The Effect of Experimental Hyperthyroidism on Characteristics of Actin–Myosin Interaction in Fast and Slow Skeletal Muscles
G. V. Kopylova1,a*, D. V. Shchepkin1, and S. Y. Bershitsky1
1Institute of Immunology and Physiology, Ural Branch of the Russian Academy of Sciences, 620049 Yekaterinburg, Russia* To whom correspondence should be addressed.
Received September 19, 2017; Revision received January 29, 2018
The molecular mechanism of the failure of contractile function of skeletal muscles caused by oxidative damage to myosin in hyperthyroidism is not fully understood. Using an in vitro motility assay, we studied the effect of myosin damage caused by oxidative stress in experimental hyperthyroidism on the actin–myosin interaction and its regulation by calcium. We found that hyperthyroidism-induced oxidation of myosin is accompanied by a decrease in the sliding velocity of the regulated thin filaments in the in vitro motility assay, and this effect is increased with the duration of the pathological process.
KEY WORDS: actin–myosin interaction, calcium regulation, myosin carbonylation, in vitro motility assayDOI: 10.1134/S000629791805005X
Abbreviations: FT3, free triiodothyronine; FT4, free thyroxine; h, Hill cooperativity coefficient; MHC, myosin heavy chains; MLC, myosin light chains; pCa, negative decimal logarithm of the calcium concentration; pCa50, calcium concentration at which half-maximal sliding velocity of thin filaments is achieved (the calcium sensitivity); Vmax, the maximal sliding velocity of thin filaments.
Contraction of striated muscles is the result of the interaction of
myosin with actin utilizing the energy of ATP hydrolysis, and it is
regulated by calcium ions via the regulatory proteins troponin and
tropomyosin. The contractile properties of muscles are determined by
the composition of the isoforms of myosin heavy chains (MHC). In the
skeletal muscle tissue of adult mammals, four genes encoding certain
MHC isoforms are expressed. One of them, isoform I, is expressed in
slow muscles, and three isoforms of type II (IIa, IIx/d and IIb) are
expressed in fast muscles [1, 2]. The expression of isoforms of skeletal myosin
changes in ontogenesis, in the postnatal period, depending on the
motion activity of muscles [1, 3], and also in myopathies. Expression of MHC isoforms
of skeletal muscles is regulated by nervous, humoral, and mechanical
factors [4, 5].
Among humoral factors, thyroid hormones play an important role in expression of MHC [6]. Their excess causes a shift in the expression of the slow isoform I of MHC towards the fast isoforms IIa, IIx/d, and IIb [6-8]. In hyperthyroidism, most of the type I muscle fibers are converted into mixed oxidative-glycolytic fibers containing IIa and IIx/d isoforms of MHC. Excess of thyroid hormones results in thyrotoxic myopathy [9]. In clinical studies, it has been found that the force of muscle contraction in hyperthyroidism falls by 40%, while muscle mass decreases by only 20% [10]. The cause of the decrease in the force is considered muscle atrophy [9], disruption of the excitation–contraction coupling and oxidative modification of proteins of the contractile apparatus [11-13].
It is known that hyperthyroidism accelerates oxidative metabolism in mitochondria and affects the antioxidant system, which increases the formation of free radicals [14]. Reactive oxygen and nitrogen species are produced in living cells and participate in the regulation of metabolism [15], but their excess leads to oxidative modification of the contractile apparatus proteins and thus can affect the contractile function of muscles [11-13, 16]. For example, treatment of both intact and permeabilized skeletal muscle fibers with nitric oxide, peroxynitrite, hydroxyl radical, or hydrogen peroxide reduces the contractile force and its calcium sensitivity, and these changes depend on the type of muscle, modification conditions, and reagents used [17-22].
The MHC and MLC (myosin light chains) are one of the principal targets of oxidative modifications [23], where the major one is the carbonylation of myosin [24, 25], which disrupts its functional properties and, as a result, the contractile characteristics of muscles. Yamada et al. [12, 13] showed that in hyperthyroidism the reason for the decrease in the force generated by bundles of intact fibers from m. soleus and diaphragm of the rat is the oxidative modification of MHC, namely, their carbonylation.
The molecular mechanism of contractile muscle dysfunction caused by oxidative damage to myosin in hyperthyroidism is not entirely clear. Apart from the direct effect on the force-generating ability of myosin, carbonylation can suppress the contractile function via cooperative mechanisms that are important for the regulation of the actin–myosin interaction by calcium. Murphy et al. [19] found that on the treatment of EDL (extensor digitorum longus) muscle fibers with hydrogen peroxide, the calcium sensitivity of the contractile force decreases. The experiments were carried out on fibers under conditions where apart from myosin, other proteins of the contractile apparatus could also be subjected to oxidation by hydrogen peroxide. The use of isolated proteins makes it possible to determine the functional significance of oxidative modifications of a particular protein. We investigated the effect of myosin carbonylation induced by oxidative stress in hyperthyroidism on the calcium regulation of its interaction with a thin filament using an in vitro motility assay.
MATERIALS AND METHODS
Animals. The effect of oxidative damage to myosin on the contractile function of skeletal muscles was investigated on myosin from muscles of healthy rabbits and rabbits with drug-induced thyrotoxicosis. All procedures involving animal care and handling were performed according to the institutional guidelines set forth by the Animal Care and Use Committee at the Institute of Immunology and Physiology, Ural Branch of the Russian Academy of Sciences, and Directive 2010/63/EU of the European Parliament.
Drug-induced thyrotoxicosis. Hyperthyroidism in 2-month-old Californian rabbits was achieved by intramuscular injection of L-thyroxine in a daily dose of 0.2 mg/kg body weight for two weeks [26]. There were three animals used in both control and experimental groups. The content of free triiodothyronine (FT3) and thyroxine (FT4) in the blood of the animals was determined weekly in the Laboratory of Biochemistry of the Diagnostic Center (Yekaterinburg). After the first week of injections, the level of FT3 in the blood plasma increased from 7.4 to 21.4 nM, and further injections did not change its content. The titer of FT4 increased from 17.6 to 37.3 nM by the end of the first week, and to the end of the second week it increased to 49.3 nM. The body weight of the experimental rabbits decreased by 10%, and the heart rate increased from 120-130 to ~250 beats/min. These symptoms correspond to the literature data for rabbits with similar drug-induced thyrotoxicosis [27], according to which a 50% increase in the level of FT3 in the blood is a confirmation of the hyperthyroidism state. Thus, after the first week of injections of L-thyroxine, the state of hyperthyroidism was reached.
Protein isolation. Myosin from m. psoas and m. soleus was obtained by a standard method [28] and stored in 50% glycerol at –20°C. The composition of MHC and MLC isoforms was determined by SDS-PAGE [29, 30]. Actin was isolated by the method of Pardee and Spudich [31] from acetone powder obtained from m. psoas of healthy rabbits. Actin was polymerized and stained with TRITC-phalloidin (Sigma-Aldrich Inc., Germany) in 1 : 2 molar ratio (actin/TRITC-phalloidin). The regulatory proteins troponin and tropomyosin were prepared by the methods Potter [32] and Smile [33], respectively, from acetone powder prepared separately from fast and slow skeletal muscles. The regulatory proteins were frozen and stored at –86°C. In experiments in the in vitro motility assay with myosin from fast and slow muscles, the regulatory proteins corresponding to myosin isoforms were used.
Assessment of the carbonyl group content. Carbonylation of amino acid residues of proteins is an indicator of their oxidative damage [24, 25]. The method for estimating the oxidative modification of proteins is based on the interaction of oxidized amino acid residues with 2,4-dinitrophenylhydrazine (2,4-DNPH) to form 2,4-dinitrophenylhydrazones. The content of the carbonyl groups in myosin was determined spectrophotometrically with the Carbonyl Colorimetric Assay Kit (Cayman Chemical, USA) according to the protocol proposed by Araujo et al. [34, 35]. The myosin samples were incubated with 10 mM 2,4-DNPH in 2.5 M HCl for an hour at room temperature. Then, 20% TCA was added, thoroughly mixed, and the sample incubated on ice for 5 min. After centrifugation at 5000g for 5 min, the precipitate was washed three times with a mixture of ethanol and ethyl acetate (1 : 1) and then dissolved in 6 M guanidine hydrochloride and centrifuged. The optical density of the solution was measured at 360 nm [36]. Determination of the content of carbonyl groups was carried out three times with each sample of myosin (9 times in total).
Experiments in the in vitro motility assay. Experiments in the in vitro motility assay were performed at 30°C as described previously [37]. Myosin at concentration of 200 μg/ml in AB buffer (25 mM KCl, 25 mM imidazole, 4 mM MgCl2, 1 mM EGTA and 10 mM DTT, pH 7.5) containing 0.5 M KCl was infused into a flow cell with a nitrocellulose-coated surface and incubated for 2 min, and the cell was washed with AB buffer containing 0.5 M KCl and then 0.5 mg/ml BSA was added for 1 min. Then, 50 μg/ml actin in AB buffer with 2 mM ATP was added to the cell, and the sample was incubated for 5 min to block the non-functional myosin heads [26, 37]. Next, 10 nM filamentous actin (F-actin) stained with TRITC-phalloidin, 100 nM tropomyosin, and 100 nM troponin were added, and the sample was incubated for 5 min. For initiation of thin filament sliding, the final AB buffer containing in addition 0.5 mg/ml BSA, 20 mM DTT, 2 mM ATP, 3.5 mg/ml glucose, 0.02 mg/ml catalase, 0.15 mg/ml glucose oxidase, 0.5% methylcellulose, 100 nM tropomyosin, and 100 nM troponin was added to prevent the dissociation of regulatory proteins from thin filaments, as well as an appropriate calcium concentration calculated using the Maxchelator program (http://maxchelator.stanford.edu). Experiments with F-actin were carried out under the same conditions, except that troponin, tropomyosin, and Ca2+ were not added.
The sliding velocity of fluorescence stained TRITC-phalloidin filaments was measured using the GMimPro program [38]. The dependence of the filament sliding velocity on the calcium concentration was analyzed using the Hill equation:
V = Vmax(1 + 10h(pCa – pCa50))–1,
where V and Vmax are the filament sliding velocity and the maximal velocity at saturating calcium concentration, respectively; pCa50 (calcium sensitivity) is the value at which the half-maximal velocity of the filaments is reached; h is the Hill cooperativity coefficient. The experiments were repeated three times. All values are presented as the mean ± standard deviation. Comparisons were performed according to the Mann–Whitney U test (p < 0.05).
RESULTS
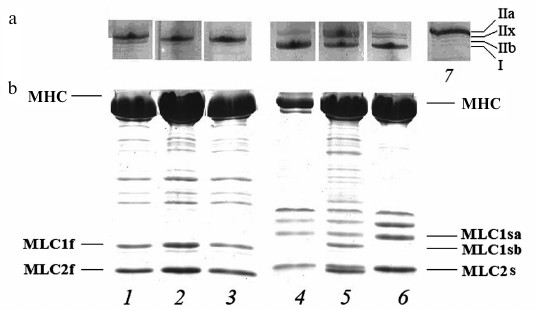
Isoform composition of myosin heavy and light chains. To estimate the effect of hyperthyroidism on the expression of skeletal myosin, the isoform composition of heavy and light chains of myosin from fast (m. psoas) and slow (m. soleus) muscles of rabbits with different degrees of hyperthyroidism was determined by gel electrophoresis (Fig. 1).
Fig. 1. Gel electrophoresis of the isoforms of the heavy (MHC) (a) and light chains (MLC) (b) of rabbit skeletal myosin: 1-3) myosin from fibers of m. psoas; 4-6) myosin from fibers of m. soleus; 7) myosin from m. tibialis anterior of euthyroid rabbits was used as marker of MHC isoforms; 1, 4) myosin from euthyroid rabbits; 2, 5) myosin from rabbits after one week of L-thyroxine injections; 3, 6) myosin from rabbits after two weeks of L-thyroxine injections. I, IIa, IIx and IIb – isoforms of MHC; MLC2s, MLC2f, MLC1f, MLC1sa, MLC1sb – light chains of skeletal myosin.
Myosin from m. psoas of healthy animals predominantly contained fast isoform of IIx MHC (90-95%), as well as 2-3% of IIb isoform and less than 1% of slow isoform I. In induced hyperthyroidism, the proportion of isoform IIx increased to 100% (Fig. 1a). The MHC from m. soleus of healthy rabbits were mainly represented by slow isoform I with an admixture of fast isoform IIa. After the first week of L-thyroxine treatment, another fast MHC isoform, IIx, appeared. In hyperthyroidism, the fraction of fast MHC isoforms increased. Expression of MLC isoforms from fast and slow skeletal muscles with an increase in the degree of hyperthyroidism did not change (Fig. 1b).
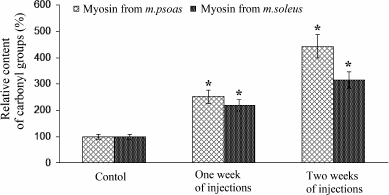
Content of carbonyl groups in myosin. In hyperthyroidism, the content of carbonyl groups in myosin of both fast and slow skeletal muscles of rabbits increased several-fold that indicates the oxidative damage of myosin (Fig. 2).
Fig. 2. The relative content of carbonyl groups in myosin of fast and slow skeletal muscles of rabbits after one and two weeks of L-thyroxine treatment, as well as of a control group. The content of carbonyl groups in myosin obtained from euthyroid rabbits is taken as 100%. Asterisks indicate significant differences from the control, p < 0.05.
Results of experiments in the in vitro motility assay. In the absence of regulatory proteins (tropomyosin and troponin), the sliding velocity of actin filaments (F-actin) over myosin from fast skeletal muscles of rabbits with different degree of hyperthyroidism did not significantly differ from that of myosin from healthy rabbits (table). The sliding velocity of F-actin over myosin from slow muscles after partial treatment with L-thyroxine did not differ from the speed on myosin from healthy rabbits but decreased 2-fold after two weeks of injections (table).
Parameters of the Hill equation of pCa–velocity
relationship
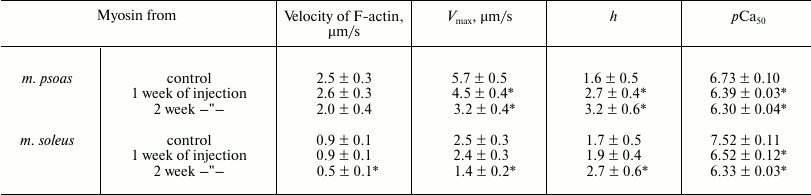
Note: Velocity of F-actin – the sliding velocity of F-actin
without troponin and tropomyosin; Vmax – the
maximal filament velocity at saturating calcium concentration; h
– the Hill cooperativity coefficient; pCa50
(the calcium sensitivity) – the value at which the half-maximal
sliding velocity of the filaments is reached.
* Significant differences from the control, p < 0.05.
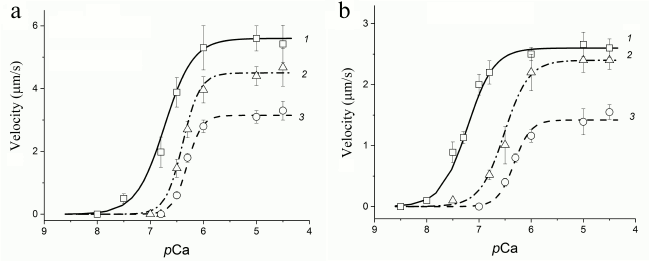
In experiments on the study of the sliding velocity of regulated thin filaments and its calcium dependence (Fig. 3), we found that with an increase in the degree of myosin carbonylation, the filament sliding velocity at saturating calcium concentration and the calcium sensitivity of the pCa–velocity relationship decreased (table and Fig. 3a), and the Hill coefficient of cooperativity for myosin both from fast and slow muscles increased (table and Fig. 3b).
Fig. 3. Curves of the pCa–velocity relationship for myosin from m. psoas (a) and m. soleus (b) of rabbits: 1) myosin obtained from euthyroid rabbits; 2, 3) myosin from rabbits after 1 and 2 weeks of L-thyroxine injection, respectively. Circles, triangles, and squares show the values of the filament sliding velocity obtained in the in vitro motility assay. The filament sliding velocity is represented as the mean ± standard deviation. The regression lines correspond to the Hill equation (see “Materials and Methods”).
DISCUSSION
Thyroid hormones play an essential role in the regulation of the development and functioning of striated muscles, especially skeletal muscles. Excess of thyroid hormones leads to oxidative stress, which causes irreversible modifications of the proteins of muscle contractile apparatus, primarily of myosin, disrupting its normal functioning [11, 12]. The indicator of oxidative damage of proteins is their carbonylation [24, 25]. We investigated the effect of intravital carbonylation of myosin induced by hyperthyroidism on the regulation by calcium of the actin–myosin interaction in fast (m. psoas) and slow (m. soleus) rabbit skeletal muscles on isolated proteins using the in vitro motility assay.
We found that the increase in the degree of hyperthyroidism increases the number of carbonyl groups in myosin both from fast and slow skeletal muscles, as well as the expression of fast isoforms of MHC (Figs. 1 and 2). The sliding velocity of thin filaments over such myosin in the in vitro motility assay decreased significantly, and the Hill cooperativity coefficient of the pCa–velocity relationship increased (table and Fig. 3). At the same time, the decrease in the sliding velocity occurred despite an increase in the expression of fast MHC isoforms, which can be explained only by the oxidative modification of myosin, since the content of carbonyl groups in it increased several-fold. Previous studies have shown that oxidation of myosin leads to multiple alterations of its heavy and light chains, which decreases the ATPase activity of myosin and worsens the characteristics of contraction of skeletal muscle fibers in hyperthyroidism [12, 13]. Oxidation involves MHC and MLC cysteine and methionine residues that are critical for interaction with actin [12, 13, 20, 23].
Cysteine residues exposed to oxidation were detected in isolated fragments of myosin when treated with peroxide [39] or peroxynitrite in solution [40]. Prochniewicz et al. [20] using electrospray-mass spectrometry (ESI-MS) and matrix-activated laser desorption/ionization (MALDI) mass spectrometry revealed that on the treatment of skeletal fibers with hydrogen peroxide, only residues of methionine (but not cysteine) are subjected to oxidation. Totally 22-25 (data from different structural models) and six methionine residues are located in myosin head and in each essential light chain, respectively, and just a few of them being subject to oxidation. Prochniewicz et al. [20] found that in the native state there are five oxidized methionine residues in each head of a myosin molecule: two in the essential light chain (Met146, Met160) and three in the heavy chain of the myosin head (Met80, Met531, and Met687). After treatment of muscle fibers with 50 mM H2O2, eight more oxidized methionine residues were detected. Three of them are in the essential light chain (Met100, Met113, and Met189), and five are in the heavy chain of myosin head (in its fragments of molecular weight 23, 20, and 50 kDa): these are Met94, Met166, Met496, Met542, and Met779. Using EPR spectroscopy, the authors showed that the oxidation of skeletal myosin does not affect its structure in rigor and contraction, but in the relaxed state it leads to the formation of myosin cross-bridges strongly bound to actin (up to 27-35%). Prochniewicz et al. [20] showed the degradation of contractile performance of muscle during oxidative stress by the violation of ATP-dependent cycle of myosin interaction with actin, namely the transition from the state of weak binding of myosin head to actin to the state of strong binding.
In studies conducted on myosin II from Dictyostelium, EPR spectroscopy was used to show that the oxidation of methionine residues in the catalytic domain of myosin head does not affect the secondary structure of the myosin molecule but changes the conformational dynamics of its actin-binding surface. It was found that the deterioration of the functional characteristics of myosin is affected by the oxidation of Met394 only, but not any other methionine residues [41, 42].
We found that the oxidation of myosin confirmed by the degree of its carbonylation significantly reduces the maximum sliding velocity of thin filaments in the in vitro motility assay and increases the Hill cooperativity coefficient of pCa–velocity relationship. Note that the filament sliding velocity in the in vitro motility assay to some extent characterizes the force-generating ability of myosin due to the resistance inevitably existing in this assay [43].
When increasing the degree of hyperthyroidism of the rabbits, we also observed a decrease in the calcium sensitivity of the velocity of thin filaments. We cannot give any definite answer to the question whether this change in calcium sensitivity is related to the carbonylation of myosin or the change in the composition of isoforms of heavy chains of myosin. Treatment of animals with L-thyroxine, as might be expected increased the proportion of fast myosin isoforms in both types of muscles (Fig. 1 and table). After a two-week treatment, the sensitivity of myosin from fast and slow muscles dropped by 0.43 and 1.2 pCa units, respectively. According to our results and the data of Schiaffino and Reggiani [2], the slow isoforms of myosin have greater Ca2+ sensitivity, and the difference in the sensitivity of myosins from healthy rabbits was ~0.8 pCa unit.
Many works have been devoted to the study of the effect of protein oxidation of the contractile apparatus of striated muscles on the calcium regulation of the contractile function of skeletal muscles [18-22, 44-46]. The results of these studies are contradictory. In some experiments on intact fibers exposed to an excess of reactive oxygen species due to their treatment with hydrogen peroxide, and also as a result of the enhanced formation of free radicals due to experimentally induced fatigue, a decrease in the calcium sensitivity of force was demonstrated [19-21, 44-46]. According to other data, no changes in the calcium sensitivity of force were detected on isolated myofibrillar proteins and permeabilized fibers treated with 10 mM hydrogen peroxide for 5 min [18, 22, 46].
The discrepancy between the data on the calcium sensitivity of force due to the treatment of both muscle fibers [18-22, 44-46] and isolated myofibrillar proteins [47] can be explained by the results of Gross and Lehman [48], who showed that the degree of protein modification depends on the time and type of impact, as well as on the stage of the cross-bridge cycle, i.e. availability of essential amino acid residues for oxidation. Prochniewicz et al. found that an increase in the time and dose of treatment leads to a decrease in force developed by the fibers and its calcium sensitivity [20].
The results of our study show that the degree of damage to skeletal muscle myosin is largely determined by the duration of application of the oxidizing factor. First of all, the force-generating function of myosin suffers. In the in vitro experiments with myosin isolated from muscles of animals with hyperthyroidism, a significant decrease in the sliding velocity of thin filaments was found in comparison to myosin from muscles of healthy animals, and this decrease becomes more pronounced with increased duration of pathogenic exposure.
Acknowledgments
We thank Mr. D. V. Zhalobin for help with the experiments.
This work was supported by grant No. 16-14-10044 from the Russian Science Foundation.
REFERENCES
1.Pette, D., and Staron, R. S. (2000) Myosin
isoforms, muscle fiber types, and transitions, Microsc. Res.
Tech., 50, 500-509.
2.Schiaffino, S., and Reggiani, C. (2011) Fiber types
in mammalian skeletal muscles, Physiol. Rev., 91,
1447-1531.
3.Galler, S., Schmitt, T. L., and Pette, D. (1994)
Stretch activation, unloaded shortening velocity, and myosin heavy
chain isoforms of rat skeletal muscle fibres, J. Physiol.,
478, 513-521.
4.Nwoye, L., Mommaerts, W. F., Simpson, D. R.,
Seraydarian, K., and Marusich, M. (1982) Evidence for a direct action
of thyroid hormone in specifying muscle properties, Am. J.
Physiol., 242, 401-408.
5.Diffee, G. M., Haddad, F., Herrick, R. E., and
Baldwin, K. M. (1991) Control of myosin heavy chain expression:
interaction of hypothyroidism and hindlimb suspension, Am. J.
Physiol., 261, 1099-1106.
6.Larsson, L., Li, X., Teresi, A., and Salviati, G.
(1994) Effects of thyroid hormone on fast- and slow-twitch skeletal
muscles in young and old rats, J. Physiol., 481,
149-161.
7.Caiozzo, V. J., Herrick, R. E., and Baldwin, K. M.
(1991) Influence of hyperthyroidism on maximal shortening velocity and
myosin isoform distribution in skeletal muscles, Am. J.
Physiol., 261, 285-295.
8.Caiozzo, V. J., Herrick, R. E., and Baldwin, K. M.
(1992) Response of slow and fast muscle to hypothyroidism: maximal
shortening velocity and myosin isoforms, Am. J. Physiol.,
263, 86-94.
9.Ramsay, I. D. (1966) Muscle dysfunction in
hyperthyroidism, Lancet, 2, 931-934.
10.Nørrelund, H., Hove, K. Y.,
Brems-Dalgaard, E., Jurik, A. G., Nielsen, L. P., Nielsen, S.,
Jorgensen, J. O., Weeke, J., and Moller, N. (1999) Muscle mass and
function in thyrotoxic patients before and during medical treatment,
Clin. Endocrinol. (Oxf.), 51, 693-699.
11.Yamada, T., and Wada, M. (2004) Effects of
thyroid hormone on sarcoplasmic reticulum Ca2+ uptake and
contractile properties in rat soleus muscle, Jpn. J. Phys. Fitness
Sports Med., 53, 509-518.
12.Yamada, T., Mishima, T., Sakamoto, M., Sugiyama,
M., Matsunaga, S., and Wada, M. (2006) Oxidation of myosin heavy chain
in force production in hyperthyroid rat soleus, J. Appl.
Physiol., 100, 1520-1526.
13.Yamada, T., Mishima, T., Sakamoto, M., Sugiyama,
M., Matsunga, S., and Wada, M. (2007) Myofibrillar oxidation and
contractile dysfunction in hyperthyroid rat diaphragm, J. Appl.
Physiol., 102, 1850-1855.
14.Venditti, P., and Di Meo, S. (2006) Thyroid
hormone-induced oxidative stress, Cell. Mol. Life Sci.,
63, 414-434.
15.Reid, M. B. (2001) Redox modulation of skeletal
muscle contraction: what we know and what we don’t, J. Appl.
Physiol., 90, 724-731.
16.Asayama, K., and Kato, K. (1990) Oxidative
muscular injury and its relevance to hyperthyroidism, Free Radic.
Biol. Med., 8, 293-303.
17.Andrade, F. H., Reid, M. B., Allen, D. G., and
Westerblad, H. (1998) Effect of hydrogen peroxide and dithiothreitol on
contractile function of single skeletal muscle fibres from the mouse,
J. Physiol., 509, 565-575.
18.Plant, D. R., Lynch, G. S., and Williams, D. A.
(2000) Hydrogen peroxide modulates Ca2+-activation of single
permeabilized fibres from fast- and slow-twitch skeletal muscles of
rats, J. Muscle Res. Cell Motil., 21, 747-752.
19.Murphy, R. M., Dutka, T. L., and Lamb, G. D.
(2008) Hydroxyl radical and glutathione interactions alter calcium
sensitivity and maximum force of the contractile apparatus in rat
skeletal muscle fibres, J. Physiol., 586, 2203-2216.
20.Prochniewicz, E., Lowe, D. A., Spakowicz, D. J.,
Higgins, L., O’Conor, K., Thompson, L. V., Ferrington, D. A., and
Thomas, D. D. (2008) Functional, structural, and chemical changes in
myosin associated with hydrogen peroxide treatment of skeletal muscle
fibers, Am. J. Physiol. Cell Physiol., 294, 613-626.
21.Lamb, G. D., and Westerblad, H. (2011) Acute
effects of reactive oxygen and nitrogen species on the contractile
function of skeletal muscle, J. Physiol., 589,
2119-2127.
22.Dutka, T. L., Verburg, E., Larkins, N., Hortemo,
K. H., Lunde, P. K., Sejersted, O. M., and Lamb, G. D. (2012)
ROS-mediated decline in maximum Ca2+-activated force in rat
skeletal muscle fibers following in vitro and in vivo
stimulation, PLoS One, 7, e35226.
23.Zergeroglu, M. A., McKenzie, M. J., Shanely, R.
A., Van Gammeren, D., DeRuisseau, K. C., and Powers, S. K. (2003)
Mechanical ventilation-induced oxidative stress in the diaphragm, J.
Appl. Physiol., 95, 1116-1124.
24.Dalle-Donne, I., Rossi, R., Giustarini, D.,
Milzani, A., and Colombo, R. (2003) Protein carbonyl groups as
biomarkers of oxidative stress, Clin. Chim. Acta, 329,
23-38.
25.Stadtman, E. R., and Levine, R. L. (2003) Free
radical-mediated oxidation of free amino acids and amino acid residues
in proteins, Amino Acids, 25, 207-218.
26.Noguchi, T., Camp, P., Jr., Alix, S. L., Gorga,
J. A., Begin, K. J., Leavitt, B. J., Ittleman, F. P., Alpert, N. R.,
LeWinter, M. M., and Van’Buren, P. (2003) Myosin from failing and
non-failing human ventricles exhibit similar contractile properties,
J. Mol. Cell. Cardiol., 35, 91-97.
27.Harrison, A. R., Lee, M. S., and McLoon, L. K.
(2010) Effects of elevated thyroid hormone on adult rabbit extraocular
muscles, Invest. Ophthalmol. Vis. Sci., 51, 183-191.
28.Margossian, S. S., and Lowey, S. (1982)
Preparation of myosin and its subfragments from rabbit skeletal muscle,
Methods Enzymol., 85, 55-71.
29.Talmadge, R. J., and Roy, R. R. (1993)
Electrophoretic separation of rat skeletal muscle myosin heavy chain
isoforms, J. Appl. Physiol., 75, 2337-2340.
30.Laemmli, U. K. (1970) Cleavage of structural
proteins during the assembly of the head of bacteriophage T4,
Nature, 227, 680-685.
31.Pardee, J. D., and Spudich, J. A. (1982)
Purification of muscle actin, Methods Enzymol., 85,
164-179.
32.Potter, J. D. (1982) Preparation of troponin and
its subunits, Methods Enzymol., 85, 241-263.
33.Smillie, L. B. (1982) Preparation and
identification of alpha- and beta-tropomyosins, Methods
Enzymol., 85, 234-241.
34.Araujo, A. S., Ribeiro, M. F., Enzveiler, A.,
Schenkel, P., Fernandes, T. R., Partata, W. A., Irigoyen, M. C.,
Llesuy, S., and Bello-Klein, A. (2006) Myocardial antioxidant enzyme
activities and concentration and glutathione metabolism in experimental
hyperthyroidism, Mol. Cell. Endocrinol., 249,
133-139.
35.Araujo, A. S., Schenkel, P., Enzveiler, A. T.,
Fernandes, T. R., Partata, W. A., Llesuy, S., Ribeiro, M. F., Khaper,
N., Singal, P. K., and Bello-Klein, A. (2008) The role of redox
signaling in cardiac hypertrophy induced by experimental
hyperthyroidism, J. Mol. Endocrinol., 41, 423-430.
36.Reznick, A. Z., and Packer, L. (1994) Carbonyl
assay for determination of oxidatively modified proteins, Meth.
Enzymol., 233, 357-363.
37.Matyushenko, A. M., Shchepkin, D. V., Kopylova,
G. V., Popruga, K. E., Artemova, N. V., Pivovarova, A. V., Bershitsky,
S. Y., and Levitsky, D. I. (2017) Structural and functional effects of
cardiomyopathy-causing mutations in the troponin T-binding region of
cardiac tropomyosin, Biochemistry, 56, 250-259.
38.Mashanov, G. I., and Molloy, J. E. (2007)
Automatic detection of single fluorophores in live cells, Biophys.
J., 92, 2199-2211.
39.Penheiter, A. R., Bogoger, M., Ellison, P. A.,
Oswald, B., Perkins, W. J., Jones, K. A., and Cremo, C. R. (2007)
H2O2-induced kinetic and chemical modifications
of smooth muscle myosin: correlation to effects of
H2O2 on airway smooth muscle, J. Biol.
Chem., 282, 4336-4344.
40.Tiago, T., Simao, S., Aureliano, M.,
Martín-Romero, F. J., and Gutierrez-Merino, C. (2006) Inhibition
of skeletal muscle S1-myosin ATPase by peroxynitrite,
Biochemistry, 45, 3794-3804.
41.Klein, J. C., Moen, R. J., Smith, E. A., Titus,
M. A., and Thomas, D. D. (2011) Structural and functional impact of
site-directed methionine oxidation in myosin, Biochemistry,
50, 10318-10327.
42.Moen, R. J., Cornea, S., Oseid, D. E., Binder, B.
P., Klein, J. C., and Thomas, D. D. (2014) Redox-sensitive residue in
the actin-binding interface of myosin, Biochem. Biophys. Res.
Commun., 453, 345-349.
43.Gordon, A. M., Homsher, E., and Regnier, M.
(2000) Regulation of contraction in striated muscle, Physiol.
Rev., 80, 853-924.
44.Andrade, F. H., Reid, M. B., and Westerblad, H.
(2001) Contractile response of skeletal muscle to low peroxide
concentrations: myofibrillar calcium sensitivity as a likely target for
redox-modulation, FASEB J., 15, 309-311.
45.Bruton, J. D., Place, N., Yamada, T., Silva, J.
P., Andrade, F. H., Dahlstedt, A. J., Zhang, S. J., Katz, A., Larsson,
N. G., and Westerblad, H. (2008) Reactive oxygen species and
fatigue-induced prolonged low-frequency force depression in skeletal
muscle fibres of rats, mice and SOD2 overexpressing mice, J.
Physiol., 586, 175-184.
46.Lamb, G. D., and Posterino, G. S. (2003) Effects
of oxidation and reduction on contractile function in skeletal muscle
fibres of the rat, J. Physiol., 546, 149-163.
47.Snook, J. H., Li, J., Helmke, B. P., and
Guilford, W. H. (2008) Peroxynitrite inhibits myofibrillar protein
function in an in vitro assay of motility, Free Radic. Biol.
Med., 44, 14-23.
48.Gross, S. M., and Lehman, S. L. (2013)
Accessibility of myofilament cysteines and effects on ATPase depend on
the activation state during exposure to oxidants, PLoS One,
8, e69110.