New Sorbent on the Basis of Covalently Immobilized Lysozyme for Removal of Bacterial Lipopolysaccharide (Endotoxin) from Biological Fluids
P. A. Levashov1,2,a*, D. A. Matolygina1,2, E. D. Ovchinnikova3, I. Yu. Adamova4, O. A. Dmitrieva3, A. V. Nuzhdina2, N. S. Pokrovsky2,5, and N. L. Eremeev1
1Lomonosov Moscow State University, Faculty of Chemistry, 119234 Moscow, Russia2Bauman Moscow State Technical University, Interindustry Engineering Center for Composite Materials, 105005 Moscow, Russia
3National Medical Research Center of Cardiology, Institute of Experimental Cardiology, Ministry of Healthcare of the Russian Federation, 121359 Moscow, Russia
4POKARD Ltd, 121359 Moscow, Russia
5Lomonosov Moscow State University, Faculty of Fundamental Medicine, 119991 Moscow, Russia
* To whom correspondence should be addressed.
Received July 23, 2018; Revised September 9, 2018; Accepted September 9, 2018
It was demonstrated for the first time that immobilized lysozyme can efficiently remove Escherichia coli and Pseudomonas aeruginosa lipopolysaccharides (endotoxins) from solutions. Experimentally confirmed sorption capacity for the developed sorbent was at least 400 ng of endotoxin per ml sorbent. The new sorbent is compatible with the whole human blood and can be potentially used in extracorporeal therapy in the treatment of sepsis.
KEY WORDS: endotoxin sorption, sepsis, immobilized lysozymeDOI: 10.1134/S0006297919010048
Sepsis is a life-threatening infectious disease accompanied by the multiple organ failure. It is caused by the penetration and circulation of pathogenic microorganisms and their toxins in the human bloodstream. The problem of sepsis treatment has become a serious challenge to modern medicine because of the emergence of new antibiotic-resistant bacterial strains [1]. Every year, more than 5 million people die from sepsis worldwide [2]. One of the key tasks in the treatment of sepsis is removal from the bloodstream of bacterial lipopolysaccharides (endotoxins) that affect the immune system and significantly worsen patient’s condition [3, 4]. Currently used methods for the endotoxin removal include extracorporeal treatment of patient’s blood plasma using chromatographic polymer materials [5-7]. Promising clinical results have been obtained by the use of sorbents based on immobilized polymyxin B (51% patient survival vs. 30% in the control group) [7, 8]. Polymyxin B is a natural antibiotic isolated from the spore-forming bacteria Paenibacillus polymyxa (Bacillus polymyxa). It represents a mixture of six peptide-like components with closely related structure that contain both standard and nonstandard amino acids residues [9].
The development of new effective sorption materials for the endotoxin removal still remains a topical problem. Another important task is synthesis of materials compatible not only with the plasma, but also with the whole blood, in order to expand the possibilities of their medical application.
In our previous works, we have shown that chicken egg lysozyme is able to efficiently adsorb on the various microbial cells of, i.e., able to bind to the cell surface components [10, 11]. Therefore, we hypothesized that immobilized lysozyme could be a suitable sorbent for removal of endotoxins from biological fluids. At the moment, there is no information on a practical application of immobilized lysozyme as a sorbent; we also failed to find any published data on the lysozyme ability to bind bacterial endotoxins. The purpose of this work was to synthesize affinity sorbent by covalently immobilizing lysozyme on an insoluble polymer matrix and to study the hemocompatibility of this sorbent and its ability to bind endotoxins.
MATERIALS AND METHODS
Reagents and equipment. The following reagents were used: lysozyme from chicken eggs, Micrococcus luteus (lyophilized cells), BrCN, dioxane, ethanolamine, EDTA, MES, sodium acetate, Tris, Coomassie R-250, dithiothreitol, 2-mercaptoethanol, and Bromophenol blue from Sigma (USA); KOH, NaOH, H3BO3, KH2PO4, K2HPO4, HCl, TEMED, and ammonium persulfate from Helicon (Russia); LAL (Limulus amoebocyte lysate) chromogenic endpoint assay from Hycult Biotech (Netherlands); acrylamide, N,N'-methylene bis-acrylamide from Panreac (Spain); electrophoresis protein markers (phosphorylase B, BSA, ovalbumin, carbonic anhydrase, soybean trypsin inhibitor, lysozyme) from BioRad (USA); Sandoglobulin (human immunoglobulin) from Sandoz (Germany); enzyme immunoassay kit for quantitative determination of total IgG from Vector-Best (Russia); Escherichia coli endotoxin standard from Charles River Laboratories (USA); Pseudomonas aeruginosa endotoxin standard (Pyrogenal) from Medgamal (Russia); water for LAL test from Pirotest (Russia); polymer matrix WorkBeads 200SEC from Bio-Works (Sweden); heparin (pharmacological grade) from Bioiberica (Spain). Bidistilled water was used unless otherwise stated. The following equipment was used in the work: UV-1800 spectrophotometer (Shimadzu, Japan); microplate photometer Multiskan FC (Thermo Scientific, USA); centrifuge Minispin (Eppendorf, Germany); TV-80-1 air circulation oven with thermostat control (MedLife, Russia); LT-105a water bath with thermostat control (LOIP, Russia); Elite3 hematology analyzer (Erba Mannheim, Czechia); OH-PA64 analytical balance (Ohaus, USA); OS-20 shaker (BioSan, Latvia); electrophoresis chamber VE-10 (Helicon, Russia), and AKTA Start chromatography system (GE Healthcare, Sweden).
Lysozyme immobilization. Lysozyme was immobilized using the standard activation procedure with BrCN [12]. About 30 ml of WorkBeads 200 SEC resin was washed on a glass filter at 5°C with 150 ml of water and then with 150 ml of solution containing 4 M KOH and 1.6 M KH2PO4. The beads were transferred to 30 ml of 4 M KOH/1.6 M KH2PO4, and 3.6 ml of BrCN solution in dioxane (1 g/ml) was added. The mixture was incubated on ice with stirring for 10 min. Activated resin was transferred to a glass filter and washed at 5°C with 150 ml of water and then with 150 ml of 0.2 M H3BO3-NaOH buffer, pH 8.0. The beads were then transferred to a closed container, and 30 ml of lysozyme solution (10 mg/ml in 0.2 M H3BO3-NaOH buffer, pH 8.0) was added. The mixture was incubated with stirring at 20°C for 3 h. At the end of immobilization, absorption of the supernatant was measured at 280 nm to determine the unbound lysozyme and calculate the reaction yield. The resulting sorbent was washed with 150 ml of water on a glass filter; 30 ml of 1 M ethanolamine solution (pH 8.0, adjusted with HCl) was added, followed by incubation for 2 h at 20°C. The sorbent was washed on a glass filter with 150 ml of water and then with 150 ml of 10 mM KH2PO4-K2HPO4 buffer containing 130 mM NaCl, pH 7.0.
Endotoxin sorption on the immobilized lysozyme. Endotoxin sorption was performed using the batch procedure. For this, 100 μl of the sorbent was placed in plastic tubes and washed with 20 volumes of water for the LAL test. Concentrated solutions of endotoxins were diluted with 10 mM KH2PO4-K2HPO4 buffer (pH 7.0) containing 130 mM NaCl to a final concentration of 20 or 50 ng/ml and added to the sorbent at a 10 : 1 (v/v) ratio. The resulting mixture was incubated for 30 and 60 min at 20°C on a shaker. After incubation, aliquots of the supernatant were diluted 200 times with water for the LAL test. The endotoxin concentration was determined from its activity in the samples by the LAL chromogenic endpoint assay [13]. As negative controls, endotoxin was adsorbed on the unmodified WorkBeads 200 SEC resin and on the beads activated with BrCN and then blocked with ethanolamine without lysozyme immobilization.
Storage of immobilized lysozyme. The obtained sorbent was stored at 5°C as 50% slurry (v/v) in 10 mM KH2PO4-K2HPO4 buffer (pH 7.0) containing 130 mM NaCl and 0.3% NaN3.
Sorbent hemocompatibility assay. Donor’s blood was collected in 50-ml tubes with pre-added heparin (final concentration, 2.5 U/ml). All procedures with the sorbent were carried out at 20°C. A 200-μl sample of the sorbent in a microcolumn with a 133-μm filter was washed with 2 ml of physiological saline (0.15 M NaCl). A silicone tube (diameter, 0.5 mm) was placed on the bottom of the column to increase the time of sorbent contact with the blood. Then, the sorbent was pre-treated with heparin by stirring with a 2× volume of physiological saline containing heparin (100 units heparin per 1 ml sorbent) for 10 min. Pre-treatment with heparin is commonly recommended for the systems used in extracorporeal therapy [14]. The blood (4 ml) was loaded on the column and let to pass through the sorbent by gravity (contact time, 25 to 30 min). A column without any sorbent was used as a negative control. After chromatography, the blood was collected in 15-ml tubes, and EDTA was added to a final concentration of 10 mM. The samples were placed in 1-ml tubes and assayed for the erythrocyte count, platelet count, lymphocyte count, hemoglobin concentration, and hematocrit with a hematology analyzer.
Cell-based assay of soluble lysozyme activity. Dried M. luteus cells (5 mg) were mixed with 10 ml of 0.01 M Tris-MES-acetate buffer (pH 8.5) at 20°C. Before the assay, the obtained M. luteus cell suspension was centrifuged for 5 min at 500 rpm at 5°C and the cells were then resuspended in 0.01 M Tris-MES-acetate buffer (pH 8.5). The bacteriolytic activity of lysozyme was determined by the turbidimetric method as a decrease in the optical density of the cell suspension at 650 nm. The rate of the optical density decrease is directly proportional to the rate of cell lysis [15, 16]. The measurements were carried out at 37°C in 0.01 M Tris-MES-acetate buffer (pH 8.5). The number of bacterial cells added to the reaction mixture was selected in such a way that the initial optical density was 0.50-0.55. The measurements were carried out in 0.5-ml cuvettes with stoppers. Lysozyme concentration was 0.1-2 μg/ml. After the enzyme was added to the cuvette, the optical density was recorded for 5-7 min, and the initial rate of the optical density decrease was determined within the 2-3 min period. The control experiments were performed without the addition of an enzyme and used for the correction of the lysis rate. The rate of enzymatic cell lysis was proportional to the enzyme concentration in a range up to 2 μg/ml.
Cell-based assay of immobilized lysozyme activity. The reaction mixture was prepared as described above for the soluble lysozyme. The decrease in the cell suspension optical density was measured at 650 nm in the same buffer mixture and at the same temperature. Immobilized lysozyme was added to the mixture at the concentration of 20-70 μl/ml solution. The reaction mixture (10 ml) was incubated in test tubes in a thermostat on a rotary shaker at 10 rpm (at speeds over 14 rpm, cells can be disrupted in the absence of lysozyme, probably, due to the mechanical action of resin beads). Samples (1 ml) were taken from the mixture every 2 min and placed in tubes; the beads with the immobilized enzyme were allowed to settle (0.5 min), and the optical density of the supernatant (cells suspension without immobilized enzyme) was measured. The change in optical density over time (10-15 min) was plotted, and the rate of the optical density decrease was determined from the graph slope. The control experiments were performed by adding the resin without immobilized lysozyme and used for the correction of the lysis rate. The rate of the enzymatic cell lysis was proportional to the amount of immobilized enzyme preparation in a range up to 70 μl/ml mixture.
Assay of lysozyme leakage from the sorbent. Two milliliters of 50% sorbent slurry was placed in a column (5 cm × 1 cm2) and let to drain by gravity. The flowthrough was collected and assayed for the bacteriolytic activity as described above for the soluble lysozyme. The calibration curve for the dependence of the enzyme activity on the lysozyme concentration in the storage buffer was plotted and used for determining the amount of lysozyme in the flowthrough [10].
Hemolysis in the presence of free lysozyme. The blood from donors was prepared as described in the section “Sorbent hemocompatibility assay”. A 2-ml blood sample was mixed with 50 μl of physiological saline (negative control) or lysozyme solution in physiological saline (2, 4, 8, or 16 mg/ml). All experiments were done in four replicates. The samples were incubated in a thermostat for 120 min with a gentle rocking at 37°C. At the end of incubation, the plasma and the blood elements were separated by centrifugation at 3000g. The percentage of hemolysis was determined according to Jager [17].
Binding of blood plasma proteins by the sorbent. Chromatography was performed at 20°C at a flow rate of 0.5 ml/min. The column (1 cm × 1 cm2) with the sorbent was washed with 15 ml of 10 mM KH2PO4-K2HPO4 buffer (pH 7.0) containing 130 mM NaCl. Ten milliliters of human blood plasma was passed through the sorbent and collected by monitoring the optical density at 280 nm. The column was then washed with 15 ml of the same buffer. The first elution was performed with 7 ml of 0.2 M Gly-HCl buffer (pH 2.5) and the sorbent was then washed with 10 ml of 10 mM KH2PO4-K2HPO4 buffer (pH 7.0) containing 130 mM NaCl. The second elution was performed with 7 ml of 0.1 M NaHCO3-NaOH buffer (pH 10.0). The eluates were collected as 1-ml fractions. The fractions of blood plasma before and after chromatography were assayed for the total protein concentration using the micro-biuret method [18] with a modified Benedict reagent [19]. The IgG concentration in the samples was determined by the enzyme immunoassay method. The protein composition of the eluates was studied by electrophoresis in a 4-22% gradient polyacrylamide gel [20] under denaturing conditions in the presence or absence of reducing agents.
RESULTS AND DISCUSSION
Synthesis and characterization of the sorbent with immobilized lysozyme. The yield of lysozyme immobilization was 96 ± 1%, the amount of immobilized lysozyme was 9.6 mg/ml beads.
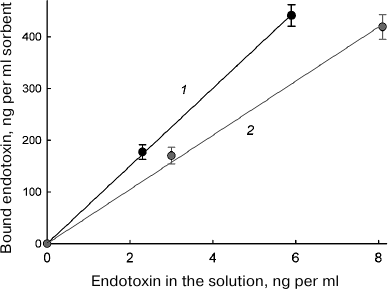
Endotoxin binding by the sorbent. The results of binding of bacterial endotoxins (lipopolysaccharides) of E. coli (Enterobacteriaceae family) and P. aeruginosa (Pseudomonadaceae family) by immobilized lysozyme are presented in Table 1. The table shows the data obtained for the 30-min contact between the endotoxin solution and the sorbent (similar results were obtained for the 60-min interaction). No endotoxin adsorption was observed in the control experiments with WorkBeads 200SEC without immobilized lysozyme. In all the cases, more than 80% endotoxin was removed from the solution. Since the amount of the adsorbed endotoxins did not increase after 30 min of contact with the sorbent, we assumed that the system has reached the equilibrium, so that the adsorption isotherm (dependence of the amount of bound endotoxin on the concentration of free endotoxin) could be plotted. As seen from the figure, this dependence could be described by a linear function of the Henry isotherm equation:
where S is adsorbed endotoxin (ng/ml sorbent), Cf is the concentration of free endotoxin in solution (ng/ml), and A is a dimensionless coefficient. Using simple transformations, we obtained an expression for the dependence of the fraction of removed endotoxin on the volume of the endotoxin solution and the volume of the sorbent:
where EF is the fraction of the removed endotoxin from the entire solution in %, V is the entire solution volume in ml, and Vs is the sorbent volume in ml.
Table 1. Binding of endotoxins by the
sorbent
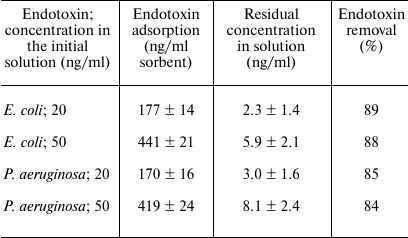
Note: The endotoxin solution in the experiments was added to the sorbent
at a 10 : 1 (v/v) ratio. All experiments are done in five replicates.
The error values were calculated from the Student’s
t-distribution for the confidence interval of 95%.
Dependence of the amount of endotoxin bound on the sorbent on the concentration of free endotoxin in the solution: 1) E. coli endotoxin; 2) P. aeruginosa endotoxin
By approximating the experimental data, we calculated the A coefficient values that equaled 75 and 52 for the adsorption of E. coli and P. aeruginosa endotoxins, respectively, assuming that the endotoxin concentration in the solution is given in ng/ml and the amount of the adsorbed endotoxin in given in ng per ml sorbent. Table 2 shows theoretically calculated values for the endotoxin fraction removed from 1 liter of solution using 50, 100, and 200 ml of the sorbent, (i.e., volumes commonly used in medical procedures). As can be seen from Table 2, in all cases, the efficiency of endotoxin removal was high and ranged from 72 to 94%.
Table 2. Calculated efficiency of endotoxin
removal from 1 liter of solution by different volumes of the
sorbent*
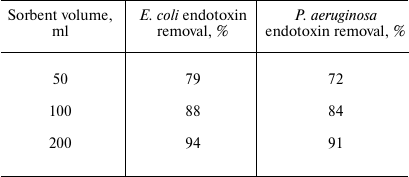
* Under assumption of linear nature of the adsorption isotherm (which
according to the data in Table 1 and our
calculations, is be at least up to the concentrations of E. coli
and P. aeruginosa endotoxins in the initial solution of 26 and
29 ng/ml, respectively, in the case of 50 ml of sorbent).
The levels of endotoxins in patients’ blood reported in different studies vary within a fairly wide range, which might be associated with the difficulties in accurate determination of endotoxin concentration in biological fluids. Endotoxins are measured by their biological activity, which varies for different microbial strains and different measurement methods. In addition, endotoxin molecules can form complexes with other substances, which can also distort the results [21-23]. Nevertheless, it is possible to find data that can be reasonably evaluated. For example, in a number of studies, the average endotoxin levels in the blood plasma were reported as 0.034 ng/ml in patients who survived sepsis due to treatment and 0.126 ng/ml in those who died [24]. Therefore, the new sorbent can efficiently bind endotoxin from the solution at substantially higher concentrations.
Stability of adsorption capacity during storage. The sorbent with immobilized lysozyme retained its adsorption capacity for at least 3 months when stored as suspension in a buffer solution at 5°C.
Verification of the sorbent hemocompatibility. The results of the hemocompatibility assay of the obtained sorbent are shown in Table 3. No significant changes in all the studied parameters were observed, except in the platelet concentration. However, these changes did not exceed the limits of the norm [25]. Therefore, it can be argued that immobilized lysozyme is compatible with whole blood.
Table 3. Blood indices after contact with
the sorbent
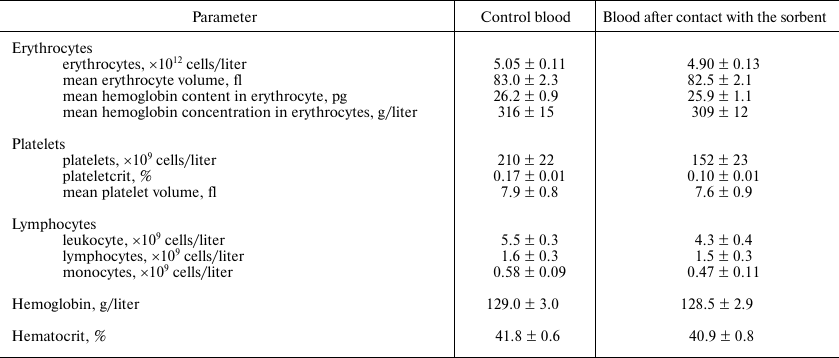
Note: 4 ml of blood was passed by gravity through 1 ml of the sorbent.
The contact time between the blood and the sorbent ranged from 25 to 30
min. All experiments were done in five replicates. The error values
were calculated from the Student’s t-distribution for the
confidence interval of 95%.
Possible lysozyme leakage from the sorbent. Washing the column with immobilized lysozyme during the first day after the sorbent synthesis revealed no leakage of free lysozyme, as determined from the absence of lysozyme activity in the wash-offs (the concentration of free lysozyme sufficient for its detection is 0.05 μg/ml). With a longer storage, an insignificant leakage of lysozyme between 0.3 and 0.5 μg from 1 ml of sorbent over 10 days was detected, which corresponded up to 0.0033% of immobilized enzyme (15 mg/ml). If we assume that the detachment of lysozyme from the matrix proceeds according to the first-order reaction (k = 300,003 days–1), the half-life of the sorbent (loss of 50% ligand) is 207,902 days or about 569.6 years. Each washing of the sorbent caused similar leakage of the enzyme. We attribute the phenomenon of lysozyme leakage to the insufficient stability of bonds formed by the BrCN activation of the matrix. However, sorbents used for extracorporeal procedures in medicine are synthesized by the same immobilization method via activation of the polysaccharide matrix with BrCN [26]. The leakage of proteins immobilized by the BrCN method has not been described in literature before, which in our opinion, may be partly due to the fact that most biochemical methods for total protein determination have detection limits above 1 μg/ml [18].
Bacteriolytic activity of immobilized lysozyme. We determined that 25 μl of immobilized lysozyme added to 1 ml of the reaction mixture lyzed the cells at a rate of (7.5 ± 0.8)·10–3 min–1 (the rate of optical density loss). Similar activity ((7.3 ± 0.6)·10–3 min–1) is displayed by 0.1 μg/ml of soluble lysozyme. A 25-μl sample of the immobilized enzyme contains ~375 μg of lysozyme, which is 3750 times greater than the amount of soluble lysozyme exhibiting the same activity. In fact, only a small fraction of the lysozyme immobilized on the polymer beads is sterically available and interacts with the substrate, which is not surprising because of the large size of the substrate (bacterial cells).
Hemolysis in the presence of soluble lysozyme. We found that addition of free soluble lysozyme to the blood samples to a final concentration of 0.05 to 0.4 mg/ml did not increase hemolysis within 2 h after addition. Therefore, even in the case of its leakage from the sorbent, lysozyme does not present a serious danger for erythrocytes.
Non-specific binding of blood plasma proteins. To investigate the possible binding of blood plasma proteins by the sorbent, we loaded 10 ml of blood plasma on a column with 1 ml of sorbent. After washing the column, bound proteins were eluted with buffers with different pH values: first, with 0.2 M Gly-HCl (pH 2.5) and then with 0.2 M NaHCO3-NaOH (pH 10.0). A narrow protein peak was eluted in the acidic buffer, while no protein elution was observed in the alkaline buffer. The pI value of lysozyme is 10.20-10.35, depending of the ionic strength [27], therefore it could be theoretically expected that immobilized lysozyme, like a positively charged ion-exchange chromatographic agent, would bind only negatively charged proteins that would elute at alkaline pH values. However, we saw a completely different picture, since bound proteins were eluted at acidic pH. When analyzed by electrophoresis under denaturing conditions, the eluted proteins produced an electrophoretic pattern identical to that of immunoglobulin G (IgG), either whole molecule or light and heavy chains, in the absence or presence of the reducing agent, respectively (data not shown). Enzyme immunoassay also confirmed that the eluted protein was IgG. Based on the gel staining, the eluted IgG was highly pure (>80% purity) with no significant amounts of admixtures. The adsorption capacity for IgG was 5.8 ± 0.7 mg/ml sorbent. The concentration of immunoglobulins in the blood plasma before chromatography was 10.2 ± 0.4 mg/ml; therefore, only 5.7% of IgG was removed from 10 ml of plasma. The loss of such portion of immunoglobulins does not pose a significant threat to the patient’s health. In some diseases immunoglobulins are purposely removed from the blood in significantly larger amounts using special sorbents [28]. In case of acute need, the loss of immunoglobulins could be compensated by injection of donor IgGs.
The fact of lysozyme binding with total IgG (i.e., not with specifically generated anti-lysozyme antibodies) has not been described before. The ability of lysozyme to bind immunoglobulins may be important from the immunological point of view, because, when bound to bacterial cells, lysozyme itself enhances their opsonization and facilitates phagocytosis. There are some speculations in literature that lysozyme can play a role of opsonin [29]; the authors suggest that sorption of lysozyme on the surface of pathogen cell can change the charge distribution on the cell surface thus attracting phagocytes to the antigen. But immunoglobulins themselves also attract immune cells, and therefore lysozyme binding to immunoglobulins, discovered by us, can explain the opsonin function of lysozyme. The phenomenon of IgG binding by lysozyme requires further studying, as it can reveal new aspects in the interactions between immune system and pathogens.
In conclusion, we showed that immobilized lysozyme is able to efficiently bind endotoxins from E. coli and P. aeruginosa. Lysozyme is a very attractive ligand from the viewpoint of biotechnology, since it is non-toxic and commercially available. Compared to lysozyme, polymyxin B is more expensive and toxic; this is why the risks of application of its soluble form in medicine have been discussed for many years [30]. Among other things, lysozyme can be immobilized on a variety of polymer materials, which distinguishes it from various low-molecular-weight ligands, including polymyxin B, whose adsorption properties largely depend on the nature of the matrix on which they are immobilized. Further optimization of structural and functional properties of sorbents with immobilized lysozyme may further improve their adsorption characteristics. The fact that the developed sorbent is compatible with whole blood extends the possibilities of its use in medicine.
Funding
The work was supported by the Federal Target Program “Research and Development in Priority Areas for the Development of the Russian Science and Technology Complex for 2014-2020”, section “Development of new medical sorbents for extracorporeal treatment of sepsis with antimicrobial activity and ability to adsorb bacterial toxins” (application code 2017-14-576-0053-142; unique project ID RFMEFI57417X0181; agreement no. 14.574.21.0181).
Conflict of Interest
Authors declare no conflict of interest in financial or any other sphere.
REFERENCES
1.Cohen, J. (2002) The immunopathogenesis of sepsis,
Nature, 420, 885-891.
2.Fleischmann, C., Scherag, A., Adhikari, N. K.,
Hartog, C. S., Tsaganos, T., Schlattmann, P., Angus, D. C., and
Reinhart, K. (2016) Assessment of global incidence and mortality of
hospital-treated sepsis. Current estimates and limitations, Am. J.
Respir. Crit. Care Med., 193, 259-272.
3.Danner, R. L., Natanson, C., Elin, R. J., Hosseini,
J. M., Banks, S., MacVittie, T. J., and Parrillo, J. E. (1990)
Pseudomonas aeruginosa compared with Escherichia coli
produces less endotoxemia but more cardiovascular dysfunction and
mortality in a canine model of septic shock, Chest, 98,
1480-1487.
4.Opal, S. M. (2010) Endotoxins and other sepsis
triggers, Contrib. Nephrol., 167, 14-24.
5.Ongkudon, C. M., Chew, J. H., Liu, B., and Danquah,
M. K. (2012) Chromatographic removal of endotoxins: a bioprocess
engineer’s perspective, ISRN Chromatogr., 2012,
1-9.
6.Esteban, E., Ferrer, R., Alsina, L., and Artigas,
A. (2013) Immunomodulation in sepsis: the role of endotoxin removal by
polymyxin B-immobilized cartridge, Mediators Inflamm.,
2013, 1-12.
7.Anspach, F. B., and Hilbeck, O. (1995) Removal of
endotoxins by affinity sorbents, J. Chromatogr. A, 711,
81-92.
8.Yaroustovsky, M., Abramyan, M., Komardina, E.,
Nazarova, H., Popov, D., Plyushch, M., Soldatkina, A., and Rogalskaya,
E. (2018) Selective LPS adsorption using polymyxin B-immobilized fiber
cartridges in sepsis patients following cardiac surgery, Shock,
49, 658-666.
9.Orwa, J. A., Govaerts, C., Busson, R., Roets, E.,
Van Schepdael, A., and Hoogmartens, J. (2001) Isolation and structural
characterization of polymyxin B components, J. Chromatogr. A,
912, 369-373
10.Sedov, S. A., Belogurova, N. G., Shipovskov, S.,
Levashov, A. V., and Levashov, P. A. (2011) Lysis of Escherichia
coli cells by lysozyme: discrimination between adsorption and
enzyme action, Colloids Surf. B Biointerfaces, 88,
131-133.
11.Matolygina, D. A., Osipova, H. E., Smirnov, S.
A., Belogurova, N. G., Eremeev, N. L., Tishkov, V. I., Levashov, A. V.,
and Levashov, P. A. (2015) Determination of bacteriolytic activity and
measurement of enzyme sorption by cells in the system of living
Lactobacillus plantarum, Mosc. Univ. Chem. Bull.,
70, 292-297.
12.Porath, J., and Axen, R. (1976) Immobilization of
enzymes to agar, agarose, and Sephadex supports, Methods
Enzymol., 44, 19-45.
13.Schaumberger, S., Ladinig, A., Reisinger, N.,
Ritzmann, M., and Schatzmayr, G. (2014) Evaluation of the endotoxin
binding efficiency of clay minerals using the Limulus amebocyte
lysate test: an in vitro study, AMB Express, 4,
1-9.
14.Schmaldienst, S., Goldammer, A., Spitzauer, S.,
Derfler, K., Horl, W. H., and Knobl, P. (2000) Local anticoagulation of
the extracorporeal circuit with heparin and subsequent neutralization
with protamine during immunoadsorption, Am. J. Kidney Dis.,
36, 490-497.
15.Levashov, P. A., Sedov, S. A., Belogurova, N. G.,
Levashov, A. V., and Shipovskov, S. (2010) Quantitative turbidimetric
assay of enzymatic gram-negative bacteria lysis, Anal. Chem.,
82, 2161-2163.
16.Matolygina, D. A., Dushutina, N. S.,
Ovchinnikova, E. D., Eremeev, N. L., Belogurova, N. G., Atroshenko, D.
L., Smirnov, S. A., Savin, S. S., Tishkov, V. I., Levashov, A. V., and
Levashov, P. A. (2018) Enzymatic lysis of living microbial cells: a
universal approach to calculating the rate of cell lysis in
turbidimetric measurements, Mosc. Univ. Chem. Bull., 73,
47-52.
17.Jager, F. C. (1968) Determination of vitamin E
requirement in rats by means of spontaneous haemolysis in vitro,
Nutr. Dieta Eur. Rev. Nutr. Diet., 10, 215-223.
18.Levashov, P. A., Sutherland, D. S., Besenbacher,
F., and Shipovskov, S. (2009) A robust method of determination of high
concentrations of peptides and proteins, Anal. Biochem.,
395, 111-112.
19.Levashov, P., Ovchinnikova, E., Afanasieva, M.,
Frid, D., Azmuko, A., Bespalova, Zh., Adamova, I., Afanaiseva, O., and
Pokrovskii, S. (2012) Affinity sorbent based on
tryptophyl-threonyl-tyrosine for binding of the immunoglobulins G:
sorption characteristics and aspects of practical application, Russ.
J. Bioorg. Chem., 8, 46-50.
20.Laemmli, U. K. (1970) Cleavage of structural
proteins during the assembly of the head of bacteriophage T4,
Nature, 227, 680-685.
21.Hurley, J. C., Nowak, P., Ohrmalm, L., Gogos,
Ch., Armaganidis, A., and Giamarellos-Bourboulis, E. J. (2015)
Endotoxemia as a diagnostic tool for patients with suspected bacteremia
caused by Gram-negative organisms: a meta-analysis of 4 decades of
studies, J. Clin. Microbiol., 53, 1183-1191.
22.Marshall, J. C., Walker, P. M., Foster, D. M.,
Harris, D., Ribeiro, M., Paice, J., Romaschin, A. D., and Derzko, A. N.
(2002) Measurement of endotoxin activity in critically ill patients
using whole blood neutrophil dependent chemiluminescence, Crit.
Care, 6, 342-348.
23.Schwarz, H., Gornicec, J., Neuper, Th.,
Parigiani, M. A., Wallner, M., Duschl, A., and Horejs-Hoec, J. (2017)
Biological activity of masked endotoxin, Sci. Rep., 7,
1-11.
24.Behre, G., Schedel, I., Nentwig, B., Wormann, B.,
Essink, M., and Hiddemann, W. (1992) Endotoxin concentration in
neutropenic patients with suspected gram-negative sepsis: correlation
with clinical outcome and determination of anti-endotoxin core
antibodies during therapy with polyclonal immunoglobulin M-enriched
immunoglobulins, Antimicrob. Agents Chemother., 36,
2139-2146.
25.Zaninetti, C., Biino, G., Noris, P., Melazzini,
F., Civaschi, E., and Balduini, C. L. (2015) Personalized reference
intervals for platelet count reduce the number of subjects with
unexplained thrombocytopenia, Haematologica, 100,
338-340.
26.Kiseleva, E. A., Afanasieva, O. I., Kosheleva, N.
A., and Pokrovsky, S. N. (1996) Immunosorbent for IgG apheresis: an
in vitro study, Transfus. Sci., 17, 519-525.
27.Wetter, L. R., and Deutsch, H. F. (1951)
Immunological studies on egg white proteins. IV. Immunochemical and
physical studies of lysozyme, J. Biol. Chem., 192,
237-242.
28.Blaha, M., Pit’ha, J., Blaha, V., Lanska,
M., Maly, J., Filip, S., and Langrova, H. (2010) Extracorporeal
immunoglobulin elimination for the treatment of severe myasthenia
gravis, J. Biomed. Biotechnol., 2010, 419520.
29.Daniel, M. P., Gaikwad, V., Verghese, M.,
Abraham, R., and Kapoor, R. (2015) Serum lysozyme (muramidase) levels
in intra-abdominal abscesses: an experimental study, Indian J.
Surg., 77, 117-119.
30.Falagas, M. E., and Kasiakou, S. K. (2006)
Toxicity of polymyxins: a systematic review of the evidence from old
and recent studies, Crit. Care, 10, 1-13.