REVIEW: Inhibitors of Glyceraldehyde 3-Phosphate Dehydrogenase and Unexpected Effects of Its Reduced Activity
V. I. Muronetz1,2,a*, A. K. Melnikova2, K. V. Barinova1, and E. V. Schmalhausen1
1Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119234 Moscow, Russia2Lomonosov Moscow State University, Faculty of Bioengineering and Bioinformatics, 119234 Moscow, Russia
* To whom correspondence should be addressed.
Received June 11, 2019; Revised August 11, 2019; Accepted August 14, 2019
The review describes the use of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) inhibitors to study the enzyme and to suppress its activity in various cell types. The main problem of selective GAPDH inhibition is a highly conserved nature of the enzyme active site and, especially, Cys150 environment important for the catalytic action of cysteine sulfhydryl group. Numerous attempts to find specific inhibitors of sperm GAPDH and enzymes from Trypanosoma sp. and Mycobacterium tuberculosis that would not inhibit GAPDH of somatic mammalian cells have failed, which has pushed researchers to search for new ways to solve this problem. The sections of the review are devoted to the studies of GAPDH inactivation by reactive oxygen species, glutathione, and glycating agents. The final section discusses possible effects of GAPDH inhibition and inactivation on glycolysis and related metabolic pathways (pentose phosphate pathway, uncoupling of the glycolytic oxidation and phosphorylation, etc.).
KEY WORDS: glyceraldehyde 3-phosphate dehydrogenase, inhibitors, oxidation, sulfhydryl group, glycation, glycolysisDOI: 10.1134/S0006297919110051
Abbreviations: DHAP, dihydroxyacetone phosphate; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GAPDS, sperm-specific glyceraldehyde 3-phosphate dehydrogenase; GSH, reduced glutathione.
The study of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) had been
started at the Department of Animal Biochemistry of the Biological
Faculty, Moscow State University, more than 60 years ago by N. K.
Nagradova [1]. Like many other investigation
topics, it was suggested by S. E. Severin in order to identify the
effects of carnosine and anserine dipeptides on various processes, such
as functioning of cells and individual organelles, metabolic pathways,
and enzyme behavior. In the first works of Nagradova, no specific
effect of the dipeptides on GAPDH was found, while their impact on
other enzymes was explained by their buffering or chelating action [2]. However, these works have served as the basis for
a long-term comprehensive study of GAPDH, first, at the Department of
Biochemistry, and then at the Department of Animal Cell Biochemistry
created by S. E. Severin at the Belozersky Institute of
Physico-Chemical Biology of the Moscow State University. More than 150
articles on GAPDH, including reviews and a monograph, have been
published over the years by the scientists of our laboratory. The
prophetic words of S. E. Severin, who paraphrased the famous saying as
“Glyceraldehyde 3-phosphate dehydrogenase is inexhaustible as an
atom”, have been confirmed by the growing interest in this enzyme
that is still studied by the students of Nagradova and other
researchers.
GAPDH catalyzes one of the reactions in glycolysis, the so-called glycolytic oxidoreduction reaction, which is an important step in both anaerobic and aerobic energy pathways. This reaction results in the formation of NADH and the macroergic compound 1,3-diphosphoglycerate, which is necessary for the synthesis of ATP in the subsequent glycolytic reactions. Under anaerobic conditions (in anaerobic microorganisms or in aerobic organisms under hypoxic conditions), glycolysis is the only source of energy, while NADH is used for the reduction of pyruvate to lactate. GAPDH is equally important for the energy supply under aerobic conditions, since in this case, pyruvate and NADH are used for ATP synthesis by the mitochondria. It should also be noted that under aerobic conditions, the uncoupling of oxidation and phosphorylation in glycolysis may have a certain sense for the efficient production of substrates for the oxidative phosphorylation in mitochondria. Such uncoupling in glycolysis can occur in the case of mild oxidation of GAPDH due to the emergence of acyl phosphatase activity of the enzyme, as was shown in our earlier studies [3-5]. Oxidized GAPDH hydrolyzes 1,3-diphosphoglycerate, making it possible to bypass the 3-phosphoglycerate kinase reaction in the presence of low ADP concentrations and to synthesize pyruvate and NADH required for the oxidative phosphorylation in mitochondria.
Despite the recognized importance of GAPDH and comprehensive studies of this enzyme (a significant part of fundamental enzymological studies have been performed on GAPDH), it has been neglected for a long time as a promising target for influencing vital functions of cells due to the following reasons. Firstly, the content of GAPDH in all cells is very high (5-15% of total soluble protein) [6]. Secondly, GAPDH is a housekeeping protein constitutively synthesized in the cells [6]. Thirdly, information on the GAPDH regulation has been virtually absent for a long time. Obviously, it makes no sense to inhibit an enzyme that in no way can be attributed to the key glycolytic enzymes (unlike phosphofructokinase) because of its very high total activity. In addition, an existence of alternative metabolic pathways, e.g., pentose phosphate pathway, limits the use of GAPDH inhibitors for reducing cell viability. Even after inhibition of this glycolytic reaction, the energy supply to the cells could be provided from other sources, primarily, oxidative phosphorylation in the mitochondria.
High GAPDH concentration, its presence in all types of cells, and constitutive synthesis have made this enzyme the main protein used to normalize the concentration of other proteins. Thousands of articles that mention GAPDH (also GAPD or GPDH) do not study this enzyme, but simply use it as a marker protein. However, information has gradually accumulated that GAPDH participates in the regulation of cell functions and changes in the activity of this enzyme can influence not only energy metabolism, but also other processes. It has become clear that the catalytic activity of GAPDH is not high, since the maximal activity of this enzyme is observed in the alkaline region (pH 9-10). At physiological pH values, GAPDH activity is much lower (~30-40% of the maximal value). In addition, the content of GAPDH can significantly change in various pathological processes due to the downregulation of GAPDH synthesis, its denaturation, and accumulation of inactive aggregated forms. All these facts have caused doubts on the validity of using GAPDH as the main marker protein [7, 8]. Of particular importance is the information on the involvement of GAPDH in the development of diseases, for example, amyloid-associated neurodegenerative disorders [9-11]. GAPDH can participate in changes in the energy supply of cells during these pathologies and can be directly involved in the formation of amyloid aggregates. These observations give new importance to the studies of GAPDH inhibitors. In this review, we present the data on the effect of various inhibitors on both GAPDH properties and metabolism as a whole.
GAPDH INHIBITORS IN THE STUDIES OF ENZYME FUNCTIONING
Studying the action of inhibitors on the catalytic properties of GAPDH had been for decades the main method for elucidating the mechanism of enzyme action, until the appearance of information on its spatial structure in the early 1970s. This topic deserves a separate review, but we should at least mention the names of biochemists of involved in these studies. The works of the Nobel Prize laureate Paul Boyer (Cardon and Boyer [12]), Daniel Koshland (Koshland [13]), Sydney Bernhard (Malhotra and Bernhard [14]) and many others have established the mechanism of the GAPDH-catalyzed reaction and formulated the general principles of functioning of this complex oligomeric enzyme. These observations have made it possible to form the concepts on the enzyme interaction with its substrates (induced-fit “hand-in-glove” model proposed by Byers and Koshland [15] and Levitzki and Koshland [16]), the role of cooperative processes in the enzyme regulation, and many other classical concepts of enzymology. Comprehensive studies of GAPDH inhibition and inactivation conducted by Nagradova and co-workers have made a significant contribution to understanding the role of individual amino acid residues in the functioning of GAPDH [17-19]. Thus, the importance of arginine residues for the regulation of GAPDH activity was demonstrated for the first time [20-22], and the role of mild oxidation of the catalytic Cys150 residue in the uncoupling of oxidation and phosphorylation in glycolysis was established [3-5]. The use of coenzyme NAD analogues allowed to obtain new information on the NAD binding to GAPDH [23, 24]. Analysis of the interaction of GAPDH enzymes from different sources with the so-called half-of-the-site reagents has revealed new mechanisms of cooperative action of active sites in the protein [17, 25, 26]. After the publication of the GAPDH spatial structure, the data obtained by inhibitor analysis were confirmed or refined by the Branlant group using site-specific mutagenesis [27, 28]. With completion of fundamental studies of the mechanism of GAPDH activity and emergence of new experimental approaches, an interest in the inhibitor analysis as the main approach of classic enzymology have faded. However, in our opinion, its combination with molecular modeling, X-ray structural analysis, and site-specific mutagenesis could solve a number of problems, such as specific features of half-site reactivity in GAPDH enzymes from various sources, identification of structural motifs involved in the cooperation of active sites, the role of the second cysteine residue (Cys154) in the active site of the enzyme, etc.
INACTIVATION OF GAPDH BY NATURAL METABOLITES – OXIDATION
AND S-GLUTATHIONYLATION
The catalytic function of Cys150 residue in the active site of GAPDH has been thoroughly studied. Cys150 interacts with NAD to form the charge-transfer complex and then is acylated by the reaction substrate, glyceraldehyde 3-phosphate. Naturally, any modification of this residue or its replacement with other amino acids leads to complete enzyme inactivation. The presence of highly reactive sulfhydryl group (SH) of Cys150 involved in the catalytic act in each of the four active sites of GAPDH makes this enzyme available for a number of modifications (alkylation, nitrosylation, oxidation, and others). In this section, we will focus only on the enzyme oxidation and S-glutathionylation; glycation, which mainly affects Lys and Arg residues, will be discussed in the final part of this review.
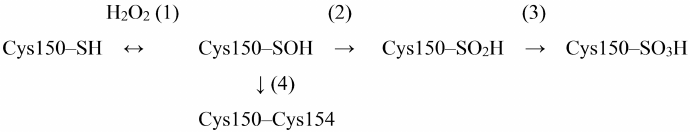
One of the most important natural ways of GAPDH inhibition is oxidation of Cys150 catalytic residue with hydrogen peroxide. The mechanism of GAPDH oxidation by hydrogen peroxide has been described in detail in the literature, including our works. The sulfhydryl group of Cys150 is sequentially oxidized with the formation of sulfenic, sulfinic, and sulfonic acids [reactions (1)-(3) in Scheme 1] [3, 29, 30].
Scheme 1. Oxidation of Cys150 by H2O2 in the active site of GAPDH
Short-term incubation (10-15 min) in the presence of low H2O2 concentrations (10-50 μM) results in the mild oxidation of the catalytic cysteine residue with the formation of cysteine-sulfenic acid (Scheme 1, reaction (1)). This stage is reversible: cysteine-sulfenic acid can be reduced to cysteine in the presence of ascorbic acid, sodium arsenite, or low-molecular-weight thiols [29, 31]. Longer incubation with H2O2 or the presence of higher H2O2 concentrations leads to irreversible oxidation of Cys150 with the formation of cysteine-sulfinic and cysteine-sulfonic acids [Scheme 1, reactions (2) and (3)].
An important feature of GAPDH is the presence of two cysteine residues in the active site: catalytic Cys150 and Cys154 not involved in catalysis. Unlike Cys150, Cys154 is screened in the active site and becomes available for oxidation only after Cys150 oxidation. The role of Cys154 is still unknown, although it is a rather conserved residue, which is present in GAPDH enzymes from various sources, with the exception of GAPDH from certain microorganisms (e.g., bacteria of the genus Thermus) (https://www.uniprot.org/uniprot/P00361). Replacing Cys154 with Ser does not significantly affect the enzyme activity, but at the same time, reduces the sensitivity of catalytic Cys150 to oxidation [32].
We hypothesized that Cys154 is necessary to prevent deep oxidation of Cys150 sulfhydryl group resulting in the irreversible enzyme inactivation. Thus, it was found that the two cysteines of the active site form a disulfide bridge under aerobic conditions in vivo [reaction (4) in Scheme 1] [33]. Apparently, Cys150 oxidation with the formation of cysteine-sulfenic acid promotes formation of the disulfide bridge with the neighboring Cys154 in accordance with the general mechanism of cysteine oxidation in proteins [34-36]. The disulfide bridge in the GAPDH active site can be reduced by dithiothreitol or β-mercaptoethanol in vitro, as well as with the participation of glutaredoxin, thioredoxin, or reduced glutathione in living systems. A combination of these processes prevents irreversible GAPDH inactivation.
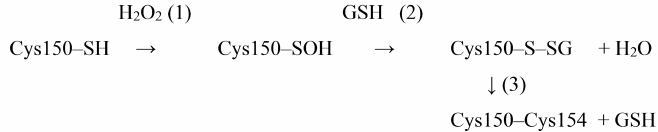
It should be noted that under normal in vivo conditions, cysteine-sulfenic acid in the GAPDH active site most likely cannot exist for a long time, since reduced glutathione (GSH), which is present in cells in high concentrations (1-5 mM), interacts with cysteine-sulfenic acid to form mixed disulfide [37, 38]:
Cys150-SOH + GSH → Cys150-SSG + H2O.
This modification, called S-glutathionylation, leads to complete enzyme inactivation; at the same time, it prevents further oxidation of cysteine-sulfenic acid with the formation of irreversible products of GAPDH oxidation. However, the possibility of irreversible GAPDH oxidation should not be completely ruled out, since the concentration of GSH can decrease under various pathological conditions.
Proteomic analysis revealed S-glutathionylated GAPDH in plant and animal tissues [39, 40]. The relationship between oxidation and S-glutathionylation of GAPDH was first demonstrated by Schuppe-Koistinen et al. [41], who showed that treatment of human endothelial cells with hydrogen peroxide leads to S-glutathionylation of GAPDH and its inactivation, while deglutathionylation of GAPDH is accompanied by the restoration of enzyme activity. Later, we confirmed the relationship between Cys150 oxidation and S-glutathionylation in our studies of purified GAPDH from rabbit muscles: the enzyme was S-glutathionylated when treated with hydrogen peroxide in the presence of GSH. The intramolecular Cys150–Cys154 disulfide bridge was revealed among the products of S-glutathionylation, in addition to the mixed disulfide GAPDH-SSG. We suggested the mixed disulfide GAPDH-SSG as an intermediate product of the reaction between Cys150-SOH and reduced glutathione [Scheme 2, reaction (2)], which then reacts with Cys154 to form a disulfide bridge [Scheme 2, reaction (3)] [38]:
Scheme 2. S-glutathionylation of GAPDH and formation of disulfide bridge in the presence of H2O2 and GSH
Thus, S-glutathionylation leads to the reversible inactivation of GAPDH. We demonstrated that in the presence of GSH excess, non-enzymatic deglutathionylation of GAPDH occurs due to the disulfide exchange reaction with GSH, which results in the restoration of enzyme activity [38]:
GAPDH-SSG + GSH → GAPDH-SH + GSSG.
In addition, deglutathionylation of GAPDH and reduction of the Cys150–Cys154 disulfide bridge can be catalyzed by glutaredoxin or thioredoxin [37, 38].
Therefore, S-glutathionylation of GAPDH is a reversible modification that is initiated by the oxidation of catalytic Cys150 residue and prevents irreversible enzyme oxidation.
ROLE OF GAPDH S-GLUTATHIONYLATION IN THE ACTIVATION OF
ANTIOXIDANT DEFENSE
The major role in protecting cells from hydrogen peroxide belongs to peroxiredoxins: the rate constant for the oxidation of SH-groups of peroxiredoxins with hydrogen peroxide is ~107 M–1·s–1 [42], which is several orders of magnitude higher than the rate constant for the oxidation of SH-groups in GAPDH (~10 M–1·s–1) [43]. However, the sensitivity of GAPDH to oxidation with hydrogen peroxide is rather high compared to most SH-containing proteins. Peroxiredoxin 2 and GAPDH are the proteins that oxidize first during incubation of the cells with hydrogen peroxide [44]. Presumably, GAPDH is oxidized when the cell antioxidant system cannot provide sufficient protection.
Peralta et al. [32] investigated the effect of Н2O2 on yeast strains expressing wild-type human GAPDH and the enzyme mutant by the non-catalytic cysteine residue (Cys156 in human GAPDH). The mutant GAPDH was resistant to oxidation and, as a consequence, to S-glutathionylation. It was shown that incubation of yeast cells expressing wild-type GAPDH with H2O2 led to the increase in the NADPH/NADP ratio; this effect was absent in the cells expressing the C156S mutant with reduced oxidation sensitivity. These studies suggested that reversible inactivation of GAPDH by S-glutathionylation may result in reversible inhibition of glycolysis and activation of the pentose phosphate pathway in response to oxidative stress. Activation of the pentose phosphate pathway increases production of coenzyme NADPH, which is necessary to recycle GSH with participation of glutathione reductase and, therefore, to maintain the antioxidant defense system. Introduction of mutations that reduce the sensitivity of GAPDH to oxidation leads to the elimination of this regulatory mechanism. Therefore, an increased sensitivity of GAPDH to oxidation is a necessary element of the antioxidant defense system of the cell. The properties of S-glutathionylation of human GAPDH C156S mutant expressed in yeast cells indicate a possible role of Cys156 in the regulation of antioxidant defense. However, to verify this assumption, it is necessary to conduct experiments not only in cell cultures, but also with isolated C156S mutant in order to compare the sensitivity of the native and mutant enzymes to oxidation by hydrogen peroxide and reactive oxygen species and to evaluate the efficiency of S-glutathionylation of the two enzyme forms, as well as the reversibility of this modification.
SPECIFIC INHIBITION OF GAPDH FROM DIFFERENT SOURCES
GAPDH inhibition as an approach to reduce cell viability has many limitations. However, in certain cases, GAPDH inhibition can efficiently disrupt the energy supply of the entire cell or its individual components. For example, inhibition of GAPDH can be effective if the cell does not have energy sources other than glycolysis. As examples of such cells, we can mention parasitic microorganisms that cause sleeping sickness [45-48] and Chagas disease [49, 50]. In addition, GAPDH inhibition can disturb energy production in cancer cells, for which glycolysis is the main source of energy. It is also assumed that GAPDH inhibition can impair energy supply of individual cell components, thereby suppressing certain cell functions. Thus, compartmentalization is characteristic of individual glycolysis enzymes, including GAPDH and 3-phosphoglycerate kinase that catalyzes subsequent glycolytic reaction coupled with ATP synthesis. In muscle cells, GAPDH binds to actin; in erythrocytes, it binds to membrane proteins through the band 3 protein, and in reticulocytes, it binds to polyribosomes. This allows glycolysis to provide energy supply for specific cellular functions that can vary in different cell types. Hence, GAPDH inhibition can impair energy supply of a specific cellular function that depend on ATP formed in glycolysis without significantly changing total ATP content in the cell [51, 52].
Therefore, it is possible that in certain cases, GAPDH inhibition can significantly reduce the viability of cells or at least suppress their functioning, despite the existence of other ATP sources (for example, mitochondria). The most striking example is the sperm of mammals. The progressive motion of a mammalian sperm cell is provided by the undulation of its flagellum. Despite the presence of mitochondria, an important source of energy for this type of movement is glycolysis that is catalyzed by GAPDH and other glycolytic enzymes associated with the sperm flagellum. We will consider these examples in more detail below.
INHIBITORS OF TRYPANOSOMAL GAPDH
GAPDH inhibitors were first used to reduce the viability of parasitic protozoa causing sleeping sickness (Trypanosoma brucei) and Chagas disease (Trypanosoma cruzi). Glycolysis is the only source of energy for the long slender form of T. brucei propagating in the bloodstream of a mammalian host; it proceeds in special organelles – glycosomes – containing a set of glycolytic enzymes [53]. This form of T. brucei lacks oxidative phosphorylation, since its mitochondria are inactive and do not contain cristae.
It was shown that the amino acid sequences of human GAPDH (https://www.uniprot.org/uniprot/P04406) and GAPDH from the glycosomes of T. cruzi (https://www.uniprot.org/uniprot/P22513) contain 178 identical residues, which corresponds to 53.1% sequence identity (Fig. 1). A similar picture is observed for GAPDH from the glycosomes of T. brucei (https://www.uniprot.org/uniprot/P22512).
Fig. 1. Alignment of GAPDH amino acid sequences from Mycobacterium tuberculosis (UniProt ID P9WN82), Ttypanosoma cruzi (UniProt ID P22513), and Homo sapiens (UniProt ID P04406). Asterisks, conserved residues; box; conserved sequence of the enzyme active site; arrow, catalytic cysteine residue.
Since 1990s, the search for specific GAPDH inhibitors has focused on NAD analogues, more precisely, on analogues of the coenzyme adenosine fragment [54, 55]. Based on the spatial structure of GAPDH enzymes from T. brucei and other representatives of this protozoan group, efficient enzyme inhibitors interacting with the NAD-binding domain have been developed that bound to the protozoan enzymes with Kd of 4-16 μM. Moreover, these inhibitors did not affect the activity of human GAPDH at concentrations below 20-40 μM [45]. The search for specific GAPDH inhibitors among analogues of GAPDH substrate 1,3-diphosphoglycerate [46] has been less successful and is still ongoing [48]. GAPDH inhibitors from natural sources are of particular interest. Virtual screening of the databases of natural compounds revealed 700 potential inhibitors for subsequent analysis [56]. Efficient inhibitors of trypanosomal GAPDH have been found, such as crassiflorone of plant origin [57], mastoparan from the venom of Brazilian wasp [58], and others. Unfortunately, no medications based on GAPDH inhibitors have been created yet. Some researchers propose the use of GAPDH inhibitors in combination with compounds that affect enzymes of other metabolic pathways, for example, trypanothione reductase [59].
SPERM-SPECIFIC GAPDH AS A POSSIBLE TARGET FOR
CONTRACEPTIVES
Development of contraceptives that could be used by men is an important and still unsolved problem. The idea of using sperm-specific GAPDH (GAPDS) as a target for contraceptive agents appeared 15 years ago after it had been found that this enzyme is essential for sperm motility. Moreover, the absence of GAPDS results in the loss of fertility in male mice [60]. It was found that, despite normal functioning of mitochondria, the progressive motion of spermatozoa is impossible without ATP that is formed in glycolysis as a result of reactions catalyzed by the sperm-specific enzymes. These observations suggested that the inhibition of GAPDS, which is bound to the fibrous sheath of the sperm flagellum along its length, could immobilize the sperm and provide the contraceptive effect. To solve this problem, recombinant forms of GAPDS were isolated [43, 61, 62], and their catalytic properties and regulatory characteristics were studied in detail [62-64]. Later, the spatial structure of GAPDS was resolved. First, the structure of the hybrid tetramer molecules composed of a dimer of rat GAPDS and a dimer of E. coli GAPDH was determined [65], and then the structure of the homotetramer of the recombinant human GAPDS was obtained [62, 66]. Most studies on the selective GAPDS inhibitors have been carried out by the group of O’Brien using high-performance experimental screening [66, 67]. Several inhibitors were found; the IC50 value for the best inhibitor was 1.2 µM. Unfortunately, this compound also inhibited somatic GAPDH, although with less efficiency. Hence, the search for selective inhibitors targeting GAPDS active site has been unsuccessful so far. Perhaps, a more promising approach might be based on the search for ligands that bind outside the active site, which we will discuss below.
INHIBITION OF SPERM-SPECIFIC GAPDH IN MELANOMA CELLS
We have shown that along with the somatic GAPDH, some melanoma cell lines contain significant amounts (approximately 50% of total GAPDH content) of the sperm-specific form of this enzyme [68]. This finding is in good agreement with numerous data on the expression of sperm-specific proteins in cancer cells. It can be assumed that expression of GAPDS in some types of cancer cells is responsible for the changes in their metabolism. As mentioned in the previous section, there is still no significant progress in the search for selective GAPDS inhibitors. However, the development of new approaches based on the search for effectors that bind outside the active site of the enzyme allows us to hope that such inhibitors will be found. Their use will allow to reduce the efficiency of glycolysis or to change the ratio of metabolic pathways in some types of cancer cells expressing GAPDS without affecting the energy metabolism of normal cells.
INHIBITORS OF METABOLISM OF TUBERCULOSIS MICOBACTERIA
The search for new compounds against the causative agent of tuberculosis (Mycobacterium tuberculosis) is an increasingly important task in the context of resistance of mycobacteria to known antibiotics. One of the targets for new drugs could be mycobacterial GAPDH, that not only catalyzes the most important glycolytic reaction, but also participates in the transport of iron by interacting with transferrin [69]. Boradia et al. suggested the existence of an additional pathway of iron acquisition by mycobacteria that includes internalization of transferrin with the assistance of GAPDH associated with the surface of mycobacterial cells. It is likely that compounds affecting the activity and other functions of GAPDH could reduce the viability of mycobacteria. However, GAPDH from M. tuberculosis (https://www.uniprot.org/uniprot/P9WN82) has approximately 49.6% identity with the human enzyme, and the structure of their active sites is virtually the same (Fig. 1).
Hence, searching for compounds that would selectively interact with the active or coenzyme-binding sites of M. tuberculosis GAPDH seems to be ineffective, as evidenced by unsuccessful attempts of using GAPDH inhibitors to suppress the viability of spermatozoa and trypanosomes.
The search for ligands that would interact with GAPDH outside the active site appears to be a more promising approach. Such compounds could prevent the binding of GAPDH to other proteins (for example, to transferrin in the case of M. tuberculosis GAPDH) or affect the catalytic activity or regulatory characteristics of the enzyme. These studies are in the very beginning; however, recent advances in molecular modeling combined with high-performance virtual and experimental screening give hope for the successful use of new types of ligands selectively affecting certain isoforms of GAPDH.
Unfortunately, inhibition of glycolysis does not completely suppress the energy supply in M. tuberculosis because of the efficient functioning of oxidative phosphorylation in mitochondria. Oxidation in mitochondria can be specifically inhibited by bedaquiline or imidazo[1,2-alpha]pyridine, whose anti-bacterial effect is due to the specific inhibition of F1Fo-ATP synthase [70, 71] or respiratory complex bc1 [72] in M. tuberculosis. However, these compounds are not always sufficient to suppress the vital functions of the mycobacteria due to the slow action, development of resistance, expression of cytochrome bd, and other factors, including the functioning of the glycolytic pathway [73, 74]. We believe that a new class of drugs against M. tuberculosis can be created based on a combination of specific inhibitors of GAPDH and oxidative phosphorylation that would suppress both pathways of energy production.
EFFECT OF GAPDH INHIBITION ON METABOLISM AND PROTEIN
GLYCATION
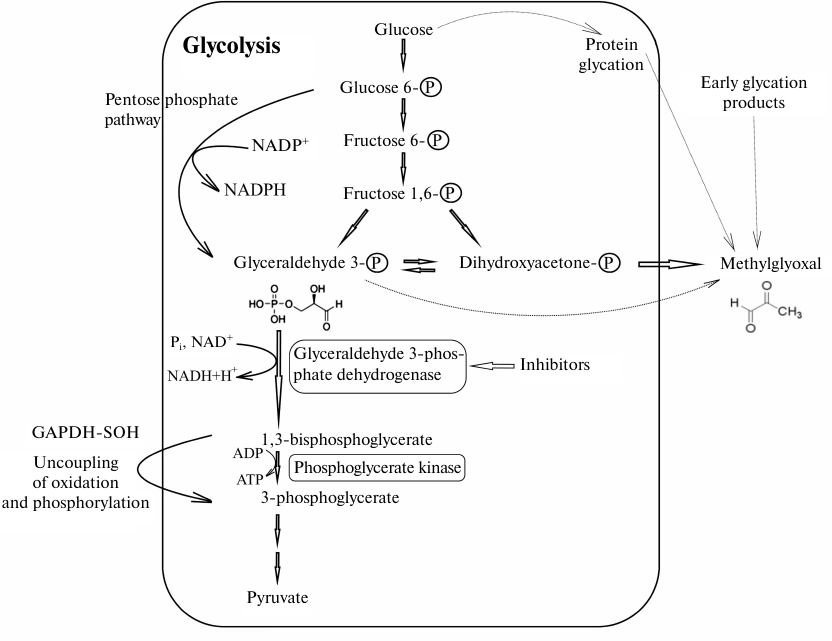
In the last section, we would like to discuss the unexpected effects of GAPDH inhibition on cell metabolism. If a decrease in the efficiency of glycolysis due to the inhibition or inactivation of GAPDH is obvious, the changes in other processes involving GAPDH are not so predictable. As noted above, the concentration of GAPDH in all types of cells is very high. Perhaps one of the reasons for the high concentration of this enzyme in the cell is the need to neutralize the effect of its highly reactive substrate, glyceraldehyde 3-phosphate. The toxic effect of glyceraldehyde 3-phosphate on proteins is due to its ability to modify the sulfhydryl groups of cysteine residues, amino groups of lysine residues, and guanidine groups of arginine residues. However, functioning cells contain almost no free glyceraldehyde 3-phosphate, since after its formation from fructose 1,6-diphosphate, glyceraldehyde 3-phosphate is mainly utilized by GAPDH in the glycolytic pathway or isomerized to dihydroxyacetone phosphate (DHAP) (Fig. 2). Even when the intensity of glycolytic reactions downstream of the GAPDH-catalyzed reaction is slowed down, no glyceraldehyde 3-phosphate in a free state is present, since it exists in the GAPDH-bound forms, such as hemithioacetal or acyl-enzyme. Under certain conditions, the intermediate product of the dehydrogenase reaction (acyl-enzyme) can be isolated [75]. Therefore, to prevent the toxic effects of glyceraldehyde 3-phosphate, the cells have to maintain high concentration of active GAPDH. Obviously, introduction of GAPDH inhibitors, as well as enzyme inactivation, primarily by oxidation of sulfhydryl groups or glycation, may lead to the accumulation of glyceraldehyde 3-phosphate and changes in the ratio of metabolic pathways shown in Fig. 2.
Fig. 2. Possible effect of GAPDH inhibition on glycolysis and associated metabolic pathways. The scheme shows main steps of glycolysis leading under aerobic conditions to the formation of pyruvate that is further used in the Krebs cycle, as well as reactions involving GAPDH. Oxidized GAPDH containing in the active site the sulfhydryl group oxidized to sulfenic acid (GAPD-SOH) has the acyl phosphatase activity and hydrolyzes 1,3-diphosphoglycerate, resulting in the futile pathway of oxidation and phosphorylation uncoupling in glycolysis. GAPDH inhibition decreases the glycolysis rate, thereby increasing the efficiency of the pentose phosphate pathway. Glycation of GAPDH with methylglyoxal leads to the enzyme inactivation and increase in the concentrations of glyceraldehyde 3-phosphate and then methylglyoxal. Thin gray lines, non-enzymatic reactions (glycation and formation of methylglyoxal from glyceraldehyde 3-phosphate and dihydroxyacetone phosphate).
We would like to pay special attention to the glycation of GAPDH, since it had been ignored for a long time. It is known that free amino groups in GAPDH can be modified by various sugars (glucose, fructose, etc.) as a result of non-enzymatic glycosylation (glycation). Modification with methylglyoxal and similar compounds, including glyceraldehyde 3-phosphate, is also referred to as glycation. Methylglyoxal can be formed from the early glycation products in a cascade of reactions or synthesized in metabolic pathways associated with glycolysis [76]. It was shown that methylglyoxal efficiently modifies amino groups of lysine residues in GAPDH, which leads to a decrease in the enzyme activity [77]. We found that GAPDH glycation by its own substrate, glyceraldehyde 3-phosphate, also results in the enzyme inactivation [78]. Naturally, GAPDH glycation by glyceraldehyde 3-phosphate at lysine and arginine residues cannot occur under normal physiological conditions. Glyceraldehyde 3-phosphate primarily binds to the sulfhydryl group of the catalytic Cys150 residue that has an enhanced reactivity due to the specific microenvironment of the active site affecting its pKa value, i.e., the first step of glycolytic oxidoreduction takes place. The high content of GAPDH that significantly exceeds the concentration of glyceraldehyde 3-phosphate and the glycolytic oxidoreduction reaction explain the absence of free glyceraldehyde 3-phosphate that could participate in glycation. However, when GAPDH is inhibited by various ligands or when its sulfhydryl groups are oxidized with reactive oxygen species, glyceraldehyde 3-phosphate is not utilized and can modify lysine and arginine residues of the enzyme, resulting in an additional decrease in the GAPDH activity. This leads to further accumulation of free glyceraldehyde 3-phosphate and activation of glycation reactions. It should be taken into account that the triose phosphate isomerase reaction is shifted towards the formation of DHAP (equilibrium constant Keq = 0.048 at 25°C) [79], which limits accumulation of glyceraldehyde 3-phosphate in the case of GAPDH inhibition. Nevertheless, even in this situation, inhibition of GAPDH results in a significant increase in concentration of glyceraldehyde 3-phosphate (6-7-fold when GAPDH is inhibited by iodoacetate), and therefore, glyceraldehyde 3-phosphate can participate in the glycation of the enzyme [80]. However, the main glycating agent is most likely methylglyoxal formed from DHAP, since its concentration in all the cases exceeds 10-fold the concentration of glyceraldehyde-3-phosphate. Obviously, GAPDH inhibition will result in the increase in methylglyoxal concentration due to its formation from DHAP.
Therefore, GAPDH inactivation or inhibition by any mechanism leads to the increase in the concentrations of free glyceraldehyde 3-phosphate and methylglyoxal, further inactivation of GAPDH, and a new round of this process. GAPDH inhibitors not only reversibly reduce the intensity of glycolysis but can also cause irreversible inactivation of GAPDH and other enzymes via glycation. These effects may be desirable consequences of the use of GAPDH inhibitors in the cases described above, such as suppression of viability of parasitic microorganisms or cancer cells and reduction of sperm motility. At the same time, it is GAPDH inhibition that can be an important reason for the appearance of glycated proteins involved in the development of pathological processes, for example, neurodegenerative diseases of the amyloid nature [76].
It is also important to take into account the effect of GAPDH inhibitors on the uncoupling of oxidation and phosphorylation in glycolysis found in our works [3-5]. The uncoupling in glycolysis takes place when Cys150 of the GAPDH active site is oxidized to sulfenic acid. The involvement of the Cys150 sulfhydryl group in the interaction with inhibitors targeting GAPDH active sites (catalytic, substrate-binding, or cofactor-binding) may prevent the formation of sulfenic acid at Cys150. In this case, the acyl phosphatase reaction (hydrolysis of 1,3-diphosphoglycerate without participation of 3-phosphoglycerate kinase and ATP synthesis) will not proceed (Fig. 2). Hydrolysis of 1,3-diphosphoglycerate by oxidized GAPDH can lead to the acceleration of pyruvate formation with a decrease in ATP production. Under aerobic conditions, glycolysis with zero ATP yield makes sense, since it provides production of NADH and pyruvate for more efficient oxidative phosphorylation in the mitochondria. However, it should be noted that sulfenic acid reacts with reduced glutathione to form mixed disulfide, which leads to the inhibition of acyl phosphatase activity [38]. Therefore, the acyl-phosphatase activity of GAPDH can be of importance when the content of GSH in the cell is significantly lowered.
The most important metabolic process associated with glycolysis is the pentose phosphate pathway (Fig. 2). The fate of glucose 6-phosphate depends on many factors, the most important of which is the concentration of NADP+ in the cell. An increase in the NADP+ concentration stimulates utilization of glucose 6-phosphate in the pentose phosphate pathway, which leads to the formation of NADPH. However, inhibition of glycolysis also results in the utilization of glucose 6-phosphate in the pentose phosphate pathway, given that this pathway was discovered using conventional glycolysis inhibitors. It should be noted that reversible GAPDH oxidation resulting in the glycolysis inhibition should increase the intensity of the pentose phosphate pathway. This process was studied in detail for S-glutathionylation of GAPDH [32]. Activation of the pentose phosphate pathway leads to the accumulation of NADPH (glutathione reductase coenzyme) and subsequent increase in the concentration of GSH that is necessary for the reactivation of GAPDH and stimulation of glycolysis. Obviously, this regulatory mechanism does not work in the case of irreversible GAPDH inhibitors. However, apart from S-glutathionylation, other methods of reversible inhibition or inactivation of GAPDH may be useful in cases where it is necessary to increase the content of NADPH and GSH. These considerations may indicate an important role of GAPDH in the regulation of cell redox status and the need to take into account the diverse effects of GAPDH inhibition on cell metabolism.
Of course, inhibition and, especially, inactivation of GAPDH can lead to more serious consequences, not limited to the effect on the cell metabolism, first of all, emergence of inactive, denatured, and aggregated forms of GAPDH that play an important role in the formation of amyloid structures in the cell. These aspects are beyond the scope of this review and were discussed in detail in our recently published articles [9, 76].
Funding. The work was supported by the Russian Science Foundation (grant no. 16-14-10027).
Conflict of interest. The authors declare no conflict of interest.
Compliance with ethical standards. This article does not contain any research using animals or people performed by any of the authors.
REFERENCES
1.Nagradova, N. K. (1956) Mechanism of action of
carnosine on glycolytic oxidation reduction combined with
phosphorylation, Biochemistry (Moscow), 21, 17-25.
2.Nagradova, N. K. (1965) The effect of histidine and
other chelating agents on the activity of 3-phosphoglyceraldehyde
dehydrogenase from rabbit muscles, Biochemistry (Moscow),
30, 50-57.
3.Schmalhausen, E. V., Nagradova, N. K.,
Boschi-Muller, S., Branlant, G., and Muronetz, V. I. (1999) Mildly
oxidized GAPDH: the coupling of the dehydrogenase and acyl phosphatase
activities, FEBS Lett., 452, 219-222.
4.Danshina, P. V., Schmalhausen, E. V., Avetisyan, A.
V., and Muronetz, V. I. (2001) Mildly oxidized
glyceraldehyde-3-phosphate dehydrogenase as a possible regulator of
glycolysis, IUBMB Life, 51, 309-314.
5.Dan’shina, P. V., Schmalhausen, E. V.,
Arutiunov, D. Y., Pleten’, A. P., and Muronetz, V. I. (2003)
Acceleration of glycolysis in the presence of the non-phosphorylating
and the oxidized phosphorylating glyceraldehyde-3-phosphate
dehydrogenases, Biochemistry (Moscow), 68, 593-600.
6.Seidler, N. W. (2013) Basic biology of GAPDH,
Adv. Exp. Med. Biol., 985, 1-36.
7.Sikand, K., Singh, J., Ebron, J. S., and Shukla, G.
C. (2012) Housekeeping gene selection advisory:
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin are
targets of miR-644a, PloS One, 7, e47510.
8.Caradec, J., Sirab, N., Revaud, D., Keumeugni, C.,
and Loric, S. (2010) Is GAPDH a relevant housekeeping gene for
normalisation in colorectal cancer experiments? Br. J. Cancer,
103, 1475-1476.
9.Muronetz, V. I., Barinova, K. V., Stroylova, Y. Y.,
Semenyuk, P. I., and Schmalhausen, E. V. (2017)
Glyceraldehyde-3-phosphate dehydrogenase: aggregation mechanisms and
impact on amyloid neurodegenerative diseases, Int. J. Biol.
Macromol., 100, 55-66.
10.Mazzola, J. L., and Sirover, M. A. (2002)
Alteration of intracellular structure and function of
glyceraldehyde-3-phosphate dehydrogenase: a common phenotype of
neurodegenerative disorders? Neurotoxicology, 23,
603-609.
11.Tatton, W. G., Chalmers-Redman, R. M., Elstner,
M., Leesch, W., Jagodzinski, F. B., Stupak, D. P., Sugrue, M. M., and
Tatton, N. A. (2000) Glyceraldehyde-3-phosphate dehydrogenase in
neurodegeneration and apoptosis signaling, J. Neural Transm.
Suppl., 60, 77-100.
12.Cardon, J. W., and Boyer, P. D. (1982) Subunit
interaction in catalysis. Some experimental and theoretical approaches
with glyceraldehyde-3-phosphate dehydrogenase, J. Biol. Chem.,
257, 7615-7622.
13.Koshland, D. E. (1977) The specificity of subunit
interactions, Biochem. Soc. Trans., 5, 605-606.
14.Malhotra, O. P., and Bernhard, S. A. (1973)
Activation of a covalent enzyme–substrate bond by noncovalent
interaction with an effector, Proc. Natl. Acad. Sci. USA,
70, 2077-2081.
15.Byers, L. D., and Koshland, D. E. (1975) The
specificity of induced conformational changes. The case of yeast
glyceraldehyde-3-phosphate dehydrogenase, Biochemistry,
14, 3661-3669.
16.Levitzki, A., and Koshland, D. E. (1976) The role
of negative cooperativity and half-of-the-sites reactivity in enzyme
regulation, Curr. Top. Cell. Regul., 10, 1-40.
17.Nagradova, N. K., Ashmarina, L. I., Asryants, R.
A., Cherednikova, T. V., Golovina, T. O., and Muronetz, V. I. (1980)
Glyceraldehyde-3-phosphate dehydrogenase: the role of subunit
interactions in enzyme functioning, Adv. Enzyme Regul.,
19, 171-204.
18.Nagradova, N. K. (2001) Interdomain interactions
in oligomeric enzymes: creation of asymmetry in homo-oligomers and role
in metabolite channeling between active centers of hetero-oligomers,
FEBS Lett., 487, 327-332.
19.Nagradova, N. K. (2001) Study of the properties
of phosphorylating D-glyceraldehyde-3-phosphate dehydrogenase,
Biochemistry (Moscow), 66, 1067-1076.
20.Asryants, R. A., Kuzminskaya, E. V., Tishkov, V.
I., Douzhenkova, I. V., and Nagradova, N. K. (1989) An examination of
the role of arginine residues in the functioning of
D-glyceraldehyde-3-phosphate dehydrogenase, Biochim. Biophys.
Acta, 997, 159-166.
21.Levashov, P. A., Schmalhausen, E. V., Muronetz,
V. I., and Nagradova, N. K. (1995) E. coli
D-glyceraldehyde-3-phosphate dehydrogenase modified by 2,3-butanedione:
manifestation of a pairwise of non-equivalence of active centers,
Biochem. Mol. Biol. Int., 37, 991-1000.
22.Nagradova, N. K., Schmalhausen, E. V., Levashov,
P. A., Asryants, R. A., and Muronetz, V. I. (1996)
D-glyceraldehyde-3-phosphate dehydrogenase. Properties of the enzyme
modified at arginine residues, Appl. Biochem. Biotechnol.,
61, 47-56.
23.Nagradova, N. K., Asryants, R. A., and Ivanov, M.
V. (1971) Interaction of 1-anilino-8-naphthalene sulfonate with yeast
glyceraldehyde-3-phosphate dehydrogenase, Experientia,
27, 1169-1170.
24.Nagradova, N. K., Asryants, R. A., and Ivanov, M.
V (1972) 1-Anilino-8-naphthalene sulfonate as a coenzyme-competitive
inhibitor of yeast glyceraldehyde-3-phosphate dehydrogenase: multiple
inhibition studies, Biochim. Biophys. Acta, 268,
622-628.
25.Golovina, T. O., Muronetz, V. I., and Nagradova,
N. K. (1978) Half-of-the-sites reactivity of rat skeletal muscle
D-glyceraldehyde-3-phosphate dehydrogenase, Biochim. Biophys.
Acta, 524, 15-25.
26.Muronets, V. I., Golovina, T. O., and Nagradova,
N. K. (1982) Use of immobilization for the study of glyceraldehyde
3-phosphate dehydrogenase. Immobilized dimers of the enzyme,
Biochemistry (Moscow), 47, 3-12.
27.Soukri, A., Mougin, A., Corbier, C., Wonacott,
A., Branlant, C., and Branlant, G. (1989) Role of the histidine 176
residue in glyceraldehyde-3-phosphate dehydrogenase as probed by
site-directed mutagenesis, Biochemistry, 28,
2586-2592.
28.Clermont, S., Corbier, C., Mely, Y., Gerard, D.,
Wonacott, A., and Branlant, G. (1993) Determinants of coenzyme
specificity in glyceraldehyde-3-phosphate dehydrogenase: role of the
acidic residue in the fingerprint region of the nucleotide binding
fold, Biochemistry, 32, 10178-10184.
29.Little, C., and O’Brien, P. J. (1969)
Mechanism of peroxide-inactivation of the sulphydryl enzyme
glyceraldehyde-3-phosphate dehydrogenase, Eur. J. Biochem.,
10, 533-538.
30.Muronetz, V. I., Melnikova, A. K., Saso, L., and
Schmalhausen, E. V. (2018) Influence of oxidative stress on catalytic
and non-glycolytic functions of glyceraldehyde-3-phosphate
dehydrogenase, Curr. Med. Chem., doi:
10.2174/0929867325666180530101057.
31.You, K. S., Benitez, L. V., McConachie, W. A.,
and Allison, W. S. (1975) The conversion of glyceraldehyde-3-phosphate
dehydrogenase to an acylphosphatase by trinitroglycerin and
inactivation of this activity by azide and ascorbate, Biochim.
Biophys. Acta, 384, 317-330.
32.Peralta, D., Bronowska, A. K., Morgan, B., Doka,
E., Van Laer, K., Nagy, P., Grater, F., and Dick, T. P. (2015) A proton
relay enhances H2O2 sensitivity of GAPDH to
facilitate metabolic adaptation, Nat. Chem. Biol., 11,
156-163.
33.Leichert, L. I., Gehrke, F., Gudiseva, H. V.,
Blackwell, T., Ilbert, M., Walker, A. K., Strahler, J. R., Andrews, P.
C., and Jakob, U. (2008) Quantifying changes in the thiol redox
proteome upon oxidative stress in vivo, Proc. Natl. Acad.
Sci. USA, 105, 8197-8202.
34.Cremers, C. M., and Jakob, U. (2013) Oxidant
sensing by reversible disulfide bond formation, J. Biol. Chem.,
288, 26489-26496.
35.Roos, G., and Messens, J. (2011) Protein sulfenic
acid formation: from cellular damage to redox regulation, Free
Radic. Biol. Med., 51, 314-326.
36.Rehder, D. S., and Borges, C. R. (2010) Cysteine
sulfenic acid as an intermediate in disulfide bond formation and
nonenzymatic protein folding, Biochemistry, 49,
7748-7755.
37.Bedhomme, M., Adamo, M., Marchand, C. H.,
Couturier, J., Rouhier, N., Lemaire, S. D., Zaffagnini, M., and Trost,
P. (2012) Glutathionylation of cytosolic glyceraldehyde-3-phosphate
dehydrogenase from the model plant Arabidopsis thaliana is
reversed by both glutaredoxins and thioredoxins in vitro,
Biochem. J., 445, 337-347.
38.Barinova, K. V., Serebryakova, M. V., Muronetz,
V. I., and Schmalhausen, E. V. (2017) S-glutathionylation of
glyceraldehyde-3-phosphate dehydrogenase induces formation of
C150–C154 intrasubunit disulfide bond in the active site of the
enzyme, Biochim. Biophys. Acta, 1861, 3167-3177.
39.Gao, X. H., Bedhomme, M., Veyel, D., Zaffagnini,
M., and Lemaire, S. D. (2009) Methods for analysis of protein
glutathionylation and their application to photosynthetic organisms,
Mol. Plant, 2, 218-235.
40.Newman, S. F., Sultana, R., Perluigi, M., Coccia,
R., Cai, J., Pierce, W. M., Klein, J. B., Turner, D. M., and
Butterfield, D. A. (2007) An increase in S-glutathionylated proteins in
the Alzheimer’s disease inferior parietal lobule, a proteomics
approach, J. Neurosci. Res., 85, 1506-1514.
41.Schuppe-Koistinen, I., Moldeus, P., Bergman, T.,
and Cotgreave, I. A. (1994) S-thiolation of human endothelial cell
glyceraldehyde-3-phosphate dehydrogenase after hydrogen peroxide
treatment, Eur. J. Biochem., 221, 1033-1037.
42.Davies, M. J. (2016) Protein oxidation and
peroxidation, Biochem. J., 473, 805-825.
43.Elkina, Y. L., Kuravsky, M. L., El’darov,
M. A., Stogov, S. V., Muronetz, V. I., and Schmalhausen, E. V. (2010)
Recombinant human sperm-specific glyceraldehyde-3-phosphate
dehydrogenase: structural basis for enhanced stability, Biochim.
Biophys. Acta, 1804, 2207-2212.
44.Baty, J. W., Hampton, M. B., and Winterbourn, C.
C. (2005) Proteomic detection of hydrogen peroxide-sensitive thiol
proteins in Jurkat cells, Biochem. J., 389, 785-795.
45.Aronov, A. M., Verlinde, C. L., Hol, W. G., and
Gelb, M. H. (1998) Selective tight binding inhibitors of trypanosomal
glyceraldehyde-3-phosphate dehydrogenase via structure-based drug
design, J. Med. Chem., 41, 4790-4799.
46.Ladame, S., Bardet, M., Perie, J., and Willson,
M. (2001) Selective inhibition of Trypanosoma brucei GAPDH by
1,3-bisphospho-D-glyceric acid (1,3-diPG) analogues, Bioorg. Med.
Chem., 9, 773-783.
47.Callens, M., and Hannaert, V. (1995) The rational
design of trypanocidal drugs: selective inhibition of the
glyceraldehyde-3-phosphate dehydrogenase in Trypanosomatidae, Ann.
Trop. Med. Parasitol., 89 (Suppl. 1), 23-30.
48.Haanstra, J. R., Gerding, A., Dolga, A. M.,
Sorgdrager, F. J. H., Buist-Homan, M., du Toit, F., Faber, K. N.,
Holzhutter, H. G., Szoor, B., Matthews, K. R., Snoep, J. L.,
Westerhoff, H. V., and Bakker, B. M. (2017) Targeting pathogen
metabolism without collateral damage to the host, Sci. Rep.,
7, 40406.
49.Pereira, J. M., Severino, R. P., Vieira, P. C.,
Fernandes, J. B., da Silva, M. F. G. F., Zottis, A., Andricopulo, A.
D., Oliva, G., and Correa, A. G. (2008) Anacardic acid derivatives as
inhibitors of glyceraldehyde-3-phosphate dehydrogenase from
Trypanosoma cruzi, Bioor. Med. Chem., 16,
8889-8895.
50.Prokopczyk, I. M., Ribeiro, J. F. R., Sartori, G.
R., Sesti-Costa, R., Silva, J. S., Freitas, R. F., Leitao, A., and
Montanari, C. A. (2014) Integration of methods in cheminformatics and
biocalorimetry for the design of trypanosomatid enzyme inhibitors,
Future Med. Chem., 6, 17-33.
51.Chu, H., Puchulu-Campanella, E., Galan, J. A.,
Tao, W. A., Low, P. S., and Hoffman, J. F. (2012) Identification of
cytoskeletal elements enclosing the ATP pools that fuel human red blood
cell membrane cation pumps, Proc. Natl. Acad. Sci. USA,
109, 12794-12799.
52.Muronetz, V. I., and Nagradova, N. K. (1990)
Interaction of glyceraldehyde-3-phosphate dehydrogenase with structural
elements of cells, Usp. Biol. Khim., 31, 115-131.
53.Opperdoes, F. R., and Borst, P. (1977)
Localization of nine glycolytic enzymes in a microbody-like organelle
in Trypanosoma brucei: the glycosome, FEBS Lett.,
80, 360-364.
54.Van Calenbergh, S., Verlinde, C. L., Soenens, J.,
De Bruyn, A., Callens, M., Blaton, N. M., Peeters, O. M., Herdewijn,
P., Rozenski, J., and Hol, W. G. J. (1995) Synthesis and
structure-activity relationships of analogs of
2′-deoxy-2′-(3-methoxybenzamido)adenosine, a selective
inhibitor of trypanosomal glycosomal glyceraldehyde-3-phosphate
dehydrogenase, J. Med. Chem., 38, 3838-3849.
55.Link, A., Heidler, P., Kaiser, M., and Brun, R.
(2009) Synthesis of a series of N6-substituted adenosines with activity
against trypanosomatid parasites, Eur. J. Med. Chem., 44,
3665-3671.
56.Herrmann, F. C., Lenz, M., Jose, J., Kaiser, M.,
Brun, R., and Schmidt, T. J. (2015) In silico identification and
in vitro activity of novel natural inhibitors of Trypanosoma
brucei glyceraldehyde-3-phosphate-dehydrogenase, Molecules,
20, 16154-16169.
57.Uliassi, E., Fiorani, G., Krauth-Siegel, R. L.,
Bergamini, C., Fato, R., Bianchini, G., Carlos Menendez, J., Molina, M.
T., Lopez-Montero, E., Falchi, F., Cavalli, A., Gul, S., Kuzikov, M.,
Ellinger, B., Witt, G., Moraes, C. B., Freitas-Junior, L. H., Borsari,
C., Costi, M. P., and Bolognesi, M. L. (2017) Crassiflorone derivatives
that inhibit Trypanosoma brucei glyceraldehyde-3-phosphate
dehydrogenase (TbGAPDH) and Trypanosoma cruzi trypanothione
reductase (TcTR) and display trypanocidal activity, Eur. J. Med.
Chem., 141, 138-148.
58.Vinhote, J. F. C., Lima, D. B., Menezes, R. R. P.
P. B., Mello, C. P., de Souza, B. M., Havt, A., Palma, M. S., Santos,
R. P. D., Albuquerque, E. L., Freire, V. N., and Martins, A. M. C.
(2017) Trypanocidal activity of mastoparan from Polybia paulista
wasp venom by interaction with TcGAPDH, Toxicon, 137,
168-172.
59.Belluti, F., Uliassi, E., Veronesi, G.,
Bergamini, C., Kaiser, M., Brun, R., Viola, A., Fato, R., Michels, P.
A., Krauth-Siegel, R. L., Cavalli, A., and Bolognesi, M. L. (2014)
Toward the development of dual-targeted glyceraldehyde-3-phosphate
dehydrogenase/trypanothione reductase inhibitors against Trypanosoma
brucei and Trypanosoma cruzi, ChemMedChem., 9,
371-382.
60.Miki, K., Qu, W., Goulding, E. H., Willis, W. D.,
Bunch, D. O., Strader, L. F., Perreault, S. D., Eddy, E. M., and
O’Brien, D. A. (2004) Glyceraldehyde 3-phosphate dehydrogenase-S,
a sperm-specific glycolytic enzyme, is required for sperm motility and
male fertility, Proc. Natl. Acad. Sci. USA, 101,
16501-16506.
61.Lamson, D. R., House, A. J., Danshina, P. V.,
Sexton, J. Z., Sanyang, K., O’Brien, D. A., Yeh, L. A., and
Williams, K. P. (2011) Recombinant human sperm-specific
glyceraldehyde-3-phosphate dehydrogenase (GAPDHS) is expressed at high
yield as an active homotetramer in baculovirus-infected insect cells,
Protein Expr. Purif., 75, 104-113.
62.Chaikuad, A., Shafqat, N., Al-Mokhtar, R.,
Cameron, G., Clarke, A. R., Brady, R. L., Oppermann, U., Frayne, J.,
and Yue, W. W. (2011) Structure and kinetic characterization of human
sperm-specific glyceraldehyde-3-phosphate dehydrogenase, GAPDS,
Biochem. J., 435, 401-409.
63.Kuravsky, M., Barinova, K., Marakhovskaya, A.,
Eldarov, M., Semenyuk, P., Muronetz, V., and Schmalhausen, E. (2014)
Sperm-specific glyceraldehyde-3-phosphate dehydrogenase is stabilized
by additional proline residues and an interdomain salt bridge,
Biochim. Biophys. Acta, 1844, 1820-1826.
64.Kuravsky, M. L., Barinova, K. V., Asryants, R.
A., Schmalhausen, E. V., and Muronetz, V. I. (2015) Structural basis
for the NAD binding cooperativity and catalytic characteristics of
sperm-specific glyceraldehyde-3-phosphate dehydrogenase,
Biochimie, 115, 28-34.
65.Frayne, J., Taylor, A., Cameron, G., and
Hadfield, A. T. (2009) Structure of insoluble rat sperm
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) via heterotetramer
formation with Escherichia coli GAPDH reveals target for
contraceptive design, J. Biol. Chem., 284,
22703-227012.
66.Dan’shina, P. V., Qu, W., Temple, B. R.,
Rojas, R. J., Miley, M. J., Machius, M., Betts, L., and O’Brien,
D. A. (2016) Structural analyses to identify selective inhibitors of
glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic
enzyme, Mol. Hum. Reprod., 22, 410-426.
67.Sexton, J. Z., Danshina, P. V., Lamson, D. R.,
Hughes, M., House, A. J., Yeh, L. A., O’Brien, D. A., and
Williams, K. P. (2011) Development and implementation of a high
throughput screen for the human sperm-specific isoform of
glyceraldehyde 3-phosphate dehydrogenase (GAPDHS), Curr. Chem.
Genomics, 5, 30-41.
68.Sevostyanova, I. A., Kulikova, K. V., Kuravsky,
M. L., Schmalhausen, E. V., and Muronetz, V. I. (2012) Sperm-specific
glyceraldehyde-3-phosphate dehydrogenase is expressed in melanoma
cells, Biochem. Biophys. Res. Commun., 427, 649-653.
69.Boradia, V. M., Malhotra, H., Thakkar, J. S.,
Tillu, V. A., Vuppala, B., Patil, P., Sheokand, N., Sharma, P.,
Chauhan, A. S., Raje, M., and Raje, C. I. (2014) Mycobacterium
tuberculosis acquires iron by cell-surface sequestration and
internalization of human holo-transferrin, Nat. Commun.,
5, 4730.
70.Andries, K., Verhasselt, P., Guillemont, J.,
Gohlmann, H. W. H., Neefs, J.-M., Winkler, H., Van Gestel, J.,
Timmerman, P., Zhu, M., Lee, E., Williams, P., de Chaffoy, D., Huitric,
E., Hoffner, S., Cambau, E., Truffot-Pernot, C., Lounis, N., and
Jarlier, V. (2005) A diarylquinoline drug active on the ATP synthase of
Mycobacterium tuberculosis, Science, 307,
223-227.
71.Koul, A., Vranckx, L., Dendouga, N., Balemans,
W., Van den Wyngaert, I., Vergauwen, K., Gohlmann, H. W., Willebrords,
R., Poncelet, A., Guillemont, J., Bald, D., and Andries, K. (2008)
Diarylquinolines are bactericidal for dormant mycobacteria as a result
of disturbed ATP homeostasis, J. Biol. Chem., 283,
25273-25280.
72.Pethe, K., Bifani, P., Jang, J., Kang, S., Park,
S., et al. (2013) Discovery of Q203, a potent clinical candidate for
the treatment of tuberculosis, Nat. Med., 19,
1157-1160.
73.Forte, E., Borisov, V. B., Falabella, M., Colaco,
H. G., Tinajero-Trejo, M., Poole, R. K., Vicente, J. B., Sarti, P., and
Giuffre, A. (2016) The terminal oxidase cytochrome bd promotes
sulfide-resistant bacterial respiration and growth, Sci. Rep.,
6, 23788.
74.Forte, E., Borisov, V. B., Vicente, J. B., and
Giuffre, A. (2017) Cytochrome bd and gaseous ligands in
bacterial physiology, Adv. Microb. Physiol., 71,
171-234.
75.Malhotra, O. P., and Bernhard, S. A. (1981) Role
of nicotinamide adenine dinucleotide as an effector in formation and
reactions of acylglyceraldehyde-3-phosphate dehydrogenase,
Biochemistry, 20, 5529-5538.
76.Muronetz, V. I., Melnikova, A. K., Seferbekova,
Z. N., Barinova, K. V., and Schmalhausen, E. V. (2017) Glycation,
glycolysis, and neurodegenerative diseases: is there any connection?
Biochemistry (Moscow), 82, 874-886.
77.Lee, H. J., Howell, S. K., Sanford, R. J., and
Beisswenger, P. J. (2005) Methylglyoxal can modify GAPDH activity and
structure, Ann. NY Acad. Sci., 1043, 135-145.
78.Muronetz, V., Barinova, K., and Schmalhausen, E.
(2017) Glycation of glyceraldehyde-3-phosphate dehydrogenase in the
presence of glucose and glyceraldehyde-3-phosphate, J. Int. Soc.
Antioxid., 2, 1-4.
79.Cornish-Bowden, A. (1981) Thermodynamic aspects
of glycolysis, Biochem. Educ., 9, 133-137.
80.Veech, R. L., Raijman, L., Dalziel, K., and
Krebs, H. A. (1969) Disequilibrium in the triose phosphate isomerase
system in rat liver, Biochem. J., 115, 837-842.