi: 10.1016/j.jaci.2014.12.1925.
45.Duffy, J., Lewis, M., Harrington, T., Baxter, R.,
Belongia, E. A., Jackson, L. A., Jacobsen, S. J., Lee, G. M., Naleway,
A. L., Nordin, J., Daley, M. F., and Vaccine Safety Datalink (2017)
Live attenuated influenza vaccine use and safety in children and adults
with asthma, Ann. Allergy Asthma Immunol., 118, 439-444,
doi: 10.1016/j.anai.2017.01.030.
46.Turner, P. J., Fleming, L., Saglani, S.,
Southern, J., Andrews, N. J., and Miller, E., SNIFFLE-4 study
investigators (2019) Safety of live attenuated influenza vaccine (LAIV)
in children with moderate to severe asthma, J. Allergy Clin.
Immunol., doi: 10.1016/j.jaci.2019.12.010.
47.Basha, S., Hazenfeld, S., Brady, R. C., and
Subbramanian, R. A. (2011) Comparison of antibody and T-cell responses
elicited by licensed inactivated- and live-attenuated influenza
vaccines against H3N2 hemagglutinin, Hum. Immunol., 72,
463-469, doi: 10.1016/j.humimm.2011.03.001.
48.Cheng, X., Zengel, J. R., Suguitan, A. L. Jr., Xu
Q, Wang, W., Lin, J., and Jin, H. (2013) Evaluation of the humoral and
cellular immune responses elicited by the live attenuated and
inactivated influenza vaccines and their roles in heterologous
protection in ferrets, J. Infect. Dis., 208, 594-602,
doi: 10.1093/infdis/jit207.
49.Cao, R. G., Suarez, N. M., Obermoser, G., Lopez,
S. M., Flano, E., Mertz, S. E., Albrecht, R. A., García-Sastre,
A., Mejias, A., Xu, H., Qin, H., Blankenship, D., Palucka, K., Pascual,
V., and Ramilo, O. (2014) Differences in antibody responses between
trivalent inactivated influenza vaccine and live attenuated influenza
vaccine correlate with the kinetics and magnitude of interferon
signaling in children, J. Infect. Dis., 210, 224-233,
doi: 10.1093/infdis/jiu079.
50.Tricco, A. C., Chit, A., Soobiah, C., Hallett,
D., Meier, G., Chen, M. H., Tashkandi, M., and Bauch, C. T., and Loeb,
M. (2013) Comparing influenza vaccine efficacy against mismatched and
matched strains: a systematic review and meta-analysis, BMC
Med., 11, 153, doi: 10.1186/1741-7015-11-153.
51.Junwei, L., Arévalo, M. T., Chen, Y.,
Chen, S., and Zeng, M. (2014) T-cell-mediated cross-strain protective
immunity elicited by prime-boost vaccination with a live attenuated
influenza vaccine, Int. J. Infect. Dis., 27, 37-43,
doi: 10.1016/j.ijid.2014.05.016.
52.Nohynek, H., Baum, U., Syrjänen, R., Ikonen,
N., Sundman, J., and Jokinen, J. (2016) Effectiveness of the live
attenuated and the inactivated influenza vaccine in two-year-olds
– a nationwide cohort study Finland, influenza season 2015/16,
Euro Surveill., 21,
doi: 10.2807/1560-7917.ES.2016.21.38.30346.
53.Ohmit, S. E., Victor, J. C., Rotthoff, J. R.,
Teich, E. R., Truscon, R. K., Baum, L. L., Rangarajan, B., Newton, D.
W., Boulton, M. L., and Monto, A. S. (2006) Prevention of antigenically
drifted influenza by inactivated and live attenuated vaccines, N.
Engl. J. Med., 355, 2513-2522.
54.Monto, A. S., Ohmit, S. E., Petrie, J. G.,
Johnson, E., Truscon, R., Teich, E., Rotthoff, J., Boulton, M., and
Victor, J. C. (2009) Comparative efficacy of inactivated and live
attenuated influenza vaccines, N. Engl. J. Med., 361,
1260-1267.
55.De Hoog, M. L. A., Venekamp, R. P., Meijer, A.,
Sanders, E. A. M., Bruijning-Verhagen, P. C. J. L. (2019) Inactivated
influenza vaccine does not reduce all cause respiratory illness in
children with pre-existing medical conditions, Vaccine,
doi: 10.1016/j.vaccine.2019.11.086.
56.Petrie, J. G., Malosh, R. E., Cheng, C. K.,
Ohmit, S. E., Martin, E. T., Johnson, E., Truscon, R., Eichelberger, M.
C., Gubareva, L. V., Fry, A. M., and Monto, A. S. (2017) The household
influenza vaccine effectiveness study: lack of antibody response and
protection following receipt of 2014-2015 influenza vaccine, Clin.
Infect. Dis., 65, 1644-1651,
doi: 10.1093/cid/cix608.
57.Castilla, J., Navascués, A.,
Fernández-Alonso, M., Reina, G., Pozo, F., Casado, I., Guevara,
M., Martínez-Baz, I., Barricarte, A., Ezpeleta, C, Primary
Health Care Sentinel Network, and Network for Influenza Surveillance in
Hospitals of Navarra (2016) Effectiveness of subunit influenza
vaccination in the 2014-2015 season and residual effect of split
vaccination in previous seasons, Vaccine, 34, 1350-1357,
doi: 10.1016/j.vaccine.2016.01.054.
58.Puig-Barberà, J., Guglieri-López,
B., Tortajada-Girbés, M., López-Labrador, F. X.,
Carballido-Fernández, M., Mollar-Maseres, J., Schwarz-Chavarri,
G., Baselga-Moreno, V., Mira-Iglesias, A., Díez-Domingo, J.,
Valencia Hospital Network for the Study of Influenza, and Respiratory
Viruses Disease (2017) Low influenza vaccine effectiveness and the
effect of previous vaccination in preventing admission with
A(H1N1)pdm09 or B/Victoria-Lineage in patients 60 years old or older
during the 2015/2016 influenza season, Vaccine, 35,
7331-7338, doi: 10.1016/j.vaccine.2017.10.100.
59.Gherasim, A., Martínez-Baz, I., Castilla,
J., Pozo, F., Larrauri, A., and the cycEVA working group (2017) Effect
of previous and current vaccination against influenza A(H1N1)pdm09,
A(H3N2), and B during the post-pandemic period 2010-2016 in Spain,
PLoS One, 12, e0179160,
doi: 10.1371/journal.pone.0179160.
60.Rondy, M., Launay, O., Castilla, J., Costanzo,
S., Puig-Barberà, J., Gefenaite, G., Larrauri, A., Rizzo, C.,
Pitigoi, D., Syrjänen, R. K., Machado, A., Kurečić
Filipović, S., Krisztina Horváth, J.,
Paradowska-Stankiewicz, I., Marbus, S., InNHOVE/I-MOVE+working group,
and Moren, A. (2017) Repeated seasonal influenza vaccination among
elderly in Europe: Effects on laboratory confirmed hospitalised
influenza, Vaccine, 35, 4298-4306,
doi: 10.1016/j.vaccine.2017.06.088.
61.He, D. H., Chiu, A. P. Y., Wu, J. T. K., and
Cowling, B. J. (2019) Pre-pandemic live-attenuated influenza vaccine,
Hong Kong Med. J., 25, (Suppl. 9), S24-S27.
62.Lomakina, N. F, Gambaryan, A. C., Boravleva, E.
Yu., Kropotkina, E. A., Kirillov, I. M., Lavrientev, M. V., and
Yamnikova, S. S. (2009) Character of apathogenic influenza A viruses
found in Moscow, Russia, Molecular Genetics, Microbiology and
Virology, (Moscow), 24, 37-45, [in Russian].
63.Heydarov, R. N., Lomakina, N. F., Boravleva, E.
Yu., Kholodilov, I. S., Gambaryan, A. S., Mikhailovich, V. M., and
Fesenko, E. E. (2017) The use of microarrays for the identification of
the origin of genes of avian influenza viruses in wild birds,
Microbiol. Independ. Res. J., 4, 21-30,
doi: 10.18527/2500-2236-2017-4-1-21-30.
64.Gambaryan, A. S., Lomakina, N. F., Boravleva, E.
Y., Kropotkina, E. A., Mashin, V. V., Krasilnikov, I. V., Klimov, A.
I., and Rudenko, L. G. (2012) Comparative safety, immunogenicity and
efficacy of several anti-H5N1 influenza experimental vaccines in a
mouse and chicken models, Influenza Other Respir. Viruses,
6, 188-195.
65.Gambaryan, A. S., Lomakina, N. F., Boravleva, E.
Y., Mochalova, L. V., Sadykova, G. K., Prilipov, A. G., Matrosovich, T.
Y., and Matrosovich, M. N. (2018) Mutations in hemagglutinin and
polymerase alter the virulence of pandemic A(H1N1) influenza virus,
Mol. Biol. (Moscow), 52, 644–658, [in
Russian].
66.Kaplun, A. P., Bezrukov, D. A., Popenko, V. I.,
and Shvets, V. I. (2011) Spherical amorphous nanoparticles from birch
bark triterpenoids – a novel type of submicronic vehicle for drug
delivery, Russ. J. Biopharmaceuticals, 3, 28-40, [in
Russian].
67.Ovcharenko, A. V., and Zhirnov, O. P., (1994)
Aprotinin aerosol treatment of influenza and para-myxovirus
bronchopneumonia of mice, Antiviral Res., 23,
107-118.
68.Gambaryan, A. S., Boravleva, E. Y., Matrosovich,
T. Y., Matrosovich, M. N., Klenk, H. D., Moiseeva, E. V., Tuzikov, A.
B., Chinarev, A. A., Pazynina, G. V., and Bovin, N. V. (2005)
Polymer-bound 6' sialyl-N-acetyllactosamine protects mice infected by
influenza virus, Antiviral Res., 68, 116-123.
69.Pandemic influenza preparedness planning,
Report on the second joint WHO/European Commission workshop,
Copenhagen, 24–26 October 2005, http://www.who.int/csr/resources/publications/influenza/WHO_CDS_CSR_GIP_2005_5.pdf.
70.Fiore, A. E., Epperson, S., Perrotta, D.,
Bernstein, H., and Neuzil, K. (2012) Expanding the recommendations for
annual influenza vaccination to school-age children in the United
States, Pediatrics, 129, Suppl. 2, S54-S62,
doi: 10.1542/peds.2011-0737C.
71.Bodewes, R., Fraaij, P. L., Kreijtz, J. H.,
Geelhoed-Mieras, M. M., Fouchier, R. A., Osterhaus, A. D., and
Rimmelzwaan, G. F. (2012) Annual influenza vaccination affects the
development of heterosubtypic immunity, Vaccine, 30,
7407-7410.
72.Bodewes, R., Fraaij, P. L., Osterhaus, A. D., and
Rimmelzwaan, G. F. (2012b) Pediatric influenza vaccination:
understanding the T-cell response, Expert Rev. Vaccines,
11, 963-971.
73.Hillaire, M. L., Osterhaus, A. D., and
Rimmelzwaan, G. F. (2011) Induction of virus-specific cytotoxic T
lymphocytes as a basis for the development of broadly protective
influenza vaccines, J. Biomed. Biotechnol., 2011,
939860.
74.Krasilnikov, I., Gambarjan, A., Mashin, V., and
Lobastova, A. (2010) Comparative study and protective properties of
live and inactivated candidate vaccines against highly pathogenic avian
influenza virus H5N1, Vopr. Virusol., 4, 16-20, [in
Russian].
75.Pelat, C., Falchi, A., Carrat, F., Mosnier, A.,
Bonmarin, I., Turbelin, C., Vaux, S., Werf, S., Cohen, J. M., Lina, B.,
Blanchon, T., and Hanslik, T. (2011) Field effectiveness of pandemic
and 2009-2010 seasonal vaccines against 2009-2010 A(H1N1) influenza:
estimations from surveillance data in France, PLoS One,
6, e19621, doi: 10.1371/journal.pone.0019621.
76.Lu, X., Edwards, L. E., Desheva, J. A., Nguyen,
D. C., Rekstin, A., Stephenson, I., Szretter, K., Cox, N. J., Rudenko,
L. G., Klimov, A., and Katz, J. M. (2006) Cross-protective immunity in
mice induced by live-attenuated or inactivated vaccines against highly
pathogenic influenza A (H5N1) viruses, Vaccine, 24,
6588-6593.
77.Kreijtz, J. H., Bodewes, R., van Amerongen, G.,
Kuiken, T., Fouchier, R. A., Osterhaus, A. D., and Rimmelzwaan, G. F.
(2007) Primary influenza A virus infection induces cross-protective
immunity against a lethal infection with a heterosubtypic virus strain
in mice, Vaccine, 25, 612-620.
78.Jang, Y. H., and Seong, B. L. (2013)
Cross-protective immune responses elicited by live attenuated influenza
vaccines, Yonsei Med. J., 54, 271-282.
79.Sun, K., Ye, J., Perez, D. R., and Metzger, D. W.
(2011) Seasonal FluMist vaccination induces cross-reactive T cell
immunity against H1N1 (2009) influenza and secondary bacterial
infections, J. Immunol., 186, 987-993.
80.Chen, G. L., Min, J. Y., Lamirande, E. W.,
Santos, C., Jin, H., Kemble, G., and Subbarao, K. (2011) Comparison of
a live attenuated 2009 H1N1 vaccine with seasonal influenza vaccines
against 2009 pandemic H1N1 virus infection in mice and ferrets, J.
Infect. Dis., 203, 930-936,
doi: 10.1093/infdis/jiq144.
81.Beyer, W. E. P., Palache, A. M., Reperant, L. A.,
Boulfich, M., and Osterhaus, A. D. M. E. (2020) Association between
vaccine adjuvant effect and pre-seasonal immunity. Systematic review
and meta-analysis of randomised immunogenicity trials comparing
squalene-adjuvanted and aqueous inactivated influenza vaccines,
Vaccine, 38, 1614-1622,
doi: 10.1016/j.vaccine.2019.12.037.