REVIEW: Enhancement of Declarative Memory: From Genetic Regulation to Non-invasive Stimulation
D. V. Bryzgalov1,a*, I. L. Kuznetsova2, and E. I. Rogaev2,3,4,5,b*
1Memory, Oscillations, Brain States (MOBS) Team, Brain Plasticity Unit, CNRS UMR 8249, ESPCI Paris, Paris, France2Vavilov Institute of General Genetics, Russian Academy of Sciences, 119991 Moscow, Russia
3Department of Psychiatry, University of Massachusetts Medical School, Worcester MA 01605, USA
4Lomonosov Moscow State University, Faculty of Biology, 119234 Moscow, Russia
5Lomonosov Moscow State University, Faculty of Bioengineering and Bioinformatics, 119234 Moscow, Russia
* To whom correspondence should be addressed.
Received June 9, 2018; Revision received June 25, 2018
The problem of memory enhancement is extremely important in intellectual activity areas and therapy of different types of dementia, including Alzheimer’s disease (AD). The attempts to solve this problem have come from different research fields. In the first part of our review, we describe the results of targeting certain genes involved in memory-associated molecular pathways. The second part of the review is focused on the deep stimulation of brain structures that can slow down memory loss in AD. The third part describes the results of the use of non-invasive brain stimulation techniques for memory modulation, consolidation, and retrieval in healthy people and animal models. Integration of data from different research fields is essential for the development of efficient strategies for memory enhancement.
KEY WORDS: memory, Alzheimer’s disease, brain stimulation, hippocampus, CREB pathwayDOI: 10.1134/S0006297918090146
Abbreviations: AD, Alzheimer’s disease; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; BDNF, brain-derived neurotrophic factor; C/EBP, CCAAT enhancer-binding protein; CREB, cAMP response element-binding protein; DBS, deep brain stimulation; dlPFC, dorsolateral prefrontal cortex; DREADD, designer receptor exclusively activated by designer drugs; (f)MRI, (functional) magnetic resonance imaging; IGF, insulin-like growth factor; IIS pathway, insulin/IGF-I-like signaling pathway; NMDA, N-methyl-D-aspartate; PKMζ, protein kinase Mζ; tACS, transcranial alternating current stimulation; tDCS, transcranial direct current stimulation; TMS, transcranial magnetic stimulation; TRE, tetracycline responsive element; TrkB, tropomyosin-related kinase B; tTa, tetracycline transactivator; WT, wild-type.
All living organisms can respond to external stimuli in accordance with
their previous experience. Memory, or the ability to encode, store, and
retrieve information, is one of the main research topics in
neuroscience. However, the definition of memory is vague and can be
interpreted differently in different areas of neuroscience. From simple
forms of memory in Protozoa to complex interactions of neural networks
in humans, from genes to behavior, memory is studied by large cohort of
scientists who do not always agree on the use of terms and approaches
(for example, see the last attempt to reformulate the definition of
memory by Kukushkin and Carew [1]). The situation
becomes even more complicated if we consider the existence of different
types of memory (declarative or procedural, short-term or long-term,
etc.) that could be affected differently in various neurodegenerative
and psychiatric disorders. For example, declarative memory is much less
impaired in the Tourette syndrome than the procedural memory [2]. However, some processes and pathologies, e.g.,
normal aging or Alzheimer’s disease (AD), cause decline in all
types of memory and, therefore, significantly affect the quality of
life. For this reason, the necessity for new approaches to the
restoration of lost memory functions becomes more urgent with time.
Memory enhancement has been a focus of numerous research groups all over the world. Such research projects offer a unique possibility for the fundamental and applied studies of memory functions. First, a large number of works have demonstrated that restoration of memory functions in AD patients significantly improves patients’ everyday life and social interactions [3] and, therefore, is extremely relevant in clinical medicine. Second, healthy population could also greatly benefit from the memory enhancement research. Memory-boosting techniques and/or drugs will be welcomed in many areas of human activity, first of all, in the intellectual activity area. Finally, we believe that memory manipulation research can significantly contribute to our understanding of how the brain works. Causal approach is one of the most powerful tools for hypothesis verification in neuroscience, and manipulations with memory will provide an invaluable insight into the brain functions.
This review is an attempt to present a vast landscape of memory enhancement research, regardless of the research area. To do so, we selected the studies on the enhancement of declarative memory that are related to the modulation of hippocampal activity. Hippocampus is one of the most investigated brain structures. Hippocampus became particularly important in memory research after the famous case of patient H. M. who suffered from severe impairments in the declarative memory consolidation after bilateral surgical removal of the hippocampus [4]. Since then, hippocampus has been considered an essential component for the formation and storage of declarative memories. Hippocampus itself and hippocampus-associated brain structures and neuronal networks (e.g., prefrontal and parietal cortical areas) remain the main subjects of the memory enhancement studies.
In the first part of this review, we will focus on the studies that describe the attempts to affect memory by regulating gene expression in animal models. The second part of the review will be dedicated to the studies on the memory restoration of AD patients by deep brain stimulation (DBS). In the third part, we will review the studies on memory enhancement in healthy subjects, including non-invasive brain stimulation techniques and successful attempts to intervene in memory consolidation and retrieval processes. With few exceptions, we will not discuss in details pharmacological methods of memory enhancement.
CHANGES IN GENE EXPRESSION CAN ENHANCE MEMORY IN ANIMAL
MODELS
The studies of genetic basis of memory usually focus on genes and products of their transcription involved in neurotransmission or de novo protein synthesis. The classic example of genetically produced memory enhancement is “Doogie” mice overexpressing the NR2B subunit of N-methyl-D-aspartate (NMDA) receptor [5]. These mice outperform their wild-type (WT) littermates in the hippocampus-dependent tests, such as novel object recognition and contextual fear conditioning tasks. Importantly, enhanced memory performance of “Doogie” mice was retained in aged mice [6]. Chemical upregulation of the NR2B subunit with magnesium L-threonate and/or D-cycloserine also resulted in memory enhancement in animals and humans [7, 8]. Most genetic manipulations that potentiate NMDA-related molecular mechanisms were found to enhance memory performance. Thus, transgenic mice overexpressing KIF17 (protein that transports NR2B along the microtubules) exhibited enhanced learning and memory in the Morris water maze task [9]. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors functionally coupled with NMDA receptors are other popular targets in the studies on memory strengthening. Chemical activation of AMPA receptors by ampakines (benzamides, benzothiadiazines, biaryl propylsulfonamides, 3-trifluoromethylpyrazoles) have been found to facilitate memory (for detailed review, see [10]). AMPA receptors are regulated by the protein kinase Mζ (PKMζ), a product of the PKCζ gene in the brain, that has been shown to affect the long-term memory storage in invertebrates and vertebrates (see review [11]). For example, overexpression of PKMζ in the rat neocortex enhanced long-term memory, whereas the dominant negative PKMζ with amino acid substitution in the active site disrupted memory [12]. In monkeys, higher levels of PKMζ were associated with more accurate recognition memory [13]. Another popular target in the memory improvement studies is cAMP response element-binding protein (CREB), a transcription factor that regulates expression of immediate early genes, such as c-Fos and zif268. In general, any manipulation that that leads to the upregulation of CREB expression results in memory enhancement. To give a few examples, local overexpression of CREB in the basolateral amygdala enhanced memory in the classic fear conditioning [14] and social defeat [15] tests. Overexpression of calcium/calmodulin-dependent protein kinase IV (CAMKIV) that directly regulates CREB in the forebrain of mice improved memory in the social recognition and Morris water maze tests [16, 17]. CCAAT/enhancer-binding protein (C/EBP), a product of the early C/EBP gene, is involved in the activation of late response genes in synaptic plasticity. Upregulation of C/EBP expression enhances memory performance. Thus, suppression of the negative C/EBP regulator, ATF4, improved performance of the experimental animals in the Morris water maze test [18].
Among many attempts to find chemical agents potentiating CREB-mediated pathways, the most productive approach to memory enhancement in AD patients involved modulation of phosphodiesterase (PDE) that negatively regulates the cAMP/CREB pathway by hydrolyzing cAMP. Hence, PDE inhibitors should enhance memory. Indeed, some PDE inhibitors, namely Rolipram and Sildenafil, have shown positive result in AD mouse models. Cilostazol a selective inhibitor of PDE3, partially prevents cognitive decline and memory loss in AD patients [19]. For more detailed information on the inhibitors on CREB-dependent pathways, see the review [20].
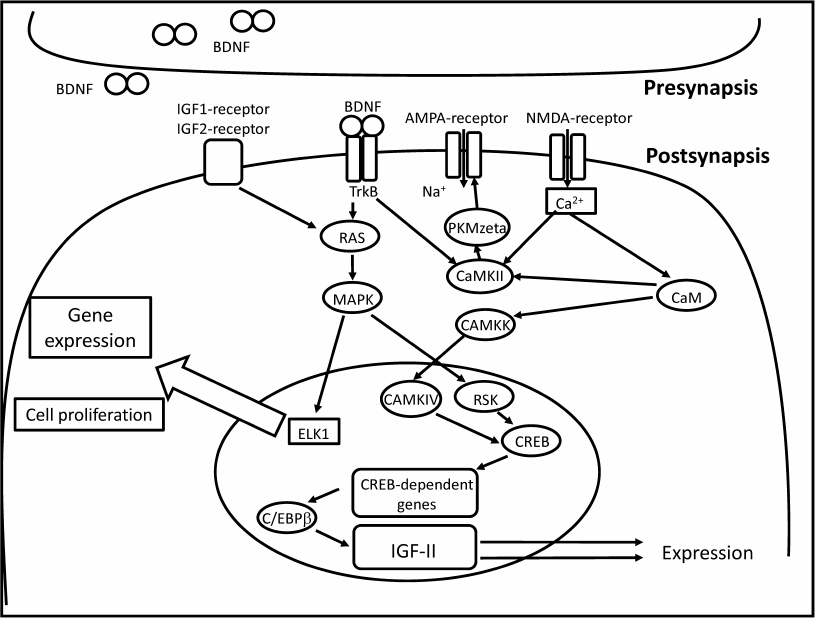
Recent studies demonstrated involvement of insulin-like growth factor 2 (IGF-2) in memory consolidation [21]. IGF-2 expression in the hippocampus is a component of canonical signaling pathway regulated by C/EBP (figure). IGF-2 affects de novo protein synthesis (supposedly, of glutamate receptor subunits) and the subsequent structural changes in the synapse [21]. Injection of recombinant IGF-2 into the hippocampus resulted in improved performance in the inhibitory avoidance and contextual fear conditioning tasks [21]; however, IGF-2 was effective only during the period of memory consolidation [21, 22]. Injections of recombinant IGF-2 in the hippocampus facilitated fear extinction [23, 24]. The authors suggested that IGF-2 regulates adult neurogenesis in the hippocampus by promoting cell proliferation and survival of immature neurons [24]. Systemic administration of IGF-2 improved memory performance in the object recognition, object placement, social recognition, and Y-maze tasks [25]. These data provide strong evidence that IGF-2 acts as an endogenous memory enhancer. IGF-1, a member of the same IGF family, did not enhance memory performance in rodents [26]. Both IGF-1 and IGF-2 belong to a complex system that regulates body size and growth during prenatal development [27]. The same system is implicated in the regulation of lifespan in vertebrates and invertebrates [28-30], which justifies its studying in the context of age-related cognitive impairments and AD-induced memory loss.
Postsynaptic pathways implicated in the IGF-mediated memory enhancement. IGFs act via the classic MAPK/ELK1 signaling cascade resulting in adult neurogenesis in the hippocampus and de novo protein synthesis (including AMPA receptor subunits) [21, 24]. Calcium influx into the cell leads to the activation calcium/calmodulin kinase (CAMKK) by calcium/calmodulin (CaM). Calcium influx increases AMPA receptor conductance via activation of calcium/calmodulin kinase II (CAMKII). CAMKK activates calcium/calmodulin kinase IV (CAMKIV) that in turn phosphorylates cAMP response element-binding protein (CREB). CREB activates transcription of immediate early genes, such as c-fos and C/EBPβ. C/EBPβ codes for CCAAT/enhancer-binding protein that regulates transcription of IGF-2
Mutations in the insulin/IGF-1-like signaling (IIS) pathway in Caenorhabditis elegans enhance different types of memory. Worms with mutations in the IGF-1 receptor (daf-2) and phosphatidylinositol 3-OH kinase (age-1) genes showed increased consistency of temperature-food association as compared to WT worms [31]. These mutants also demonstrated an increase in temperature-starvation association. Caenorhabditis elegans daf-2 mutants exhibited improved short-term memory performance in the appetitive Pavlovian associative learning paradigm, when the odorant butanone was paired with food [32]. Young daf-2 mutants’ short-term memory lasted 2 to 3 times longer than the short-term memory of WT worms; daf-2 mutants also demonstrated better long-term memory than the WT counterparts. Interestingly, no improvement in the long-term memory was found in aged daf-2 mutants, which suggest age-dependent regulation of the IIS pathway. However, aged daf-2 mutants retained ability for thermotaxis and short-term associative memory, unlike WT worms [31, 32].
Aberrant activation of the IIS pathway was found in AD, a disorder directly linked to aging and characterized by progressive memory loss [33, 34]. Both IGF-1 and IGF-2 are dysregulated in AD patients [35-38]. Aged mice overexpressing IGF-1 in the dentate gyrus of the hippocampus demonstrated better spatial memory performance in the Morris water maze test than the WT controls [39]. The authors showed that this effect depended on adult neurogenesis in the hippocampus. In other study, injection IGF-2 into the hippocampus of Tg2576 mice overexpressing amyloid precursor protein (APP) resulted in partial memory restoration in the fear conditioning and Morris water maze tasks that was accompanied by the reduction of amyloid levels in the brain [40]. Injections of recombinant IGF-2 rescued aging-related memory loss in rats [41]. These data open new possibilities for the development of new anti-AD therapies, including those targeting the IIS pathway.
It was suggested that the brain-derived neurotrophic factor (BDNF) is involved in the memory regulation. BDNF injection into the rat hippocampus resulted in the memory improvement in the spontaneous location recognition task [42]. BDNF interacts with the tropomyosin-related kinase B (TrkB) receptor. The TrkB/BDNF pathway involves CdK5 that plays an important role in learning and memory [43]. 7,8-Dihydroxiflavone (TrkB receptor agonist) enhanced both the acquisition and extinction of conditioned fear [44], while lacosamide (TrkB and BDNF suppressor) impaired both short-term and long-term memory [45] in mice. However, despite numerous reports on the BDNF role in memory enhancement, it still remains unclear if BDNF directly regulates memory and neural plasticity or it is involved in the memory-related metabolic pathways including the above-mentioned NMDA pathway.
The role of non-coding RNA in memory formation has been reported in a number of publications [46, 47]. RNA-mediated “memory transfer” was recently demonstrated in the marine mollusk Aplysia [48]. Total RNA was extracted from neurons of Aplysia subjected to sensitization training by tail electroshock and injected into neurons of naive mollusks. Snails injected with the RNA of the trained snails showed higher levels of siphon withdrawal reflex as compared to the control group. These data confirm the epigenetic hypothesis of memory formation and can be used in future studies on the possibility of memory stimulation.
DEEP BRAIN STIMULATION REDUCES MEMORY DEFICITS IN
ALZHEIMER’S DISEASE
For obvious reasons, the use of deep brain stimulation (DBS) in humans is limited to severe forms of neurologic and psychiatric diseases. For a long time, brain stimulation in humans, pioneered by Roger Penfield (1958), has been used for mapping brain areas. Much later, the efficiency of DBS was demonstrated for the treatment of many pathological conditions, including obsessive-compulsive disorder [49], major depression [50], addictions [51], Tourette syndrome [52], Parkinson’s disease [53] and Alzheimer’s disease (AD) [54]. In this review, we will focus on AD that is characterized by severe impairments in the declarative memory.
AD is a progressive neurodegenerative disease accompanied by massive loss of neurons in the brain [3]. AD patients exhibit severe progressive deficit in the declarative memory functions. Traditionally, AD treatment includes the use of various pharmacological agents or cognitive therapy; however, the outcomes of these treatments are not satisfactory enough. The first study that demonstrated DBS-induced memory improvement after DBS was published in 2008 [55]. In this study, the patient was treated for morbid obesity by brain stimulation with electrodes bilaterally implanted in the ventral hypothalamus. Unexpectedly, stimulation evoked detailed autobiographical memories. Computer-assisted tomography showed that the electrodes were placed close to the fornix, a bundle of nerve fibers that connects septal nuclei to the hippocampus. This finding was indirectly confirmed by the fact that stimulation activated hippocampus-associated neuronal networks. Continuous stimulation for three weeks increased the scores in the standard memory tests including the Wechsler Memory Scale (WMS-III). In addition, significant increase in the patient’s ability to memorize and to retrieve information was found 3 weeks and 12 months after the start of the treatment.
This serendipity discovery provided an objective for systematic study of DBS effects on the memory loss in AD [56]. Six patients with mild AD were implanted with stimulation electrodes into the ventral hypothalamus/fornix area and then received continuous stimulation for 12 months. The majority of these patients demonstrated improved standard Mini Mental State Examination (MMSE) scores. This improvement was observed mostly in the MMSE rubrics dealing with the patient’s ability to recollect already seen information. The methods for source reconstruction, such as standardized low-resolution electromagnetic tomography (sLORETA), demonstrated that stimulation activated posterior cingulate cortex and medial parietal lobe, i.e., regions closely involved in the functioning of default mode networks. Positron emission tomography revealed partial restoration of glucose metabolism in the impaired regions. The follow-up study showed that this restoration positively correlated with better outcomes in the memory and global cognition tests [57]. Furthermore, stimulations significantly slowed down neurodegeneration and even increased hippocampus volume in two patients [58]. Taken together, these results are highly encouraging, despite the lack of controls.
Another study that investigated the results of chronic fornix stimulation in a patient with mild AD was performed by Fontaine et al. [59]. One patient was implanted with the stimulation electrode in the fornix. In this study, the memory scores remain stable throughout 12 months of continuous brain stimulation, and the patient reported a feeling of improvement. However, the memory scores were below those typical for normal aging, and only slight increase in glucose metabolism in the medial temporal lobes was found (contrary to the significant increase reported in the aforementioned studies).
The first randomized double-blind clinical trial of the use of DBS in AD treatment started in 2012 [60]. Forty-two patients with mild AD were bilaterally implanted with stimulation electrodes in the fornix. No changes in the cognitive functions were reported within the first 12 months of stimulation, despite the fact that activation of glucose metabolism was observed already in the first 6 months after beginning of the treatment [61]. However, this increase disappeared within the next 6 months of treatment. Interestingly, the outcome of treatment correlated with the patient’s age: patients younger than 65 years demonstrated worse results in cognitive tests after the DBS treatment, whereas patients over 65 years of age showed improvement.
Fornix stimulation in animal models mainly produced positive effects in the recognition tests [62], as well as spatial and contextual memory tests [63, 64]. None of the targets showed side effects of anxiety, locomotor activity, or pain [64]. Rats with scopolamine-induced memory impairments stimulated with electrodes implanted in the fornix demonstrated better results in the novel object recognition test than the sham rats [62]. Stimulation also increased acetylcholine levels in the hippocampus [65] in the absence of adult neurogenesis activation [66]. DBS of the fornix improved the performance in the hippocampus-dependent memory tests in the Rett syndrome mouse model [63] and amyloid-induced AD rat model [64].
These data suggest active involvement of the hippocampus and associated neocortical regions in memory improvement caused by DBS of the fornix. The hypothesis that DBS of the entorhinal cortex, a region that has strong reciprocal connections with the hippocampus, can improve memory has been tested only in epilepsy patients. Entorhinal stimulation (40 Hz) applied while the subjects learned locations of landmarks in the virtual environment enhanced their subsequent memory of these locations: all six participants reached these landmarks more quickly and by shorter routes, as compared with locations learned without stimulation [67]. Direct hippocampal stimulation produced mixed results in the same task. Fell et al. found positive effect in the long-term verbal learning memory test when in-phase entorhinal cortex and hippocampus stimulation was applied in the epilepsy patients [68]. On the contrary, other authors observed decreased memory performance in the epilepsy patients after 50-Hz stimulation of the entorhinal cortex [69]. It is possible that such discrepancies were caused by the differences in the tasks, stimulation protocols, and number of subjects used in the studies [70]. The behavioral results obtained by Jacobs et al. [69] were recently reproduced by another research group [70]. A new breakthrough in the field was reported by researchers from Itzhak Fried’s group [71] who applied theta-burst stimulation protocol in 13 epilepsy patients performing the person recognition task. Stimulation in the right entorhinal area during learning significantly improved subsequent memory specificity for novel faces.
Memory enhancement after DBS of the entorhinal cortex may be related to adult neurogenesis. In the recent study, mice were electrically stimulated in the entorhinal cortex [72]. This stimulation promoted proliferation of granule cells in the dentate gyrus; this effect was not counterbalanced by the apoptosis of existing granule cells. Mice with activated adult neurogenesis performed better in the Morris water maze test. The improvement of spatial memory was observed ~6.5 weeks after the stimulation, i.e., the time necessary for the newborn neurons to mature and integrate into the existing neuronal circuits. Interestingly, the stimulation parameters in this study were similar to those used in DBS in PD and AD patients. In another study, stimulation of the entorhinal cortex in TgCRND8 mice (mouse model of AD) [73] reversed spatial memory deficits tested in the fear conditioning paradigm. Surprisingly, stimulation caused memory improvements in both young (6-week-old) and old (6-month-old) mice.
Another potential target for DBS treatment in AD is the cholinergic nucleus basalis of Meynert (NBM). Cholinergic projections to the cortex are the first brain structures to undergo neurodegeneration in AD [74]. The hypothesis that cognitive decline can be stopped by electrical stimulation has been tested in several studies. In the first study, unilateral stimulation of the NBM in patients with mild AD did not cause any significant cognitive improvements despite preservation of glucose metabolism in the ipsilateral temporal and parietal cortical lobes while it declined elsewhere in the cortex [75]. However, in a recent case report, a patient with severe Parkinson-dementia syndrome was bilaterally stimulated in the NBM [76]. The patient showed significantly improved performance in standard memory tasks. Based on this data, the authors started the phase I clinical trial of the NBM stimulation in early stages of Alzheimer’s dementia [77]. Six patients with mild to moderate AD where implanted with electrodes into the NBM. On average, continuous 20-Hz stimulation of the NBM resulted in stabilization of the MMSE and AD Assessment Scale (ADAG) scores within the first 12 months of stimulation. This effect was accompanied by a slight increase in glucose metabolism. On an individual level, the scores did not change for three patients; one patient improved the scores; one patient displayed slightly worsened scores, and one patient exhibited significant decrease in the memory index. These results were confirmed in a recent study by the same research group who added four more patients to the tested cohort [78]. However, the memory improvement was observed only in the cognitive subitem of ADAS, whereas the memory subitem did not show any improvements.
There are only few studies on the memory enhancement by DBS of the NBM in animal models. Thus, injection of 192 IgG-saporin to the basal forebrain of rats induced degeneration of brain neurons and worsened the outcome of the Morris water maze task [79]. DBS of the NBM restored rat performance in this hippocampus-dependent memory test to the levels observed in normal rats. Also, numerous studies have demonstrated that NBM stimulation in healthy rodents increases acetylcholine concentration in the neocortex and enhances learning-induced receptive field plasticity in the sensory cortex [80-83]. In one of the first studies of the effect of NBM stimulation on memory, rat behavior was studied using the two-way active avoidance conditioning test [84]. NBM stimulation just before the task significantly improved performance tested on the same day; however, the effect of stimulation disappeared 24 and 48 h later. Moreover, NBM stimulation after the task decreased the number of correct avoidances in the stimulated group compared to the control rats. The same effect was observed in the socially transmitted food preference test [85]. Together with the results of clinical trials, these data suggest that the effect of NBM stimulation on memory is not specific, but rather reflects facilitation of attention and sensory information processing.
APPROACHES FOR MEMORY FACILITATION IN HEALTHY SUBJECTS
Non-invasive stimulation enhances memory. DBS is a severe medical intervention, so scientists constantly improve non-invasive methods of brain stimulation that include transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), and transcranial alternating current stimulation (tACS). During the last 15 years, these methods have received significant attention in the context of memory enhancement research.
Transcranial magnetic stimulation (TMS) is a method for short-term local disruption of normal information processing by activating large groups of neurons. However, there is evidence that TMS can facilitate information processing (for comprehensive review, see [86]). Most studies reporting memory enhancement after TMS focus on the working memory [87-92], but for the purposes of this review, we will discuss other types of memory. The studies conducted in 1990s (when the spatial resolution TMS was much lower than nowadays) reported slight memory improvements in WMS-III after transcranial stimulation applied to different scalp positions in healthy subjects [93, 94]. In 2004, repetitive TMS (rTMS) of the left inferior prefrontal cortex in healthy subjects resulted in increased recognition memory in the semantic encoding task [95]. Subjects were asked to memorize words sequentially presented on the screen. During presentation of some words, rTMS was applied to the left or right inferior prefrontal cortex. Presentation of other words was accompanied with rTMS of the left parietal cortex; no stimulation was applied during presentation of the third group of words. Words encoded under stimulation of the left inferior prefrontal cortex were recognized with higher accuracy that words encoded in the absence of TMS under stimulation in the two cortical control sites.
Hawco et al. investigated the effects of rTMS of the left dorsolateral prefrontal cortex (dlPFC) on the encoding phase in the associative memory encoding task [96]. Healthy subjects were asked to memorize pairs of objects, that could be either semantically related or unrelated. Thirty minutes after completion of the encoding phase, the subjects were presented with a single object from the pair and asked to name the second object. Correct response was defined as a verbal naming of the object that was paired to the stimulus during the encoding phase. The authors found that associations for related pairs were better remembered than unrelated pairs. This effect was influenced by the time of rTMS application relatively to the moment of stimulus presentation.
Recent study of Wang et al. showed significant improvement of the associative memory after rTMS of the left parietal cortex that lasted up to five days [97]. The subjects in this study were presented with unique face/word pairs and then tested in the associative pair test five days later. Subjects who received rTMS memorized significantly higher number of combinations than those who received sham stimulation. Functional magnetic resonance imaging (fMRI) revealed positive correlation between the functional connectivity in the cortex after rTMS and memory performance. In another study, object location memory tasks were used to distinguish between general recollection success and recollection precision [98]. The participants studied trial-unique objects at randomly assigned locations. Subsequent memory testing involved object-cued recall of locations (i.e., the use of spatial memory). The subjects receiving rTMS of the posterior parietal cortex during the five days of training showed no differences in the recognition success with the control group. In contrast to general success, the recollection precision in these participants was much higher than in the control subjects. This effect lasted for at least 24 h after the last stimulation session.
To sum up the attempts on the improvement of declarative memory by TMS, we can conclude that these studies are very rare and often use non-comparable behavioral paradigms. The results of these studies are contradictory or limited to very specific types of memory; the observed effects are short-term and poorly pronounced. TMS setups are non-transportable and their efficiency depends on the possibility to conduct MRI. All these issues prevent broad implementation of this technique for memory enhancement.
Transcranial direct current stimulation (tDCS) is a simpler and more mobile technique that, theoretically, can be used in everyday life. It is believed that tDCS modulates the resting potential, or excitability, of neurons, making it closer or further away from the action potential threshold [99]. Traditionally, most memory enhancement studies use anodal tDCS that makes neurons more excitable. Thus, bilateral anodal tDCS of frontal lobes was applied in healthy subjects at the stage of slow-wave sleep (SWS) [100]. Before the sleep, these subjects had memorized associative word pairs. Pulsed tDCS during the first 30 min of SWS resulted in distinct increase in the word pair recollection compared to the control group. Importantly, the same protocol did not affect the procedural memory tested in the mirror tracing skill task. The authors speculated that tDCS facilitated declarative memory performance by affecting synaptic plasticity and memory consolidation during the sleep. Recently, this approach was successfully used for visual declarative memory enhancement in patients with moderate cognitive impairments [101].
Another study tested the effects of tDCS applied to participants in an awake state during encoding and recognition phases of word memorization tasks [102]. Anodal tDCS of the dlPFC during the encoding phase led to a higher recognition accuracy compared to sham and cathodal tDCS. Also, the authors did not observe any effect of tDCS applied during the recognition phase. The same procedure led to the increased memory performance in the non-verbal associative memory task [103]. However, another research group showed that anodal tDCS of the left dlPFC applied during the encoding phase increased the number of false alarms in the non-verbal episodic memory task, while cathodal tDCS reduced number of false alarms [104]. The authors suggested that enhancement of excitability in the dlPFC by anodal tDCS can be associated with blurred detail memory, whereas cathodal tDCS acts as a noise filter inhibiting the development of imprecise memory traces by reducing the activity of neurons in the prefrontal cortex.
Anodal tDCS of dlPFC improved recollection of words in semantic encoding task when stimulation was applied during the memory reactivation phase [105]. Participants memorized words in encoding session and then reactivated the memory of words 3 h later using the old-new recognition task under anodal, cathodal, or sham tDCS to the left dlPFC. In the other round of the old-new recognition task performed 2 h later, the subjects who underwent anodal tDCS were able to recognize more words than the control group. Cathodal (but not anodal) tDCS of the right dlPFC applied between the encoding and testing phases improved memory performance in the non-verbal face recognition memory task [106]. Several studies also showed that a single session of anodal tDCS of the frontal lobe increases percentage of correct responses in the language learning paradigm [107-109].
However, some studies failed to find any positive effect of tDCS of dlPFC on memory. For example, de Lara et al. did not observe any positive effect of anodal tDCS of left dlPFC the encoding and retrieval of verbal declarative information in the language learning paradigm [110]. Another study demonstrated that anodal tDCS of the left or right dlPFC during the encoding phase decreased the accuracy of retrieval for both verbal and nonverbal material [111]. Instead, the authors found that anodal tDCS of the right or left posterior parietal cortex improved the accuracy in the task with non-verbal material. Anodal tDCS of the parietal lobe enhanced memory enhancement in the California verbal learning task [112]. The subjects were able to recall more words after anodal tDCS of the left posterior parietal cortex applied during encoding, whereas no improvement was observed after stimulation in the testing phase.
There is a convincing body of evidence on the positive effects of tDCS in subjects with cognitive decline. Boggio et al. observed significant improvement in the visual recognition memory in AD patients after tDCS of the temporal and parietal cortices [113]. Anodal tDCS over the right temporoparietal cortex increased memory performance in the spatial associative learning paradigm in elderly [114]. Also, aged subjects demonstrated better results in the verbal episodic memory task after anodal tDCS of the left dlPFC [115, 116] or parietal cortex [115].
Similar results were obtained in primates. Thus, studies in macaques reported the positive effect of anodal tDCS of the right prefrontal cortex on the performance in the spatial associative memory task [117]. The authors demonstrated that this effect likely depends on the induction of low-frequency oscillations in the prefrontal cortex that alter the functional connectivity in the brain. Taken together, these data open new ways for safer treatment of memory disabilities using non-invasive and safe tDCS approach [118, 119].
Transcranial alternating current stimulation (tACS) is an oscillatory counterpart of tDCS. tACS modulates spontaneous neural oscillations in the brain by resonating with them and facilitating synchronization of neural activity [120]. The first study that investigated the positive effect of tACS on memory was performed by Marshall et al. who extended their approach to non-invasive brain stimulation during the SWS [100, 121]. In this study, the participants memorized pairs of words. During subsequent early nocturnal SWS, they were subjected to tACS (0.75 Hz) bilaterally applied over the frontal lobe. The procedure induced highly pronounced slow oscillations in the cortex. After waking up, the subjects were able to recall significantly more words than the control group. Interestingly, no memory improvements were observed when the subjects were stimulated during the daytime naps [122]. However, another research group found a decrease in the power of low-frequency oscillations, as well as reduction in the number of recalled words, in subjects that underwent bilateral 0.75-Hz tACS over the frontal cortex; the decrease correlated with the reduction in word pair recall after the nap. It is possible that such discrepancies could be accounted to the differences in the stimulated areas.
In the follow-up study, Marshall et al. used 0.75-Hz tACS to the frontal cortex in subjects in the state of wakefulness [123]. Surprisingly, this stimulation resulted in the increase in the power of theta-oscillations (4-8 Hz) registered by electroencephalography. Verbal learning and memory tests demonstrated boosted memory performance when tACS was applied during the encoding phase but not during the testing phase. These results clearly suggest the dependence of tACS effect on the physiological state of the tested subjects. In another study, young and elderly subjects were stimulated over the frontal lobe in the theta range (6 Hz) during the encoding phase of the associative memory task [124]. Both groups showed enhanced memory performance in the testing phase compared to the control group not receiving tACS. High-frequency (140 Hz) tACS to the frontal lobe during the encoding phase of the word-pair associative learning task resulted in partial reduction in the overnight forgetting of words [125].
Javadi et al. hypothesized that using the same tACS frequency during the encoding and testing phases is more important for the declarative memory than the stimulation frequency itself [126]. The subjects were asked to memorize 100 words and then stimulated over the left dlPFC during both encoding and testing phases at 60 or 90 Hz in various combinations. The memory performance was significantly improved if the tACS frequency during the encoding and testing phases was the same.
Numerous drugs for memory and cognitive function enhancement have been developed for the use by healthy population (e.g., methylphenidate, amphetamines, modafinil, acetylcholine esterase inhibitors, nicotine, etc.). Methylphenidate and amphetamines are especially popular among medical students for achieving high academic grades, despite numerous known negative side effects [127, 128]. Catecholaminergic, cholinergic, and melatoninergic agents are now hailed as new memory-enhancing drugs (for detailed information about “smart” drugs, see reviews [129, 130]). An important issue in the use of “smart” drugs, including anti-AD agents, is their transport through the blood-brain barrier. Because of the low efficiency of delivery to the brain, the drugs should be used in high doses. To minimize the doses in order to avoid toxic side effects, the scientists have developed a number of alternative approaches for drug delivery, e.g., nasal inhalation [131]. The most advanced direction in this area is the use of nanoparticles [132, 133]; however, nanoparticles themselves can be neurotoxic [134].
Intervention in the processes of memory consolidation and memory trace retrieval. Memory studies in rodents often focus on the hippocampus-dependent memory and spatial orientation. Hippocampal principal neurons, also called place cells, discharge in exact location of animal’s environment, which eliminates the ambiguities in the interpretation of relationships between behavior and neural activity [135]. Sequential activation of place cells reflects the trajectory of animal’s movement [136-139] and can also provide information on animal’s motivation and its emotional significance [140-142]. The theory of two-stage memory consolidation, first proposed by David Marr in 1971 and then brought to physiology by György Buzsáki in 1989, suggests that place cells become a component of activation sequences during active exploration of the environment [143, 144]. In rodents, exploratory activity is always accompanied by prominent theta-oscillations (4-12 Hz) registered in the hippocampus. The trajectories of movement are first encoded as the sequences of activation of place cells and then transferred for the long-term storage to the neocortex during the SWS. This transfer, which is considered a physiological basis of memory consolidation, is thought to be a result of fine orchestration of several events, such as hippocampal sharp-wave ripples (SWRs) (120-200 Hz), thalamic α-spindles (8-12 Hz), and cortical δ-waves (1-4 Hz). SWRs coincide with the so-called reactivation of hippocampal sequences [136-138], during which the sequences already explored during the wake period are replayed in a compressed form. In one of the studies, the researchers stimulated the medial forebrain bundle (MFB) known for its self-stimulation properties each time computer algorithm detected spontaneous sleep reactivation of a particular place cell [145]. This manipulation lead to goal-directed behavior of the animal towards the receptive field of the place cell whose reactivation coincided with the MFB stimulation. In other words, the authors were able to create artificial memory with a high temporal and spatial resolution.
The hypothesis that memory could be boosted by enhancing the coupling between the hippocampus and the cortex (according to the two-stage theory of memory consolidation) during the SWS has been tested by several research groups. Thus, δ-waves were induced in the cortex by electrical stimulation each time when the SWRs was detected by the computer algorithm in order to promote coordination between the hippocampus and the cortex [146]. The animals with boosted coordination of oscillatory events mimicking the natural sequence of sleep events were more discriminative in the novel object recognition task than the animals with random stimulation protocol or without any stimulation. Another study demonstrates that optogenetic activation of interneurons in the thalamic reticular nucleus (TRN) in-phase, but not out-of-phase, with cortical δ-oscillations up-states induced spindles and improved memory performance in the context fear conditioning test [147].
The same hypothesis has been tested in humans. Subjects were cued with an odor during the encoding stage of the declarative memory task. Re-exposure to the same odor during the SWS significantly increased memory performance at the testing stage after waking up as compared to the control group [148]. fMRI revealed that odor presentation during sleep activated hippocampus and hippocampus-associated neuronal networks. In another study, subjects performed the spatial memory task, in which presented objects were associated with sounds [149]. Memory improvement was observed in the group of subjects receiving the same sound cues during sleep, but not in the control group.
This approach was improved in [150] The participants performed word-pair associative memory task without any auditory stimulation during the encoding phase. During subsequent SWS sleep, auditory cues were played in-phase with spontaneous slow oscillations for 210 min. The subjects with the sleep sensory stimulation demonstrated 2 times better memory performance at the testing stage compared to the control group. It should be noted that sound stimulation increased the power of slow oscillations and the number of phase-coupled spindles, thereby corroborating the Marr–Buzsáki theory of two-stage memory consolidation.
Another mechanism was proposed by Bendor and Wilson [151] who trained rats in the auditory spatial association task. Presentation of auditory cues during the SWS increased the number of hippocampal replays associated with a particular cue. This result shows that sensory cueing during the sleep enhances memory by increasing the number of relevant memory reactivations.
In a very elegant study, Arzi et al. were able to create artificial memory during sleep using the sensory cueing technique [152]. The researchers paired different tones with pleasant or unpleasant odors during the sleep and then measured sniff responses to the auditory stimuli during sleep or subsequent wakefulness. It was demonstrated that the subjects learned associations between odors and sound cues during sleep and differentiated the tones according to the pleasantness of odors that was associated with them. Interestingly, subjects were not aware of the learning process.
Several research groups used the neuronal tagging technique to manipulate memory in mice. This method requires specific mouse strains carrying tetracycline transactivator (tTa) controlled by the immediate early c-Fos gene (c-Fos-tTa strains). Expression of immediate early genes is driven by the neural activity, i.e., c-Fos is expressed in neurons with elevated firing rates. Doxycycline (Dox) inhibits tTa binding to the tetracycline-responsive element (TRE), making expression of TRE-regulated genetic constructs dependent on tTa and doxycycline. In their seminal work, Garner et al. used c-Fos-tTa mouse line expressing hM3Dq designer receptor exclusively activated by a designer drug (DREADD) under control of tTa [153]. The animals were placed into the context A in order to drive expression of the hM3Dq transgene into neurons activated in that context. The animals were then injected with Dox to inhibit further hM3Dq receptor expression and with clozapine N-oxide (CNO) to stimulate activity in the pattern of neurons that expressed the receptor. The mice were then fear conditioned in a distinct context B, and 24 h later, memory performance was tested in the absence and presence of CNO. Chemogenetic activation of the neurons tagged in the context A failed to induce freezing in the same context. However, simultaneous activation of the context A neurons with CNO and context B neurons by placing the mice in context B lead to the freezing levels similar to those observed in WT mice, suggesting that the authors created a novel hybrid memory trace representation that included both A and B contexts.
This approach was refined in the study of Tonegawa et al., in which c-Fos-tTa mice were injected with TRE-ChR2 vector into the hippocampus [154]. Mice kept on a Dox-free in the were exposed to the neutral context 1, after which Dox was added to the diet resulting in the ChR2 opsin expression in the dentate gyrus neurons of the hippocampus. Mice with the tagged neurons were subjected to fear conditioning in the context 2, in which reactivation of tagged neurons by light served as a conditioning stimulus. Reactivation of these neurons in the context not associated with the negative stimulation (context 1) still produced increased freezing levels similar to those observed in the context 2, allowing the authors to claim that new artificial memory was created. This study manipulated a smaller population of neurons than the study of Garner et al. and used activation procedure with higher temporal resolution than the DREADD protocol, which could explain the success of the used approach. Later, the same optogenetic technique was successfully used to switch the valence of the emotional memory trace [155] and to restore normal behavior in stress-induced depression [156].
Memory enhancement is broad research area that unifies scientists pursuing different goals: from the development of methods for memory improvement to verification of fundamental hypotheses. Often, the methods used in healthy subjects cannot be used in diseased individuals, and vice versa. However, discoveries made in one field could bring ideas to other fields. In this review, we tried to create a common research landscape focused on facilitation of declarative memory. On one hand, our approach could help to reveal potential links which are difficult to see from a single point of view. For example, both regulation of the IIS pathway and DBS of the fornix increase adult neurogenesis that, in turn, improves memory performance in classical memory tests [24, 72]. As far as we know, the mechanisms underlying changes in the expression of IIS pathway genes upon DBS of the fornix have not been yet investigated. Studies dedicated to the elucidation of the nature of such link could significantly enrich and improve the methods for AD treatment.
On the other hand, our review demonstrates that existing models and approaches are diverse and very difficult to reconcile. The methodology of non-invasive brain stimulation exists in another paradigm and stems from completely different area of knowledge than the methodology of genetic research. For this reason, it is often impossible to compare results of studies in these two research areas, which requires development of a complex and integrative approach that would unify fundamental concepts, frameworks, and methods in the area of memory enhancement research. We hope that the technological progress, as well as ever-growing interdisciplinary communication, will make such integration possible in nearest future.
Funding
The work was supported by the Russian Science Foundation grant #14-44-00077 and National Institutes of Health, USA (# R01AG054712 to E.I.R.; study concept and supervision).
REFERENCES
1.Kukushkin, N. V., and Carew, T. J. (2017) Memory
takes time, Neuron, 95, 259-279.
2.Morand-Beaulieu, S., Leclerc, J., Valois, P.,
Lavoie, M., O’Connor, K., and Gauthier, B. (2017) A review of the
neuropsychological dimensions of tourette syndrome, Brain Sci.,
7, E106.
3.Ballard, C., Gauthier, S., Corbett, A., Brayne, C.,
Aarsland, D., and Jones, E. (2011) Alzheimer’s disease,
Lancet, 377, 1019-1031.
4.Penfield, W., and Milner, B. (1958) Memory deficit
produced by bilateral lesions in the hippocampal zone, AMA
Arch. Neurol. Psychiatry, 79, 475-497.
5.Tang, Y.-P., Shimizu, E., Dube, G. R., Rampon, C.,
Kerchner, G. A., Zhuo, M., Liu, G., and Tsien, J. Z. (1999) Genetic
enhancement of learning and memory in mice, Nature, 401,
63-69.
6.Cao, X., Cui, Z., Feng, R., Tang, Y.-P., Qin, Z.,
Mei, B., and Tsien, J. Z. (2007) Maintenance of superior learning and
memory function in NR2B transgenic mice during ageing, Eur. J.
Neurosci., 25, 1815-1822.
7.Wang, D., Jacobs, S. A., and Tsien, J. Z. (2014)
Targeting the NMDA receptor subunit NR2B for treating or preventing
age-related memory decline, Expert Opin. Ther. Targets,
18, 1121-1130.
8.Kalisch, R., Holt, B., Petrovic, P., De Martino,
B., Kloppel, S., Buchel, C., and Dolan, R. J. (2009) The NMDA agonist
D-cycloserine facilitates fear memory consolidation in humans,
Cereb. Cortex, 19, 187-196.
9.Wong, R. W.-C., Setou, M., Teng, J., Takei, Y., and
Hirokawa, N. (2002) Overexpression of motor protein KIF17 enhances
spatial and working memory in transgenic mice, Proc. Natl. Acad.
Sci. USA, 99, 14500-14505.
10.Partin, K. M. (2015) AMPA receptor potentiators:
from drug design to cognitive enhancement, Curr. Opin.
Pharmacol., 20, 46-53.
11.Borodinova, A. A., Zuzina, A. B., and Balaban, P.
M. (2017) Role of atypical protein kinases in maintenance of long-term
memory and synaptic plasticity, Biochemistry (Moscow),
82, 243-256.
12.Shema, R., Haramati, S., Ron, S., Hazvi, S.,
Chen, A., Sacktor, T. C., and Dudai, Y. (2011) Enhancement of
consolidated long-term memory by overexpression of protein
kinase M in the neocortex, Science, 331,
1207-1210.
13.Hara, Y., Punsoni, M., Yuk, F., Park, C. S.,
Janssen, W. G. M., Rapp, P. R., and Morrison, J. H. (2012) Synaptic
distributions of GluA2 and PKM in the monkey dentate gyrus and their
relationships with aging and memory, J. Neurosci., 32,
7336-7344.
14.Josselyn, S. A., Shi, C., Carlezon, W. A., Neve,
R. L., Nestler, E. J., and Davis, M. (2001) Long-term memory is
facilitated by cAMP response element-binding protein overexpression in
the amygdala, J. Neurosci., 21, 2404-2412.
15.Jasnow, A. M., Shi, C., Israel, J. E., Davis, M.,
and Huhman, K. L. (2005) Memory of social defeat is facilitated by cAMP
response element-binding protein overexpression in the amygdala,
Behav. Neurosci., 119, 1125-1130.
16.Wu, L.-J., Zhang, X.-H., Fukushima, H., Zhang,
F., Wang, H., Toyoda, H., Li, B.-M., Kida, S., and Zhuo, M. (2008)
Genetic enhancement of trace fear memory and cingulate potentiation in
mice overexpressing Ca2+/calmodulin-dependent protein kinase
IV, Eur. J. Neurosci., 27, 1923-1932.
17.Fukushima, H., Maeda, R., Suzuki, R., Suzuki, A.,
Nomoto, M., Toyoda, H., Wu, L.-J., Xu, H., Zhao, M.-G., and Ueda, K.
(2008) Upregulation of calcium/calmodulin-dependent protein kinase IV
improves memory formation and rescues memory loss with aging, J.
Neurosci., 28, 9910-9919.
18.Chen, A., Muzzio, I. A., Malleret, G., Bartsch,
D., Verbitsky, M., Pavlidis, P., Yonan, A. L., Vronskaya, S., Grody, M.
B., Cepeda, I., Gilliam, T. C., and Kandel, E. R. (2003) Inducible
enhancement of memory storage and synaptic plasticity in transgenic
mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins,
Neuron, 39, 655-669.
19.Sakurai, H., Hanyu, H., Sato, T., Kume, K.,
Hirao, K., Kanetaka, H., and Iwamoto, T. (2013) Effects of cilostazol
on cognition and regional cerebral blood flow in patients with
Alzheimer’s disease and cerebrovascular disease: a pilot study,
Geriatr. Gerontol. Int., 13, 90-97.
20.Kametani, F. (2015) Austin Alzheimer’s and
Parkinson’s disease phosphodiesterase as a drug target of
Alzheimer’s disease, Austin Alzheimers J. Park. Dis.,
2, 2-5.
21.Chen, D. Y., Stern, S. A., Garcia-Osta, A.,
Saunier-Rebori, B., Pollonini, G., Bambah-Mukku, D., Blitzer, R. D.,
and Alberini, C. M. (2011) A critical role for IGF-II in memory
consolidation and enhancement, Nature, 469, 491-497.
22.Lee, Y., Lee, Y. W., Gao, Q., Lee, Y., Lee, H.
E., and Ryu, J. H. (2015) Exogenous insulin-like growth factor 2
administration enhances memory consolidation and persistence in a
time-dependent manner, Brain Res., 1622, 466-473.
23.Agis-Balboa, R. C., Arcos-Diaz, D., Wittnam, J.,
Govindarajan, N., Blom, K., Burkhardt, S., Haladyniak, U., Agbemenyah,
H. Y., Zovoilis, A., Salinas-Riester, G., Opitz, L., Sananbenesi, F.,
and Fischer, A. (2011) A hippocampal insulin-growth factor 2 pathway
regulates the extinction of fear memories, EMBO J., 30,
4071-4083.
24.Agis-Balboa, R. C., and Fischer, A. (2014)
Generating new neurons to circumvent your fears: the role of IGF
signaling, Cell. Mol. Life Sci., 71, 21-42.
25.Stern, S. A., Kohtz, A. S., Pollonini, G., and
Alberini, C. M. (2014) Enhancement of memories by systemic
administration of insulin-like growth factor II,
Neuropsychopharmacology, 39, 2179-2190.
26.Stern, S. A., Chen, D. Y., and Alberini, C. M.
(2014) The effect of insulin and insulin-like growth factors on
hippocampus- and amygdala-dependent long-term memory formation,
Learn. Mem., 21, 556-563.
27.Fernandez, A. M., and Torres-Aleman, I. (2012)
The many faces of insulin-like peptide signalling in the brain, Nat.
Rev. Neurosci., 13, 225-239.
28.Kenyon, C., Chang, J., Gensch, E., Rudner, A.,
and Tabtiang, R. (1993) A C. elegans mutant that lives twice as
long as wild type, Nature, 366, 461-464.
29.Giannakou, M., and Partridge, L. (2007) Role of
insulin-like signalling in Drosophila lifespan, Trends
Biochem. Sci., 32, 180-188.
30.Van Heemst, D. (2010) Insulin, IGF-1 and
longevity, Aging Dis., 1, 147-157.
31.Kauffman, A. L., Ashraf, J. M., Corces-Zimmerman,
M. R., Landis, J. N., and Murphy, C. T. (2010) Insulin signaling and
dietary restriction differentially influence the decline of learning
and memory with age, PLoS Biol., 8, e1000372.
32.Murakami, H. (2005) Aging-dependent and
-independent modulation of associative learning behavior by
insulin/insulin-like growth factor-1 signal in Caenorhabditis
elegans, J. Neurosci., 25, 10894-10904.
33.Carro, E., and Torres-Aleman, I. (2004) The role
of insulin and insulin-like growth factor I in the molecular and
cellular mechanisms underlying the pathology of Alzheimer’s
disease, Eur. J. Pharmacol., 490, 127-133.
34.O’Neill, C., Kiely, A. P., Coakley, M. F.,
Manning, S., and Long-Smith, C. M. (2012) Insulin and IGF-1 signalling:
longevity, protein homoeostasis and Alzheimer’s disease,
Biochem. Soc. Trans., 40, 721-727.
35.Watanabe, T., Miyazaki, A., Katagiri, T.,
Yamamoto, H., Idei, T., and Iguchi, T. (2005) Relationship between
serum insulin-like growth factor-1 levels and Alzheimer’s disease
and vascular dementia, J. Am. Geriatr. Soc., 53,
1748-1753.
36.Aleman, A., and Torres-Aleman, I. (2009)
Circulating insulin-like growth factor I and cognitive function:
neuromodulation throughout the lifespan, Prog. Neurobiol.,
89, 256-265.
37.Hertze, J., Nagga, K., Minthon, L., and Hansson,
O. (2014) Changes in cerebrospinal fluid and blood plasma levels of
IGF-II and its binding proteins in Alzheimer’s disease: an
observational study, BMC Neurol., 14, 64.
38.Lane, E. M., Hohman, T. J., and Jefferson, A. L.
(2017) Insulin-like growth factor binding protein-2 interactions with
Alzheimer’s disease biomarkers, Brain Imaging Behav.,
11, 1779-1786.
39.Hu, A., Yuan, H., Wu, L., Chen, R., Chen, Q.,
Zhang, T., Wang, Z., Liu, P., and Zhu, X. (2016) The effect of
constitutive over-expression of insulin-like growth factor 1 on the
cognitive function in aged mice, Brain Res., 1631,
204-213.
40.Pascual-Lucas, M., Viana da Silva, S., Di Scala,
M., Garcia-Barroso, C., Gonzalez-Aseguinolaza, G., Mulle, C., Alberini,
C. M., Cuadrado-Tejedor, M., and Garcia-Osta, A. (2014) Insulin-like
growth factor 2 reverses memory and synaptic deficits in APP transgenic
mice, EMBO Mol. Med., 6, 1246-1262.
41.Steinmetz, A. B., Johnson, S. A., Iannitelli, D.
E., Pollonini, G., and Alberini, C. M. (2016) Insulin-like growth
factor 2 rescues aging-related memory loss in rats, Neurobiol.
Aging, 44, 9-21.
42.Bekinschtein, P., Kent, B. A., Oomen, C. A.,
Clemenson, G. D., Gage, F. H., Saksida, L. M., and Bussey, T. J. (2013)
BDNF in the dentate gyrus is required for consolidation of
“pattern-separated” memories, Cell Rep., 5,
759-768.
43.Lai, K. O., Wong, A. S., Cheung, M. C., Xu, P.,
Liang, Z., Lok, K. C., Xie, H., Palko, M. E., Yung, W. H., Tessarollo,
L., Cheung, Z. H., and Ip, N. Y. (2012) TrkB phosphorylation by Cdk5 is
required for activity-dependent structural plasticity and spatial
memory, Nat. Neurosci., 15, 1506-1515.
44.Andero, R., Heldt, S. A., Ye, K., Liu, X.,
Armario, A., and Ressler, K. J. (2011) Effect of 7,8-dihydroxyflavone,
a small-molecule TrkB agonist, on emotional learning, Am. J.
Psychiatry, 168, 163-172.
45.Shishmanova-Doseva, M., Peychev, L., Koeva, Y.,
Terzieva, D., Georgieva, K., and Peychev, Z. (2018) Chronic treatment
with the new anticonvulsant drug lacosamide impairs learning and memory
processes in rats: a possible role of BDNF/TrkB ligand receptor system,
Pharmacol. Biochem. Behav., 169, 1-9.
46.Tan, M. C., Widagdo, J., Chau, Y. Q., Zhu, T.,
Wong, J. J.-L., Cheung, A., and Anggono, V. (2017) The activity-induced
long non-coding RNA Meg3 modulates AMPA receptor surface
expression in primary cortical neurons, Front. Cell. Neurosci.,
11, 124.
47.Fiumara, F., Rajasethupathy, P., Antonov, I.,
Kosmidis, S., Sossin, W. S., and Kandel, E. R. (2015) MicroRNA-22 gates
long-term heterosynaptic plasticity in Aplysia through
presynaptic regulation of CPEB and downstream targets, Cell
Rep., 11, 1866-1875.
48.Bedecarrats, A., Chen, S., Pearce, K., Cai, D.,
and Glanzman, D. L. (2018) RNA from trained Aplysia can induce
an epigenetic engram for long-term sensitization in untrained
Aplysia, eNeuro, 5, e0038-18.2018.
49.Blomstedt, P., Sjoberg, R. L., Hansson, M.,
Bodlund, O., and Hariz, M. I. (2013) Deep brain stimulation in the
treatment of obsessive-compulsive disorder, World Neurosurg.,
80, e245-e253.
50.Schlaepfer, T. E., and Bewernick, B. H. (2013)
Deep brain stimulation for major depression, Handb. Clin.
Neurol., 116, 235-243.
51.Luigjes, J., van den Brink, W., Feenstra, M., van
den Munckhof, P., Schuurman, P. R., Schippers, R., Mazaheri, A., De
Vries, T. J., and Denys, D. (2012) Deep brain stimulation in addiction:
a review of potential brain targets, Mol. Psychiatry, 17,
572-583.
52.Baldermann, J. C., Schuller, T., Huys, D.,
Becker, I., Timmermann, L., Jessen, F., Visser-Vandewalle, V., and
Kuhn, J. (2016) Deep brain stimulation for tourette-syndrome: a
systematic review and meta-analysis, Brain Stimul., 9,
296-304.
53.Pozzi, N. G., and Pacchetti, C. (2017) Back to
the future: 30th anniversary of deep brain stimulation for
Parkinson’s disease, Funct. Neurol., 32, 5-6.
54.Hardenacke, K., Shubina, E., Buhrle, C. P., Zapf,
A., Lenartz, D., Klosterkotter, J., Visser-Vandewalle, V., and Kuhn, J.
(2013) Deep brain stimulation as a tool for improving cognitive
functioning in Alzheimer’s dementia: a systematic review,
Front. Psychiatry, 4, 159.
55.Hamani, C., McAndrews, M. P., Cohn, M., Oh, M.,
Zumsteg, D., Shapiro, C. M., Wennberg, R. A., and Lozano, A. M. (2008)
Memory enhancement induced by hypothalamic/fornix deep brain
stimulation, Ann. Neurol., 63, 119-123.
56.Laxton, A. W., Tang-Wai, D. F., McAndrews, M. P.,
Zumsteg, D., Wennberg, R., Keren, R., Wherrett, J., Naglie, G., Hamani,
C., Smith, G. S., and Lozano, A. M. (2010) A phase I trial of deep
brain stimulation of memory circuits in Alzheimer’s disease,
Ann. Neurol., 68, 521-534.
57.Smith, G. S., Laxton, A. W., Tang-Wai, D. F.,
McAndrews, M. P., Diaconescu, A. O., Workman, C. I., and Lozano, A. M.
(2012) Increased cerebral metabolism after 1 year of deep brain
stimulation in Alzheimer disease, Arch. Neurol., 69,
1141-1148.
58.Sankar, T., Chakravarty, M. M., Bescos, A., Lara,
M., Obuchi, T., Laxton, A. W., McAndrews, M. P., Tang-Wai, D. F.,
Workman, C. I., Smith, G. S., and Lozano, A. M. (2015) Deep brain
stimulation influences brain structure in Alzheimer’s disease,
Brain Stimul., 8, 645-654.
59.Fontaine, D., Deudon, A., Lemaire, J. J.,
Razzouk, M., Viau, P., Darcourt, J., and Robert, P. (2013) Symptomatic
treatment of memory decline in Alzheimer’s disease by deep brain
stimulation: a feasibility study, J. Alzheimer’s Dis.,
34, 315-323.
60.Ponce, F. A., Asaad, W. F., Foote, K. D.,
Anderson, W. S., Rees Cosgrove, G., Baltuch, G. H., Beasley, K.,
Reymers, D. E., Oh, E. S., Targum, S. D., Smith, G. S., Lyketsos, C.
G., and Lozano, A. M. (2016) Bilateral deep brain stimulation of the
fornix for Alzheimer’s disease: surgical safety in the ADvance
trial, J. Neurosurg., 125, 75-84.
61.Lozano, A. M., Fosdick, L., Chakravarty, M. M.,
Leoutsakos, J.-M., Munro, C., Oh, E., Drake, K. E., Lyman, C. H.,
Rosenberg, P. B., Anderson, W. S., Tang-Wai, D. F., Pendergrass, J. C.,
Salloway, S., Asaad, W. F., Ponce, F. A., Burke, A., Sabbagh, M., Wolk,
D. A., Baltuch, G., Okun, M. S., Foote, K. D., McAndrews, M. P.,
Giacobbe, P., Targum, S. D., Lyketsos, C. G., and Smith, G. S. (2016) A
phase II study of fornix deep brain stimulation in mild
Alzheimer’s disease, J. Alzheimer’s Dis., 54,
777-787.
62.Hescham, S., Lim, L. W., Jahanshahi, A.,
Steinbusch, H. W. M., Prickaerts, J., Blokland, A., and Temel, Y.
(2013) Deep brain stimulation of the forniceal area enhances memory
functions in experimental dementia: the role of stimulation parameters,
Brain Stimul., 6, 72-77.
63.Hao, S., Tang, B., Wu, Z., Ure, K., Sun, Y., Tao,
H., Gao, Y., Patel, A. J., Curry, D. J., Samaco, R. C., Zoghbi, H. Y.,
and Tang, J. (2015) Forniceal deep brain stimulation rescues
hippocampal memory in Rett syndrome mice, Nature, 526,
430-434.
64.Zhang, J.-G., Zhang, C., Hu, W.-H., Wu, D.-L.,
and Zhang, K. (2015) Behavioral effects of deep brain stimulation of
the anterior nucleus of thalamus, entorhinal cortex and fornix in a rat
model of Alzheimer’s disease, Chin. Med. J. (Engl.),
128, 1190-1195.
65.Hescham, S., Jahanshahi, A., Schweimer, J. V.,
Mitchell, S. N., Carter, G., Blokland, A., Sharp, T., and Temel, Y.
(2016) Fornix deep brain stimulation enhances acetylcholine levels in
the hippocampus, Brain Struct. Funct., 221,
4281-4286.
66.Hescham, S., Temel, Y., Schipper, S., Lagiere,
M., Schonfeld, L.-M., Blokland, A., and Jahanshahi, A. (2017) Fornix
deep brain stimulation induced long-term spatial memory independent of
hippocampal neurogenesis, Brain Struct. Funct., 222,
1069-1075.
67.Suthana, N., Haneef, Z., Stern, J., Mukamel, R.,
Behnke, E., Knowlton, B., and Fried, I. (2012) Memory enhancement and
deep-brain stimulation of the entorhinal area, N. Engl. J. Med.,
366, 502-510.
68.Fell, J., Staresina, B. P., Do Lam, A. T. A.,
Widman, G., Helmstaedter, C., Elger, C. E., and Axmacher, N. (2013)
Memory modulation by weak synchronous deep brain stimulation: a pilot
study, Brain Stimul., 6, 270-273.
69.Jacobs, J., Miller, J., Lee, S. A., Coffey, T.,
Watrous, A. J., Sperling, M. R., Sharan, A., Worrell, G., Berry, B.,
Lega, B., Jobst, B. C., Davis, K., Gross, R. E., Sheth, S. A., Ezzyat,
Y., Das, S. R., Stein, J., Gorniak, R., Kahana, M. J., and Rizzuto, D.
S. (2016) Direct electrical stimulation of the human entorhinal region
and hippocampus impairs memory, Neuron, 92, 983-990.
70.Hansen, N., Chaieb, L., Derner, M., Hampel, K.
G., Elger, C. E., Surges, R., Staresina, B., Axmacher, N., and Fell, J.
(2018) Memory encoding-related anterior hippocampal potentials are
modulated by deep brain stimulation of the entorhinal area,
Hippocampus, 28, 12-17.
71.Titiz, A. S., Hill, M. R. H., Mankin, E. A.,
Aghajan, Z. M., Eliashiv, D., Tchemodanov, N., Maoz, U., Stern, J.,
Tran, M. E., Schuette, P., Schuette, P., Behnke, E., Suthana, N. A.,
and Fried, I. (2017) Theta-burst microstimulation in the human
entorhinal area improves memory specificity, Elife, 6,
e29515.
72.Stone, S. S. D., Teixeira, C. M., DeVito, L. M.,
Zaslavsky, K., Josselyn, S. A., Lozano, A. M., and Frankland, P. W.
(2011) Stimulation of entorhinal cortex promotes adult neurogenesis and
facilitates spatial memory, J. Neurosci., 31,
13469-13484.
73.Xia, F., Yiu, A., Stone, S. S. D., Oh, S.,
Lozano, A. M., Josselyn, S. A., and Frankland, P. W. (2017) Entorhinal
cortical deep brain stimulation rescues memory deficits in both young
and old mice genetically engineered to model Alzheimer’s disease,
Neuropsychopharmacology, 42, 2493-2503.
74.Mufson, E. J., Counts, S. E., Perez, S. E., and
Ginsberg, S. D. (2008) Cholinergic system during the progression of
Alzheimer’s disease: therapeutic implications, Expert Rev.
Neurother., 8, 1703-1718.
75.Turnbull, I. M., McGeer, P. L., Beattie, L.,
Calne, D., and Pate, B. (1985) Stimulation of the basal nucleus of
Meynert in senile dementia of Alzheimer’s type. A preliminary
report, Appl. Neurophysiol., 48, 216-221.
76.Freund, H.-J., Kuhn, J., Lenartz, D., Mai, J. K.,
Schnell, T., Klosterkoetter, J., and Sturm, V. (2009) Cognitive
functions in a patient with Parkinson-dementia syndrome undergoing deep
brain stimulation, Arch. Neurol., 66, 781-785.
77.Kuhn, J., Hardenacke, K., Shubina, E., Lenartz,
D., Visser-Vandewalle, V., Zilles, K., Sturm, V., and Freund, H.-J.
(2015) Deep brain stimulation of the nucleus basalis of Meynert in
early stage of Alzheimer’s dementia, Brain Stimul.,
8, 838-839.
78.Baldermann, J. C., Hardenacke, K., Hu, X.,
Köster, P., Horn, A., Freund, H. J., Zilles, K., Sturm, V.,
Visser-Vandewalle, V., Jessen, F., Maintz, D., and Kuhn, J. (2017)
Neuroanatomical characteristics associated with response to deep brain
stimulation of the nucleus basalis of meynert for Alzheimer’s
disease, Neuromodulation, 21, 184-190.
79.Lee, J. E., Jeong, D. U., Lee, J., Chang, W. S.,
and Chang, J. W. (2016) The effect of nucleus basalis magnocellularis
deep brain stimulation on memory function in a rat model of dementia,
BMC Neurol., 16, 6.
80.Casamenti, F., Deffenu, G., Abbamondi, A. L., and
Pepeu, G. (1986) Changes in cortical acetylcholine output induced by
modulation of the nucleus basalis, Brain Res. Bull., 16,
689-695.
81.Buzsaki, G., Bickford, R. G., Ponomareff, G.,
Thal, L. J., Mandel, R., and Gage, F. H. (1988) Nucleus basalis and
thalamic control of neocortical activity in the freely moving rat,
J. Neurosci., 8, 4007-4026.
82.Bjordahl, T. S., Dimyan, M. A., and Weinberger,
N. M. (1998) Induction of long-term receptive field plasticity in the
auditory cortex of the waking guinea pig by stimulation of the nucleus
basalis, Behav. Neurosci., 112, 467-479.
83.Kilgard, M. P., Pandya, P. K., Vazquez, J., Gehi,
A., Schreiner, C. E., and Merzenich, M. M. (2001) Sensory input directs
spatial and temporal plasticity in primary auditory cortex, J.
Neurophysiol., 86, 326-338.
84.Montero-Pastor, A., Vale-Martinez, A.,
Guillazo-Blanch, G., and Marti-Nicolovius, M. (2004) Effects of
electrical stimulation of the nucleus basalis on two-way active
avoidance acquisition, retention, and retrieval, Behav. Brain
Res., 154, 41-54.
85.Boix-Trelis, N., Vale-Martinez, A.,
Guillazo-Blanch, G., Costa-Miserachs, D., and Marti-Nicolovius, M.
(2006) Effects of nucleus basalis magnocellularis stimulation on a
socially transmitted food preference and c-Fos expression, Learn.
Mem., 13, 783-793.
86.Luber, B., and Lisanby, S. H. (2014) Enhancement
of human cognitive performance using transcranial magnetic stimulation
(TMS), Neuroimage, 85, 961-970.
87.Kirschen, M. P., Davis-Ratner, M. S., Jerde, T.
E., Schraedley-Desmond, P., and Desmond, J. E. (2006) Enhancement of
phonological memory following transcranial magnetic stimulation (TMS),
Behav. Neurol., 17, 187-194.
88.Cattaneo, Z., Vecchi, T., Pascual-Leone, A., and
Silvanto, J. (2009) Contrasting early visual cortical activation states
causally involved in visual imagery and short-term memory, Eur. J.
Neurosci., 30, 1393-1400.
89.Yamanaka, K., Yamagata, B., Tomioka, H.,
Kawasaki, S., and Mimura, M. (2010) Transcranial magnetic stimulation
of the parietal cortex facilitates spatial working memory:
near-infrared spectroscopy study, Cereb. Cortex., 20,
1037-1045.
90.Bagherzadeh, Y., Khorrami, A., Zarrindast, M. R.,
Shariat, S. V., and Pantazis, D. (2016) Repetitive transcranial
magnetic stimulation of the dorsolateral prefrontal cortex enhances
working memory, Exp. Brain Res., 234, 1807-1818.
91.Albouy, P., Weiss, A., Baillet, S., and Zatorre,
R. J. (2017) Selective entrainment of theta oscillations in the dorsal
stream causally enhances auditory working memory performance,
Neuron, 94, 193-206.e5.
92.Chung, S. W., Rogasch, N. C., Hoy, K. E.,
Sullivan, C. M., Cash, R. F. H., and Fitzgerald, P. B. (2018) Impact of
different intensities of intermittent theta burst stimulation on the
cortical properties during TMS-EEG and working memory performance,
Hum. Brain Mapp., 39, 783-802.
93.Pascual-Leone, A., Houser, C. M., Reese, K.,
Shotland, L., Grafman, J., Sato, S., Valls-Sole, J., Brasil-Neto, J.
P., Wassermann, E. M., Cohen, L. G., and Hallet, M. (1993) Safety of
rapid-rate transcranial magnetic stimulation in normal volunteers,
Electroencephalogr. Clin. Neurophysiol., 89, 120-130.
94.Wassermann, E. M., Grafman, J., Berry, C.,
Hollnagel, C., Wild, K., Clark, K., and Hallett, M. (1996) Use and
safety of a new repetitive transcranial magnetic stimulator,
Electroencephalogr. Clin. Neurophysiol., 101,
412-417.
95.Kohler, S., Paus, T., Buckner, R. L., and Milner,
B. (2004) Effects of left inferior prefrontal stimulation on episodic
memory formation: a two-stage fMRI-rTMS study, J. Cogn.
Neurosci., 16, 178-188.
96.Hawco, C., Armony, J. L., Daskalakis, Z. J.,
Berlim, M. T., Chakravarty, M. M., Pike, G. B., and Lepage, M. (2017)
Differing time of onset of concurrent TMS-fMRI during associative
memory encoding: a measure of dynamic connectivity, Front. Hum.
Neurosci., 11, 404.
97.Wang, J. X., Rogers, L. M., Gross, E. Z., Ryals,
A. J., Dokucu, M. E., Brandstatt, K. L., Hermiller, M. S., and Voss, J.
L. (2014) Targeted enhancement of cortical-hippocampal brain networks
and associative memory, Science, 345, 1054-1057.
98.Nilakantan, A. S., Bridge, D. J., Gagnon, E. P.,
Van Haerents, S. A., and Voss, J. L. (2017) Stimulation of the
posterior cortical-hippocampal network enhances precision of memory
recollection, Curr. Biol., 27, 465-470.
99.Paulus, W. (2011) Transcranial electrical
stimulation (tES – tDCS; tRNS, tACS) methods,
Neuropsychol. Rehabil., 21, 602-617.
100.Marshall, L. (2004) Transcranial direct current
stimulation during sleep improves declarative memory, J.
Neurosci., 24, 9985-9992.
101.Ladenbauer, J., Ladenbauer, J., Kulzow, N., de
Boor, R., Avramova, E., Grittner, U., and Floel, A. (2017) Promoting
sleep oscillations and their functional coupling by transcranial
stimulation enhances memory consolidation in mild cognitive impairment,
J. Neurosci., 37, 7111-7124.
102.Javadi, A. H., and Walsh, V. (2012)
Transcranial direct current stimulation (tDCS) of the left dorsolateral
prefrontal cortex modulates declarative memory, Brain Stimul.,
5, 231-241.
103.Leshikar, E. D., Leach, R. C., McCurdy, M. P.,
Trumbo, M. C., Sklenar, A. M., Frankenstein, A. N., and Matzen, L. E.
(2017) Transcranial direct current stimulation of dorsolateral
prefrontal cortex during encoding improves recall but not recognition
memory, Neuropsychologia, 106, 390-397.
104.Zwissler, B., Sperber, C., Aigeldinger, S.,
Schindler, S., Kissler, J., and Plewnia, C. (2014) Shaping memory
accuracy by left prefrontal transcranial direct current stimulation,
J. Neurosci., 34, 4022-4026.
105.Javadi, A. H., and Cheng, P. (2013)
Transcranial direct current stimulation (tDCS) enhances reconsolidation
of long-term memory, Brain Stimul., 6, 668-674.
106.Smirni, D., Turriziani, P., Mangano, G. R.,
Cipolotti, L., and Oliveri, M. (2015) Modulating memory performance in
healthy subjects with transcranial direct current stimulation over the
right dorsolateral prefrontal cortex, PLoS One, 10,
e0144838.
107.Floel, A., Rosser, N., Michka, O., Knecht, S.,
and Breitenstein, C. (2008) Noninvasive brain stimulation improves
language learning, J. Cogn. Neurosci., 20, 1415-1422.
108.Hammer, A., Mohammadi, B., Schmicker, M.,
Saliger, S., and Munte, T. F. (2011) Errorless and errorful learning
modulated by transcranial direct current stimulation, BMC
Neurosci., 12, 72.
109.Nikolin, S., Loo, C. K., Bai, S., Dokos, S.,
and Martin, D. M. (2015) Focalised stimulation using high definition
transcranial direct current stimulation (HD-tDCS) to investigate
declarative verbal learning and memory functioning, Neuroimage,
117, 11-19.
110.De Lara, G. A., Knechtges, P. N., Paulus, W.,
and Antal, A. (2017) Anodal tDCS over the left DLPFC did not affect the
encoding and retrieval of verbal declarative information, Front.
Neurosci., 11, 452.
111.Manuel, A. L., and Schnider, A. (2016) Effect
of prefrontal and parietal tDCS on learning and recognition of verbal
and non-verbal material, Clin. Neurophysiol., 127,
2592-2598.
112.Jones, K. T., Gozenman, F., and Berryhill, M.
E. (2014) Enhanced long-term memory encoding after parietal
neurostimulation, Exp. Brain Res., 232, 4043-4054.
113.Boggio, P. S., Khoury, L. P., Martins, D. C.,
Martins, O. E., de Macedo, E. C., and Fregni, F. (2009) Temporal cortex
direct current stimulation enhances performance on a visual recognition
memory task in Alzheimer disease, J. Neurol. Neurosurg.
Psychiatry, 80, 444-447.
114.Floel, A., Suttorp, W., Kohl, O., Kurten, J.,
Lohmann, H., Breitenstein, C., and Knecht, S. (2012) Non-invasive brain
stimulation improves object-location learning in the elderly,
Neurobiol. Aging, 33, 1682-1689.
115.Manenti, R., Brambilla, M., Petesi, M.,
Ferrari, C., and Cotelli, M. (2013) Enhancing verbal episodic memory in
older and young subjects after non-invasive brain stimulation,
Front. Aging Neurosci., 5, 49.
116.Sandrini, M., Manenti, R., Brambilla, M.,
Cobelli, C., Cohen, L. G., and Cotelli, M. (2016) Older adults get
episodic memory boosting from noninvasive stimulation of prefrontal
cortex during learning, Neurobiol. Aging, 39,
210-216.
117.Krause, M. R., Zanos, T. P., Csorba, B. A.,
Pilly, P. K., Choe, J., Phillips, M. E., Datta, A., and Pack, C. C.
(2017) Transcranial direct current stimulation facilitates associative
learning and alters functional connectivity in the primate brain,
Curr. Biol., 27, 3086-3096.e3.
118.Hampstead, B. M., Sathian, K., Bikson, M., and
Stringer, A. Y. (2017) Combined mnemonic strategy training and
high-definition transcranial direct current stimulation for memory
deficits in mild cognitive impairment. Alzheimer’s dement,
Transl. Res. Clin. Interv., 3, 459-470.
119.Murugaraja, V., Shivakumar, V., Sivakumar, P.
T., Sinha, P., and Venkatasubramanian, G. (2017) Clinical utility and
tolerability of transcranial direct current stimulation in mild
cognitive impairment, Asian J. Psychiatr., 30,
135-140.
120.Herrmann, C. S., Rach, S., Neuling, T., and
Struber, D. (2013) Transcranial alternating current stimulation: a
review of the underlying mechanisms and modulation of cognitive
processes, Front. Hum. Neurosci., 7, 279.
121.Marshall, L., Helgadottir, H., Molle, M., and
Born, J. (2006) Boosting slow oscillations during sleep potentiates
memory, Nature, 444, 610-613.
122.Garside, P., Arizpe, J., Lau, C.-I., Goh, C.,
and Walsh, V. (2015) Cross-hemispheric alternating current stimulation
during a nap disrupts slow wave activity and associated memory
consolidation, Brain Stimul., 8, 520-527.
123.Kirov, R., Weiss, C., Siebner, H. R., Born, J.,
and Marshall, L. (2009) Slow oscillation electrical brain stimulation
during waking promotes EEG theta activity and memory encoding, Proc.
Natl. Acad. Sci. USA, 106, 15460-15465.
124.Antonenko, D., Faxel, M., Grittner, U.,
Lavidor, M., and Floel, A. (2016) Effects of transcranial alternating
current stimulation on cognitive functions in healthy young and older
adults, Neural Plast., 2016, 1-13.
125.Ambrus, G. G., Pisoni, A., Primaßin, A.,
Turi, Z., Paulus, W., and Antal, A. (2015) Bi-frontal transcranial
alternating current stimulation in the ripple range reduced overnight
forgetting, Front. Cell. Neurosci., 9, 374.
126.Javadi, A.-H., Glen, J. C., Halkiopoulos, S.,
Schulz, M., and Spiers, H. J. (2017) Oscillatory reinstatement enhances
declarative memory, J. Neurosci., 37, 9939-9944.
127.Franke, A., Northoff, R., and Hildt, E. (2015)
The case of pharmacological neuroenhancement: medical, judicial and
ethical aspects from a german perspective, Pharmacopsychiatry,
48, 256-264.
128.Micoulaud-Franchi, J.-A., MacGregor, A., and
Fond, G. (2014) A preliminary study on cognitive enhancer consumption
behaviors and motives of French Medicine and Pharmacology students,
Eur. Rev. Med. Pharmacol. Sci., 18, 1875-1878.
129.Fond, G., Micoulaud-Franchi, J.-A., Brunel, L.,
Macgregor, A., Miot, S., Lopez, R., Richieri, R., Abbar, M., Lancon,
C., and Repantis, D. (2015) Innovative mechanisms of action for
pharmaceutical cognitive enhancement: a systematic review,
Psychiatry Res., 229, 12-20.
130.Coghill, D. R., Seth, S., Pedroso, S., Usala,
T., Currie, J., and Gagliano, A. (2014) Effects of methylphenidate on
cognitive functions in children and adolescents with
attention-deficit/hyperactivity disorder: evidence from a systematic
review and a meta-analysis, Biol. Psychiatry, 76,
603-615.
131.Kamei, N., Tanaka, M., Choi, H., Okada, N.,
Ikeda, T., Itokazu, R., and Takeda-Morishita, M. (2017) Effect of an
enhanced nose-to-brain delivery of insulin on mild and progressive
memory loss in the senescence-accelerated mouse, Mol. Pharm.,
14, 916-927.
132.Dominguez, A., Suarez-Merino, B., and
Goni-de-Cerio, F. (2014) Nanoparticles and blood-brain barrier: the key
to central nervous system diseases, J. Nanosci. Nanotechnol.,
14, 766-779.
133.Leszek, J., Md Ashraf, G., Tse, W. H., Zhang,
J., Gasiorowski, K., Avila-Rodriguez, M. F., Tarasov, V. V., Barreto,
G. E., Klochkov, S. G., Bachurin, S. O., and Aliev, G. (2017)
Nanotechnology for Alzheimer disease, Curr. Alzheimer Res.,
14, 1182-1189.
134.Pandey, A., Malek, V., Prabhakar, V., Kulkarni,
Y. A., and Gaikwad, A. B. (2015) Nanoparticles: a neurotoxicological
perspective, CNS Neurol. Disord. Drug Targets, 14,
1317-1327.
135.O’Keefe, J., and Nadel, L. (1978) The
Hippocampus as a Cognitive Map, Clarendon Press, Oxford.
136.Foster, D. J., and Wilson, M. A. (2006) Reverse
replay of behavioural sequences in hippocampal place cells during the
awake state, Nature, 440, 680-683.
137.Jackson, J. C., Johnson, A., and Redish, A. D.
(2006) Hippocampal sharp waves and reactivation during awake states
depend on repeated sequential experience, J. Neurosci.,
26, 12415-12426.
138.Diba, K., and Buzsaki, G. (2007) Forward and
reverse hippocampal place-cell sequences during ripples, Nat.
Neurosci., 10, 1241-1242.
139.Davidson, T. J., Kloosterman, F., and Wilson,
M. A. (2009) Hippocampal replay of extended experience, Neuron,
63, 497-507.
140.Moita, M. A. P. (2004) Putting fear in its
place: remapping of hippocampal place cells during fear conditioning,
J. Neurosci., 24, 7015-7023.
141.Wu, C.-T., Haggerty, D., Kemere, C., and Ji, D.
(2017) Hippocampal awake replay in fear memory retrieval, Nat.
Neurosci., 20, 571-580.
142.Girardeau, G., Inema, I., and Buzsaki, G.
(2017) Reactivations of emotional memory in the
hippocampus–amygdala system during sleep, Nat. Neurosci.,
20, 1634-1642.
143.Marr, D. (1971) Simple memory: a theory for
archicortex, Philos. Trans. R. Soc. Lond. B Biol. Sci.,
262, 23-81.
144.Buzsaki, G. (1989) Two-stage model of memory
trace formation: a role for “noisy” brain states,
Neuroscience, 31, 551-570.
145.De Lavilleon, G., Lacroix, M. M., Rondi-Reig,
L., and Benchenane, K. (2015) Explicit memory creation during sleep
demonstrates a causal role of place cells in navigation, Nat.
Neurosci., 18, 493-495.
146.Maingret, N., Girardeau, G., Todorova, R.,
Goutierre, M., and Zugaro, M. (2016) Hippocampo-cortical coupling
mediates memory consolidation during sleep, Nat. Neurosci.,
19, 959-964.
147.Latchoumane, C.-F. V., Ngo, H.-V. V., Born, J.,
and Shin, H.-S. (2017) Thalamic spindles promote memory formation
during sleep through triple phase-locking of cortical, thalamic, and
hippocampal rhythms, Neuron, 95, 424-435.e6.
148.Rasch, B., Buchel, C., Gais, S., and Born, J.
(2007) Odor cues during slow-wave sleep prompt declarative memory
consolidation, Science, 315, 1426-1429.
149.Rudoy, J. D., Voss, J. L., Westerberg, C. E.,
and Paller, K. A. (2009) Strengthening individual memories by
reactivating them during sleep, Science, 326,
1079-1079.
150.Ngo, H.-V. V., Martinetz, T., Born, J., and
Molle, M. (2013) Auditory closed-loop stimulation of the sleep slow
oscillation enhances memory, Neuron, 78, 545-553.
151.Bendor, D., and Wilson, M. A. (2012) Biasing
the content of hippocampal replay during sleep, Nat. Neurosci.,
15, 1439-1444.
152.Arzi, A., Shedlesky, L., Ben-Shaul, M., Nasser,
K., Oksenberg, A., Hairston, I. S., and Sobel, N. (2012) Humans can
learn new information during sleep, Nat. Neurosci., 15,
1460-1465.
153.Garner, A. R., Rowland, D. C., Hwang, S. Y.,
Baumgaertel, K., Roth, B. L., Kentros, C., and Mayford, M. (2012)
Generation of a synthetic memory trace, Science, 335,
1513-1516.
154.Ramirez, S., Liu, X., Lin, P.-A., Suh, J.,
Pignatelli, M., Redondo, R. L., Ryan, T. J., and Tonegawa, S. (2013)
Creating a false memory in the hippocampus, Science, 341,
387-391.
155.Redondo, R. L., Kim, J., Arons, A. L., Ramirez,
S., Liu, X., and Tonegawa, S. (2014) Bidirectional switch of the
valence associated with a hippocampal contextual memory engram,
Nature, 513, 426-430.
156.Ramirez, S., Liu, X., MacDonald, C. J., Moffa,
A., Zhou, J., Redondo, R. L., and Tonegawa, S. (2015) Activating
positive memory engrams suppresses depression-like behaviour,
Nature, 522, 335-339.