Role of Caspases in the Cytotoxicity of NK-92 Cells in Various Models of Coculturing with Trophoblasts
Yu. P. Milyutina1,a*, V. A. Mikhailova1, K. M. Pyatygina1, E. S. Demidova1, D. A. Malygina1, T. E. Tertychnaia1, A. V. Arutjunyan1, D. I. Sokolov1, and S. A. Selkov1
1Ott Institute of Obstetrics, Gynecology, and Reproductology, 199034 St. Petersburg, Russia* To whom correspondence should be addressed.
Received April 17, 2019; Revised June 18, 2019; Accepted June 22, 2019
Studies of interactions between natural killer (NK) cells and trophoblasts and identification of conditions for the NK cells to perform their cytotoxic function are of fundamental and practical importance for understanding their role in the development of pathological processes and complications during pregnancy. In this study, we examined changes in the content of caspases and studied activation of these enzymes in Jeg-3 trophoblasts in various models of their coculturing with NK-92 cells and demonstrated the necessity of direct contact between these cell populations for the activation of caspase-8 and caspase-3 in the trophoblasts. Contact coculturing of the two cell lines resulted in the appearance of the cytotoxic protein granzyme B in Jeg-3 cells that was accompanied by a decrease in the content of this enzyme in NK-92 cells. Distant coculturing of NK-92 and Jeg-3 cells did not trigger initiator and effector caspases characteristic for the apoptosis development in Jeg-3 cells. The observed decrease in the content of procaspases in the trophoblasts may be associated with alternative non-apoptotic functions of these enzymes.
KEY WORDS: caspases, granzyme B, trophoblast, natural killers, apoptosisDOI: 10.1134/S0006297919100079
Abbreviations: CFSE, 5(6)-carboxyfluorescein diacetate N-succinimidyl ester; dNK cell, decidual natural killer cell; FITC, fluorescein isothiocyanate; IFN-γ, interferon γ; pNK cell, peripheral blood NK cell; TNF-α, tumor necrosis factor α.
Apoptosis plays a vital role in normal pregnancy and placenta formation.
Either inhibition or hyperactivation of apoptosis as a result of
disruption of its regulatory mechanisms could cause the development of
obstetric complications and underlie pathogenesis of different diseases
of the fetus [1]. Currently, studies of apoptosis
in the placenta present a number of challenges associated with
acquiring primary biological material and estimating the contribution
of different cell populations to this process [2,
3]. That is why studies using various models of
interaction between trophoblasts and cells actively participating in
placental development (e.g., immune cells providing immunological
maternal tolerance to paternal antigens expressed by the trophoblasts)
are so important [4]. Considering that one of the
functions of natural killer (NK) cells involves destruction of
allogeneic cells, interaction between trophoblasts and NK cells has
attracted significant attention of researchers. A great body of
information on decidual NK cells (dNK) is now available, although the
origin of these cells is still poorly understood [5].
dNK cells contain large amounts of cytotoxic proteins, such as the pore-forming protein perforin, granulysin, and specific serine proteases, including granzymes A and B [6-9], and produce factors that mediate trophoblast invasion and angiogenesis. On the other hand, they can limit the extent of trophoblast invasion via cytotoxic action due to the secretion of tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) [10, 11]. The inhibitory effect of trophoblasts is considered nowadays as one of the main factors altering the cytotoxicity of NK cells in the placenta. A decrease in the cytotoxicity of NK cells to the level that does not cause trophoblast cytolysis is a required condition for normal invasion. However, the results of studies on the changes in the cytotoxic activity of NK cells caused by their interaction with various cells in the area of maternal-fetal interface are contradictory [6, 12-14].
There are several methods for evaluating the cytotoxic activity of NK cells. One of them involves activation of intracellular cysteine proteases (caspases) [15]. Considering the key role of caspases in the apoptotic cell death, as well as their participation in other biological processes (e.g., cell differentiation) [16], it seemed especially interesting to examine the pattern of caspase activation in the trophoblasts after their contact with NK cells in vitro.
The objective of this study was to evaluate the content of cytotoxic proteins in the NK-92 cells and to analyze activation of the initiator caspase-8 and effector caspase-3 in Jeg-3 trophoblasts in various coculturing models.
MATERIALS AND METHODS
Cells. Jeg-3 cell line (ATCC, USA) displaying morphological, phenotypical, and functional characteristics of invasive trophoblasts from the first trimester of pregnancy was used in the study [17, 18]. The cells were cultured in DMEM complete growth medium supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin, 0.5 mM L-glutamine, 1% essential amino acids in MEM (Minimum Essential Medium), and 1 mM sodium pyruvate (Sigma-Aldrich, USA). The cell monolayer was detached with a mixture of Versene and trypsin (1 : 1; Biolot, Russia). NK-92 cells (ATCC) displaying basic phenotypic and functional characteristics of activated NK cells [19, 20] were cultured in α-modification Minimum Essential Medium (α-MEM) containing 12.5% inactivated FBS, 12.5% inactivated horse serum, 0.02 mM folic acid, 0.2 mM myo-inositol, 2 mM L-glutamine, 100 μg/ml streptomycin, 100 U/ml penicillin, 10 mM HEPES, 0.1 mM 2-mercaptoethanol (Sigma-Aldrich), and 500 IU/ml recombinant IL-2 (Roncoleukin; Biotekh, Russia). The cells were cultured in an incubator in a humid atmosphere containing 5% CO2 at 37°C. Cell viability was examined using Trypan blue and maintained at >96%.
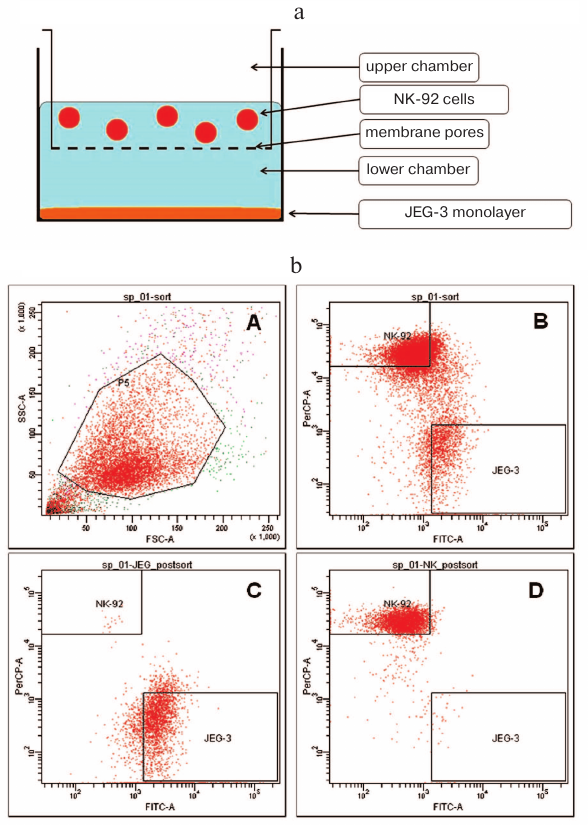
Distant coculturing of NK cells and trophoblasts. In order to prevent direct contact between NK-92 and Jeg-3 cells, we used the transwell experimental model [21] using 6-well plates with polycarbonate membrane inserts (pore diameter, 1 μm; membrane surface area, 4.8 cm2) (BD, USA) that ensured cell–cell interactions via soluble factors only. Jeg-3 cells were seeded at a concentration of 1.2·106 cells per well in 8 ml of DMEM supplemented with 10% FBS and incubated for 24 h to form a confluent monolayer in the lower chamber (well in a 6-well plate). On the second day, 4 ml of the medium was removed from each well and polycarbonate membrane inserts were placed into the wells. NK-92 cells in 4 ml of α-MEM supplemented with 12.5% FBS were added to each insert (1.8·106 cells per insert) (Fig. 1a). The cells were incubated for another 24 h in a humid atmosphere containing 5% CO2 at 37°C. Individually cultured NK-92 and Jeg-3 cells were used as controls.
Fig. 1. Strategy used for conducting experiments on distant and contact coculturing of Jeg-3 and NK-92 cells. a) Schematic representation of experimental setup for the distant coculturing of Jeg-3 and NK-92 cells in a 6-well plate. b) Gating strategy for the NK-92 and Jeg-3 cells used for their sorting following contact coculturing: A) presort of NK-92 cells treated with anti-CD45(PerCP) antibodies and CFSE (FITC)-labeled Jeg-3 cells; density plot in FSC–SSC coordinates, gate P5 contains NK-92 and Jeg-3 cells; B) density plot in PerCP–FITC coordinates. Quadrant NK-92 contains NK-92 cells labeled with CD45(PerCP). Quadrant Jeg-3 contains Jeg-3 cells labeled with CFSE (FITC); C) postsort of Jeg-3 cells in PerCP–FITC coordinates; D) postsort of NK-92 cells in PerCP–FITC coordinates.
Contact coculturing of NK cells and trophoblasts. Jeg-3 cells were cultured in 75-cm2 flasks (BD) in complete DMEM at a concentration 2·106 cells/ml for 24 h to 50% confluency and then stained with 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE) according to the manufacturer’s instructions (Sigma, USA). Next, NK-92 cells were introduced at a concentration of 3·106 cells per flask with the CFSE-stained cells and incubated for 24 h in a humid atmosphere containing 5% CO2 at 37°C. Following incubation, the cells were collected by treating with Versene solution. NK-92 and Jeg-3 cells cultured separately under identical conditions were used as controls. Experiments with contact and distant coculturing were carried out four times each; in each experiment, four replicates of cocultured and control intact cells were used.
Cell sorting. After contact coculturing, NK-92 and Jeg-3 cells were sorted with a FacsAriaIII flow cytometer (BD). Cell suspension in the Versene solution was washed three times with warm DMEM using centrifugation at 200g. Next, the suspension was treated with antibodies against CD45 (PerCP) in accordance with the manufacturer’s instructions (BD). Non-specific binding was evaluated using antibodies for the isotopic control (BD). The cells were sorted according to the Purity protocol (Fig. 1b) with the flow cell tip diameter of 85 μm. NK-92 cells were identified as CD45-positive (CD45+)(PerCP); Jeg-3 cells – as CFSE (FITC)-labeled. The cells were sorted based on the forward and side scattering in the FITC and PerCP channels. Fluorescence gating was set based on the analysis of samples treated with the isotype control. Target cells for sorting (presort) were identified based on the analysis the samples treated with the antibodies. To control the quality of sorting of cells from different populations, the postsort procedure was employed according to the manufacturer’s instructions. In all cases, the purity of sorted NK-92 and Jeg-3 cells was no less than 99.0 ± 1.0%. The fraction of viable cells after sorting was evaluated by the 7-AAD staining (Biolegend, USA) and comprised 91.6 ± 2.9%.
Immunoblotting. The cells were washed three times with cold 0.01 M phosphate buffer (pH 7.4) and lysed in RIPA buffer (50 mM Tris-HCl, pH 8.1, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, 1 mM EDTA, 150 mM NaCl) containing protease inhibitor cocktail (Roche, Switzerland). The samples were centrifuged for 10 min at 16,000g to remove cell debris. Total protein concentration in the samples was estimated using Bradford protein assay [22]. The lysates were fractionated by electrophoresis in 10% polyacrylamide gel according to Laemmli and transferred onto a PVDF membrane. The amount of procaspases and active caspases was evaluated using specific primary antibodies against caspase-8 [caspase-8 (1C12) mouse Ab, 1 : 1000; Cell Signaling, USA] and caspase-3 (caspase-3 rabbit Ab, 1 : 1000; Cell Signaling). Granzyme B and perforin in the lytic granules were stained with anti-granzyme B monoclonal mouse antibody (1 : 1000; Biolegend) and anti-human perforin mouse antibody (1 : 1000; Bioscience Inc., USA), respectively. After incubation with HRP-conjugated goat anti-rabbit or goat-anti-mouse antibodies (1 : 1000; BioRad, USA), the signals were recorded using enhanced chemiluminescence (ECL GE, Sweden). The obtained data were normalized to either β-actin (rabbit Ab against β-actin; Cell Signaling), or glyceraldehyde-3-phosphate dehydrogenase [GAPDH (14C10) rabbit Ab, 1 : 1000; Cell Signaling]. Caspase-3 activation was assessed as a ratio of the active caspase-3 fragment (p17) produced as a result of protein cleavage to the endogenous level of inactive caspase-3 proenzyme (p35). Caspase-8 activation was calculated as a ratio of its active fragment (p18) to the procaspase-8 (p57) and expressed in arbitrary units.
Statistical data processing was carried out using STATISTICA 10.0 program. The results were compared using the non-parametric Mann–Whitney U-criterion or t-test for independent samples. The data were tested for the normality of distribution using the Shapiro–Wilk test; and the uniformity of dispersion was assessed with the Levene’s test. Variables with normal distribution are presented as mean ± standard deviation (M ± sd). In the absence of normal distribution, medians were used (25th-75th percentiles).
RESULTS
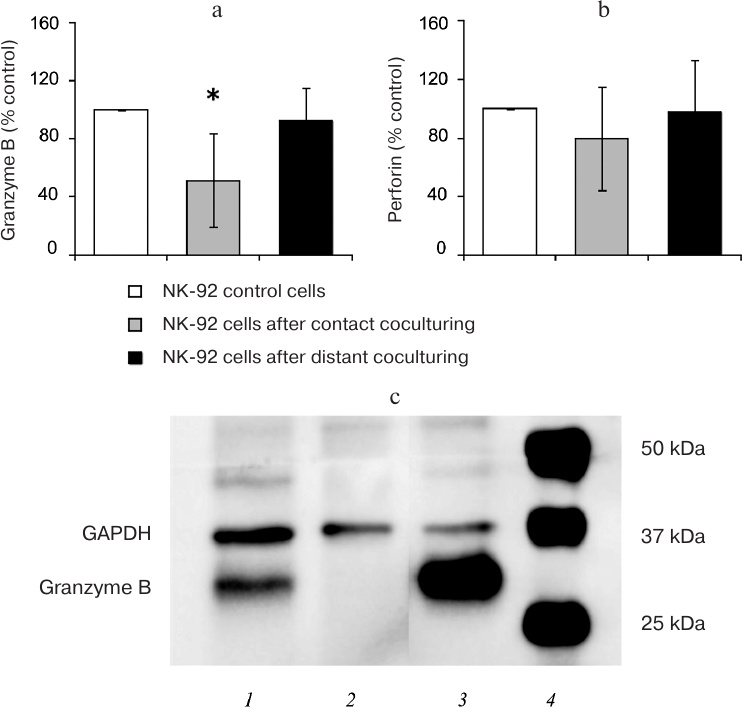
The data presented in Fig. 2 demonstrate that the amount of cytotoxic protein granzyme B was reduced in the lysates of NK-92 cells (n = 6) after contact coculturing, while the content of perforin remained the same as in the control NK-92 cells. No differences were observed between the content of granzyme B and perforin in control cells and NK-92 cells after distant coculturing with Jeg-3 cells (n = 6). Immunoblot staining revealed the presence of granzyme B in Jeg-3 cells following contact, but not distant coculturing with NK-92 cells.
Fig. 2. Analysis of the cytotoxic protein content. Quantitative assessment of the granzyme B (a) and perforin (b) content in the lysates of NK-92 cells following contact and distant coculturing with Jeg-3 cells and intact NK-92 cells (control, 100%); * p < 0.05, difference with the control. c) Immunostaining of Jeg-3 cells for granzyme B: 1) Jeg-3 cells after contact coculturing with NK-92 cell lines; 2) intact Jeg-3 cells (control); 3) intact NK-92 cells; 4) molecular weight markers.
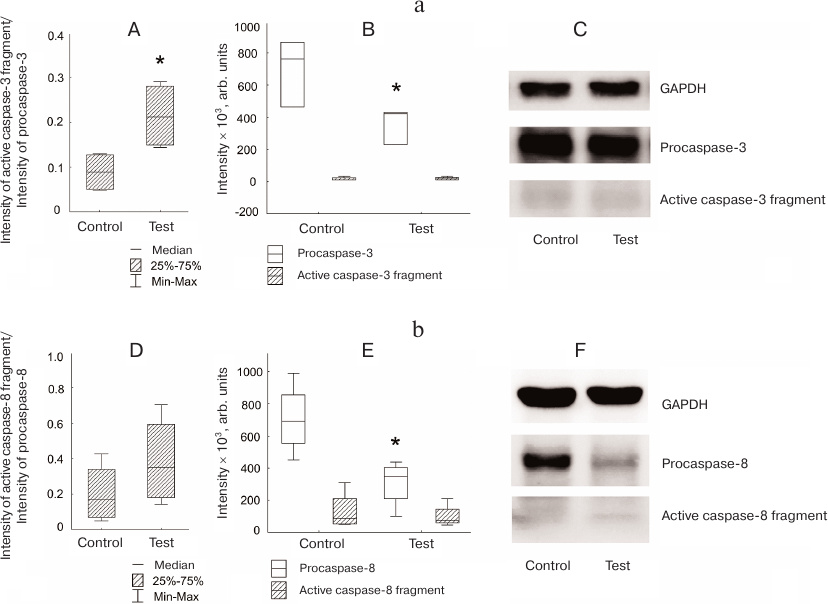
The amount of procaspase-3 in the Jeg-3 cells after distant coculturing with NK-92 cells was lower than in the intact Jeg-3 cells (control) (p < 0.05). Despite the difference in the ratio of the active caspase-3 fragment to procaspase-3 in the control and cocultured Jeg-3 cells, no significant differences were observed in the levels of active caspase-3 fragment in the lysates of Jeg-3 cells after distant coculturing with NK-92 cells and control Jeg-3 cells (n = 4) (Fig. 3a). The content of procaspase-8 in the Jeg-3 cells distant coculturing with NK-92 cells for 24 h was reduced in comparison with the intact Jeg-3 cells. The content of active caspase-8 fragment in the Jeg-3 cells after their distant coculturing with NK-92 cells was the same as in the control Jeg-3 cells. No differences were observed in the activation of caspase-8 expressed as a ratio of the intensity of the active caspase-8 fragment staining to the intensity of procaspase-8 staining in Jeg-3 cells after distant coculturing with NK-92 cells and intact Jeg-3 cells (n = 4) (Fig. 3b).
Fig. 3. Activation of caspases in the Jeg-3 cells following distant coculturing with NK-92 cells. a) Caspase-3 activation in Jeg-3 cells: A) ratio of the intensity of active caspase-3 fragment staining to the intensity of procaspase-3 staining in intact Jeg-3 cells (control) and Jeg-3 cells after distant coculturing with NK-92 cells (test); B) effect of NK-92 cells on the intensity of caspase-3 active fragment and procaspase-3 staining in Jeg3 cells after distant coculturing; C) immunoblot of caspase-3 in Jeg-3 cells. b) Caspase-8 activation in Jeg-3 cells: D) ratio of the intensity of active caspase-8 fragment staining to the intensity of procaspase-8 staining in intact Jeg-3 cells (control) and Jeg-3 cells after distant coculturing with NK-92 cells (test); E) effect of NK-92 cells on the intensity of caspase-8 active fragment and procaspase-8 staining in Jeg3 cells after distant coculturing; F) immunoblot of caspase-8 in Jeg-3 cells; * p < 0.05, difference with the control.
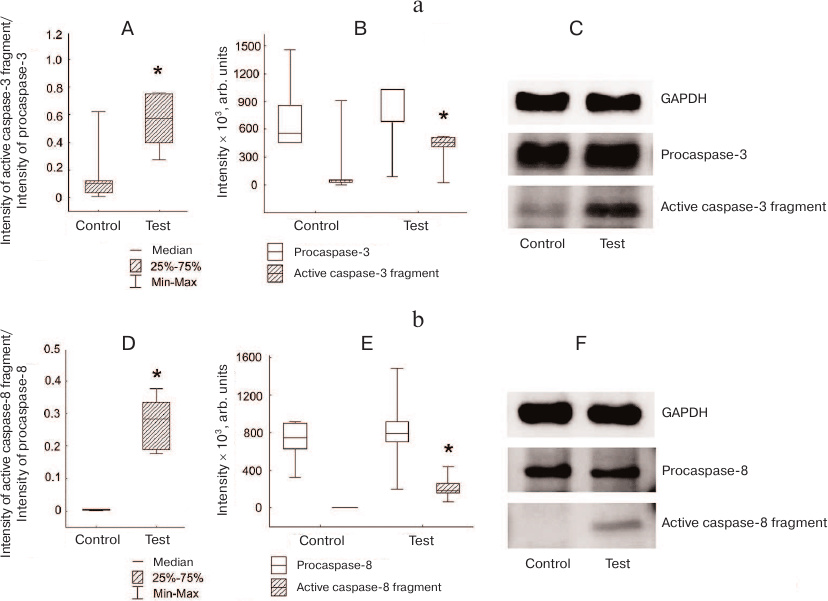
Increased caspase-3 activation was observed in Jeg-3 cells after contact coculturing with NK-92 cells in comparison with intact Jeg-3 cells. The ratio between the amounts (staining intensities) of active caspase-3 fragment and procaspase-3 was higher in Jeg-3 cell after contact coculturing with NK-92 cells than in intact Jeg-3 cells (p < 0.05) (n = 4). Unlike distant coculturing, changes in this parameter were associated with the increased level of caspase-3 active fragment in Jeg-3 cells after contact coculturing with NK-92 cells in comparison with the control Jeg-3 cells. No significant difference in the intensity of procaspase-3 band in the control and cocultured Jeg-3 cells was observed (n = 4) (Fig. 4a).
Fig. 4. Activation of caspases in Jeg-3 cells following contact coculturing with NK-92 cells. a) Caspase-3 activation in Jeg-3 cells: A) ratio of the intensity of active fragment of caspase-3 staining to the intensity of procaspase-3 staining in intact Jeg-3 cells (control) and Jeg-3 cells after contact coculturing with NK-92 cells (test); B) effect of NK-92 cells on the intensity of caspase-3 active fragment and procaspase-3 staining in Jeg3 cells after contact coculturing; C) immunoblot of caspase-3 in Jeg-3 cells. b) Caspase-8 activation in Jeg-3 cells: D) ratio of the intensity of active caspase-8 fragment staining to the intensity of procaspase-8 staining in intact Jeg-3 cells (control) and Jeg-3 cells after contact coculturing with NK-92 cells (test); E) effect of NK-92 cells on the intensity of caspase-8 active fragment and procaspase-8 staining in Jeg3 cells after contact coculturing; F) immunoblot of caspase-8 in Jeg-3 cells; * p < 0.05, difference with the control.
Simultaneously with the appearance of the active caspase-3 fragment, we observed an increase in the degree of activation of caspase-8 (ratio of active caspase-8 fragment to procaspase-8) in Jeg-3 cells after contact coculturing with NK-92 cells in comparison to intact Jeg-3 cells (p < 0.05) (n = 4). The change in this ratio was associated only with the appearance of the caspase-8 fragment p18 in the cocultured cells (p < 0.05), since no differences were observed between the intensity of procaspase-8 staining in Jeg-3 cells after contact coculturing with NK-92 cells and in intact Jeg-3 cells (n = 4) (Fig. 4b).
DISCUSSION
Protection of semi-allogeneic trophoblasts from death caused by their interaction with cytotoxic cells of the maternal immune system is a strategic physiological task accomplished by the organism during pregnancy. That is why trophoblasts possess a wide variety of protective mechanisms aimed at maintaining their viability. Mutual effects of trophoblasts and NK cells can be evaluated when the cells are brought into contact. The immunoregulatory (mainly immunosuppressive) role of compounds synthesized by trophoblasts (syncytin-1, NKG2D receptor ligand, and others) toward the maternal immune system has been demonstrated recently [23, 24]. It was also found that trophoblasts produce IL-10 [25, 26] and TGFβ [27].
NK cells, peripheral blood NK (pNK) cells, and NK-92 cell used in this study become activated upon interaction with other cells in which they are recognized as target cells. Numerous cytotoxic proteins are released into the cytoplasm of the target cell as a result of the immunological synapse formation, which, in turn, induces apoptosis [28-30]. It is possible that NK-92 cells can exhibit their cytotoxic effect on trophoblasts during contact coculturing. This suggestion was corroborated by the fact that granzyme B was observed in Jeg-3 cells following their contact culturing with NK-92 cells. The presence of granzyme B in Jeg-3 cells is accompanied by a decrease in its content in NK-92 cells, which indicates targeted secretion of this enzyme.
In addition, NK cells can activate apoptosis in the target cells by introducing death receptors, such as proteins of the tumor necrosis factor receptor family (TNFR) including Fas (CD95), to the cell surface [31-33]. We demonstrated that contact coculturing of NK-92 and Jeg-3 cells resulted in the activation of the contact-dependent apoptosis mediated by the initiator caspase-8 in the Jeg-3 cells. It was shown that the trophoblasts express Fas and other death receptors (TRAIL-R1 and TRAIL-R2) on their surface [34]. Caspase-8 has been initially characterized as an initiator of the external apoptotic pathway. Later, observations of caspase-8 activation in many types of tumors have resulted in the discovery of its alternative non-apoptotic functions [35, 36]. Caspase-8 is completely activated only after a two-step process involving initial dimerization of procaspase-8. In this step, the enzyme displays low catalytic activity toward a narrow range of substrates. Full activation requires the second proteolytic step. Autoprocessing produces small p10 subunit and intermediate p43 subunit (DEDs-p18). It is followed by the cleavage of the death effector domains (DEDs) resulting in the release of the large p18 subunit with the cysteine-containing active site (as detected by immunoblotting technique) [37]. This increases the range of enzyme substrates, including caspase-3 [38]. The presence of p18 subunits in the cells is usually considered as a marker of caspase-8 activation. Many non-apoptotic functions of caspase-8 depend on the phosphorylation of tyrosine 380 residue located in the linker between the large and small enzyme subunits, which promotes cell resistance to the CD95-induced apoptosis. It has been suggested that the suppression of apoptosis in this case is due to the inhibition of caspase-8 cleavage, which is manifested by a decrease in the formation of p18 or its complete disappearance [37, 39]. It was shown that caspase-8 activation accompanied by the expression of phosphatidylserine on the cytoplasmic membrane outer side is not always associated with the cell death. A similar process in trophoblasts could be a consequence of cell fusion and formation of syncytial layer [40], as demonstrated in the in vitro JAR choriocarcinoma model [41]. However, JAR cells (similarly to Jeg-3 cells) do not undergo massive syncytialization as, for example, BeWo cells [42-44]. Furthermore, it was shown for the forskolin-mediated fusion of BeWo cells, syncytialization is accompanied by a decrease of the levels of procaspase-3 (after 24-h culturing) and procaspase-8 (after 48-h culturing) due to the downregulated expression of these proteins. This was supported by the observed decrease in the transcription of the respective mRNA during syncytium formation and by the absence of active fragments of caspase-3 and caspase-8 [42]. We also detected the decrease in the levels of procaspase-3 and procaspase-8 and the absence of active fragments of the respective caspases in Jeg-3 cells following distant coculturing with NK-92 cells. Based on these facts, we suggest that the process of syncytium formation by Jeg-3 cells can be enhanced by distant coculturing with NK-92 cell, but this hypothesis requires further investigation. The levels of procaspase-3 and procaspase-8 in the experiments on the contact coculturing of Jeg-3 and NK-92 cells were the same as in the control Jeg-3 cells. It is possible that NK-92 cells stimulate the cleavage of procaspase-8 in Jeg-3 cells during contact coculturing, likely via activation of death receptors. At the same time, it was demonstrated that Jeg-3 cells contain large amounts of mRNA for the TRAIL-R3 receptor, which does not have functional death domains and operates as a “bait” [45]. It was also found that choriocarcinoma cells are resistant to TRAIL [46]. Hence, these observations require further investigation as well.
Each step of the apoptotic cascade in trophoblasts is strictly controlled by endogenous inhibitors. Some of these inhibitors demonstrate significant homology with caspase-8, which allows them to block its activation and prevent receptor-dependent apoptosis [1]. A number of researchers consider that the cleavage of procaspase detected by immunoblotting does not imply that its activation [47]. They emphasize that the absolute requirement of activation is dimerization, and that the cleavage in the absence of dimerization does not result in activation [47-49]. That is why evaluation of caspase-8 activity by immunoblotting technique should involve assessment of caspase-3 activation in addition to the detection of the cleaved fragment p18. It is also important to examine the enzymatic activity of this protein using specific substrates [50]. However, we could not use this assay because of the cross-reactivity of caspase-8 and granzyme B towards the majority of commonly used substrates containing the Ile-Glu-Thr-Asp sequence. Moreover, the mechanisms of apoptosis inactivation in trophoblasts take place downstream of caspase-8 activation [51]. At the same time, the cleavage of caspase-3 in Jeg-3 cells following their contact coculturing with the NK-92 cells is an indicator of irreversible apoptosis development. This fact can cast some doubt on the suggestion that apoptosis inactivation in our experiments on contact coculturing was mediated by trophoblasts. Caspase-3 activation can be associated not only with the action of caspase-8 but results from other events. For example, it can be activated directly by the lytic granule proteins delivered from NK cells as a result of immunological synapse formation [52]. As mentioned above, the substrate specificity of granzyme B is similar to that of caspase-8; hence, granzyme B can cleave zymogen forms of other caspases (e.g., caspase-3) [53-55]. It can also activate caspases via mitochondrial pathway by stimulating the release of cytochrome c and other apoptogenic molecules to the cytoplasm with subsequent activation of caspase-9 and then caspase-3 [56]. Nevertheless, the observed markers of apoptosis in the trophoblasts in the presence of NK cells require detailed investigation because trophoblasts have a mechanism protecting them from NK cells that involves expression of HLA-G and HLA-E [57]. Trophoblasts also express death receptor ligand both on the surface and in soluble form [1]. Besides, it should be taken into account that NK-92 cells used in this in vitro model differ from pNK or dNK cells.
Taking this into consideration, we based our conclusions on our studies of pNK cells [58] and results reported by other researchers [59] that suggest the possibility of extrapolation of this type of data to the maternal NK cells, because NK-92 cells display main phenotypical and functional characteristics of human NK cells.
It is possible that the cytotoxic response of NK-92 cells toward the trophoblasts could be associated with the insufficiency of inhibitory signaling from Jeg-3 cells. It has been found that NK-92 cells do not express receptors of the KIR family on their surface [60]. However, low-level expression of KIR2DL4 and ILT-2/CD85 that recognize directly the trophoblast HLA-G has been observed in NK-92 cells, and this expression was upregulated by coculturing with the BeWo trophoblasts [4]. HLA-E receptors NKG2A and NRG2B are actively expressed by NK-92 cells [60]. Hence, despite the fact that NK-92 cells are considered as cells with enhanced cytotoxic activity and high level of cytotoxic proteins, their functioning can be inhibited by trophoblasts via various mechanisms.
No changes in the content of cytotoxic proteins in the NK-92 cells were observed during their distant coculturing with Jeg-3 cells. It was shown that changes in the array of cytokines secreted by dNK cells occurred only during their contact but not distant coculturing with trophoblasts [61]. Accumulation of granulysin from dNK cell in the EVT cells (the most invasive cells of embryonic origin) was demonstrated following their contact coculturing, which caused activation of apoptosis in the EVT cells [52]. The importance of cell–cell contacts (and not only soluble factors) in the functioning of trophoblasts has been also reported in other studies [62]. The data obtained in this work confirm the role of contact interaction between the trophoblasts and NK cells.
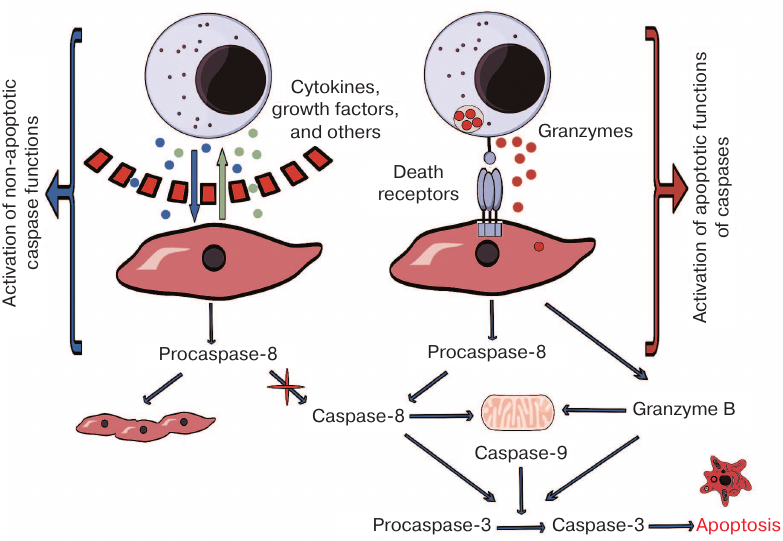
The outcomes of the interaction between NK-92 and Jeg-3 cells in the presence or absence of direct contact between them, as well as the suggested mechanisms of caspase activation in Jeg-3 trophoblasts, are presented in Fig. 5. Our results indicate that contact coculturing of Jeg-3 and NK-92 cells in vitro is characterized by the granzyme B secretion by NK-92 cells, which results in the activation of caspases and could cause the death of trophoblasts. The pattern of changes in the levels of caspase-3 and caspase-8 in the absence of direct contact between the populations of these cells suggests possible activation of processes associated with the syncytium formation by Jeg-3 cells.
Fig. 5. Mechanism of caspase activation in Jeg-3 cells following in vitro distant or contact coculturing with NK-92 cells.
Funding. This work was supported by the Science School grant NSh-2873.2018.7 and State Budget Project no. AAAA-A19-119021290116-1.
Conflict of interest. The authors declare no conflict of interest in financial or any other sphere.
Ethical approval. This article does not contain description of any studies with participants of humans or animals performed by any of the authors.
REFERENCES
1.Straszewski-Chavez, S. L., Abrahams, V. M., and
Mor, G. (2005) The role of apoptosis in the regulation of trophoblast
survival and differentiation during pregnancy, Endocr. Rev.,
26, 877-897; doi: 10.1210/er.2005-0003.
2.Romanski, A., Uherek, C., Bug, G., Seifried, E.,
Klingemann, H., Wels, W. S., Ottmann, O. G., and Tonn, T. (2016)
CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell
resistance in B-cell malignancies, J. Cell. Mol. Med.,
20, 1287-1294; doi: 10.1111/jcmm.12810.
3.Crespo, A. C., Strominger, J. L., and Tilburgs, T.
(2016) Expression of KIR2DS1 by decidual natural killer cells increases
their ability to control placental HCMV infection, Proc. Natl. Acad.
Sci. USA, 113, 15072-15077; doi:
10.1073/pnas.1617927114.
4.Liu, Y., Zhang, L., Gao, M., Zhang, F., Xu, X.,
Liu, X., and Hu, X. (2013) Changes of inhibitory receptors on NK-92
cells and HLA-G on BeWo cells with Toxoplasma gondii infection,
Inflammation, 36, 1440-1447; doi:
10.1007/s10753-013-9684-1.
5.Matson, B. C., and Caron, K. M. (2014) Uterine
natural killer cells as modulators of the maternal–fetal
vasculature, Int. J. Dev. Biol., 58, 199-204; doi:
10.1387/ijdb.140032kc.
6.Manaster, I., and Mandelboim, O. (2010) The unique
properties of uterine NK cells, Am. J. Reprod. Immunol.,
63, 434-444; doi: 10.1111/j.1600-0897.2009.00794.x.
7.Vacca, P., Pietra, G., Falco, M., Romeo, E.,
Bottino, C., Bellora, F., Prefumo, F., Fulcheri, E., Venturini, P. L.,
Costa, M., Moretta, A., Moretta, L., and Mingari, M. C. (2006) Analysis
of natural killer cells isolated from human decidua: evidence that 2B4
(CD244) functions as an inhibitory receptor and blocks NK-cell
function, Blood, 108, 4078-4085; doi:
10.1182/blood-2006-04-017343.
8.Koopman, L. A., Kopcow, H. D., Rybalov, B., Boyson,
J. E., Orange, J. S., Schatz, F., Masch, R., Lockwood, C. J.,
Schachter, A. D., Park, P. J., and Strominger, J. L. (2003) Human
decidual natural killer cells are a unique NK cell subset with
immunomodulatory potential, J. Exp. Med., 198, 1201-1212;
doi: 10.1084/jem.20030305.
9.King, A., Wooding, P., Gardner, L., and Loke, Y. W.
(1993) Expression of perforin, granzyme A and TIA-1 by human uterine
CD56+ NK cells implies they are activated and capable of
effector functions, Hum. Reprod., 8, 2061-2067; doi:
10.1093/oxfordjournals.humrep.a137982.
10.Lash, G. E., Robson, S. C., and Bulmer, J. N.
(2010) Review: functional role of uterine natural killer (uNK) cells in
human early pregnancy decidua, Placenta, 31 (Suppl.),
87-92; doi: 10.1016/j.placenta.2009.12.022.
11.Ivanisevic, M., Segerer, S., Rieger, L., Kapp,
M., Dietl, J., Kammerer, U., and Frambach, T. (2010) Antigen-presenting
cells in pregnant and non-pregnant human myometrium, Am. J. Reprod.
Immunol., 64, 188-196; doi:
10.1111/j.1600-0897.2010.00858.x.
12.Kopcow, H. D., Allan, D. S., Chen, X., Rybalov,
B., Andzelm, M. M., Ge, B., and Strominger, J. L. (2005) Human decidual
NK cells form immature activating synapses and are not cytotoxic,
Proc. Natl. Acad. Sci. USA, 102, 15563-15568; doi:
10.1073/pnas.0507835102.
13.Redzovic, A., Laskarin, G., Dominovic, M.,
Haller, H., and Rukavina, D. (2013) Mucins help to avoid alloreactivity
at the maternal fetal interface, Clin. Dev. Immunol.,
2013, 542152; doi: 10.1155/2013/542152.
14.Sun, J., Yang, M., Ban, Y., Gao, W., Song, B.,
Wang, Y., Zhang, Y., Shao, Q., Kong, B., and Qu, X. (2016) Tim-3 is
upregulated in NK cells during early pregnancy and inhibits NK
cytotoxicity toward trophoblast in galectin-9 dependent pathway,
PLoS One, 11, e0147186; doi:
10.1371/journal.pone.0147186.
15.Langhans, B., Ahrendt, M., Nattermann, J.,
Sauerbruch, T., and Spengler, U. (2005) Comparative study of NK
cell-mediated cytotoxicity using radioactive and flow cytometric
cytotoxicity assays, J. Immunol. Methods, 306, 161-168;
doi: 10.1016/j.jim.2005.08.010.
16.Shalini, S., Dorstyn, L., Dawar, S., and Kumar,
S. (2015) Old, new and emerging functions of caspases, Cell Death
Differ., 22, 526-539; doi: 10.1038/cdd.2014.216.
17.Jingting, C., Yangde, Z., Yi, Z., Huining, L.,
Rong, Y., and Yu, Z. (2007) Heparanase expression correlates with
metastatic capability in human choriocarcinoma, Gynecol. Oncol.,
107, 22-29; doi: 10.1016/j.ygyno.2007.05.042.
18.Kohler, P. O., and Bridson, W. E. (1971)
Isolation of hormone-producing clonal lines of human choriocarcinoma,
J. Clin. Endocrinol. Metab., 32, 683-687; doi:
10.1210/jcem-32-5-683.
19.Gong, J. H., Maki, G., and Klingemann, H. G.
(1994) Characterization of a human cell line (NK-92) with phenotypical
and functional characteristics of activated natural killer cells,
Leukemia, 8, 652-658.
20.Komatsu, F., and Kajiwara, M. (1998) Relation of
natural killer cell line NK-92-mediated cytolysis (NK-92-lysis) with
the surface markers of major histocompatibility complex class I
antigens, adhesion molecules, and Fas of target cells, Oncol.
Res., 10, 483-489.
21.Coulomb-L’Hermine, A., Larousserie, F.,
Pflanz, S., Bardel, E., Kastelein, R. A., and Devergne, O. (2007)
Expression of interleukin-27 by human trophoblast cells,
Placenta, 28, 1133-1140; doi:
10.1016/j.placenta.2007.06.004.
22.Bradford, M. M. (1976) A rapid and sensitive
method for the quantitation of microgram quantities of protein
utilizing the principle of protein–dye binding, Anal.
Biochem., 72, 248-254.
23.Hedlund, M., Stenqvist, A. C., Nagaeva, O.,
Kjellberg, L., Wulff, M., Baranov, V., and Mincheva-Nilsson, L. (2009)
Human placenta expresses and secretes NKG2D ligands via exosomes that
down-modulate the cognate receptor expression: evidence for
immunosuppressive function, J. Immunol., 183, 340-351;
doi: 10.4049/jimmunol.0803477.
24.Lokossou, A. G., Toudic, C., and Barbeau, B.
(2014) Implication of human endogenous retrovirus envelope proteins in
placental functions, Viruses, 6, 4609-4627; doi:
10.3390/v6114609.
25.Hakam, M. S., Miranda-Sayago, J. M., Hayrabedyan,
S., Todorova, K., Spencer, P. S., Jabeen, A., Barnea, E. R., and
Fernandez, N. (2017) Preimplantation factor (PIF) promotes HLA-G, -E,
-F, -C expression in JEG-3 choriocarcinoma cells and endogenous
progesterone activity, Cell. Physiol. Biochem., 43,
2277-2296; doi: 10.1159/000484378.
26.Hanna, N., Hanna, I., Hleb, M., Wagner, E.,
Dougherty, J., Balkundi, D., Padbury, J., and Sharma, S. (2000)
Gestational age-dependent expression of IL-10 and its receptor in human
placental tissues and isolated cytotrophoblasts, J. Immunol.,
164, 5721-5728.
27.Knofler, M., and Pollheimer, J. (2012) IFPA award
in placentology lecture: molecular regulation of human trophoblast
invasion, Placenta, 33, 55-62; doi:
10.1016/j.placenta.2011.09.019.
28.Rousalova, I., and Krepela, E. (2010) Granzyme
B-induced apoptosis in cancer cells and its regulation (review),
Int. J. Oncol., 37, 1361-1378.
29.Thiery, J., Keefe, D., Saffarian, S.,
Martinvalet, D., Walch, M., Boucrot, E., Kirchhausen, T., and
Lieberman, J. (2010) Perforin activates clathrin- and dynamin-dependent
endocytosis, which is required for plasma membrane repair and delivery
of granzyme B for granzyme-mediated apoptosis, Blood,
115, 1582-1593; doi: 10.1182/blood-2009-10-246116.
30.Thiery, J., Keefe, D., Boulant, S., Boucrot, E.,
Walch, M., Martinvalet, D., Goping, I. S., Bleackley, R. C.,
Kirchhausen, T., and Lieberman, J. (2011) Perforin pores in the
endosomal membrane trigger the release of endocytosed granzyme B into
the cytosol of target cells, Nat. Immunol., 12, 770-777;
doi: 10.1038/ni.2050.
31.Lieberman, J. (2003) The ABCs of granule-mediated
cytotoxicity: new weapons in the arsenal, Nat. Rev. Immunol.,
3, 361-370; doi: 10.1038/nri1083.
32.Orange, J. S., and Ballas, Z. K. (2006) Natural
killer cells in human health and disease, Clin. Immunol.,
118, 1-10; doi: 10.1016/j.clim.2005.10.011.
33.Hazeldine, J., and Lord, J. M. (2013) The impact
of ageing on natural killer cell function and potential consequences
for health in older adults, Ageing Res. Rev., 12,
1069-1078; doi: 10.1016/j.arr.2013.04.003.
34.Hammer, A., and Dohr, G. (2000) Expression of
Fas-ligand in first trimester and term human placental villi, J.
Reprod. Immunol., 46, 83-90.
35.Salvesen, G. S., and Walsh, C. M. (2014)
Functions of caspase 8: the identified and the mysterious, Semin.
Immunol., 26, 246-252; doi: 10.1016/j.smim.2014.03.005.
36.Feltham, R., Vince, J. E., and Lawlor, K. E.
(2017) Caspase-8: not so silently deadly, Clin. Transl.
Immunol., 6, e124; doi: 10.1038/cti.2016.83.
37.Cursi, S., Rufini, A., Stagni, V., Condo, I.,
Matafora, V., Bachi, A., Bonifazi, A. P., Coppola, L., Superti-Furga,
G., Testi, R., and Barila, D. (2006) Src kinase phosphorylates
caspase-8 on Tyr380: a novel mechanism of apoptosis suppression,
EMBO J., 25, 1895-1905; doi:
10.1038/sj.emboj.7601085.
38.Powley, I. R., Hughes, M. A., Cain, K., and
MacFarlane, M. (2016) Caspase-8 tyrosine-380 phosphorylation inhibits
CD95 DISC function by preventing procaspase-8 maturation and cycling
within the complex, Oncogene, 35, 5629-5640; doi:
10.1038/onc.2016.99.
39.Cohen, G. M. (1997) Caspases: the executioners of
apoptosis, Biochem. J., 326, Pt. 1, 1-16.
40.Gauster, M., and Huppertz, B. (2010) The paradox
of caspase 8 in human villous trophoblast fusion, Placenta,
31, 82-88; doi: 10.1016/j.placenta.2009.12.007.
41.Adler, R. R., Ng, A. K., and Rote, N. S. (1995)
Monoclonal antiphosphatidylserine antibody inhibits intercellular
fusion of the choriocarcinoma line, JAR, Biol. Reprod.,
53, 905-910; doi: 10.1095biolreprod53.4.905.
42.Wei, B. R., Xu, C., and Rote, N. S. (2012)
Increased resistance to apoptosis during differentiation and
syncytialization of BeWo choriocarcinoma cells, Adv. Biosci.
Biotechnol., 3, 805-813; doi: 10.4236/abb.2012.326100.
43.Borges, M., Bose, P., Frank, H. G., Kaufmann, P.,
and Potgens, A. J. (2003) A two-colour fluorescence assay for the
measurement of syncytial fusion between trophoblast-derived cell lines,
Placenta, 24, 959-964; doi:
10.1016/S0143-4004(03)00173-5.
44.Al-Nasiry, S., Spitz, B., Hanssens, M., Luyten,
C., and Pijnenborg, R. (2006) Differential effects of inducers of
syncytialization and apoptosis on BeWo and JEG-3 choriocarcinoma cells,
Hum. Reprod., 21, 193-201; doi:
10.1093/humrep/dei272.
45.Phillips, T. A., Ni, J., Pan, G., Ruben, S. M.,
Wei, Y. F., Pace, J. L., and Hunt, J. S. (1999) TRAIL (Apo-2L) and
TRAIL receptors in human placentas: implications for immune privilege,
J. Immunol., 162, 6053-6059.
46.Yusuf, K., Smith, S. D., Sadovsky, Y., and
Nelson, D. M. (2002) Trophoblast differentiation modulates the activity
of caspases in primary cultures of term human trophoblasts, Pediatr.
Res., 52, 411-415; doi:
10.1203/00006450-200209000-00018.
47.Van Raam, B. J., and Salvesen, G. S. (2012)
Proliferative versus apoptotic functions of caspase-8: hetero or homo:
the caspase-8 dimer controls cell fate, Biochim. Biophys. Acta,
1824, 113-122; doi: 10.1016/j.bbapap.2011.06.005.
48.Keller, N., Grutter, M. G., and Zerbe, O. (2010)
Studies of the molecular mechanism of caspase-8 activation by solution
NMR, Cell Death Differ., 17, 710-718; doi:
10.1038/cdd.2009.155.
49.Pop, C., Fitzgerald, P., Green, D. R., and
Salvesen, G. S. (2007) Role of proteolysis in caspase-8 activation and
stabilization, Biochemistry, 46, 4398-4407; doi:
10.1021/bi602623b.
50.Carrillo, I., Droguett, D., Castillo, C., Liempi,
A., Munoz, L., Maya, J. D., Galanti, N., and Kemmerling, U. (2016)
Caspase-8 activity is part of the BeWo trophoblast cell defense
mechanisms against Trypanosoma cruzi infection, Exp.
Parasitol., 168, 9-15; doi:
10.1016/j.exppara.2016.06.008.
51.Barnhart, B. C., and Peter, M. E. (2002) Two
faces of caspase-8, Nat. Immunol., 3, 896-898; doi:
10.1038/ni1002-896.
52.Nakashima, A., Shiozaki, A., Myojo, S., Ito, M.,
Tatematsu, M., Sakai, M., Takamori, Y., Ogawa, K., Nagata, K., and
Saito, S. (2008) Granulysin produced by uterine natural killer cells
induces apoptosis of extravillous trophoblasts in spontaneous abortion,
Am. J. Pathol., 173, 653-664; doi:
10.2353/ajpath.2008.071169.
53.Sweeney, E. A., Inokuchi, J., and Igarashi, Y.
(1998) Inhibition of sphingolipid induced apoptosis by caspase
inhibitors indicates that sphingosine acts in an earlier part of the
apoptotic pathway than ceramide, FEBS Lett., 425,
61-65.
54.Estebanez-Perpina, E., Fuentes-Prior, P.,
Belorgey, D., Braun, M., Kiefersauer, R., Maskos, K., Huber, R., Rubin,
H., and Bode, W. (2000) Crystal structure of the caspase activator
human granzyme B, a proteinase highly specific for an Asp-P1 residue,
Biol. Chem., 381, 1203-1214; doi:
10.1515/BC.2000.148.
55.Watt, W., Koeplinger, K. A., Mildner, A. M.,
Heinrikson, R. L., Tomasselli, A. G., and Watenpaugh, K. D. (1999) The
atomic-resolution structure of human caspase-8, a key activator of
apoptosis, Structure, 7, 1135-1143.
56.Afonina, I. S., Cullen, S. P., and Martin, S. J.
(2010) Cytotoxic and non-cytotoxic roles of the CTL/NK protease
granzyme B, Immunol. Rev., 235, 105-116; doi:
10.1111/j.0105-2896.2010.00908.x.
57.Morandi, F., and Pistoia, V. (2014) Interactions
between HLA-G and HLA-E in physiological and pathological conditions,
Front. Immunol., 5, 394; doi:
10.3389/fimmu.2014.00394.
58.Mikhailova, V. A., Bazhenov, D. O., Viazmina, L.
P., Agnaeva, A. O., Bespalova, O. N., Sel’kov, S. A., and
Sokolov, D. I. (2019) Cytotoxic activity of peripheral blood NK cells
towards trophoblast cells during pregnancy, Bull. Exp. Biol.
Med., 166, 567-573; doi: 10.1007/s10517-019-04393-4.
59.Park, D. W., Lee, H. J., Park, C. W., Hong, S.
R., Kwak-Kim, J., and Yang, K. M. (2010) Peripheral blood NK cells
reflect changes in decidual NK cells in women with recurrent
miscarriages, Am. J. Reprod. Immunol., 63, 173-180; doi:
10.1111/j.1600-0897.2009.00777.x.
60.Maki, G., Klingemann, H. G., Martinson, J. A.,
and Tam, Y. K. (2001) Factors regulating the cytotoxic activity of the
human natural killer cell line, NK-92, J. Hematother. Stem. Cell.
Res., 10, 369-383; doi: 10.1089/152581601750288975.
61.Lash, G. E., Naruse, K., Robson, A., Innes, B.
A., Searle, R. F., Robson, S. C., and Bulmer, J. N. (2011) Interaction
between uterine natural killer cells and extravillous trophoblast
cells: effect on cytokine and angiogenic growth factor production,
Hum. Reprod., 26, 2289-2295; doi:
10.1093/humrep/der198.
62.Zhang, Y., Qu, D., Sun, J., Zhao, L., Wang, Q.,
Shao, Q., Kong, B., Zhang, Y., and Qu, X. (2016) Human trophoblast
cells induced MDSCs from peripheral blood CD14(+) myelomonocytic cells
via elevated levels of CCL2, Cell. Mol. Immunol.,
13, 615-627; doi: 10.1038/cmi.2015.41.