Regulation of Malate Dehydrogenases and Glutamate Dehydrogenase of Mammalian Brain by Thiamine in vitro and in vivo#
O. A. Mezhenska1, V. A. Aleshin2,3, T. Kaehne4, A. V. Artiukhov2,3, and V. I. Bunik2,3,a*
1Palladin Institute of Biochemistry, National Academy of Sciences of Ukraine, 01601 Kyiv, Ukraine2Lomonosov Moscow State University, Faculty of Bioengineering and Bioinformatics, 119991 Moscow, Russia
3Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119991 Moscow, Russia
4Institute of Experimental Internal Medicine, Otto-von-Guericke University, 39120 Magdeburg, Germany
# This paper is dedicated to 80th anniversary of the Biochemistry Department of Lomonosov Moscow State University (see vol. 84, no. 11, 2019).
* To whom correspondence should be addressed.
Received August 5, 2019; Revised September 24, 2019; Accepted September 24, 2019
To study the mechanisms of the non-coenzyme action of thiamine and its diphosphate (ThDP) on brain proteins, proteins of acetone extract of bovine brain synaptosomes or the homogenate of rat brain cortex were subjected to affinity chromatography on thiamine-modified Sepharose. In the step-wise eluates by thiamine (at pH 7.4 or 5.6), NaCl, and urea, the occurrence of glutamate dehydrogenase (GDH) and isoenzymes of malate dehydrogenase (MDH) along with the influence of thiamine and/or ThDP on the enzymatic activities were characterized using mass spectrometry and kinetic experiments. Maximal activation of the malate dehydrogenase reaction by thiamine is observed after the protein elution with the acidic thiamine solution, which does not elute the MDH1 isoenzyme. Effects of exogenous thiamine or ThDP on the GDH activity may depend on endogenous enzyme regulators. For example, thiamine and/or ThDP activate the brain GDH in eluates from thiamine-Sepharose but inhibit the enzyme in the crude preparations applied to the sorbent. Inhibition of GDH by ThDP is observed using the ADP-activated enzyme. Compared to the affinity chromatography employing the elution by thiamine at pH 7.4, the procedure at pH 5.6 decreases the activation of GDH by thiamine (but not ThDP) in the eluates with NaCl and urea. Simultaneously, the MDH2 content and total GDH activity are higher after the affinity elution at pH 5.6 than at pH 7.4, suggesting the role of the known interaction of GDH with MDH2 in stabilizing the activity of GDH and in the regulation of GDH by thiamine. The biological potential of thiamine-dependent regulation of the brain GDH is confirmed in vivo by demonstration of changes in regulatory properties of GDH after administration of a high dose of thiamine to rats. Bioinformatics analysis of the thiamine-eluted brain proteins shows a specific enrichment of their annotation terms with “phosphoprotein”, “acetylation”, and “methylation”. The relationship between thiamine and the posttranslational modifications in brain may contribute to the neuroprotective effects of high doses of thiamine, including the regulation of oxidation of the major excitatory neurotransmitter in brain – glutamate.
KEY WORDS: thiamine, malate dehydrogenase, glutamate dehydrogenase, thiamine-Sepharose, phosphoprotein, acetylation, methylationDOI: 10.1134/S0006297920010034
Abbreviations: GDH, glutamate dehydrogenase; MDH, malate dehydrogenase; MS, mass spectrometry; ThDP, thiamine diphosphate; TIC, total ion current.
Thiamine (vitamin B1) is a major vitamin of B-group that is widely used
in medical practice due to its neurotrophic action and stimulating
effect on the central glucose metabolism [1-3]. Mostly, these actions are ascribed to the vitamin
diphosphorylated derivative, thiamine diphosphate (ThDP), which is an
essential coenzyme of such enzymes of central metabolism as
transketolase and 2-oxo acid dehydrogenases. However, also other,
so-called non-coenzyme, actions of thiamine and its natural derivatives
have acquired increasing attention recently, especially in view of
unprecedented growth of neurodegenerative diseases [4-7]. Affecting not only the
ThDP-dependent enzymes, but also the coupled ones, the thiamine
compounds may provide for systemic regulation of metabolism. The
pleiotropic action of thiamine compounds better explains some
experimental results on the regulation by these compounds of
acetylcholine production that are not fully consistent with the
coenzyme action of ThDP only [5, 6]. It is worth noting that the pleiotropic action of
genes [8] or protein regulators of metabolism, such
as transcriptional regulators p53 or Nrf-2 [9], is
well-known. However, regarding the low molecular mass regulators, such
pleiotropic action is traditionally considered only as a source of side
effects [10]. Nevertheless, there is growing
interest in an opportunity to increase efficacy of pharmacological
treatments by drugs whose action is mediated by multiple targets [11]. In this regard, study of molecular mechanisms of
analogous regulation by natural compounds including vitamins is of
special interest [6, 12].
This work develops a recently described approach to the investigation of multiple targets of thiamine; it uses affinity chromatography of brain proteins on a sorbent covalently modified by thiamine, followed by mass spectrometric identification of the eluted proteins [6]. Compared to our previous study, where proteins of rat brain (so-called thiamine proteome), bound to the thiamine-modified Sepharose (further: thiamine-Sepharose), were eluted by non-specific agents, this work employs several modifications. They are: pH-dependent affinity elution of thiamine-Sepharose-bound proteins by thiamine; modification of the sorbent by thiamine that excludes multiple orientations of the bait on the sorbent; and comparative study of the protein elution profiles upon affinity chromatography of the proteins of either synaptosomal fraction of bovine brain or homogenate of rat brain cortex. As a result, new data on the pathways and molecular mechanisms of the non-coenzyme action of thiamine and its natural derivatives are obtained.
MATERIALS AND METHODS
Materials. Reagents from the following providers were used: ThDP, 2-oxoglutarate (disodium salt), NADH (disodium salt), ADP, GTP, CHAPS, protease inhibitors AEBSF, aprotinin, bestatin, E-64, leupeptin, and pepstatin A from Sigma (USA); thiamine, polyethylene glycol 6000, glucose, and Tris-HCl from Serva (Germany); glycerol from Biomedicals, LLC (USA); NAD+ from Gerbu (Germany). Physiological Krebs–Ringer solution comprised 118 mM NaCl, 2.34 mM KH2PO4, 4.6 mM KCl, 1.19 mM MgSO4, 2.42 mM CaCl2, 24.9 mM NaHCO3, and 10 mM glucose, pH 7.4. Milli-Q-deionized water and reagents of the highest available purity were used.
Animal experiments. The rat brain proteins were isolated from Wistar rats. The animals were kept under standard conditions of the vivarium of Lomonosov Moscow State University, at temperature 21 ± 2°C and air humidity 53 ± 5%, with the water and food access ad libitum. The animals were exposed to a 12/12 h day cycle (light phase from 9 am till 9 pm of Moscow time). The rats were killed by decapitation using a guillotine. Bovine brains were obtained from a local slaughterhouse (Kyiv, Ukraine) from animals 1.5-3.0 years old.
The rat brain cortex homogenates for affinity chromatography were from female Wistar rats of 250-300 g; action of a high dose of thiamine was studied using male Wistar rats of 300-350 g (age of 3-4 months). Thiamine (400 mg per 1 kg body mass) was administered to experimental animals i.p. in the evening time (5-7 pm). Water solution of 200 mg/ml of thiamine hydrochloride, with pH adjusted to 6.7-6.9 with NaOH, was used in the experimental group. Control rats obtained the same volume of 0.9% NaCl. Insulin syringes were used for the injections according to published recommendations [13]. Decapitation was done 24 h after the injections.
Affinity chromatography. Acetone powder of synaptosomal proteins from the bovine brain was obtained according to a published procedure [14] and stored frozen at –70°C. The acetone powder proteins were extracted with the Krebs–Ringer buffer as describer earlier [6]. Homogenates of the rat brain cortex were obtained as described earlier [15].
Affinity sorbent thiamine-N-4-azobenzoyl-ε-hydrazide-Sepharose 4B (thiamine-Sepharose) was synthesized according to Klyashchitsky et al. [16]. The extract of the acetone powder of the synaptosomal proteins of bovine brain or the homogenates of the rat brain cortex were applied on a column filled with the thiamine-Sepharose and equilibrated with the Krebs–Ringer buffer at a flow rate of 0.2 ml/min. Washing with the Krebs–Ringer buffer was done at the same rate until the non-bound or poorly bound proteins were removed, as judged by a decrease of optical density at 280 nm down to the baseline.
The proteins bound to the thiamine-Sepharose were eluted step-wise. Specific elution in the first (affinity) step was done using solutions of 5- or 10-mM thiamine in the Krebs–Ringer buffer, adjusted to pH 7.4 or pH 5.6. Tris-HCl solution (10 mM, pH 7.4) with 1 M NaCl or 2 M urea was applied at the second and third steps, correspondingly, as described earlier [6]. The eluted proteins were concentrated with simultaneous buffer exchange to 10 mM Tris-HCl, pH 7.4, using Amicon Ultra 15 centrifuge units with membrane cut-off of 30 kDa. The sorbent was regenerated by washing with 8 M urea followed by deionized water and stored in 0.02% solution of sodium azide.
Enzyme assays and sources. Activity of the NADH-dependent dehydrogenases, i.e., malate dehydrogenases (MDH) and glutamate dehydrogenase (GDH), was detected by changes in the absorbance of NADH at 340 nm. The elution profiles were characterized by the enzyme assays at the saturating substrate concentrations. To investigate the influence of thiamine or ThDP on the enzymatic activities, the previously determined enzyme-specific conditions maximizing the influence were used [6]. Specific details of the assays are provided in the corresponding tables and figure legends.
For the study of the combined action of ThDP and ADP on GDH, the enzyme was partially purified from the rat brain. The centrifugation-precipitated fraction of the rat brain homogenate was resuspended in sonication buffer and sonicated as described earlier [17]. The sonicated suspension was extracted by 1% CHAPS. The supernatant obtained after a 40 min centrifugation at 11,000g, 4°C was adjusted to pH 6.15 and supplemented with 0.16 volume of 35% polyethylene glycol 6000 in a drop-wise manner. The suspension was centrifuged for 15 min at 18,500g, 4°C, and the supernatant was used as a source of partially purified GDH.
To assess the influence of thiamine administration on kinetic properties of the rat brain GDH and MDH, the brain homogenates from the animals of one experimental group in one series were combined. Each series comprised three control animals and three animals after the thiamine administration. In total, three independent series of animal experiments (18 animals) were performed. The brain cortex homogenates were prepared according to the earlier protocol [15], and insoluble fraction was separated by centrifugation for 30 min at 20,000g, 4°C. To remove enzyme regulators of low molecular mass that were present in the homogenates, the supernatant was subjected to rapid gel-filtration on a HiTrapTM desalting column, 5 ml (GE Healthcare, Sweden) according to the manufacturer’s protocol. Proteins were eluted by 100 mM Tris-HCl, pH 7.5. The results of kinetic experiments in each animal series (n = 3) were combined and approximated using the built-in models of GraphPad Prism v. 8.0. 2-Oxoglutarate and glutamate saturations were approximated by the model of complete substrate inhibition: v = Vmax . [S]/(Km + [S](1 + [S]/Ki)), where v – the reaction rate at a substrate concentration [S], Vmax – maximal reaction rate, Km – Michaelis constant, Ki – the substrate inhibition constant. The saturation of GDH by the allosteric inhibitor (GTP) or activators (ADP and leucine) is characterized by cooperative subunit interactions. Therefore, these dependencies were approximated by the 4PL (four-parameter logistic regression) model assuming the Hill coefficient to be not equal to 1.
Determination of protein concentration. Protein was determined by the method of Lowry et al. [18].
Analysis of the thiamine-dependent proteomes in the studied samples. Proteins eluted from the thiamine-Sepharose, constituting the so-called thiamine-dependent proteome, were subjected to denaturing SDS electrophoresis followed by trypsinolysis and peptide analysis by LC-MS/MS as described earlier [6]. Identification of peptides employed the NCBI database. Due to insufficient annotation of the bovine genome, identification of the bovine proteins used the SwissProt database for all organisms. The rat proteins were identified using the SwissProt database for this species. Bioinformatics analysis of the proteomes eluted from the thiamine-Sepharose was done by DAVID v. 6.8 as previously described [6].
Relative abundance of GDH and MDH peptides in samples. Semi-quantitative estimation of the enzyme abundance in the analyzed samples was based on the proportionality of the protein abundance to the number of its peptides identified in a preparation [17]. To compare the abundance in different samples, the numbers of identified peptides of an enzyme were normalized to total ion current (TIC) in the sample upon its MS analysis, because TIC is proportional to the total level of the sample peptides. TIC was obtained from the data provided by Skyline [19]. For convenience of the presentation, the normalized data used TIC × 10–10.
RESULTS AND DISCUSSION
Affinity chromatography of bovine brain synaptosomal proteins on thiamine-Sepharose. Comparison of the activity and number of GDH peptides from the acetone extract of synaptosomal proteins of bovine brain at different steps of affinity chromatography, depending on pH (7.4 or 5.6) of the first step employing affinity elution with thiamine, is shown in Table 1. The MS data indicate that in both cases GDH is well eluted by urea: a significant number of GDH peptides is present in this eluate independent of the GDH elution on the previous steps (Table 1). However, in the urea eluate, which is obtained after the affinity elution with an acidic (pH 5.6) thiamine solution, the specific and total activity of GDH are at least an order of magnitude higher than the values in the urea eluate after the affinity elution with a slightly alkaline (pH 7.4) thiamine solution. Besides, with the affinity elution by the acidic thiamine solution, the total GDH activity in the eluates of the three steps shows a good correspondence to the enzyme abundance in these eluates according to MS data: a higher total activity is observed in the fraction(s) where more peptides of GDH are identified. The correspondence is absent when the affinity elution is done with a slightly basic (pH 7.4) thiamine solution. In this case, the total GDH activity in the thiamine eluate comprises a major part of the total GDH eluted from the thiamine-Sepharose. In addition, specific activity of GDH eluted with a slightly alkaline solution of thiamine is 2-fold higher than the activity obtained after the elution at acidic pH. However, the MS data in Table 1 indicate that the GDH abundance in the former case (one unique peptide of GDH) is significantly lower than in the latter case (six unique peptides of GDH). Thus, affinity elution of the GDH protein from the thiamine-Sepharose is much more efficient at pH 5.6 than at pH 7.4, but the total and specific activities of GDH are higher after the elution at pH 7.4 than at pH 5.6 (Table 1).
Table 1. Activity of glutamate dehydrogenase
(GDH) and the number of unique GDH peptides in eluates from
thiamine-Sepharose obtained upon affinity chromatography of the acetone
extract of synaptosomal proteins of bovine brain
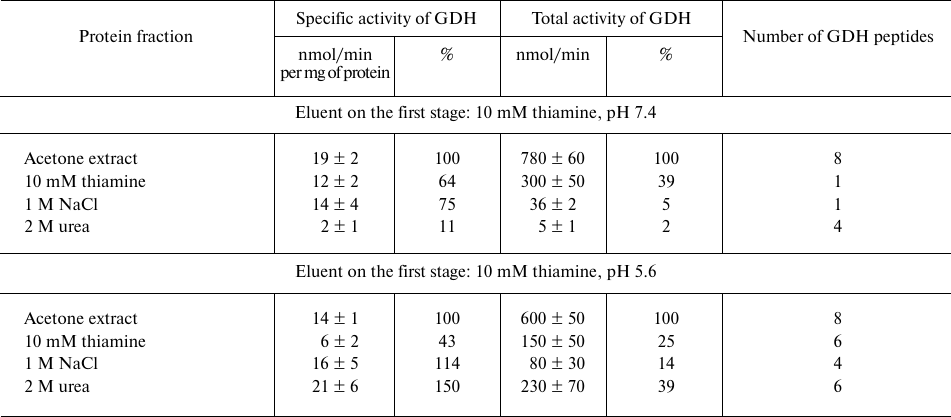
Note: The activity of eluted proteins was determined after replacing the
eluents with 10 mM Tris-HCl (pH 7.4). The assay medium comprised 100 mM
Tris-HCl, pH 7.5, and saturating substrates: 2.5 mM 2-oxoglutarate, 0.2
mM NADH, 50 mM NH4Cl. Relative activities are indicated as a
percentage of the levels in the initial preparations.
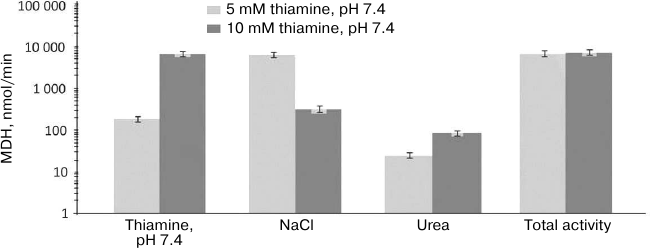
Analogous comparison of the MDH activity and unique peptides of the MDH isoenzymes eluted from the thiamine-Sepharose is shown in Table 2. It is worth noting in this regard that the MDH reaction catalyzed by the cytoplasmic (MDH1) and mitochondrial (MDH2) isoenzymes of MDH are regulated by thiamine compounds in opposite ways [6]. The MDH elution by acidic (pH 5.6) solution of thiamine significantly decreases the MDH reaction rate. Under these conditions, less than 10% of the total MDH activity applied to the column is eluted, whereas a slightly alkaline solution of thiamine significantly (1.5-2.5-fold) increases both the specific and total MDH activity compared to the values of the applied sample. Nevertheless, the MS data indicate that the MDH2 isoenzyme prevails in the eluate with the acidic thiamine solution, whereas elution of the MDH1 isoenzyme requires the slightly alkaline solution of thiamine (Table 2). Accordingly, the MDH1 isoenzyme is eluted with NaCl or urea only when its affinity elution by thiamine was inefficient, i.e., after the thiamine elution at pH 5.6. In contrast, the MDH2 isoenzyme is eluted by NaCl and urea after the thiamine elution at both pH values. Thus, at pH 7.4 the thiamine solution elutes from the thiamine-Sepharose all bound MDH1 isoenzyme, while at pH 5.6 only the MDH2 isoenzyme is eluted by thiamine (Table 2). Dependence of the elution of the MDH isoenzymes on the pH of the thiamine solution is in good accord with the different reactivity of the structures and functions of the isoenzymes to changes in pH [20, 21]. Increasing the thiamine concentration during the affinity elution increases the MDH activity eluted at this step, concomitantly decreasing the MDH activity eluted on the next stage employing NaCl (Fig. 1). This finding shows the specificity of the eluting action of thiamine, not only pH. However, the total MDH activity in the urea eluate does not depend on the thiamine concentration at the first step of elution (Fig. 1). One may therefore suggest that the urea-eluted MDH (mostly this is MDH2; Table 2) has a different binding mode to the thiamine-Sepharose compared to that of the MDH isoenzymes eluted by thiamine and NaCl.
Table 2. Malate dehydrogenase (MDH) activity
and number of unique peptides of the cytoplasmic (MDH1) and
mitochondrial (MDH2) isoenzymes in eluates from thiamine-Sepharose
obtained upon affinity chromatography of the acetone extract of
synaptosomal proteins of bovine brain
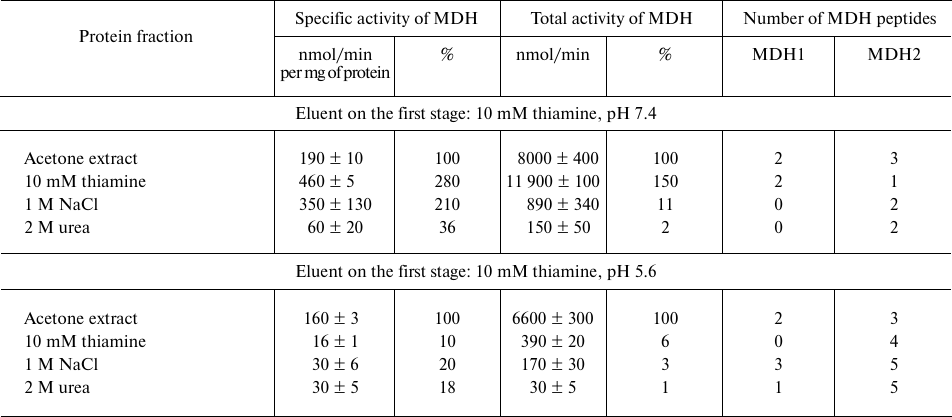
Note: The activity of eluted proteins was determined after replacing the
eluents with 10 mM Tris-HCl (pH 7.4) in assay medium comprising 20 mM
potassium phosphate buffer (pH 7.2) and saturating substrates: 0.3 mM
oxaloacetate, 0.14 mM NADH. Relative activities are indicated as a
percentage of the levels in the initial preparations.
Fig. 1. Dependence of total malate dehydrogenase (MDH) activity in fractions obtained during affinity chromatography of synaptosomal proteins of bovine brain on thiamine-Sepharose on thiamine concentration in the eluent (pH 7.4) at the first elution step. Results of at least two independent chromatographies with elution by the 5- and 10-mM thiamine solutions are averaged.
GDH and MDH2 are known to interact, with their complex having functional significance, particularly for regulation of these enzymes by low molecular mass compounds [22-24]. Our results suggest that formation of the complex with MDH2 may stabilize the activity of GDH during elution from the thiamine-Sepharose by NaCl and urea. In fact, the total and specific GDH activities in these fractions are higher in the presence of MDH2, which is observed after the first elution step is done at pH 5.6 (Tables 1 and 2). The GDH-mediated binding of MDH2 to the thiamine-Sepharose may result in the coelution of GDH and MDH2 by urea and in the different type of the binding to the thiamine-Sepharose of the urea-eluted MDH2 (Fig. 1).
Affinity chromatography on thiamine-Sepharose of homogenate of rat brain cortex. Dependence of the GDH and MDH activities eluted from the thiamine-Sepharose on the protein composition of the sample is supported by the difference in the elution profiles of the activities observed in the analyses of the different preparations of the brain proteins. Compared to the GDH and MDH activities in the synaptosomal fraction of the bovine brain proteins applied to the thiamine-Sepharose, the specific GDH and MDH activities in the thiamine (pH 7.4) eluate exhibit a decrease (Table 1) and more than a 2-fold increase, correspondingly (Table 2). In contrast, compared to the GDH and MDH activities in the brain homogenate applied to the thiamine-Sepharose, in the thiamine (pH 7.4) eluate the specific GDH activity is significantly (3-fold) increased, while the specific MDH activity does not largely change (from 8 to 11 nmol/min per mg of protein) (Table 3). Besides, the elution of GDH and MDH2 in the brain homogenates is maximal under the action of NaCl (nine GDH peptides and three MDH2 peptides), with this fraction possessing the highest specific activities of these interacting enzymes (Table 3). Such relationship between the activities and protein composition of the eluate depends on the chromatographed sample type. When the synaptosomal fraction of the bovine brain proteins is subjected to the affinity chromatography with the affinity elution at pH 7.4, then the major part of the GDH protein is eluted by urea (Table 1), with MDH2 well resolved from MDH1 (Table 2). Thus, both the interaction of GDH with the isoenzymes of MDH and coelution of GDH and MDH2 significantly depend on the composition of the sample subjected to the affinity chromatography, probably due to the sample differences in their sets of proteins and low molecular mass regulators.
Table 3. Activities and unique peptides of
glutamate dehydrogenase (GDH) and the isoenzymes of malate
dehydrogenases (MDH) – cytoplasmic (MDH1) and mitochondrial
(MDH2) – in the eluates from thiamine-Sepharose obtained upon
affinity chromatography of homogenates of rat brain cortex
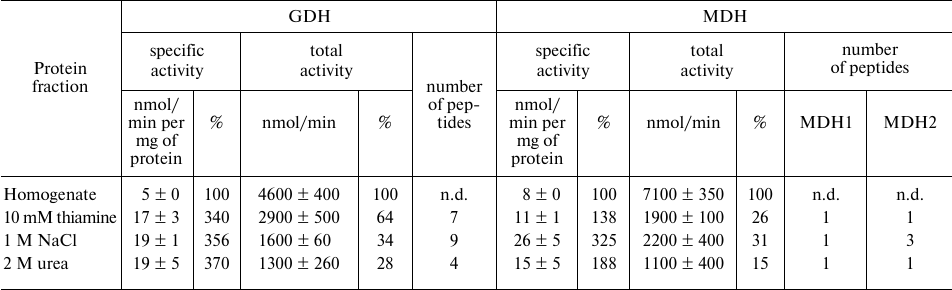
Note: Activities of eluted proteins were determined after replacing the
eluents with 10 mM Tris-HCl (pH 7.4). The GDH assay medium comprised
100 mM Tris-HCl (pH 7.5), 2.5 mM 2-oxoglutarate, 0.2 mM NADH, 50 mM
NH4Cl; the MDH assay medium comprised 20 mM potassium
phosphate buffer (pH 7.2), 0.3 mM oxaloacetate, 0.14 mM NADH. Relative
activities are indicated as percentage of the levels in the initial
preparations; n.d., not determined.
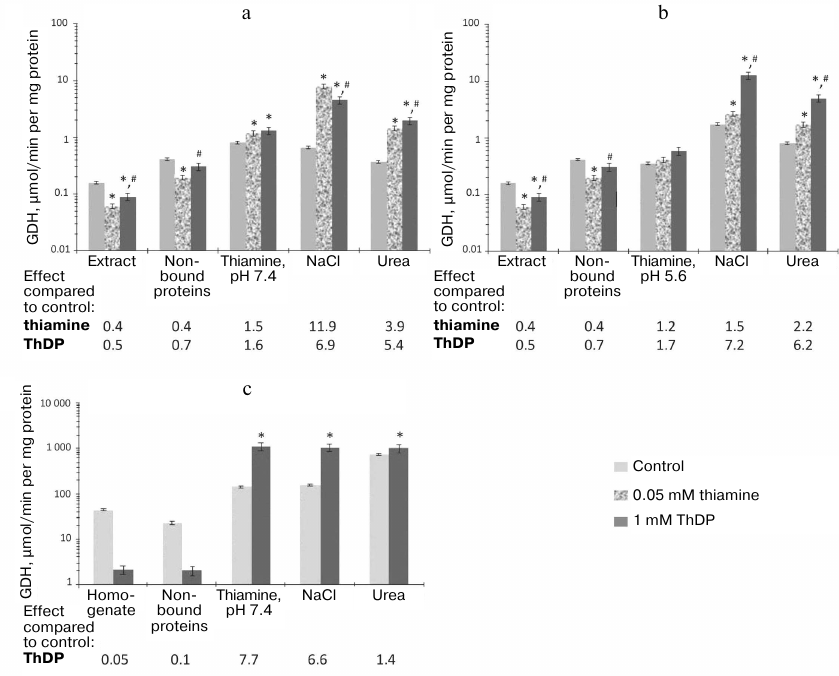
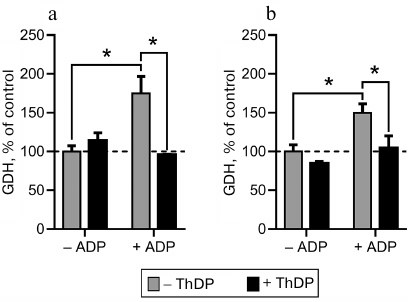
Regulation of GDH and MDH activities by thiamine compounds in vitro. The influence of thiamine compounds on GDH and MDH has been investigated using earlier data on the acting concentrations and conditions maximizing the effects on the studied enzymes [6]. For comparison, fixed concentrations of thiamine (0.05 mM) and ThDP (1 mM) were used in the ratio mimicking that in vivo. Figure 2 demonstrates the influence of thiamine and/or ThDP on the brain GDH activity in the samples applied to and eluted from thiamine-Sepharose. It is seen that in the original preparations, thiamine and ThDP inhibit the GDH activity, whereas after the chromatography the enzyme is activated by these compounds. Analogous difference in the effects of ThDP on GDH had been observed earlier, although the effects were much smaller: ThDP activated the purified GDH 1.2-fold but inhibited GDH in mitochondrial extracts 1.3-fold [6]. A much more significant influence of ThDP on GDH activity, observed after the affinity chromatography in this work (Fig. 2), suggests a role of protein dilution or chromatography-removed endogenous factors in the enzyme regulation by ThDP. For instance, one may expect that the endogenous content of the GDH activator ADP may be much higher in the brain homogenate than in the acetone extract of synaptosomal fraction of brain proteins. As seen from Fig. 3, ThDP interferes with the activation of GDH by ADP. Thus, the ThDP inhibition in the samples applied to the affinity chromatography may depend on the activating effect of endogenous ADP on GDH. Decreased ThDP inhibition of the enzyme within the brain synaptosomal fraction (Fig. 2a), compared to the brain homogenate (Fig. 2c), agrees with the expectation of a much lower level of endogenous ADP in the partially purified fraction compared to that in the brain homogenate, where the inhibition by ThDP of GDH in much more pronounced (Fig. 2c). On the other hand, the high level of the ThDP activation after the affinity chromatography could be expected in view of a stimulated dissociation of GTP, which is a highly efficient inhibitor of GDH. However, model studies with purified GDH did not reveal a decrease in the inhibitory action of GTP by thiamine or ThDP in the used interval of conditions (up to 1 mM ThDP or thiamine at 1 μM GTP and 2-oxoglutarate varied from 0.1 to 10 mM). Apart from the influence of endogenous regulators, different regulation of the synaptosomal and total brain GDH by ThDP (Fig. 2) cannot be excluded. For instance, this functional difference may be supported by specific posttranslational modifications or alternative splicing of synaptosomal and total GDH.
Fig. 2. Influence of thiamine and/or thiamine diphosphate (ThDP) on the activity of glutamate dehydrogenase (GDH) from bovine or rat brain in eluates from thiamine-Sepharose. a, b) Affinity chromatography of acetone extract of bovine brain synaptosomes, using 10 mM thiamine solution, pH 7.4 (a) or 5.6 (b) at the first step; c) affinity chromatography of the rat brain homogenate using 10 mM thiamine solution, pH 7.4, at the first step. All eluted proteins were transferred to 10 mM Tris-HCl buffer (pH 7.4) using ultrafiltration. The effects of thiamine and ThDP were detected using non-saturated NADH in the assay medium comprising 100 mM Tris-HCl (pH 7.5), 2.5 mM 2-oxoglutarate, 0.02 mM NADH, 50 mM NH4Cl. The results are averaged from 2-4 independent experiments; *, # significant (p < 0.05) differences from the control activity and activity in the presence of 0.05 mM thiamine, respectively, according to Student’s t-test.
Fig. 3. Interdependent influence of thiamine diphosphate (ThDP) and ADP on glutamate dehydrogenase (GDH) activity. The activity was measured in Krebs–Ringer buffer (pH 7.4) under substrate saturating conditions (2.5 mM 2-oxoglutarate, 0.2 mM NADH, 50 mM NH4Cl). ADP and ThDP were added to 1.7 and 1 mM, respectively. Commercial GDH from bovine liver (a) and GDH partially purified from rat brain (b) were used; * significant (p < 0.05) differences according to two-way ANOVA with Sidak’s post hoc test.
Worth noting, a lower inhibition by ThDP of GDH in original samples is accompanied by a lower activation by ThDP of GDH eluted by thiamine solution of pH 7.4 (Fig. 2). In fact, a 2-fold inhibition of GDH by ThDP in the synaptosomal fraction of the bovine brain proteins is followed by a 1.5-fold activation of the GDH eluted by thiamine at pH 7.4 (Fig. 2a). At the same time, in the rat brain homogenates ThDP causes a 20-fold inhibition of GDH, whose affinity elution by thiamine (pH 7.4) results in an 8-fold activation by ThDP (Fig. 2c). The results thus indicate that the allosteric effects of ThDP, both the inhibitory and activatory ones, are expressed more strongly with GDH within the rat brain homogenate than with GDH within the acetone extract of the synaptosomal fraction of bovine brain (Fig. 2).
Figure 2 also shows that after the chromatography of the acetone extract the activation of GDH by ThDP is significantly higher after elution by NaCl and urea (a 5-7-fold activation) than at the affinity elution step by thiamine (a 1.5-1.7-fold activation). At the same time, the GDH activity level in the thiamine eluate is lower than that of the ThDP-activated GDH (Fig. 2). Thus, the low ThDP activation of GDH in the thiamine eluate cannot be due to the already activated state of the thiamine-eluted GDH, which could comprise a tightly bound thiamine. A similar (1.2-fold) level of the enzyme activation by ThDP was also observed in previous work with purified GDH or GDH in mitochondrial extracts [6]. In contrast to the affinity chromatography of the synaptosomal fraction of bovine brain proteins, the chromatography of the brain homogenate reveals a maximal (8-fold) activation of GDH by ThDP in the fraction eluted by thiamine (Fig. 2c). Because in this case thiamine elutes a major part of GDH (64% of the total GDH activity in the applied sample; Table 1), the activation of GDH by ThDP seems to depend on the concentration of GDH and/or its heterological complexes. Investigation of the action of thiamine on GDH of synaptosomal fraction eluted by NaCl and urea (Fig. 2, a and b) confirms this supposition. In contrast to the activation of GDH by ThDP, which does not depend on pH of the thiamine elution, the activation of GDH by thiamine in the eluates by NaCl and urea is significant (up to 12-fold) only after the thiamine elution at pH 7.4 (Fig. 2a). Under these conditions, GDH eluted by NaCl and urea comprises significantly less of the total GDH than the same fractions after the affinity elution by thiamine at pH 5.6. As shown above, the latter eluates also contain more MDH2 (Table 2). The simultaneous presence in the eluates of GDH and MDH2 stimulates formation of their complex. However, the specific and total MDH activities, as well as the activation of MDH by thiamine, are significantly lower in this case compared to the eluates by NaCl and urea after the affinity elution by thiamine at pH 7.4 (Table 2). These results suggest that after the chromatography on the thiamine-Sepharose the activation of GDH by thiamine is not pronounced under the conditions that promote formation of the complex between GDH and MDH2, i.e., after the affinity elution at pH 5.6. In contrast, the activation of GDH by thiamine is maximal in the fractions with the low total activity of GDH and low content of MDH2, both occurring in the eluates by NaCl and urea after the affinity elution at pH 7.4 (Tables 1 and 2). It may thus be suggested that NaCl and urea may destabilize and/or partially denature GDH in these fractions, with both thiamine and MDH2 counteracting such action.
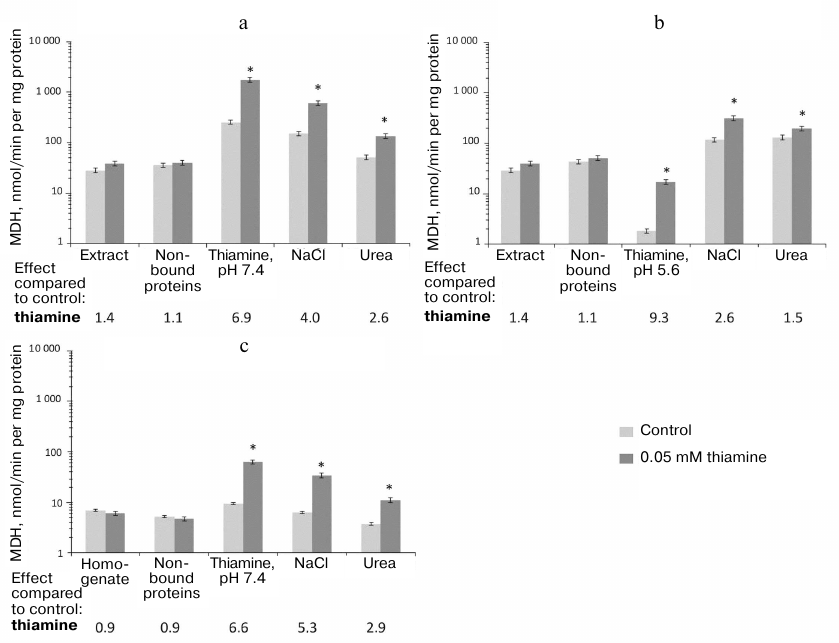
Stimulation by thiamine of MDH activity in the studied samples of brain proteins is not as complicated as the action of thiamine on GDH. Thiamine does not affect the MDH activity of the original preparations subjected to the affinity chromatography (Fig. 4). After the chromatography of either the synaptosomal proteins of bovine brain or rat brain homogenate, the maximal effect of thiamine on the MDH activity is observed in the fraction after affinity elution by thiamine. The thiamine effect is significantly reduced in the following fractions eluted by NaCl and especially in the urea eluate (Fig. 4). Obviously, the non-specific elution by NaCl or urea partially desensitizes MDH to the allosteric action of thiamine.
Fig. 4. Influence of thiamine (0.05 mM) on the malate dehydrogenase (MDH) activity of bovine and rat brains in eluates from thiamine-Sepharose. a, b) Affinity chromatography of the acetone extract of the bovine brain synaptosomes using 10 mM thiamine solution, pH 7.4 (a) or 5.6 (b), as the first eluent; c) affinity chromatography of the rat brain homogenate using 10 mM thiamine solution, pH 7.4, as the first eluent. All eluted proteins were transferred to 10 mM Tris-HCl buffer (pH 7.4) using ultrafiltration. The effects of thiamine and ThDP were detected in Krebs–Ringer buffer (pH 7.4) comprising 0.01 mM oxaloacetate and 0.14 mM NADH. The results are averaged from 2-5 independent experiments; * significant (p < 0.05) differences of the activity in the presence of 0.05 mM thiamine from the control activity according to Student’s t-test.
The lack of a detectable action of thiamine on MDH in the samples before the affinity chromatography may be due to the opposite effects of thiamine on the MDH isoenzymes (inhibition of MDH1 and activation of MDH2) shown previously [6]. Indeed, Fig. 4a and Table 2 show that maximal (9-fold) activation of MDH reaction by thiamine is observed in the fraction where MDH2 prevails over MDH1, i.e., after the affinity elution by the acidic thiamine solution (Table 2), whereas the co-elution of MDH1 and MDH2 (after the affinity elution by the slightly alkaline thiamine solution; Table 2) decreases the activation of MDH by thiamine to 7-fold. Thus, in the fraction comprising both isoenzymes of MDH, the apparent activation by thiamine of MDH2 may be reduced due to the inhibition by thiamine of MDH1 [6]. However, one should also take into account that a significant part of MDH does not bind to the thiamine-Sepharose even when the acetone extract of synaptosomal fraction, which contains much less protein than the rat brain homogenate, is applied to the sorbent (Fig. 4). It may therefore be suggested that only some isoforms of the MDH1 and MDH2 isoenzymes, e.g., those which differ in specific posttranslational modifications, interact with the thiamine sorbent.
Investigation of the influence of a high thiamine dose administered to rats on regulation of GDH and MDH in brain homogenates. As shown above, the thiamine-dependent regulation of the enzymatic activities may differ dependent on the enzyme preparation (Figs. 2 and 4). For instance, in the brain homogenates and fraction of synaptosomal proteins GDH is inhibited by thiamine, whereas MDH is not affected. However, after the affinity chromatography of these preparations, both GDH and MDH are activated by thiamine (Figs. 2 and 4), with the MDH reaction rate increasing even when the two isoenzymes are present (Tables 1 and 3), one of them (MDH1) inhibited by thiamine [6]. In view of this complexity of the thiamine-dependent regulation in vitro, we assessed its biological significance by investigating regulatory properties of the enzymes after the rats obtained a high dose of thiamine. For this study, GDH and MDH of the brain homogenates were released from the endogenous low molecular mass regulators by rapid gel-filtration without significant dilution of the homogenates.
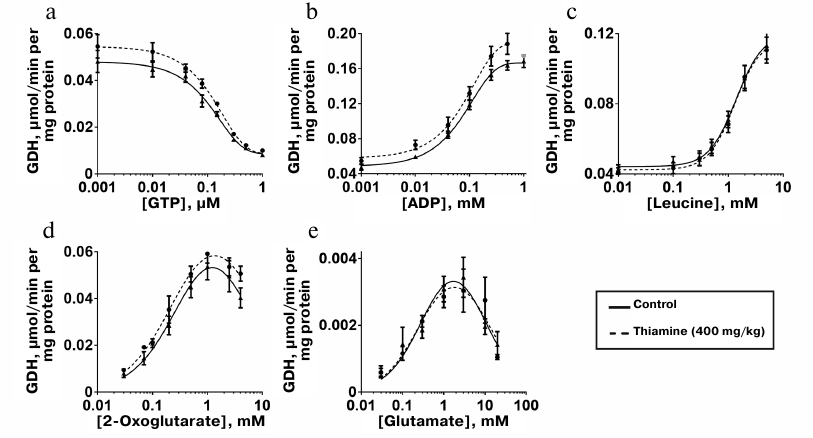
Kinetic dependencies of the GDH activity on concentrations of the low molecular mass regulators of the enzyme, such as allosteric effectors GTP, ADP, and leucine, and the substrates of the opposite directions of the catalyzed reactions, 2-oxoglutarate and glutamate, are shown in Fig. 5. Comparison of the kinetic curves indicates that the administration of thiamine increases the amplitude of the inhibition of GDH by GTP (Fig. 5a) and maximal reaction rate upon the activation of GDH by ADP (Fig. 5b) and 2-оxoglutarate (Fig. 5d). At the same time, the thiamine administration in vivo does not change the regulation of the brain GDH by leucine (Fig. 5c) or glutamate (Fig. 5e). Hence, the increased amplitudes of the response of GDH to GTP, ADP, and 2-oxoglutarate are not due to increased GDH expression. This notion is supported by the MS quantification of the GDH peptides in the investigated homogenates. It should be noted that only the change in the GTP inhibition amplitude is statistically significant (Table 4). This may be due to a higher complexity of the dependencies of GDH activity on ADP and 2-oxoglutarate, where the activation is followed by inhibition at high concentrations of ADP (after the administration of thiamine) and 2-oxoglutarate. However, the kinetic parameters of the 2-oxoglutarate saturation, i.e., KmOG and KiOG (Table 4), are in accordance with a decreased substrate inhibition seen in Fig. 5d. The observed coupling in the regulatory changes corresponds to the known interrelationships between the regulation of GDH by GTP or ADP and the substrate inhibition [25]. Because a number of GDH residues in the regulatory sites, including a lysine residue participating in the GTP binding, are subjected to posttranslational acetylation [25], one may suppose that the administration of thiamine changes the regulatory properties of GDH (Fig. 5 and Table 4) through such posttranslational modifications. For instance, conformational changes after the binding of thiamine and/or its regulatory derivatives to GDH in vivo may change the availability of certain lysine residues to acylation or deacylation.
Fig. 5. Influence of thiamine administration (400 mg/kg) on regulatory properties of GDH from rat brain. Dependencies of the enzyme activity on the allosteric regulators GTP (a), ADP (b), leucine (c), and the substrates 2-oxoglutarate (d) and glutamate (e) were investigated. The data for dependences obtained in three independent animal experiments were averaged and approximated using the built-in models of GraphPad Prism v. 8.0 as described in “Materials and Methods”. The approximation parameters are given in Table 4.
Table 4. Kinetic parameters of regulation of
GDH from rat brain cortex of control rats and rats after administration
of a high (400 mg/kg) dose of thiamine
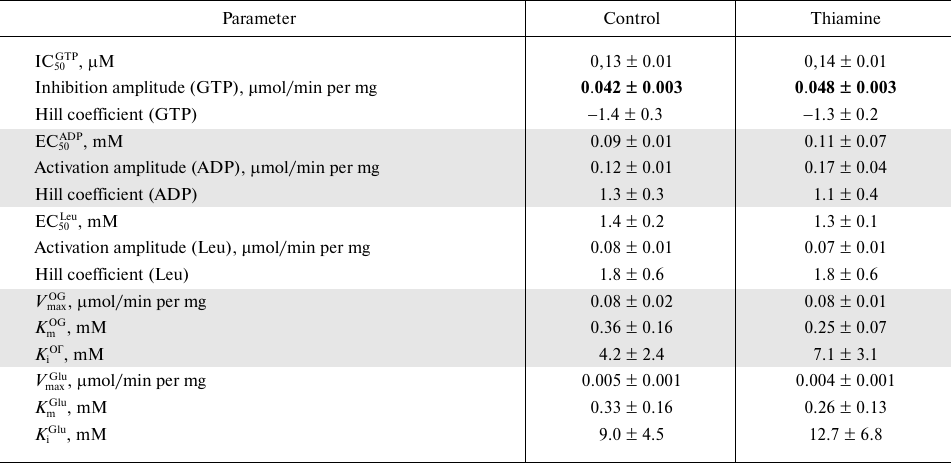
Note: Parameters of non-linear regression of the kinetic dependencies
presented in Fig. 5 were obtained using the
built-in models of GraphPad Prism 8.0 (see “Materials and
Methods”). The upper-case indices of the shown parameters refer
to the regulator. OG, 2-oxoglutarate. Significant differences between
the kinetic parameters of the approximated dependences, determined by F-criterion,
are shown in bold.
Analogous analysis of the influence of the administration of a high dose of thiamine on MDH in rat brain homogenates after rapid gel-filtration does not reveal significant changes in the dependencies of the MDH reaction rate on the saturations with oxaloacetate (0.01-1.00 mM) or malate (0.1-10.0 mM). As discussed above, only a minor part of the brain MDH interacts with the thiamine-Sepharose (Table 2), and the MDH isoenzymes are regulated by thiamine compounds in opposite ways [6]. Therefore, our kinetic analysis of total MDH within the rat brain homogenate only shows that expression of the isoforms and balance of the thiamine-dependent regulation of MDH1 and MDH2 isoenzymes are not significantly changed by the thiamine administration in vivo.
Comparative analysis of the proteomes eluted from thiamine-Sepharose. Compared to the previous study of the affinity chromatography of the thiamine-dependent proteins, which employed Sepharose modified by thiamine or its functional (thiazolium) part, this work used a different modification procedure that excluded potential variability in the conjugation of thiamine to the linker. To increase the elution specificity, we also used thiamine solution as the affinity eluent. Finally, to extend our knowledge on the pathways employing the non-coenzyme action of thiamine, whose proteins could have been lost upon the purification of the earlier analyzed synaptosomal proteins of the rat brain [6], we have subjected to the affinity chromatography complete homogenates of the rat brain. Results of the bioinformatic analysis of the sets of proteins that are eluted upon the affinity chromatography of the synaptosomal proteins of bovine brain or of the rat brain homogenates are shown in Table 5. Comparison of these results and those obtained in the earlier analysis of the thiamine-dependent proteomes of the synaptosomal proteins of the rat brain [6] indicates that introduction of the affinity elution by thiamine changes relative enrichment of the annotation terms “phosphoprotein” and “acetylation” in the characteristics of the proteins eluted from the thiamine-Sepharose. In our previous investigation [6], where elution by thiamine of the rat brain synaptosomal proteins was not used, the term “acetylation” was characterized by a significantly higher enrichment (P = 10–17, according to DAVID analysis) than the term “phosphoprotein” (P = 10–6). In the current study, where the synaptosomal proteins of the bovine brain are subjected to the affinity elution by thiamine, both terms show a similar degree of enrichment (P = 10–35-10–36), whereas in the affinity chromatography of the rat brain homogenates the term “phosphoprotein” is even enriched with a higher significance (P = 10–76) than the term “acetylation” (P = 10–61) (Table 5). Nevertheless, the specificity of the term “acetylation” as a characteristic of the thiamine-dependent proteome is confirmed by the comparison of the major annotation terms in the sets of proteins in the affinity and non-specific eluates. In the thiamine eluates of the synaptosomal proteins of the bovine brain the terms “phosphoprotein” and “acetylation” show a similar enrichment significance (P = 10–35-10–36), whereas in the eluates by non-specific agents (NaCl and urea) the relative enrichment of the term “acetylation” decreases from the second to the fourth position with the simultaneous increase in the enrichment of the term “phosphoprotein” (P = 10–46). Thus, compared to the previous usage of the non-specific elution [6], the affinity elution by thiamine of the synaptosomal brain proteins bound to the thiamine-Sepharose increased the relative abundance of proteins annotated by the term “phosphoprotein”, confirming specific association of the thiamine-dependent pathways with the term “acetylation”. Both these terms are associated with the thiamine-dependent proteomes independent of the animal species (ox or rat) and the preparation subjected to the affinity chromatography (synaptosomal fraction or brain homogenate) (Table 5) [6]. The bioinformatics analysis using DAVID v. 6.8 (Table 5) also shows that the synaptosomal proteins of bovine brain that interact with the thiamine-Sepharose are enriched with the term “methylation” (positions 5 and 6) much less than the proteins of the rat brain homogenate (position 3). Because the term “methylation” was not significant in the previous analysis of the thiamine-dependent proteomes of the synaptosomal proteins of the rat brain [6], one may conclude that enrichment of this term in the rat brain homogenates is not due to the species-specific effects, but points to the loss of the corresponding proteins when the synaptosomal fraction is purified.
Table 5. Major annotation terms that are
significantly enriched in the proteomes eluted from thiamine-Sepharose
with thiamine or non-specific agents (NaCl and urea) during affinity
chromatography of acetone extract of synaptosomal proteins of bovine
brain (“Ox”) or homogenates of rat brain cortex
(“Rat”)
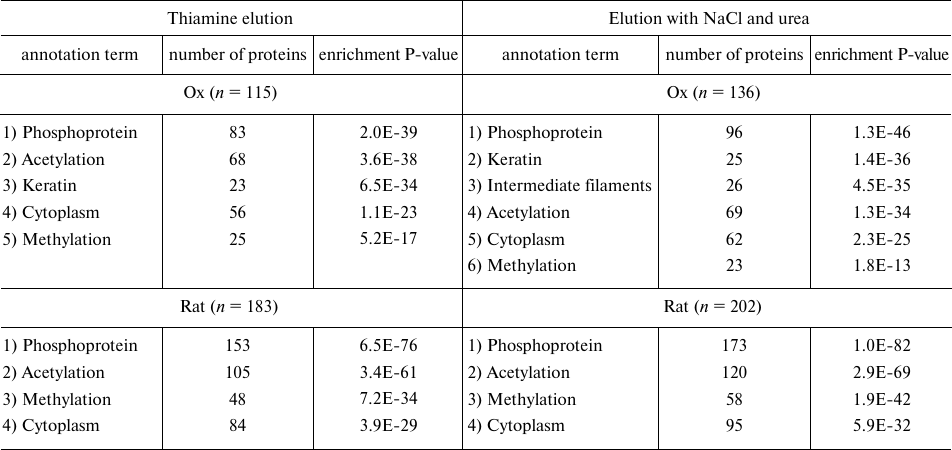
Note: Representative results of the bioinformatics analysis by DAVID (v.
6.8) of the sets of proteins eluted during affinity chromatography of
acetone extract of synaptosomal proteins of bovine brain (two
independent identifications) and of rat cortex homogenates (three
independent identifications) are shown; n, total number of
proteins in the analyzed proteomes; enrichment P-value defines the
probability of occurrence of an annotation term in the analyzed sample
of n proteins compared to a random occurrence of this term in
the corresponding genome.
Our comparison of the interaction with thiamine-Sepharose of either the different fractions of the brain proteins (complete homogenate or acetone extract of synaptosomes) or the different organisms (ox or rat) points to a universal regulation by thiamine compounds of such enzymes of central brain metabolism as the dehydrogenases of glutamate and malate. The biological significance of the regulatory action of thiamine and/or ThDP is confirmed by changes in the regulatory properties of GDH in the rat brain after administration of a high dose of thiamine. Bioinformatics analysis of the sets of proteins eluted from the thiamine-Sepharose under different conditions points to their specific enrichment by the terms “acetylation” and “methylation”.
Funding. The work of a PhD student of the Palladin Institute of Biochemistry (Kyiv, Ukraine), O. A. Mezhenska (experimental results in Figs. 1, 2, 4 and Tables 1-3 excluding MS data) within this project performed at Lomonosov Moscow State University was supported by the Russian Foundation for Basic Research in 2015 (grant no. 15-34-50124) among projects done by young investigators under supervision of candidates and doctors of sciences in scientific institutes of the Russian Federation. Experiments shown in Figs. 3 and 5, the analysis (including the bioinformatics analysis) of the affinity chromatography and mass spectrometry data, and preparation of the data for publication were supported by the Russian Science Foundation (grant no. 18-14-00116).
Acknowledgements. The authors acknowledge the work of Dr. A. V. Graf and Dr. M. V. Maslova (Moscow State University) with animals used in this study.
Conflict of interest. The authors declare no conflict of interest.
Ethical statements. All the international, national, and institutional principles of the animal care and usage were satisfied. In particular, all the animal experiments were done according to the EU directive 2010/63/EU. The experiments were approved by the Bioethics Committee of Lomonosov Moscow State University.
REFERENCES
1.Frank, L. L. (2015) Thiamin in clinical practice,
JPEN J. Parenter. Enteral. Nutr., 39, 503-520,
doi: 10.1177/0148607114565245.
2.Tylicki, A., Lotowski, Z., Siemieniuk, M., and
Ratkiewicz, A. (2018) Thiamine and selected thiamine antivitamins
– biological activity and methods of synthesis, Biosci.
Rep., 38, BSR20171148, doi: 10.1042/BSR20171148.
3.Pavlovic, D. M. (2019) Thiamine deficiency and
benfotiamine therapy in brain diseases, Am. J. Biomed. Sci.
Res., 3, 1-5, doi: 10.34297/AJBSR.2019.03.000621.
4.Bunik, V. I., and Aleshin, V. A. (2017) Analysis of
the protein binding sites for thiamin and its derivatives to elucidate
molecular mechanisms of the non-coenzyme action of thiamin (vitamin
B1), Studies Nat. Prod. Chem., 53, 375-429,
doi: 10.1016/B978-0-444-63930-1.00011-9.
5.Aleshin, V. A., Mkrtchyan, G. V., and Bunik, V. I.
(2019) Mechanisms of the non-coenzyme action of thiamine: protein
targets and medical implications, Biochemistry (Moscow),
84, 1051-1075, doi: 10.1134/S0006297919080017.
6.Mkrtchyan, G., Aleshin, V., Parkhomenko, Y.,
Kaehne, T., Luigi Di Salvo, M., Parroni, A., Contestabile, R., Vovk,
A., Bettendorff, L., and Bunik, V. (2015) Molecular mechanisms of the
non-coenzyme action of thiamin in brain: biochemical, structural and
pathway analysis, Sci. Rep., 5, 12583,
doi: 10.1038/srep12583.
7.Tsepkova, P. M., Artiukhov, A. V., Boyko, A. I.,
Aleshin, V. A., Mkrtchyan, G. V., Zvyagintseva, M. A., Ryabov, S. I.,
Ksenofontov, A. L., Baratova, L. A., Graf, A. V., and Bunik, V. I.
(2017) Thiamine induces long-term changes in amino acid profiles and
activities of 2-oxoglutarate and 2-oxoadipate dehydrogenases in rat
brain, Biochemistry (Moscow), 82, 723-736,
doi: 10.1134/S0006297917060098.
8.He, X., and Zhang, J. (2006) Toward a molecular
understanding of pleiotropy, Genetics, 173, 1885-1891,
doi: 10.1534/genetics.106.060269.
9.Morales-Gonzalez, J. A., Madrigal-Santillan, E.,
Morales-Gonzalez, A., Bautista, M., Gayosso-Islas, E., and
Sanchez-Moreno, C. (2015) What is known regarding the participation of
factor Nrf-2 in liver regeneration? Cells, 4, 169-177,
doi: 10.3390/cells4020169.
10.Muller, S. (2017) DNA damage-inducing compounds:
unraveling their pleiotropic effects using high throughput sequencing,
Curr. Med. Chem., 24, 1558-1585,
doi: 10.2174/0929867324666170124143710.
11.Leeuw, E. P. H., Lee, S. H., Kim, W. H., Kwasny,
S. M., Opperman, T. J., and Lillehoj, H. S. (2018) Pleiotropic
anti-infective effects of defensin-derived antimicrobial compounds,
Avian Dis., 62, 381-387,
doi: 10.1637/11912-061118-Reg.1.
12.Gomes, M. B., and Negrato, C. A. (2014)
Alpha-lipoic acid as a pleiotropic compound with potential therapeutic
use in diabetes and other chronic diseases, Diabetol. Metab.
Syndr., 6, 80, doi: 10.1186/1758-5996-6-80.
13.Turner, P. V., Pekow, C., Vasbinder, M. A., and
Brabb, T. (2011) Administration of substances to laboratory animals:
equipment considerations, vehicle selection, and solute preparation,
J. Am. Assoc. Lab. Anim. Sci., 50, 614-627.
14.Postoyenko, V. A., Parkhomenko, Y. M., Vovk, A.
I., Khalmuradov, A. G., and Donchenko, G. V. (1987) Isolation and some
properties of thiamine-binding protein from the rat brain synaptosomes,
Biokhimiya, 52, 1792-1797.
15.Graf, A., Kabysheva, M., Klimuk, E., Trofimova,
L., Dunaeva, T., Zundorf, G., Kahlert, S., Reiser, G., Storozhevykh,
T., Pinelis, V., Sokolova, N., and Bunik, V. (2009) Role of
2-oxoglutarate dehydrogenase in brain pathologies involving glutamate
neurotoxicity, J. Mol. Catal. B Enzym., 61, 80-87,
doi: 10.1016/j.molcatb.2009.02.016.
16.Klyashchitsky, B. A., Pozdnev, V. F., Mitina, V.
K., Voskoboev, A. I., and Chernikevich, I. P. (1980) Isolation and
purification of biopolymers by biospecific affinity chromatography. V.
Affinity chromatography of pyruvate decarboxylase from brewer’s
yeast, Russ. J. Bioorg. Chem., 6, 1572-1579.
17.Bunik, V., Kaehne, T., Degtyarev, D.,
Shcherbakova, T., and Reiser, G. (2008) Novel isoenzyme of
2-oxoglutarate dehydrogenase is identified in brain, but not in heart,
FEBS J., 275, 4990-5006,
doi: 10.1111/j.1742-4658.2008.06632.x.
18.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and
Randall, R. J. (1951) Protein measurement with the Folin phenol
reagent, J. Biol. Chem., 193, 265-275.
19.MacLean, B., Tomazela, D. M., Shulman, N.,
Chambers, M., Finney, G. L., Frewen, B., Kern, R., Tabb, D. L.,
Liebler, D. C., and MacCoss, M. J. (2010) Skyline: an open source
document editor for creating and analyzing targeted proteomics
experiments, Bioinformatics, 26, 966-968,
doi: 10.1093/bioinformatics/btq054.
20.Wood, D. C., Jurgensen, S. R., Geesin, J. C., and
Harrison, J. H. (1981) Subunit interactions in mitochondrial malate
dehydrogenase. Kinetics and mechanism of reassociation, J. Biol.
Chem., 256, 2377-2382.
21.Bleile, D. M., Schulz, R. A., Harrison, J. H.,
and Gregory, E. M. (1977) Investigation of the subunit interactions in
malate dehydrogenase, J. Biol. Chem., 252, 755-758.
22.Fahien, L. A., Kmiotek, E., and Smith, L. (1979)
Glutamate dehydrogenase–malate dehydrogenase complex, Arch.
Biochem. Biophys., 192, 33-46,
doi: 10.1016/0003-9861(79)90069-9.
23.Fahien, L. A., Kmiotek, E. H., MacDonald, M. J.,
Fibich, B., and Mandic, M. (1988) Regulation of malate dehydrogenase
activity by glutamate, citrate, alpha-ketoglutarate, and multienzyme
interaction, J. Biol. Chem., 263, 10687-10697.
24.Fahien, L. A., MacDonald, M. J., Teller, J. K.,
Fibich, B., and Fahien, C. M. (1989) Kinetic advantages of
hetero-enzyme complexes with glutamate dehydrogenase and the
alpha-ketoglutarate dehydrogenase complex, J. Biol. Chem.,
264, 12303-12312.
25.Bunik, V., Artiukhov, A., Aleshin, V., and
Mkrtchyan, G. (2016) Multiple forms of glutamate dehydrogenase in
animals: structural determinants and physiological implications,
Biology (Basel), 5, 53,
doi: 10.3390/biology5040053.