Thymoquinone Induces Mitochondrial Damage and Death of Cerebellar Granule Neurons
E. V. Stelmashook1, N. S. Chetverikov2, S. A. Golyshev3, E. E. Genrikhs1, and N. K. Isaev1,2,a,b*
1Research Center of Neurology, 125367 Moscow, Russia2Lomonosov Moscow State University, Biological Faculty, 119234 Moscow, Russia
3Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119991 Moscow, Russia
* To whom correspondence should be addressed.
Received July 11, 2019; Revised November 11, 2019; Accepted November 13, 2019
Thymoquinone (TQ) exhibits a wide spectrum of biological activities. Most studies on the neurotoxic action of TQ have been carried out in cancer cell lines. Here, we studied the toxic effect of TQ in primary neuronal cultures in vitro. Incubation with 0.04-0.05 mM TQ for 24 h induced the death of cultured cerebellar granule neurons (CGNs) in a dose-dependent manner. Neuronal death was preceded by an increase in the reactive oxygen species (ROS) generation, as demonstrated using CellROX Green and MitoSOX Red. Confocal and electron microscopy showed that incubation with 0.05 mM TQ for 5 h induced changes in the intracellular localization of mitochondria and mitochondria hypertrophy and cell swelling. The antioxidant N-acetyl-L-cysteine (2 mM) protected CGNs from the toxic action of TQ. Taken together, these facts suggest that TQ is toxic for normal neurons, while ROS-induced changes in the mitochondria can be one of the major causes of the TQ-induced neuronal damage and death.
KEY WORDS: thymoquinone, mitochondria, cerebellar granule neurons, N-acetyl-L-cysteineDOI: 10.1134/S0006297920020078
Abbreviations: CGN, cerebellar granule neuron; NAC, N-acetyl-L-cysteine; ROS, reactive oxygen species; TQ, thymoquinone.
Thymoquinone (2-methyl-5-isopropyl-1,4-benzoquinone, TQ) is one of the
main constituents and an active ingredient of the volatile oil of
Nigella sativa seeds [1]. This quinone
exhibits a wide spectrum of beneficial biological activities (e.g.,
immune enhancing, as well as anti-inflammatory and antimutagenic
activities) [2-4]. TQ scavenges
superoxide, hydroxyl radical, and singlet oxygen [5]. It exhibits protective effects by suppressing
lipid peroxidation during ischemia-reperfusion injury in the rat
hippocampus [6] and prevents β-amyloid
(Abeta)-induced neurotoxicity in cultured rat primary neurons [7] by inhibiting mitochondrial dysfunction and
oxidative stress [8]. TQ protects cultured SH-SY5Y
cells and rat hippocampus against arsenic-induced mitochondrial
dysfunction and cytotoxicity [9, 10] and prevents 1-methyl-4-phenylpyridinium-induced
dopaminergic cell death in the primary mesencephalic cell culture [11]. TQ-containing mitochondria-targeted antioxidants
display neuroprotective properties [12, 13]. There is a growing interest in the therapeutic
potential of TQ in different research fields, particularly, in cancer
therapy. Recent studies have shown that TQ exhibits cytotoxic effects
in several cancer cell lines [14-16], including those derived from nervous tissue [17-20]. The oxidant/antioxidant
effects of TQ depend on its concentration. As a quinone, TQ can be
reduced by various reductases with the formation of semiquinone (one
reduction) or thymohydroquinone (two reductions). While the latter was
reported to have the antioxidant properties, semiquinone acts as a
prooxidant by generating reactive oxygen species (ROS) [14, 21]. TQ induces apoptotic
death in cancer cells through the oxidative stress [14]. However, most studies on the TQ toxicity have
been carried out in cancer cell lines, while there are only few studies
performed in cultured neurons of the central nervous system. Here, we
investigated the toxic action of TQ in primary neuronal cultures in
vitro.
MATERIALS AND METHODS
Reagents. Unless otherwise noted, all media and supplements used in the experiments were purchased from Biochrom KG (Germany). CellROX Green, MitoSOX Red, and rhodamine 123 were from Invitrogen (USA). Thymoquinone and other reagents were from Sigma Chemicals (Germany).
Rat primary cerebellar neuronal cultures. Primary cultures were prepared from the cerebella of 7- to 8-day-old Wistar rats as described previously [22]. The cerebella were washed with Ca2+- and Mg2+-free PBS (Dulbecco) and incubated in the same solution containing 0.05% trypsin and 0.02% EDTA (Invitrogen) for 15 min at 37°C. After incubation, the tissue was washed twice with PBS and dissociated by repeated pipetting in a nutrient medium of the following composition: Eagle’s minimum essential medium (MEM, 90%), fetal calf serum (10%), glutamine (2 mM), and HEPES (10 mM). After mild centrifugation, the cells were resuspended in a required volume of the nutrient medium of the same composition containing 25 mM KCl. Cell suspension was added to poly-L-lysine-coated 96-well plates (0.1 ml), 35-mm Petri dishes (0.2 ml), or coverslips in a 40-mm Petri dishes (0.8 ml) and kept in a CO2-incubator (5% CO2, 36.5 ± 0.5°C). The cells were cultured for up to 6-7 days in vitro without medium replacement.
Pharmacological treatment. The viability of rat cerebellar granule neurons (CGNs) was studied in Eagle’s MEM with 1% B-27 supplement minus antioxidants, 0.5 mM glutamine, 25 mM KCl, 10 mM HEPES, and 2 g/liter NaHCO3, which was supplemented with thymoquinone (0.01-0.06 mM) and other reagents depending on the experiment. Evaluation of free radical content and visualization of mitochondria with rhodamine 123 were carried out in balanced salt solution of the following composition (mM): NaCl (154), KCl (25), CaCl2 (2.3), MgCl2 (1), NaHCO3 (3.6), Na2HPO4 (0.35), glucose (5.6), HEPES (10), pH 7.3. The concentration of KCl in the incubation solution was the same as in the culture medium to prevent a decrease in the intracellular calcium levels and initiation of apoptosis [23].
Assessment of neuronal viability. The viability of CGNs in the experiments on the TQ toxicity and protective effect of N-acetyl-L-cysteine (NAC) was determined as described previously [22]. After incubation with TQ (0.01-0.05 mM, 24 h), the cells were fixed with ethanol/formaldehyde/acetic acid (7 : 2 : 1 v/v), stained with trypan blue, and analyzed under an inverted phase-contrast microscope Olympus CKX41 (Japan) equipped with a CC12 camera for light microscopy. The percentage of survived neurons was estimated by counting morphologically intact CGN nuclei in five adjacent fields of view along the well diameter using a ×40 objective, which provided an accurate estimate of cell survival. The viability of cells in the untreated control cultures was taken as 100%; the viability of treated cultures was counted as a percentage of the control value. This method is more accurate than the MTT assay, but much more laborious.
Evaluation of reactive oxygen species (ROS) content. CellROX Green and MitoSOX Red are fluorescent dyes used for measuring ROS content in the cells. These dyes can penetrate into the cell and fluoresce only in the oxidized state. Five hours after the beginning of the experiment, the cultures were stained with CellROX Green (0.005 mM) or MitoSOX Red (0.005 mM) for 30 min at 36.5 ± 0.5°C and then washed thrice with balanced salt solution. The dyes were added at a time when the cultures showed marked morphological changes in the mitochondria that were detected with rhodamine 123. CellROX Green fluorescence was excited by blue light at 485 nm and recorded at 530 nm using a SpectraMax M2 microplate fluorescent scanner (Molecular Devices, USA); MitoSOX Red fluorescence was excited by green light at 510 nm and recorded at 580 nm.
Visualization of mitochondria. After incubation with 0.05 mM TQ for 5 h, the cells were loaded with 0.005 mM rhodamine 123 for 15 min at 36.5 ± 0.5°C, followed by triple washing in balanced salt solution. The mitochondria were visualized using an Olympus IX71 confocal microscope (Japan) with a spinning-disk, ×100 objective, and 488-nm OBIS (USA) laser controlled by the Coherent Connection 3 program. Fluorescence images of the cells for mitochondria imaging were captured with emission at >500 nm and excitation at 488 nm
Electron microscopy. Cell grown on poly-L-lysine-coated coverslips in 40-mm Petri dishes were used for electron microscopy studies [24]. The cells grown for 6-7 days in vitro were exposed to 0.05 mM TQ for 4 to 6 h and then fixed in aqueous solution of 2.5% glutaraldehyde (SPI Inc., USA), 100 mM sodium cacodylate for 48 h at 4°C [25]. The fixed cells were washed twice with fresh 100 mM sodium cacodylate solution and post-fixed with 1% osmium tetroxide dissolved in 100 mM sodium cacodylate for 60 min at 4°C. The samples were then dehydrated in a series of increasing ethanol concentrations. Dehydration included en-bloc staining with 2% uranyl acetate in 70% ethanol for 1 h at 4°C. Ethanol was followed by acetone and increasing series of acetone resin mixes and two changes of pure freshly made resin. Finally, the coverslips were placed in silicone rubber molds filled with pure Spi-pon 812 resin (SPI Inc.) and cured at 70°C for 72 h. The cured blocks were trimmed with a razor blade and 90-nm ultrathin sections were prepared with an Ultracut E ultramicrotome (Reichert-Jung, Germany) equipped with an Ultra 45 diamond knife (Diatome, Switzerland). The sections were mounted on formvar-coated copper slot-grids and additionally contrasted with 2% aqueous uranyl acetate for 40 min and with lead citrate for 3 min. The samples were studied and photographed under a JEM-1400 electron microscope (JEOL, Japan) operating at 100 kV and equipped with a Quemesa CCD camera (Olympus Soft Imaging Solutions, Japan).
Statistical analysis. All data were obtained from 9-12 individual cultures in 3-4 independent experiments. Variation of the examined parameters had normal distribution and was analyzed using the t-test or the one-way ANOVA with Neuman–Keuls and Bonferroni post hoc tests. The value p < 0.05 was considered as statistically significant; the results are presented as mean ± SEM.
RESULTS
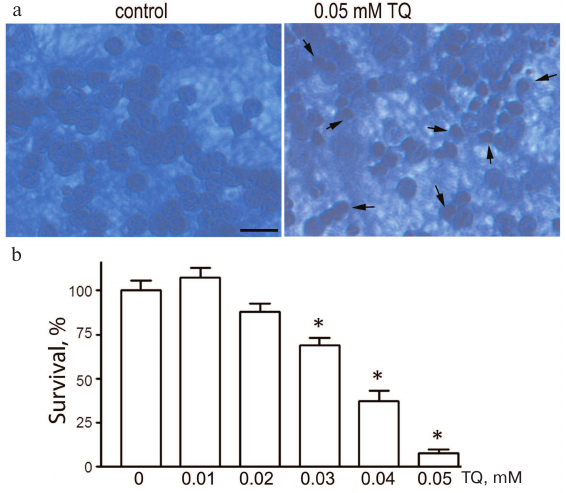
Thymoquinone toxicity was investigated in the 0.01-0.05 mM concentration range. Incubation of cultured CGNs with TQ (0.04-0.05 mM) for 24 h led to the appearance of pyknotic nuclei, which reliably indicated cell death. The loss of cell viability was evaluated by counting morphologically intact CGNs (Fig. 1). The toxicity of TQ depends on its concentration in the culture medium. For further experiments, we selected TQ concentrations that were toxic to cancer cells according to the earlier published studies.
Fig. 1. TQ effect on the CGN viability. a) Cell cultures fixed with ethanol/formaldehyde/acetic acid (7 : 2 : 1) and stained with trypan blue. Pyknotic nuclei are indicated by arrows. Scale bar, 15 μm. b) Quantitative evaluation of neuronal survival by counting morphologically intact nuclei of CGNs; *p < 0.01, statistically significant difference with the control (0 mM TQ). (Colored versions of Figs. 1 and 5 are available in electronic version of the article on the site http://protein.bio.msu.ru/biokhimiya/)
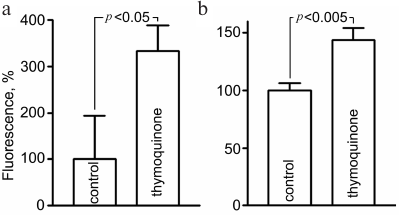
Evaluation of oxidative stress. Assessment of ROS level using fluorescent probes showed that 5-h exposure to 0.05 mM TQ led to an increase in CellROX Green fluorescence in live CGNs to 334 ± 50% and MitoSOX Red fluorescence to 144 ± 10% (Fig. 2). MitoSOX Red permeates live cells where it selectively targets mitochondria. It is rapidly oxidized by superoxide, and the dye is highly fluorescent upon binding to nucleic acids. CellROX Green is a cell-permeant dye which exhibits bright green photostable fluorescence upon oxidation with ROS.
Fig. 2. Increase in the CellROX Green (a) and MitoSOX Red (b) fluorescence as indication of free radical generation in CGNs after 5-h treatment with 0.05 mM TQ.
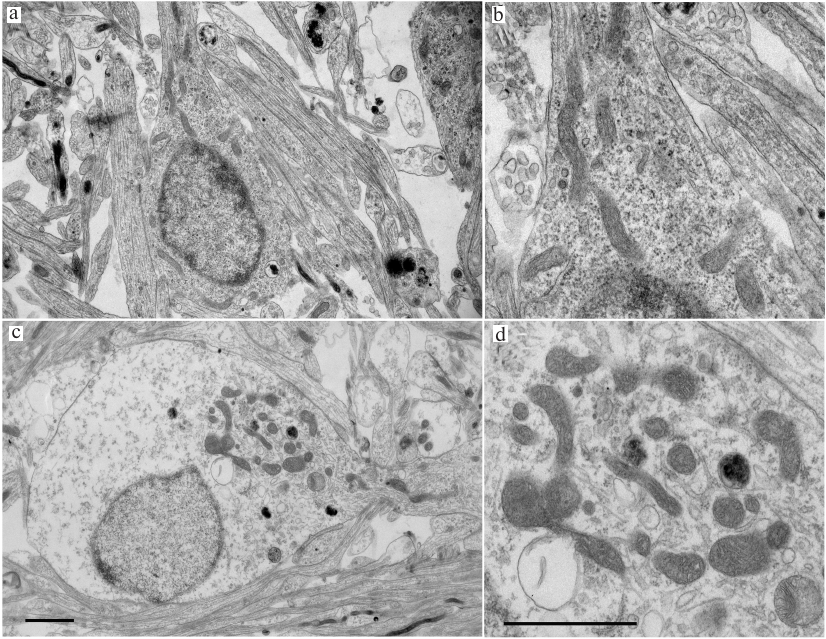
Electron microscopy. Morphological changes in the TQ-treated CGNs were studied by electron microscopy. The control cells cultured under normal conditions had round body shape; most cell volume was occupied by a nucleus with diffuse chromatin and small clumps of condensed chromatin. The ultrastructure of mitochondria was typical for normal cells, with highly organized cytoplasm and mitochondria of regular size (not enlarged) with slightly oval or elongated shape. The cristae were clearly seen, and the mitochondrial matrix had a higher electron density than the surrounding cytoplasm (Fig. 3). Most mitochondria were located around the nucleus. In neurons treated with TQ for 5 h, the mitochondria were hypertrophied, but the electron density of their matrix did not change significantly. The mitochondria themselves were gathered in a group in the cytoplasm. The electron density of the cytoplasm and the nuclei of the TQ-treated neurons was noticeably lower than in the control, which was apparently due to cell swelling (Fig. 3).
Fig. 3. Transmission electron micrograph of cultured CGNs. a, b) Control culture. Mitochondria and other compartments appear undamaged; c, d) cells after exposure to 0.05 mM TQ for 5 h. Mitochondria are swollen and grouped at the cytoplasm periphery in the vicinity of outgoing protrusion. There is a noticeable swelling of the cell body and the nucleus. Scale bar, 1.5 μm.
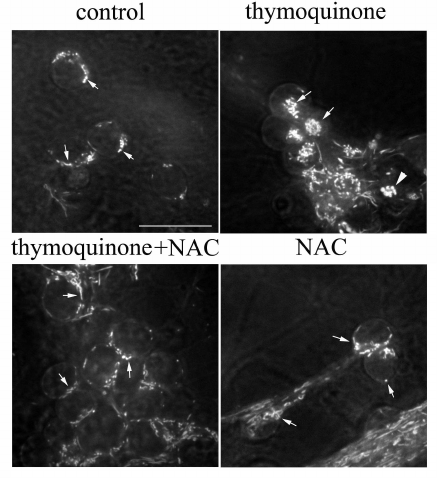
NAC protects against TQ toxicity. Mitochondria were stained with rhodamine 123 (dye that emits green fluorescence when excited with blue light) and studied by confocal microscopy. The mitochondria of control cells actively accumulated rhodamine 123. They had a uniform thickness and rod-like shape and were positioned around the nucleus (Fig. 4). In neurons treated with 0.05 mM TQ for 5 h, the mitochondria were concentrated in a group in the cytoplasm; some of them were swollen. Treatment of CGNs with the antioxidant NAC (2 mM) prevented abnormal repositioning and swelling of the mitochondria (Fig. 4).
Fig. 4. NAC prevents TQ-induced (0.05 mM, 5 h) alterations in the mitochondrial localization and swelling of these organelles. CGNs were stained with rhodamine 123; arrows show mitochondria; triangle indicates swollen mitochondria. Scale bar, 15 μm.
Counting neurons with normal morphology in histological specimens is a very laborious method for assessing neuronal viability, but it is much more accurate than the MTT assay. For this reason, we used neuron counting to assess with a high accuracy the combined effect of studied substances.
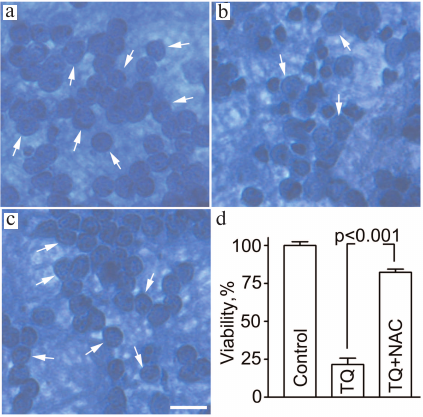
Assessment of cell viability by counting neurons with normal morphology in histological preparations demonstrated that 24 ± 5% of cells survived after exposure to TQ (0.05 mM, 24 h). Simultaneous administration of 2 mM NAC protected the neurons from the deleterious action of TQ and increased neuronal survival to 82 ± 2% (Fig. 5).
Fig. 5. NAC attenuates the toxic effect of TQ in CGNs. The cells were fixed with ethanol/formaldehyde/acetic acid (7 : 2 : 1) and stained with trypan blue. Normal CGN nuclei are indicated by arrows. a) Control; b) TQ; c) TQ + NAC. Scale bar, 15 μm. d) Quantitative evaluation of neuronal survival.
DISCUSSION
TQ possesses a wide spectrum of biological activities and exhibits antioxidant, neuroprotective, antiproliferative, anti-metastatic, cytotoxic, pro-apoptotic, and natural killer-dependent cytotoxic effects [26-30]. The anti-tumor effects of TQ are mainly associated with the induction of the G2/M cell cycle arrest and initiation of apoptotic pathways. TQ inhibits autophagy, angiogenesis, cell invasion and migration and enhances the efficacy of chemotherapeutic drugs [31]. Several studies have demonstrated that TQ shows therapeutic effects in tumors of the central nervous system. TQ inhibited the growth of human medulloblastoma cells by inducing oxidative stress and caspase-dependent apoptosis while suppressing NF-κB signaling and IL-8 expression [32]. It also reduced migration and invasion of human glioblastoma cells [33]. Most research on the neurotoxic action of TQ has been carried out in cancer cell lines; there are only a few studies performed in normal cultured neurons of the central nervous system. In our study, we used dissociated neuronal cultures isolated from the rat cerebellum. A significant advantage of such neuronal culture is morphological and neurochemical uniformity of neurons, which makes it a good model for studying pathological processes, including cytotoxicity [22, 25, 34, 35]. Administration of 0.03-0.05 mM TQ induced the concentration-dependent cell death in the neuronal cultures. The cytotoxic action of TQ on neurons might be associated with the disruption of ionic balance in the cytoplasm and cell swelling, since the study of the neuronal ultrastructure revealed a decrease in the electron density of the cytoplasm and the nuclei of the TQ-treated cells. The range of the cytotoxic TQ concentration has been estimated in earlier studies in human medulloblastoma, mouse neuroblastoma (Neuro-2a), and human glioblastoma cells [18, 32, 33]. It was also observed that TQ at a concentration of 0.05 mM demonstrated the cytotoxic effect on glioblastoma cells without significantly affecting the viability of astrocytes or fibroblasts [33]. The toxicity of 0.05 mM TQ has been shown for medulloblastoma cells as well: TQ increased the level of ROS responsible for the apoptosis induction in these cells [32]. Using CellROX Green and MitoSOX Red as probes for estimating the content of intracellular free radicals, we showed that TQ caused an excessive formation of free radicals in the CGNs. The fact that the antioxidant NAC protected neurons from the TQ-induced death also confirms the involvement of oxidative stress in the mechanism of neuronal damage caused by TQ. MitoSOX Red is a fluorescent dye specifically targeted to mitochondria in live cells that can be used for evaluation of superoxide production by these organelles [36, 37]. Mitochondria not only are important producers of ROS in the cell, but also one of the main targets of these chemically active molecules. TQ-induced production of free radicals in neuronal cultures was accompanied by changes in the localization of mitochondria. Typically, the mitochondria of CGNs are positioned around the nucleus, whereas TQ caused an unusual accumulation of these organelles in a limited region of the cytoplasm. In addition, TQ treatment led to the increase in the size and swelling of these organelles. The presence of the antioxidant NAC in the culture medium prevented all changes caused by the toxic action of TQ, which indicates a direct role of free radicals in the mitochondrial damage caused by TQ. It should be noted that TQ in the concentrations toxic to neurons induces cytotoxicity and trigger mitochondrial dysfunction in bladder cancer cells [38]. Interestingly, one of the mechanisms of the protective effect of TQ is its antioxidant action [39]. However, it is known that many antioxidants in high concentrations exhibit the prooxidant properties and can cause oxidative stress and cell death. The manifestation of the prooxidant properties depends on the chemical nature of the antioxidant, presence of variable valence metals, and concentrations used. By the way of illustration, the strong antioxidant SkQ1 [10(6′-plastoquinonyl)decyltriphenylphosphonium] in high concentrations displays the properties of a prooxidant [40].
Taken together, these facts lead us to suggest that TQ can be toxic for normal neurons, while TQ-induced ROS-dependent processes of mitochondrial destruction can be one of the major causes of neuronal damage and TQ-induced death.
Conflict of interest. The authors declare no conflict of interest in financial or any other area.
Compliance to ethical norms. All experimental protocols were approved by the Animal Ethics Committee of the Research Center of Neurology (Protocol registration no. 2-5/16) and were in accordance with the Council Directive 2010/63EU of the European Parliament and the Council of September 22, 2010 on the protection of animals used for scientific purposes.
REFERENCES
1.Burits, M., and Bucar, F. (2000) Antioxidant
activity of Nigella sativa essential oil, Phytother.
Res., 14, 323-328.
2.Hajhashemi, V., Ghannadi, A., and Jafarabadi, H.
(2004) Black cumin seed essential oil, as a potent analgesic and
anti-inflammatory drug, Phytother. Res., 18, 195-199;
doi: 10.1002/ptr.1390.
3.Ahmed, A. M., Al-Olayan, E. M., Aboul-Soud, M. A.,
and Al-Khedhairy, A. A. (2010) The immune enhancer, thymoquinone, and
the hope of utilizing the immune system of Aedes caspius against
disease agents, Afr. J. Biotechnol., 9, 3183-3195.
4.Khader, M., Bresgen, N., and Eckl, P. M. (2010)
Antimutagenic effects of ethanolic extracts from selected Palestinian
medicinal plants, J. Ethnopharmacol., 127, 319-324; doi:
10.1016/j.jep.2009.11.001.
5.Al-Majed, A. A., Al-Omar, F. A., and Nagi, M. N.
(2006) Neuroprotective effects of thymoquinone against transient
forebrain ischemia in the rat hippocampus, Eur. J. Pharmacol.,
543, 40-47; doi: 10.1016/j.ejphar.2006.05.046.
6.Hosseinzadeh, H., Parvardeh, S., Asl, M. N.,
Sadeghnia, H. R., and Ziaee, T. (2007) Effect of thymoquinone and
Nigella sativa seeds oil on lipid peroxidation level during global
cerebral ischemia-reperfusion injury in rat hippocampus,
Phytomedicine, 14, 621-627; doi:
10.1016/j.phymed.2006.12.005.
7.Alhebshi, A. H., Gotoh, M., and Suzuki, I. (2013)
Thymoquinone protects cultured rat primary neurons against amyloid
β-induced neurotoxicity, Biochem. Biophys. Res. Commun.,
433, 362-367; doi: 10.1016/j.bbrc.2012.11.139.
8.Khan, A., Vaibhav, K., Javed, H., Khan, M. M.,
Tabassum, R., Ahmed, M. E., Srivastava, P., Khuwaja, G., Islam, F.,
Siddiqui, M. S., Safhi, M. M., and Islam, F. (2012) Attenuation of
Aβ-induced neurotoxicity by thymoquinone via inhibition of
mitochondrial dysfunction and oxidative stress, Mol. Cell
Biochem., 369, 55-65; doi: 10.1007/s11010-012-1368-x.
9.Firdaus, F., Zafeer, M. F., Anis, E., Ahmad, F.,
Hossain, M. M., Ali, A., and Afzal, M. (2019) Evaluation of
phytomedicinal efficacy of thymoquinone against arsenic-induced
mitochondrial dysfunction and cytotoxicity in SH-SY5Y cells,
Phytomedicine, 54, 224-230; doi:
10.1016/j.phymed.2018.09.197.
10.Firdaus, F., Zafeer, M. F., Waseem, M., Ullah,
R., Ahmad, M., and Afzal, M. (2018) Thymoquinone alleviates arsenic
induced hippocampal toxicity and mitochondrial dysfunction by
modulating mPTP in Wistar rats, Biomed. Pharmacother.,
102, 1152-1160; doi: 10.1016/j.biopha.2018.03.159.
11.Radad, K. S., Al-Shraim, M. M., Moustafa, M. F.,
and Rausch, W. D. (2015) Neuroprotective role of thymoquinone against
1-methyl-4-phenylpyridinium-induced dopaminergic cell death in primary
mesencephalic cell culture, Neurosciences (Riyadh), 20,
10-16.
12.Genrikhs, E. E., Stelmashook, E. V., Popova, O.
V., Kapay, N. A., Korshunova, G. A., Sumbatyan, N. V., Skrebitsky, V.
G., Skulachev, V. P., and Isaev, N. K. (2015) Mitochondria-targeted
antioxidant SkQT1 decreases trauma-induced neurological deficit in rat
and prevents amyloid-β-induced impairment of long-term
potentiation in rat hippocampal slices, J. Drug Target.,
23, 347-352; doi: 10.3109/1061186X.2014.997736.
13.Isaev, N. K., Stelmashook, E. V., Genrikhs, E.
E., Korshunova, G. A., Sumbatyan, N. V., Kapkaeva, M. R., and
Skulachev, V. P. (2016) Neuroprotective properties of
mitochondria-targeted antioxidants of the SkQ-type, Rev.
Neurosci., 27, 849-855; doi: 10.1515/revneuro-2016-0036.
14.El-Najjar, N., Chatila, M., Moukadem, H.,
Vuorela, H., Ocker, M., Gandesiri, M., Schneider-Stock, R., and
Gali-Muhtasib, H. (2010) Reactive oxygen species mediate
thymoquinone-induced apoptosis and activate ERK and JNK signaling,
Apoptosis, 15, 183-195; doi:
10.1007/s10495-009-0421-z.
15.Park, E. J., Chauhan, A. K., Min, K. J., Park, D.
C., and Kwon, T. K. (2016) Thymoquinone induces apoptosis through
downregulation of c-FLIP and Bcl-2 in renal carcinoma Caki cells,
Oncol. Rep., 36, 2261-2267; doi:
10.3892/or.2016.5019.
16.Assaf, M. D., Semaan, J., El-Sabban, M.,
Al-Jaouni, S. K., Azar, R., Kamal, M. A., and Harakeh, S. (2018)
Inhibition of proliferation and induction of apoptosis by thymoquinone
via modulation of TGF family, p53, p21 and Bcl-2α in leukemic
cells, Anticancer Agents Med. Chem., 18, 210-215; doi:
10.2174/1871520617666170912133054.
17.Gurung, R. L., Lim, S. N., Khaw, A. K., Soon, J.
F., Shenoy, K., Mohamed Ali, S., Jayapal, M., Sethu, S., Baskar, R.,
and Hande, M. P. (2010) Thymoquinone induces telomere shortening, DNA
damage and apoptosis in human glioblastoma cells, PLoS One,
5, e12124; doi: 10.1371/journal.pone.0012124.
18.Paramasivam, A., Sambantham, S., Shabnam, J.,
Raghunandhakumar, S., Anandan, B., Rajiv, R., Vijayashree
Priyadharsini, J., and Jayaraman, G. (2012) Anti-cancer effects of
thymoquinone in mouse neuroblastoma (Neuro-2a) cells through caspase-3
activation with down-regulation of XIAP, Toxicol. Lett.,
213, 151-149; doi: 10.1016/j.toxlet.2012.06.011.
19.Paramasivam, A., Raghunandhakumar, S.,
Priyadharsini, J. V., and Jayaraman, G. (2015) In vitro
anti-neuroblastoma activity of thymoquinone against Neuro-2a cells via
cell-cycle arrest, Asian Pac. J. Cancer Prev., 16,
8313-8319; doi: 10.7314/apjcp.2015.16.18.8313.
20.Elmaci, I., and Altinoz, M. A. (2016)
Thymoquinone: an edible redox-active quinone for the pharmacotherapy of
neurodegenerative conditions and glial brain tumors. A short review,
Biomed. Pharmacother., 83, 635-640; doi:
10.1016/j.biopha.2016.07.018.
21.Mansour, M. A., Nagi, M. N., El-Khatib, A. S.,
and Al-Bekairi, A. M. (2002) Effects of thymoquinone on antioxidant
enzyme activities, lipid peroxidation and DT-diaphorase in different
tissues of mice: a possible mechanism of action, Cell Biochem.
Funct., 20, 143-151; doi: 10.1002/cbf.968.
22.Isaev, N. K., Genrikhs, E. E., Aleksandrova, O.
P., Zelenova, E. A., and Stelmashook, E. V. (2016) Glucose deprivation
stimulates Cu2+ toxicity in cultured cerebellar granule
neurons and Cu2+-dependent zinc release, Toxicol.
Lett., 250-251, 29-34; doi:
10.1016/j.toxlet.2016.04.002.
23.Galli, C., Meucci, O., Scorziello, A., Werge, T.
M., Calissano, P., and Schettini, G. (1995) Apoptosis in cerebellar
granule cells is blocked by high KCl, forskolin, and IGF-1 through
distinct mechanisms of action: the involvement of intracellular calcium
and RNA synthesis, J. Neurosci., 15, 1172-1179.
24.Stelmashook, E. V., Genrikhs, E. E., Mukhaleva,
E. V., Kapkaeva, M. R., Kondratenko, R. V., Skrebitsky, V. G., and
Isaev, N. K. (2019) Neuroprotective effects of methylene blue in
vivo and in vitro, Bull. Exp. Biol. Med., 167,
455-445; doi: 10.1007/s10517-019-04548-3.
25.Isaev, N. K., Avilkina, A., Golyshev, S. A.,
Genrikhs, E. E., Alexandrova, O. P., Kapkaeva, M. R., and Stelmashook,
E. V. (2018) N-acetyl-L-cysteine and Mn2+ attenuate
Cd2+-induced disturbance of the intracellular free calcium
homeostasis in cultured cerebellar granule neurons, Toxicology,
393, 1-8; doi: 10.1016/j.tox.2017.10.017.
26.Ebrahimi, S. S., Oryan, S., Izadpanah, E., and
Hassanzadeh, K. (2017) Thymoquinone exerts neuroprotective effect in
animal model of Parkinson’s disease, Toxicol. Lett.,
276, 108-114; doi: 10.1016/j.toxlet.2017.05.018.
27.Majdalawieh, A. F., Fayyad, M. W., and Nasrallah,
G. K. (2017) Anti-cancer properties and mechanisms of action of
thymoquinone, the major active ingredient of Nigella sativa,
Crit. Rev. Food Sci. Nutr., 57, 3911-3928; doi:
10.1080/10408398.2016.1277971.
28.Ullah, I., Ullah, N., Naseer, M. I., Lee, H. Y.,
and Kim, M. O. (2012) Neuroprotection with metformin and thymoquinone
against ethanol-induced apoptotic neurodegeneration in prenatal rat
cortical neurons, BMC Neurosci., 13, 11; doi:
10.1186/1471-2202-13-11.
29.Kanter, M. (2011) Protective effects of
thymoquinone on the neuronal injury in frontal cortex after chronic
toluene exposure, J. Mol. Histol., 42, 39-46; doi:
10.1007/s10735-010-9305-3.
30.Kanter, M. (2008) Nigella sativa and
derived thymoquinone prevents hippocampal neurodegeneration after
chronic toluene exposure in rats, Neurochem. Res.,
33, 579-588.
31.Farkhondeh, T., Samarghandian, S., Hozeifi, S.,
and Azimi-Nezhad, M. (2017) Therapeutic effects of thymoquinone for the
treatment of central nervous system tumors: a review, Biomed.
Pharmacother., 96, 1440-1444; doi:
10.1016/j.biopha.2017.12.013.
32.Ashour, A. E., Ahmed, A. F., Kumar, A., Zoheir,
K. M., Aboul-Soud, M. A., Ahmad, S. F., Attia, S. M., Abd-Allah, A. R.,
Cheryan, V. T., and Rishi, A. K. (2016) Thymoquinone inhibits growth of
human medulloblastoma cells by inducing oxidative stress and
caspase-dependent apoptosis while suppressing NF-κB signaling and
IL-8 expression, Mol. Cell Biochem., 416, 141-155; doi:
10.1007/s11010-016-2703-4.
33.Kolli-Bouhafs, K., Boukhari, A., Abusnina, A.,
Velot, E., Gies, J. P., Lugnier, C., and Ronde, P. (2012) Thymoquinone
reduces migration and invasion of human glioblastoma cells associated
with FAK, MMP-2 and MMP-9 down-regulation, Invest. New Drugs,
30, 2121-2131; doi: 10.1007/s10637-011-9777-3.
34.Thangnipon, W., Kingsbury, A., Webb, M., and
Balazs, R. (1983) Observations on rat cerebellar cells in vitro:
influence of substratum, potassium concentration and relationship
between neurons and astrocytes, Brain Res., 313, 177-189;
doi: 10.1016/0165-3806(83)90215-8.
35.Costa, L. G., Tagliaferri, S., Roque, P. J., and
Pellacani, C. (2016) Role of glutamate receptors in tetrabrominated
diphenyl ether (BDE-47) neurotoxicity in mouse cerebellar granule
neurons, Toxicol. Lett., 241, 159-166; doi:
10.1016/j.toxlet.2015.11.026.
36.Robinson, K. M., Janes, M. S., Pehar, M.,
Monette, J. S., Ross, M. F., Hagen, T. M., Murphy, M. P., and Beckman,
J. S. (2006) Selective fluorescent imaging of superoxide in vivo
using ethidium-based probes, Proc. Natl. Acad. Sci. USA,
103, 15038-15043; doi: 10.1073/pnas.0601945103.
37.Johnson-Cadwell, L. I., Jekabsons, M. B., Wang,
A., Polster, B. M., and Nicholls, D. G. (2007) “Mild
uncoupling” does not decrease mitochondrial superoxide levels in
cultured cerebellar granule neurons but decreases spare respiratory
capacity and increases toxicity to glutamate and oxidative stress,
J. Neurochem., 101, 1619-1631.
38.Zhang, M., Du, H., Huang, Z., Zhang, P., Yue, Y.,
Wang, W., Liu, W., Zeng, J., Ma, J., Chen, G., Wang, X., and Fan, J.
(2018) Thymoquinone induces apoptosis in bladder cancer cell via
endoplasmic reticulum stress-dependent mitochondrial pathway, Chem.
Biol. Interact., 292, 65-75; doi:
10.1016/j.cbi.2018.06.013.
39.Gokce, E. C., Kahveci, R., Gokce, A., Cemil, B.,
Aksoy, N., Sargon, M. F., Kisa, U., Erdogan, B., Guvenc, Y., Alagoz,
F., and Kahveci, O. (2016) Neuroprotective effects of thymoquinone
against spinal cord ischemia-reperfusion injury by attenuation of
inflammation, oxidative stress, and apoptosis, J. Neurosurg.
Spine, 24, 49-59; doi:
10.3171/2015.10.SPINE15612.
40.Stelmashook, E. V., Genrikhs, E. E., Kapkaeva, M.
R., Zelenova, E. A., and Isaev, N. K. (2017) N-Acetyl-L-cysteine in the
presence of Cu2+ induces oxidative stress and death of
granule neurons in dissociated cultures of rat cerebellum,
Biochemistry (Moscow), 82, 1176-1182; doi:
10.1134/S0006297917100108.