REVIEW: Exogenous 8-Oxo-7,8-dihydro-2′-deoxyguanosine: Biomedical Properties, Mechanisms of Action, and Therapeutic Potential
A. V. Chernikov1, S. V. Gudkov2,3,4, A. M. Usacheva1, and V. I. Bruskov1,5*
1Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences, 142292 Pushchino, Moscow Region, Russia; E-mail: bruskov_vi@rambler.ru, anatole@inbox.ru2Moscow Regional Research and Clinical Institute (MONIKI), 129110 Moscow, Russia
3Prokhorov Institute of General Physics, Russian Academy of Sciences, 119991 Moscow, Russia
4Lobachevsky State University of Nizhnii Novgorod, 603950 Nizhnii Novgorod, Russia
5Pushchino State Research Institute for Natural Sciences, 142290 Pushchino, Moscow Region, Russia
* To whom correspondence should be addressed.
Received July 12, 2017
8-Oxo-7,8-dihydroguanine (8-oxo-G) is a key biomarker of oxidative damage to DNA in cells, and its genotoxicity is well-studied. In recent years, it has been confirmed experimentally that free 8-oxo-G and molecules containing it are not merely inert products of DNA repair or degradation, but they are actively involved in intracellular signaling. In this review, data are systematized indicating that free 8-oxo-G and oxidized (containing 8-oxo-G) extracellular DNA function in the body as mediators of stress signaling and initiate inflammatory and immune responses to maintain homeostasis under the action of external pathogens, whereas exogenous 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dGuo) exhibits pronounced antiinflammatory and antioxidant properties. This review describes known action mechanisms of oxidized guanine and 8-oxo-G-containing molecules. Prospects for their use as a therapeutic target are considered, as well as a pharmaceutical agent for treatment of a wide range of diseases whose pathogenesis is significantly contributed to by inflammation and oxidative stress.
KEY WORDS: exogenous 8-oxo-7,8-dihydro-2′-deoxyguanosine, free 8-oxo-7,8-dihydroguanine, oxidized extracellular DNA, inflammation, small GTPasesDOI: 10.1134/S0006297917130089
Abbreviations: A, adenine; BER, base excision repair; DAMP, damage-associated molecular patterns; ecDNA, extracellular DNA; G, guanine; MAPK, mitogen-activated protein kinases; OGG1, 8-oxoguanine-DNA-glycosylase 1; 8-oxo-dGuo, 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-hydroxydeoxyguanosine); 8-oxo-G, 8-oxo-7,8-dihydroguanine (8-hydroxyguanine); 8-oxo-Guo, 8-oxo-7,8-dihydroguanosine (8-hydroxyguanosine); RNS, reactive nitrogen species; ROS, reactive oxygen species; TLR, Toll-like receptors.
In cells of living organisms, metabolic processes lead to constant
generation of reactive oxygen species (ROS) and reactive nitrogen
species (RNS), which in low concentrations perform physiological
functions, including signaling and regulatory ones. Under the influence
of environmental factors (ionizing and nonionizing radiation, toxins,
xenobiotics, etc.), ROS/RNS can be generated in amounts exceeding
protective abilities of the antioxidant and repair systems of the
organism (oxidative stress), which results in damage to
biomacromolecules [1]. More than 30 products of
oxidatively generated modification of nucleobases are known, among
which 8-oxo-7,8-dihydroguanine (8-oxo-G) is the most widespread and the
best-studied. 8-Oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dGuo)
was first identified in vitro in the early 1980s by Japanese
researchers [2, 3], and during
the subsequent 30 years the properties of 8-oxo-dGuo and its role in
DNA have been considered in thousands of publications. Among the
natural nucleobases, guanine (G) within DNA has the lowest redox
potential (1.29 mV with respect to the normal hydrogen electrode
(NHE) [4]) and is easily oxidized in position C8,
producing 8-oxo-G [5]. The redox potential of
8-oxo-G is still lower (0.74 mV) [4]), which
results in its further oxidation to spiroiminodihydantoin (Sp),
iminoallantoin (Ia), and guanidinohydantoin (Gh) [6]. The generation of 8-oxo-G (and of its modification
products) is the most frequent type of oxidative damage of nucleic
acids [7], and 8-oxo-G is considered to be one of
the main biomarkers of oxidative stress [8].
According to modern evaluations, the steady-state level of 8-oxo-G in
nuclear DNA varies from 0.3 to 6 bases per 106 guanine
residues [9], whereas in mitochondrial DNA there
are 1-3 bases per 105 G [10]. On
average, 105 8-oxo-G residues are produced in a cell every
day [4, 11]. It was shown that
8-oxo-G has mutagenic properties; during DNA replication, it produces
in the syn-conformation the mismatch 8-oxo-G:A, which results in
the G→T transversion [12]. Oxidation of the
base in 2′-deoxyguanosine strengthens the glycoside bond [13], and the damage must be repaired enzymatically.
Most frequently, 8-oxo-G is recognized in double-helical DNA, and in
eukaryotes it is excised from the DNA under the influence of a highly
specific 8-oxoguanine DNA glycosylase 1 (OGG1) through the base
excision repair (BER) mechanism. Minor pathways of 8-oxo-G repair in
DNA include nucleotide excision repair (NER), mismatch repair (MMR),
nucleotide incision repair (NIR), and correction of mismatches
(proofreading) by DNA polymerases [14]. MMR is
catalyzed by glycosylase MYH (an Escherichia coli MutY homolog),
which eliminates adenine from the mismatched 8-oxo-G:A [15]. The cytoplasmic pool of dGTP (which is oxidized
more easily than G in DNA) is removed by a highly specific hydrolase
from the Nudix family (MTH1/2 in humans, which is an E. coli
MutT homolog). This hydrolase hydrolyzes 8-oxo-dGTP to 8-oxo-dGDP,
which is not rephosphorylated. 8-Oxo-dGDP is dephosphorylated to
8-oxo-dGMP and then to 8-oxo-dGuo by a nucleotidase or phosphatase [16]. It has been shown that exogenous 8-oxo-dGuo is
not phosphorylated to 8-oxo-dGMP and is not degraded to 8-oxo-G, i.e.
it is not used by the cell repeatedly for synthesis of nucleotides [17, 18]. Cell membranes are
easily permeable for 8-oxo-dGuo, but they are impermeable for
8-oxo-dGTP [19]. Upon removal from DNA,
8-oxo-G/8-oxo-dGuo enters the bloodstream and then is eliminated from
the body with urine. The average background values of 8-oxo-G and
8-oxo-dGuo concentrations determined by liquid
chromatography–tandem mass spectrometry (LC-MS/MS) in healthy
adults are 0.21 and 0.016 ng/ml in blood plasma, 0.85 and
0.01 ng/ml in saliva, and 12.2 and 3.8 ng/ml in urine,
respectively [20]. In DNA, 8-oxo-G in the absence
of repair can cause the arrest of the cell cycle and apoptosis [21]. Enzymatic repair of oxidative DNA base damage,
its mechanisms, and its biological consequences have been considered in
several reviews [22-24].
Earlier, oxidative modification of guanine in DNA and RNA was believed to be exceptionally genotoxic and mutagenic, and thus causing very harmful damage to nucleic acids, but this concept is now reconsidered [25, 26]. It is now thought that easily oxidized G and 8-oxo-G in RNA and in untranscribed parts of genomic DNA (including telomeres and adjacent regions) can act as intracellular antioxidants, a buffer capable of intercepting highly reactive free radicals and damping their chemical activity. It is known that the amount of RNA in the cell is fourfold higher than the amount of DNA, and 8-oxo-G is detected in 30-70% of mRNA [27]. It seems that the mammalian organism is tolerant to high stationary levels of 8-oxo-G in the genomic DNA in cells: in hepatocytes of 14-month-old mice with knockout of the OGG1-encoding gene, the 8-oxo-G contents in nuclear DNA and mitochondrial DNA were, respectively, seven and ~20 times higher than in wild-type control mice; however, this did not lead to dysfunction of mitochondria or increase in the frequency of tumor formation in tissues [28]. Moreover, Ogg1−/− mice are resistant to development of inflammation [29]. 8-Oxo-G plays an important role in chromatin relaxation and initiation of DNA transcription due to the nicking action of OGG1 during 8-oxo-G repair [30] and, as discriminated from 8-oxoadenine and thymine glycol, it has virtually no effect on the activities of RNA polymerases [31]. It seems that there is an optimal tissue-dependent intracellular level of genomic 8-oxo-G that is necessary for normal physiological processes in the organism [25].
BIOMEDICAL PROPERTIES OF EXOGENOUS 8-oxo-dGuo, FREE 8-oxo-G, AND
OXIDIZED EXTRACELLULAR DNA
Biomedical properties of exogenous 8-oxo-dGuo. The properties and biological functions have been studied for a long time only for 8-oxo-G/8-oxo-dGuo produced in biological systems endogenously under the influence of ROS in conditions of oxidative stress. The properties, functions, and action mechanisms of exogenous 8-oxo-G/8-oxo-dGuo that entered the cell/organism from outside an organism became a subject of studies only during recent years. In 2004, authors from South Korea showed in the pioneering work [32] that exogenous 8-oxo-dGuo (0.1-0.2 mM) protects in vitro thymidine both free and within oligodeoxynucleotides (0.01 mM and 1 µM, respectively) against oxidative degradation and supercoiled circular plasmid DNA against chain break and relaxation under the action of Fenton’s reagent (0.1 mM copper sulfate, 0.1 mM ascorbic acid, 5 mM hydrogen peroxide, 10 mM Tris-HCl, pH 7.4, at 20 and 37°C for 20 and 120 min, respectively). 8-Oxo-dGuo was shown to be about two times more efficient as a scavenger of hydroxyl radicals than mannitol, sodium formate, tert-butanol, sodium azide, and dimethylsulfoxide (DMSO) [32]. In one work [33], the ability of 8-oxo-dGuo to scavenge OH radicals was quantitatively evaluated using EPR. In the presence of 5,5-dimethylpyrrolin-N-oxide (DMPO, 1 mM) as a spin trap, the Fenton reaction (0.05 mM ferrum sulfate, 1 mM hydrogen peroxide, 50 mM sodium phosphate, pH 7.4, 37°C) was accompanied by generation of a signal specific for the DMPO–OH complex; 8-oxo-dGuo decreased the intensity of this signal dose-dependently and eliminated it completely at concentrations > 5 µg/ml (17.7 µM). In another work [34], the antioxidant activities of 8-oxo-dGuo, 8-oxo-Guo, Trolox (Tro), ascorbate (Asc), uric acid (UA), N-acetyl-L-cysteine (NAC), and superoxide dismutase (SOD) were compared in the following model chemical systems: oxidation of 2′,7′-dichlorofluorescein (DCHF) by the Fenton’s reagent (0.75 mM ferric chloride, 6 mM hydrogen peroxide, phosphate-buffered saline, pH 7.0, 37°C) (a) or by 0.5 mM peroxynitrite (b), oxidation of low-density lipoproteins (LDL; 200 µg protein/ml) by singlet oxygen (c), inhibition of generation of superoxide anion-radicals on oxidation of hypoxanthine catalyzed by xanthine oxidase (d). The antioxidant activity of the compounds decreased in the series:
Based on these results, it was supposed that 8-oxo-dGuo entering in vivo due to DNA repair into the cytoplasm and further into blood plasma and urine can play a positive role in the organism and possibly display antioxidant properties and protect adjacent macromolecules against damage by radicals.
Like many antioxidants and some natural purine nucleosides [35], 8-oxo-dGuo displays radioprotective properties [36]. 8-Oxo-dGuo (60 mg/kg) given per os to C57Bl/J mice before a total single exposure to γ-radiation at dose 2-7 Gy decreased in splenocytes the generation of ROS/RNS and the level of cellular lesions caused by them; in particular, it decreased the amount of nitrotyrosine in proteins. In spleen lymphocytes, it decreased the content of transcription factors – of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and of activator protein-1 (AP-1), as well as of proinflammatory cytokines associated with the signaling cascade of transforming growth factor-β (TGF-β).
Photoprotective properties of 8-oxo-dGuo were studied in vitro on immortalized human HaCaT line keratinocytes and in vivo on SKH-1 hairless mice [34]. 8-Oxo-dGuo (15-30 µg/ml) was added into the culture medium or was applicated as an ointment (1 mg/ml) onto the dorsal region of the animals’ skin 1 h before exposure to mid-wavelength UV (UVB, 312 nm) at dose 15 or 180 mJ/cm2, respectively. In the keratinocytes incubated with 8-oxo-dGuo, the ROS production under the influence of UVB was decreased dose-dependently (to 2.5-fold), as well as the ROS-stimulated expression of proteins: mitogen-activated protein kinases (MAPK) (ERK, JNK, p38), transcription factors (c-Jun, ATF-2), and matrix metalloproteinases (MMP-1 and -9). By the end of two days after the exposure, 8-oxo-dGuo abolished in the mice the skin eczematous reaction and hyperplasia of the epidermis nearly completely, and during a few hours it significantly decreased in the treated mice the generation of hydrogen peroxide, the level of protein carbonyls, and expression of some MAPKs (JNK, p38) and transcription factors (c-Jun, ATF-2) and MMPs (MMP-9 and -13). 2′-Deoxyguanosine (dGuo) at the same concentrations did not cause such effects, or they were negligibly small.
Antioxidants frequently have an antiinflammatory effect [37]; therefore, the influence of exogenous 8-oxo-dGuo was studied on the course of pathologic states and diseases in the genesis of which inflammation plays an essential role.
Antiallergic and immunosuppressive effects of 8-oxo-dGuo were studied on mice sensitized with ovalbumin [38, 39]. 8-Oxo-dGuo was introduced to animals per os (6-60 mg/kg) 6 h before repeated injection of the antigen, and this was associated with a dose-dependent decrease in respiratory pathway resistance, number of leukocytes in bronchoalveolar lavage liquid (LL) (including eosinophil number – up to 2.5-fold), in the level of IgE antibodies specific to ovalbumin (up to 2-fold). Histologic signs of inflammation and pulmonary tissue restructuring (hypertrophy of bronchial smooth muscle cells, hyperplasia of mucosa-secreting goblet cells, collagen deposits, leukocytic infiltration of peribronchial and perivascular regions) were decreased. 8-Oxo-dGuo dose-dependently (2-fold and stronger) lowered in LL the levels of proinflammatory cytokines (interleukins IL-4, -5, -13, interferon-γ (IFN-γ) and of tumor necrosis factor-α (TNF-α)), which were increased on the allergic reaction background. 8-Oxo-G used at the same doses did not cause these effects, or they were insignificant. Antiallergic effect of 8-oxo-dGuo was also observed when it was given to mice (30 mg/kg, per os, daily for three days) after development of allergic reaction to ovalbumin: in pulmonary tissue there was a decrease in deposits of collagen, fibronectin, laminin and in the number of mast and goblet cells, in blood plasma the titers decreased of ovalbumin-specific IgE, in bone marrow mast cells the expression decreased of mRNA of proinflammatory cells and chemokines of the NF-κB/AP-1 signaling pathway (IL-4, -6, -13, TGF-β, TNF-α, RANTES, CCL5) [40].
The antiinflammatory activity of 8-oxo-dGuo was also studied in vivo in another model [19]. Mice were injected intraperitoneally (i.p.) with bacterial lipopolysaccharide (LPS) and 4 h before they were injected with 8-oxo-dGuo (i.p., 60 mg/kg), which suppressed the development of LPS-induced inflammatory reactions. Blood plasma was isolated 2 h after the LPS injection, and 8-oxo-dGuo was shown to dose-dependently decrease the level of proinflammatory cytokines (IL-4, -18, -12p70, TNF-α) with efficacy equal or higher than the efficacy of acetylsalicylic acid at the same concentration. 8-Oxo-Guo (60 mg/kg) significantly decreased the levels of IL-18 and TNF-α, whereas the effects of dGuo and Guo were insignificant. 8-Oxo-dGuo decreased neutrophilic infiltration of pulmonary tissue and increased survival of mice up to 40% at 36 h after the LPS injection under conditions of toxic shock (survival in the control was 0, and 10% in the aspirin-treated mice).
8-Oxo-dGuo manifested antiinflammatory action on nervous tissue of mice injected with LPS into the brain striatum and in vitro upon addition of LPS into microglia culture line BV2 cells [41, 42]. 8-Oxo-dGuo injected into the striatum 1 h before the injection of LPS virtually normalized in the tissue the expression of proinflammatory enzymes cyclooxygenase-2 (COX-2) and inducible NO synthase (iNOS); on addition into the cultural medium, 8-oxo-dGuo (to 100 µg/ml) significantly decreased in the medium the levels of IL-6, TNF-α, and IFN-γ, and in the BV2 cells it decreased nearly 2-fold the activity of COX-2, decreased NO production about 2.7-fold, and lowered dose-dependently ROS production with efficacy comparable with the action of 10 mM NAC.
In acute experimental autoimmune encephalomyelitis (EAE) in mice, an experimental model of multiple sclerosis, 8-oxo-dGuo decreased parameters of disease severity [43]. Injection to mice of 8-oxo-dGuo (i.p. 60 mg/kg) during five days after the induction of EAE relieved clinical symptoms (decreased by ~20% the mean clinical index of the disease as compared to the control), in the nervous tissue decreased by ~25% the number and activity of mast cells, lowered migration into the tissue of their circulating precursors, and increased the population of immunosuppressive regulatory T-lymphocytes (Treg). In the nervous tissue of the experimental mice, 8-oxo-dGuo decreased the levels of proinflammatory cytokines (TNF-α, IL-6, -17), chemokines (CCL2/CCR2), and cellular adhesion molecules (VCAM-1, PECAM-1) and leukotrienes.
Atherosclerotic lesions of blood vessels associated with formation of atheromatous plaques is accompanied by chronic inflammation of the vascular intima and migration and anomalous proliferation of smooth muscle cells of the vascular wall, i.e. processes regulated by ROS-mediated signaling cascades [44]. In DNA of cells isolated from human atherosclerotic plaques (macrophages and smooth muscle and endothelial cells), the level of 8-oxo-G was many times higher than in DNA of the vascular wall cells of normal arteries [45]. The possibility of antiatherosclerotic action of exogenous 8-oxo-dGuo was studied on mice with knockout of the gene encoding apolipoprotein E (ApoE). In these mice, one of the common carotid arteries was partially ligated, and then the mice received a lipid-enriched diet for two weeks [46]. 8-Oxo-dGuo (30 mg/kg) was given to mice per os daily beginning from two days before the ligation. In the experimental mice, plaque areas were significantly lower, vascular lumen in distal parts of the carotid artery was increased, triglyceride concentration in blood plasma was slightly but significantly decreased, infiltration of plaques with monocytes/macrophages and foam cells was lowered, accumulation of extracellular matrix components in the plaques was decreased, and the generation of ROS including superoxide anion-radicals by vascular wall cells was decreased.
The influence of exogenous 8-oxo-dGuo was studied on the course of some gastrointestinal tract (GIT) diseases where oxidative stress and inflammation play a key role in pathogenesis [47]. Acute gastritis was caused in rats by water-immersion restraint stress (WIRS): the animals in cramped wire cages were partially immersed in warm water for 10 h [33]. In the gastric mucosa of the stress-subjected rats, multiple foci of erosion and ulceration, hemorrhages, oedema, and ischemic necrosis were observed. In animals that received 8-oxo-dGuo (50 mg/kg) per os once 1 h before the stress initiation, pathologic changes in the mucosa were significantly less pronounced, and the therapeutic efficiency of 8-oxo-dGuo was comparable with that of Rebamipide, which is widely used in clinical practice as a gastroprotector. On the background of 8-oxo-dGuo, the levels of some proinflammatory cytokines and signaling proteins (TNF-α, vascular endothelial growth factor (VEGF)) in the gastric mucosa were significantly decreased. In a model of chronic gastritis in mice induced by infecting with Helicobacter pylori and salt-enriched diet, 8-oxo-dGuo consumption with potable water ad libitum significantly attenuated signs of inflammation and atrophy of the gastric mucosa and prevented its dysplasia and precancer transformation [47]. Acute colitis in mice was initiated by addition of dextran sulfate (5%) into potable water for one week; some of the animals during this period were injected with 8-oxo-dGuo (i.p. 50-150 mg/kg) [48]. Inflammation of the large intestine was accompanied by a decrease in the weight of the mice, reduction of intestine length, anal hemorrhages and hematochezia, destruction of mucosa, and increase in it of inflammation mediators (COX-2, iNOS, TNF-α, IL-6). 8-Oxo-dGuo dose-dependently (significantly at high concentrations) decreased the expression of the above-described pathologic changes in the large intestine.
In humans, chronic systemic inflammation underlies metabolic syndrome (MS), which is a complex of hormonal and metabolic disturbances on the background of abdominal obesity. This complex involves a change in glucose tolerance, insulin-resistance, and compensatory hyperinsulinemia, hypertriglyceridemia, etc. [49]. MS is a risk factor for development of type 2 diabetes mellitus and cardiovascular diseases. The influence of exogenous 8-oxo-dGuo on the development of MS was studied in mice of the KKAy and db/db strains that are genetically predisposed to obesity and diabetes mellitus [50]. Starting from the age of 6 weeks, the mice received daily intragastric injections of 8-oxo-dGuo (60 mg/kg) for 2 months; every 2 weeks, biological samples were taken for analysis. In the control mice, signs of metabolic disturbances appeared by the 8th week and then increased: excessive body weight, increased fasting serum glucose and insulin levels, dyslipidemia (increased levels in blood of triglycerides and LDL cholesterol), steatosis, increase in blood of inflammation markers (TNF-α, IL-6, MMP-9), and decrease in adiponectin level (an antiinflammatory hormone of fatty tissue). In the animals treated with 8-oxo-dGuo, the severity of metabolic disturbances (hyperglycemia, insulin-resistance, morphologic disorders in the liver) was significantly less pronounced, and the systemic inflammation signs were suppressed (blood contents of cytokines diminished, and adiponectin level increased).
A relation between chronic inflammation and appearance of malignant tumors was noted even in the nineteenth century by Rudolf Virchow. Now it is thought that inflammation mediates up to 20% of clinical cases of cancer by influencing all phases of tumorigenesis: mutagenesis and instability of genome of cells with their subsequent malignization, suppression of immune response of the organism, tumor formation and growth, tumoral angiogenesis and invasiveness, and dissemination of metastases [51]. Having in mind antiinflammatory properties of 8-oxo-dGuo and its structural similarity with purine analogs 6-thioguanine (6-TG) and 6-mercaptopurine (6-thioinosine), which are known anticarcinogenic agents, possible antitumoral effects of exogenous 8-oxo-dGuo were studied. Studies in vitro revealed [52] that incubation of human myelosarcoma line KG-1 cells with 8-oxo-dGuo (50-200 µg/ml) for 96 h led to a sharp decrease in the number of viable cells in the MTT-test (down to ~25% at the highest concentration of 8-oxo-dGuo). The KG-1 line cells are characterized by absence of the OGG1 enzyme activity as a result of the point mutation Arg(229)Gln in the gene encoding it, whereas the normal activities of other enzymes of 8-oxoguanine repair, MYH and MTH1, are retained [52, 53]. Under the influence of 8-oxo-dGuo, cells die through apoptosis, which is indicated by increase in the number of hypodiploid cells (Sub-G1) and by the degree of DNA fragmentation in them, loss by the cells (by 40% of their total number) of the mitochondrial transmembrane potential Δφm, cytochrome c release from mitochondria, decrease in level of anti-apoptogenic protein BCL-2, and increase in caspase-8, -9, and -3 in the cells. Moreover, 8-oxo-dGuo can inhibit the growth of the KG-1 cells in the G1 phase of the cell cycle [54]. Tumor cells of other lines (H9, CEM-CM3, Molt-4) with low OGG1 activity also were sensitive to the cytotoxic effect of exogenous 8-oxo-dGuo, whereas leukemic cells of a line with normal activity of the enzyme (U937) were tolerant to 8-oxo-dGuo [52]. 8-Oxo-dGuo and dGuo were injected (i.p. 3.3-330 mg/kg daily for two weeks) to mice with transplanted tumor of human myeloleukosis lines KG-1 and U937 [55]. 8-Oxo-dGuo suppressed dose-dependently the tumor growth induced by KG-1 and reduced tumor volume (~3.5-fold compared with the control mice injected with saline) and weight (~3-fold), but it did not influence tumor growth in mice inoculated with U937 cells. 2′-Deoxyguanosine (dGuo) displayed a weak and statistically insignificant action in the first case (KG-1) and did not influence tumor growth in the second case (U937), whereas 6-TG (33 mg/kg daily) virtually completely inhibited tumor progression in both cases. The cytotoxic action of 8-oxo-dGuo on KG-1 cells in vivo is based on induction of apoptosis and inhibition of the cell cycle, which is indicated by increased level of proapoptotic proteins (caspase-8, -9 and -3), histologic signs of apoptosis, increased fragmentation of DNA, and differently directed changes in the level of proteins regulating the cell cycle rate (lowering of cyclins D3 and E, kinases CDK2 and 4, and increase in cyclin-dependent inhibitors of kinases p16 and p27). In another work [48], antitumor properties of 8-oxo-dGuo were studied in vivo in a model of colorectal cancer associated with ulcerative colitis. Colorectal cancer was induced in mice by injection of azoxymethane (AOM; 10 mg/kg) and the subsequent maintenance of the animals for 1 week on water with 2.5% dextran sulfate. During the following 20 weeks, the mice received 8-oxo-dGuo with food (50 and 100 mg/kg daily). It was shown that on the background of 8-oxo-dGuo treatment, the number and size of tumor foci formed in the large intestine of mice were decreased, tumor progression was inhibited at the stage of dysplasia and adenoma (in control mice without 8-oxo-dGuo adenocarcinoma developed), in tissue levels of some inflammation-related cytokines, chemokines, and proteins were decreased (in particular, TNF-α, P-selectin, monocytic chemotactic protein-1 (MCP-1), matrix metalloproteinases (MMP-2, -3, -9, -11), NADPH oxidase type 4 (NOX4), COX-2), lipid peroxidation was decreased (malonic dialdehyde (MDA) content), as well as expression of β-catenin, a protein of the Wnt signaling pathway involved in the regulation of carcinogenesis.
Biological features of free 8-oxo-G. Oxidation in DNA of guanine to 8-oxo-G mediated through ROS (especially through hydroxyl radicals and singlet oxygen as the most reactive) and its subsequent enzymatic repair initiated by OGG1 generates free intracellular 8-oxo-G. Although in vitro 8-oxo-G plays in solution the role of an antioxidant and scavenger of free radicals [32], free 8-oxo-G generated in biological systems as a result of repair (or injected from the outside) displays, unlike 8-oxo-dGuo, pronounced proinflammatory features. Acting as an alarmin, 8-oxo-G triggers an organism’s protective reactions for maintaining homeostasis, especially by stimulating the immune system and activating inflammatory reactions.
Intranasal application of 8-oxo-G to mice (50 ng/kg) and subsequent full-transcriptome RNA sequencing and quantitative PCR of pulmonary tissue showed that the 8-oxo-G caused selective changes in the expression of genes related to different intracellular proinflammatory signaling pathways and biological processes [56]. A single injection of 8-oxo-G activated signaling cascades leading to increased production of cytokines, chemokines, and interleukins associated with innate immunity reactions and cellular homeostasis [57]. Multiple injections to mice of 8-oxo-G at the same dose increased the number of transcripts involved in transduction pathways of cadherin-, integrin-, TGF-β-, and Wnt-mediated signals associated with biological adhesion, intercellular communications, and histological remodeling of pulmonary tissue [58]. In control experiments, injections to mice of G, dGuo, 8-oxo-dGuo, 8-oxo-A, and FapyG (2,6-diamino-4-hydroxy-5-formamidopyrimidine) did not elicit these effects [57, 58]. 8-Oxo-G acts as a connecting link between the damaging effects of environmental agents and the organism’s inflammation-like defense reactions directed for maintaining homeostasis of the organism’s internal milieu.
Exogenous 8-oxo-dGuo, free 8-oxo-G, and cell aging. The geroprotective effect of exogenous 8-oxo-dGuo was shown on Chinese hamster cell culture, and this effect depended on the proliferative state of the culture [59]. The addition of exogenous 8-oxo-dGuo (1-1000 nM) to young cells (5 days of culture) did not influence the level of 8-oxo-G in the cellular DNA, whereas in “old” cells (17 days) it sharply (4-5-fold) decreased it. Exogenous 8-oxo-dGuo (1 µM) diminished the density of the “old” cell culture.
Daily addition of 8-oxo-G (but not of G, dGuo, 8-oxo-dGuo, FapyG, A, dAdo, or 8-oxo-A) to final concentration of 0.1-10 µM into the culture medium of human diploid lung fibroblasts (HDLF) and of mouse embryonic lung fibroblasts (MELF) decreased cell proliferation (up to irreversible blockade of the cell cycle in the G1 phase) and accelerated replicative cell aging (reaching the Hayflick limit) [60]. In the fibroblasts, lipofuscin and senescence-associated β-galactosidase (SA-βGal) accumulated, but neither telomere shortening nor increase in mutation rate was observed. Exhaustion in cells of the OGG1 pool using small interfering RNAs (siRNA) prevented the accelerated senescence of the cells caused by 8-oxo-G. In in vivo experiments, Balb/c mice received multiple intranasal injections with 8-oxo-G (0.5 µg/kg), and in lung tissue cells activation of small GTPases of the Ras family was observed, as well as a differential expression of genes related with aging and cell cycle regulation. The authors suggested that the accelerated cell aging under the influence of 8-oxo-G during DNA repair under conditions of oxidative stress can be a defense mechanism preventing undesirable proliferation of cells and decreasing risk of their malignant transformation and tumorigenesis.
Biomedical features of oxidized extracellular DNA. During recent years, interest has grown in extracellular nucleic acids, DNA and RNA, which are freely circulating in blood plasma and other biological fluids [61, 62] and, in particular, in oxidized extracellular DNA (ecDNA) containing 8-oxo-G [63]. EcDNA can enter blood due to cell death in the organism’s tissues because of apoptosis and necrosis, after the death of blood cells, viruses, and bacteria, and due to a spontaneous release by living cells of exosomes and de novo synthesized DNA (metabolic DNA). EcDNA is different from genomic DNA by the presence of motifs enriched with unmethylated CpG-dinucleotides and of single- and double-strand breaks; the physiological concentration of ecDNA in blood of healthy adults is 1-40 ng/ml [64]. In healthy subjects, ecDNA is usually bound to the cell surface, the size of ecDNA fragments in blood is ~23 kb and more, and 8-oxo-G content in ecDNA extracted from the culture medium of normal endotheliocytes was <1 residue of 8-oxo-G per 107 G; in chronic diseases, ecDNA freely circulates in blood, usually as nucleosomes or as components of vesicles, and the size of the fragments can both significantly decrease (to 100 bp) and increase, and the 8-oxo-G level in ecDNA can increase to 50-400 8-oxo-G per 106 G [65, 66]. Introduction into the incubation medium of different human cells, such as umbilical vein endothelial cells (HUVEC) [67], mesenchymal stem cells (MSC) [66], and mammary gland carcinoma cells (MCF-7) [68], of fragments of genomic DNA (20-50 ng/ml) oxidized in vitro and containing 8-oxo-G caused a significant and rapid (~30 min) “explosive” growth of ROS biosynthesis followed by a decrease to the initial (or lower) values, and also a transient increase in the number of single- or double-strand breaks in DNA. ROS level in the cells increased, in particular due to a large increase in the amount of mRNA of the gene encoding the NOX4 enzyme [67], which in turn activated the redox-sensitive transcriptional nuclear E2-related factor 2 (NRF2) and the related regulatory cascade that controls the cell response to oxidative stress (including expression of genes KEAP1, SOD1, BCL2) and stimulates the activity of antioxidant systems of the cell [66]. Incubation of human fibroblasts with DNA oxidized in vitro (20 ng/ml, ~400 8-oxo-G per 106 G) induced a pronounced adaptive response of the cells to subsequent stress (poor culture medium or exposure to X-rays at a dose of 1.2 Gy), increasing fibroblast survival, decreasing in them the number of double-stranded DNA breaks, decreasing the number of apoptotic cells, and stimulating transcriptional factor NRF2 activity and suppressing NF-κB – all this led to a 2-fold decrease in the production by the cells of the proinflammatory cytokines TNF-α and IL-6 [64]. Exposure at a dose of 0.1 Gy or treatment with in vitro oxidized human DNA or ecDNA isolated from the cultural medium of irradiated cells caused in HUVEC endotheliocytes similar changes in cell morphology and in contents of ROS and NO, i.e. the effect of DNA containing 8-oxo-G on the cells was similar to the “bystander effect” [67, 69]. The cell response to the presence of oxidized DNA depended on the level in it of 8-oxo-G [67, 70], on the position of 8-oxo-G in the chain, chain length, and the nucleotide sequence [71]. According to data of different authors, the oxidation of G inside a CpG motif (i.e. the presence in ecDNA of the dinucleotide CpOxoG) could strengthen or weaken the activity of oxidized DNA [71-73]. The degradation of oxidized DNA to the nucleotide level inactivated its immunostimulatory effect on mouse macrophages [71].
Mitochondrial DNA (mtDNA) of eukaryotes is oxidized more easily than nuclear DNA because it is located closer to the respiratory chain, which is a source of ROS, and also due to the absence of histone proteins and to the low efficiency of the mtDNA enzymatic repair system. Therefore, the background level of oxidized bases in mtDNA is several times higher than in nuclear DNA [74]. The transfection of LPS-primed macrophages from mouse bone marrow with oxidized mtDNA (2 µg/ml) that contained 8-oxo-G (to 5 ng/ml) activated intracellular inflammasomes and nearly a 2-fold growth (as compared to intact mtDNA) of the inflammatory interleukin IL-1β production by the cells was observed, but in the presence of free 8-oxo-dGuo (200 µM) the effect was completely inhibited [75]. Both the native and oxidized extracellular mtDNA (7-10 µg) have an immunostimulatory effect on human and mouse plasmacytoid dendritic cells (pDC) in vitro and in vivo, respectively, enhancing the generation by these leukocytes of proinflammatory cytokines and chemokines (IL-8, TNF-α, CXCL1). The stimulatory activity of mtDNA containing 8-oxo-G is significantly higher than the activity of native mtDNA [76]. MtDNA of human neutrophils oxidized by ROS during NETosis and containing 8-oxo-G displays proinflammatory properties both in vitro on coincubation (250 ng) with human peripheral blood mononuclear cells, increasing their expression of mRNA of cytokines IL-1β, -6, and TNF-α, and in vivo on parenteral injection into mice (1 µg), inducing interferon-stimulated genes (ISG) MX1, IRF7, and IFIT1 [77]. Highly purified native mtDNA (A260/A280 = 1.91 and A260/A230 = 1.67) is unable to activate human neutrophils [78]. Intraarticular injection into mice of free mtDNA (1-5 µg) or of a 20-meric oligonucleotide containing one 8-oxo-G residue (10 nmol), but not free nuclear DNA, resulted in the development of arthritis [73, 79]. In sinovial fluid (SF) of impaired joints, extracellular mtDNA and 8-oxo-dGuo (>1.5 ng/ml) are detected in the great majority of patients with rheumatoid arthritis as differentiated from healthy subjects. The level of 8-oxo-dGuo in the SF positively correlates with the presence in the patients’ blood of the rheumatoid factor (RF), which is a diagnostic marker indicating risk of development of systemic manifestations of the disease [73, 79].
Both genomic and mitochondrial DNA isolated from organisms of different biological species (human, mouse, herring) and oxidized by exposure to UV-radiation displayed interferonogenic properties in vitro depending on the 8-oxo-G content in the DNA. Thus, it seems that the key factor that determines proinflammatory properties of ecDNA is the degree of its oxidation, and not its origin [77].
ACTION MECHANISMS OF EXOGENOUS 8-oxo-dGuo, FREE 8-oxo-G, AND
OXIDIZED EXTRACELLULAR DNA
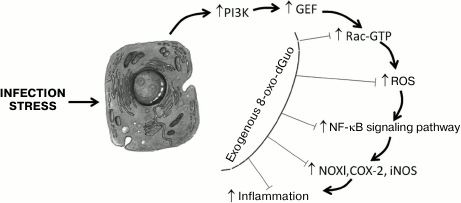
Mechanisms of antiinflammatory action of exogenous 8-oxo-dGuo. The antiinflammatory action of 8-oxo-dGuo is realized through various pathways. First, 8-oxo-dGuo is a highly effective scavenger of ROS, which are proinflammatory mediators in different types of cells [80]. Due to scavenging of ROS, 8-oxo-dGuo not only prevents the immediate damaging action of ROS on biological macromolecules, but it also inhibits the key link of some proinflammatory signaling cascades (e.g. ROS directly stimulate MAP kinases (ERK, JNK, and p38) in cells, which activates transcription factor AP-1, and then increases the expression of matrix metalloproteinases [81, 82]). This mechanism of the antiinflammatory action of 8-oxo-dGuo was shown in models of photoinduced skin lesions and of stress-caused gastritis in mice [33, 34]. The most important other universal mechanism of the protective action of 8-oxo-dGuo mediated through Rac proteins (isoforms Rac1 and Rac2) was demonstrated in some cell models in vitro, as well as in animals [19, 33, 38, 41, 42, 50]. Rac (Ras-related C3 botulinum toxin substrate) is a monomeric 20-30 kDa protein of the GTPase Rho family, which belongs to the superfamily of small low molecular weight GTP-binding G-proteins – Ras (Rat sarcoma viral oncogene homolog). Rac proteins are activated by many agonists, including phosphatidyl inositol 3-kinase (PI3K), TNF-α, and growth factors. Rac proteins are involved in regulation of important biological processes, including the cell migration, cytoskeletal dynamics, adhesion, proliferation, apoptosis, tumorigenesis, and gene expression [83]. In a cell, Rac proteins function as a molecular binary “switch” responsible for transitions between inactive (GDP-bound) and active (GTP-bound) conformations. This process is regulated by proteins (guanine-nucleotide exchange factors (GEFs)) that promote the replacement of GDP by GTP. GEFs for Rac (Rac-GEFs), which include proteins VAV2, TIAM1, TRIO, etc., catalyze the dissociation of GDP by modifying the nucleotide-binding site of Rac. It is just the active form of Rac that ensures the realization of its biological functions by transducing a signal from membrane receptors and triggering Rac-dependent signaling cascades whose molecular targets are some NOX isoforms, actin, protein kinases JNK and p38, transcription factor NF-κB, etc. [84]. Directly or indirectly through protein kinases JNK/p38, Rac influences the activity of a group of signal transducers and activators of transcription (STATs), which include polypeptides playing an important role in the cell response to stimulation by proinflammatory cytokines (IL-6, -10, -11, IFN-γ, oncostatin M, etc.) and growth factors (EGF, TGF, G-CSF, leptin, etc.) and in inflammation-induced cellular carcinogenesis [85, 86]. STAT3 mediates cell and organ responses to an extremely wide spectrum of signals [87]. The action on cytoplasmic membrane of oxidative stressors (ROS, proinflammatory cytokines) triggers in the cell a reaction cascade (Fig. 1): PI3K is activated, which in turn activates Rac-GEF, which increases GTP binding in the nucleotide-exchanging center of Rac. The activated Rac stimulates both NF-κB- and STAT3-dependent signaling pathways. In the first case, Rac binds subunit p67(phox) of the NOX enzymatic complex, which sharply strengthens the production by the enzyme of ROS, activating transcription factor NF-κB and thus increasing the expression of proinflammatory genes COX2, NOX1, iNOS, etc. In the other case, the activated Rac forms a complex with STAT3, stimulates its phosphorylation on Tyr and Ser residues, and induces transcriptional activity of STAT3, which is associated with inflammation-caused carcinogenesis. 8-Oxo-dGuo was shown to dose-dependently prevent the activation of Rac, making difficult its binding to GTP, possibly due to a competitive inhibition mechanism. However, 8-oxo-dGuo does not influence the functional state of Rac-GEF and PI3K [33]. It was shown by molecular modeling that 8-oxo-dGuo corresponds well to the topology of the Rac1 nucleotide-binding site and forms hydrogen bonds with adjacent amino acid residues Lys116, Asp118, and Ala159, producing a Rac1•8-oxo-dGuo•GEF triple complex and preventing the dissociation of GEF that blocks the activation of Rac1 [46]. 8-Oxo-dGuo suppresses Rac activity more efficiently than 8-oxo-Guo and 8-oxo-GTP, whereas 8-oxo-G, dGuo, Guo, and Ado lack this ability [19, 38, 41, 88, 89].
Fig. 1. Possible mechanism of the influence of exogenous 8-oxo-dGuo on the inflammatory cascade initiated by infection or stress. Under the influence of an external biological, chemical, or physical pathogen, kinase PI3K and protein Rac-GEF are activated successively. The activated Rac-GEF catalyzes GDP→GTP translocation in the nucleotide-binding site of Rac. The activated Rac (Rac-GTP) stimulates NOX, which generates ROS. A high level of ROS activates transcription factor NF-κB, which strengthens the expression of redox-dependent inflammation mediators – NOX1, COX-2, iNOS, etc. 8-Oxo-dGuo prevents the binding of GTP with Rac, presumably through competitive inhibition, and thus prevents the development of the inflammatory cascade and of tissue lesion mediated by oxidative stress. Adapted from [33] with changes.
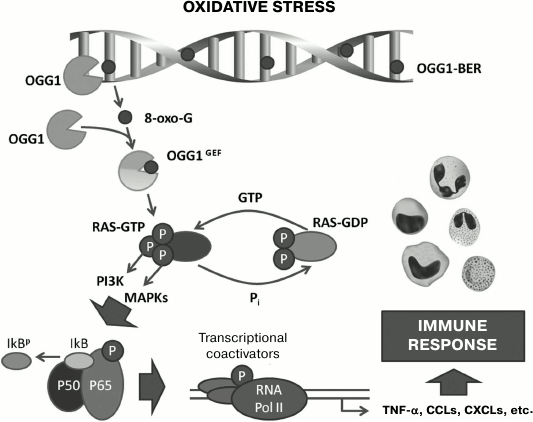
Mechanisms of proinflammatory action of free 8-oxo-G. Proinflammatory action of 8-oxo-G is also mediated through small GTPases (Fig. 2). It was shown that free 8-oxo-G that is a result of base excision repair produces with high affinity (dissociation constant Kd ≈ 0.56 nM) in the cytoplasm a complex with the OGG1 molecule binding to a site beyond the catalytic center of the enzyme [90]. 8-Oxo-G induces a conformational change in OGG1 that allows the OGG1•8-oxo-G complex to physically interact with GTPases of the Ras superfamily, activating them by facilitating the replacement of GDP by GTP in the nucleotide-binding site of the protein, i.e. functioning as GEF [91]. The activated (GTP-bound) Ras liberates OGG1•8-oxo-G. 8-Oxo-G and OGG1 do not interact with Ras separately, and the OGG1 binding to 8-oxo-G is highly specific: 8-oxo-dGuo, FapyG, G, 8-oxo-A, or uric acid does not form a complex with OGG1 [90, 92]. OGG1 complexing with free 8-oxo-G increases the AP-lyase activity of the enzyme [93]. DNA glycosylases NEIL1 and NEIL2 (Nei endonuclease VIII-like DNA glycosylases) involved in repair of oxidized bases during replication and transcription of DNA do not influence Ras proteins. Increase in Ras-GTP level is caused by both free 8-oxo-G generated in the cell endogenously during DNA repair and the exogenous compound, and the minimal concentration of 8-oxo-G required for recording the effect on cell culture line MRC5 is 100 nM [90]. On intranasal injection into mice of 8-oxo-G solution, an effect can be recorded already at dose 5 ng/kg, which can be reached in vivo during excision repair of DNA [94]. Functioning of OGG1•8-oxo-G as a GEF has been demonstrated in vitro, in cellulo, and in animal models of some pulmonary diseases [92]. Small GTPases activated by 8-oxo-G include “canonic” Ras, such as Harvey sarcoma viral oncogene homolog (H-Ras), Kirsten sarcoma viral oncogene homolog (K-Ras), and neuroblastoma Ras viral oncogene homolog (N-Ras) [90, 91, 94], Rac1 [92], and RhoA (Ras homolog gene family, member A) [95]. Addition to cells of 8-oxo-dGuo decreases the level of Rac1-GTP in them [92], which is consistent with results of independent works [19, 38, 41, 88].
Fig. 2. Schematic description of proinflammatory effect of free 8-oxo-G produced by OGG1 during base excision repair (BER) in DNA. In DNA under conditions of oxidative stress, 8-oxo-G is generated, and it is released during repair by DNA glycosylase OGG1. The free 8-oxo-G enters the cytoplasm, where it binds to OGG1 with production of the OGG1•8-oxo-G complex, which functions as a GEF and activates small GTPases (Ras, Rho, Rac), stimulating in them GDP→GTP nucleotide exchange. Activated small GTPases induce the expression of proinflammatory genes, which provide triggering and realization of innate and acquired immunity reactions. Adapted and changed from [94].
The activation of Ras triggers the chain of intracellular signal transmission through cascades connected with kinase PI3K and mitogen stress-related kinase 1 (MSK1) and also through the three-component mitogen-activated protein kinase cascade through the successive phosphorylation of RAF1 kinases (virus-induced rapidly accelerated fibrosarcoma murine leukemia viral oncogene homolog 1), MEK1/2 kinases (MAPK/ERK kinase 1/2), ERK1/2 kinases (extracellular signal-regulated kinase 1/2) [90, 91, 94]. This results in the activation of IKK, kinase of the nuclear factor κB inhibitor (IκB); IκB is phosphorylated, ubiquitinylated, and cleaved in proteasomes, releasing homo- and heterodimers of the NF-κB proteins including RELA (p65), which are translocated into the cell nucleus. Transcriptional factors NF-κB are activated by Ras-GTP, and this activation leads to increase in the expression by cells of proinflammatory chemokines (of families C-C and C-X-C) and cytokines (IL-1β, TNF-α) and to accumulation of neutrophils in the tissue [94, 96].
Rac1-GTP is responsible for a transient local increase in intracellular generation of ROS (mainly H2O2) by membrane-bound NOX4. The depletion of Rac1 or NOX4 under the influence of siRNA leads to a significant decrease in the production of ROS, as compared to the control, after the addition of 8-oxo-G to the cells [92]. It is known that there is a causal relationship between increased level of ROS in the tissue and induction of an inflammatory reaction [97].
RhoA activation causes an increase in the synthesis of α-smooth muscle actin (α-SMA) and its polymerization with generation of “stress” fibers and increase in the fraction of insoluble α-SMA [95].
Thus, 8-oxo-G through small GTPases via the signaling cascades of Ras–kinases (MAPK, PI3K, MSK1)–NF-κB and Rac1–NOX4–ROS activates in organs and tissues the expression of proinflammatory mediators and reactions of innate immunity. It is thought that free 8-oxo-G constantly produced in the cell during the repair of oxidative damage of DNA acts as a secondary messenger that acts synergically with signaling processes induced by oxidative stress [98]. Activating Ras and subsequent signaling pathways, OGG1•8-oxo-G serves as a secondary source of ROS and thus amplifies the primary redox-signal and provides reliable triggering the nonspecific immunity reactions in response to external stimuli [99].
It should be noted that some small GTPases including Ras and Rho contain redox-sensitive motifs near the nucleotide-binding site, and this allows ROS or RNS to stimulate the nucleotide exchange reaction (GDP→GTP), acting like a GEF [100]. It seems that signaling and regulatory functions of ROS are partially mediated through small GTPases [94].
In endotoxin-treated mouse cells, OGG1 stimulated inflammatory response, acting as a coactivator of transcription factor STAT1 and demonstrating its own transcriptional activity, thus increasing the expression of proinflammatory mediators (COX-2 and iNOS) [101].
Ogg1−/− mice not expressing OGG1 display increased resistance to inflammation induced by various agents (oxidative stress, bacterial infection, LPS, allergens) and attenuated reactions of innate and acquired immunity [29, 94]. The weak immune reaction to LPS in these animals is associated with the decreased level of proinflammatory cytokines/chemokines in their blood plasma, notwithstanding the signs of acute oxidative stress in their tissues. On the contrary, Neil2−/− mice are very sensitive to inflammation induced by LPS, oxidative stress, and TNF-α, whereas exhaustion of the NEIL2 cell pool initiates nonspecific inflammation [102]. Thus, OGG1 displays a proinflammatory and NEIL2 an antiinflammatory effect, maintaining and balancing in tandem the inflammatory response of the cell to external pathogens.
Mechanisms of proinflammatory action of oxidized extracellular DNA. It seems that the immunostimulatory effects of oxidized DNA can be realized on its interaction with a receptor or sensor on the cell surface or within the cell. Membrane sensors of DNA include some of the Toll-like receptors (TLR) and cytoplasmic receptors comprise proteins RIG1, IFI16, AIM2, DAI, etc. [103]. It was shown that DNA fragments containing CpG-sequences can bind to receptor TLR9, which leads to activation of transcription factor NF-κB, synthesis of proinflammatory cytokines and chemokines, and generation of ROS [104, 105]. TLR9 is located in endolysosomes and triggers a cascade of inflammatory reactions, the process in which the adaptor protein MyD88 (myeloid differentiation primary-response protein 88) plays an important role [106]. A 20-meric oligodeoxynucleotide containing an 8-oxo-G residue increased approximately twofold the production of TNF-α and IL-6 induced by CpG-containing DNA fragments in primary cultures of macrophages obtained from the spleen of wild-type C57Bl/6 mice; this effect was completely absent in macrophages isolated from mice with knockout of the gene encoding TLR9 (TLR9–/–), which is evidence of direct participation of TLR9 in the reception of oxidized DNA [71]. Incubation for 3 h of HUVEC endotheliocytes with oxidized ecDNA enriched in CpG-dinucleotides (~10 kb, 50 ng/ml) increased several times the expression level of mRNA of protein TLR9 (the effect was suppressed by chloroquine, an inhibitor of TLR receptors) and of intracellular nucleic acid sensors AIM2 and RIG1 [107]. The cell reaction to oxidized DNA depends on the level of its oxidative modification; therefore, it is possible that the reception of oxidized DNA is realized by a set of sensors capable of binding oxidized DNA or by a specialized sensor that discriminates the level of DNA damage [67].
MtDNA contains unmethylated CpG-dinucleotides, and it was shown that in humans circulating mtDNA can, through TLR9, stimulate polymorphonuclear neutrophils, inducing their migration and degranulation and thus provoking systemic inflammation [108]. In mice, mtDNA through TLR9 activates in macrophages the signaling cascade of MAP kinase p38 and increases the production of proinflammatory cytokines IL-1β, IL-6, and TNF-α [109], and in cardiomyocytes mtDNA increases the level of mRNA of genes IL1b and IL6 [110]. The action of both native and oxidized mtDNA on TLR9 is confirmed by abolishment of their immunostimulatory effect on human leukocytes (pDC) in the presence of a specific competitive inhibitor of TLR9, the oligonucleotide TTAGGG [76].
The activation of inflammasomes is another mechanism for realizing the proinflammatory effect of cytoplasmic and extracellular DNA [111]. During apoptosis, oxidized mtDNA containing 8-oxo-G enters the cytoplasm, where it interacts with inflammasomes of the NLRP3 family (nucleotide binding domain and leucine-rich repeat pyrin domain containing 3), which are responsible for innate immunity reactions and proinflammatory response of phagocytes to the action of pathogens [75]. Oxidized mtDNA binds directly to receptor protein NLRP3, which results in activation of the protein complex of inflammasomes, including caspases 1 and 5, and in increase in production of interleukins IL-1β and IL-18 by the apoptotic cells. The DNA-sensor function of inflammasomes is realized without the involvement of TLRs [111].
The proinflammatory properties of the double-strand cytoplasmic (including mitochondrial) DNA can also be realized by its binding to 2′-3′-cyclic GMP-AMP synthase (cGAS), which leads to synthesis of cyclic dinucleotide guanosine monophosphate–adenosine monophosphate (cGAMP) and activates protein STING (stimulator of interferon genes) located on the endoplasmic reticulum, inducing the synthesis by the cell of first group interferons (type I IFN), in particular IFN-β [112, 113]. The cGAS–cGAMP–STING signaling cascade participates in the detection by mouse splenocytes of the 8-oxo-G-containing oxidized mtDNA from human neutrophils when it is injected parenterally into the animals [77].
MEDICAL POTENTIAL OF EXOGENOUS 8-oxo-dGuo, FREE 8-oxo-G, AND
OXIDIZED EXTRACELLULAR DNA
The above-described works showed that free 8-oxo-dGuo has high biological activity: it displays antioxidant, antiinflammatory, and antiallergic effects in vitro, in cellulo, and in vivo; therefore, it is a promising therapeutic agent for treatment of diseases whose pathogenesis involves oxidative stress and chronic inflammation, such as atherosclerosis, arthritis, osteoporosis, gastritis, bronchial asthma, diabetes mellitus, neurodegenerative diseases, malignant neoplasms, etc. It is necessary to administer the preparation from the outside because the rate of endogenous generation of 8-oxo-dGuo in the human body is low: it is reported [19] to be on average 0.55 µg/kg daily, which is insufficient for therapeutic effect. It seems that a small amount of 8-oxo-dGuo is produced in the human gastrointestinal tract during digestion of thermally treated food containing DNA, because guanine in DNA is oxidized under the action of heat [114].
Exogenous 8-oxo-dGuo is not mutagenic: it penetrates across cell membranes but does not enter the nucleus [19]; it is not rephosphorylated to 8-oxo-dGTP in the cell and is not incorporated into DNA [17, 18]. In the available literature, there is no value of LD50 for 8-oxo-dGuo, but some observations indicate low toxicity of this compound. Thus, 8-oxo-dGuo at concentrations up to 500 µg/ml had no cytotoxic action on the microglial BV2 line cells during coincubation for 72 h [41, 42]. Daily introduction of 8-oxo-dGuo (300 µg/ml) for 3 days into the incubation medium of mast cells isolated from mouse bone marrow did not increase the number of double-strand breaks in DNA, which was assessed by the level of expression of phosphorylated histone γ-H2AX [40]. Daily injections of 8-oxo-dGuo (330 mg/kg daily) for two weeks did not influence the weight of thymus-less Balb/c mice, whereas in mice injected with 6-TG in a lower dose (33 mg/kg daily), the body weight decreased by 35% [55]. No histological changes were observed in the tissues of lungs, liver, and intestine in mice injected with 8-oxo-dGuo in doses of 60 and 100 mg/kg daily [38, 39, 48].
Large-scale preclinical trials of 8-oxo-dGuo seem promising for determination of pharmacological properties and toxicological characteristics of this compound, its pharmacokinetics and metabolism, possible accumulation, development of tolerance and addiction, with the purpose of searching for possible advantages of the drug in comparison with presently used pharmaceutical agents.
Under normal conditions, the inflammatory reaction triggered by 8-oxo-G (OGG1•8-oxo-G) can be a component of the organism’s physiological response to deleterious agents and the organism’s defense against external pathogens. However, if the external influence is added to already existing pathological conditions (immunosuppression, allergy, aging) when inflammatory processes are not controlled in due manner, the proinflammatory signaling initiated by 8-oxo-G can lead to aggravation of existing diseases. Neutrophils recruited by chemokines/cytokines into the inflammation focus generate ROS/RNS, which induce lesions in DNA, and the repair of the DNA might form a vicious circle supporting chronic inflammation: OGG1•8-oxo-G → Ras-GTP → proinflammatory signaling → chemokines/cytokines → ROS/RNS → 8-oxo-G [56]. It is possible that the signaling cascade activated by 8-oxo-G can be connected etiologically with somatic inflammatory diseases. Thus, it can be concluded that an important therapeutic target for prophylaxis and treatment of such diseases might be modulation of: (i) OGG1 activity [115]; (ii) basal level of 8-oxo-G in DNA; (iii) GEF-like activity of the OGG1•8-oxo-G complex. The age-related decrease in OGG1 activity in tissues is presumably a manifestation of cellular defense against exacerbation of pathophysiological inflammatory processes [98]. The strategy of searching for the manner of switching 8-oxo-G repair from OGG1 onto NEIL1/2 seems promising, because these enzymes do not interact physically with Ras GTPases [90], and NEIL2 displays in mice an antiinflammatory effect [102].
The high biological activity of ecDNA molecules is determined by the presence in them of unmethylated CpG-dinucleotides and 8-oxo-dGuo. DNA containing CpG belongs to so-called “pathogen-associated molecular patterns” (PAMPs), which signal about an infectious invasion and initiate the inflammatory and immune response of the organism [116]. Oxidized DNA seems to belong to the related group of “damage-associated molecular patterns” (DAMPs), which consists of endogenous molecules signaling about any deleterious influence [117]. Oxidized DNA can induce inflammatory reactions in vitro and in vivo [73] as it is and can also act in synergy with CpG-containing DNA, increasing the immunostimulatory features of the latter [71]. The biological action of 8-oxo-G-containing ecDNA on different types of cells is different and depends on the level of its oxidation [67], but in general its action resembles the action of ionizing radiation: low doses of ecDNA stimulate the biosynthesis of ROS and appearance of breaks in DNA, inhibit cell division along with formation of adaptive response – the activation of transcriptional factor NRF2, increase in expression of the genes SOD1 and BCL2, suppression of NF-κB activity, and increase in stress resistance of cells; high doses of oxidized ecDNA are toxic for cells [65]. In clinical practice, it would be useful to take in consideration the role of CpG-containing oxidized ecDNA as a mediator of stress-signalization during the development of the adaptive response of cells to deleterious factors. Since it has been shown that tumor cells are more sensitive to the action of oxidized DNA than normal cells and their adaptive response to radio- and chemotherapy is pronounced [68], it is obvious that oxidized DNA released from tumor cells killed by therapy will stimulate intact malignant cells and increase their survival. On the other hand, an increase in the stress resistance of cells by low doses of oxidized DNA can be favorable for cultivation of stem and differentiated cells aimed for cellular therapy and tissue engineering [65]. Considering that the effect of oxidized ecDNA on tissues is similar to the “bystander effect”, it might be used for treatment of malignancies in combination with ionizing radiation (or instead of it) for reducing the radiation load on the patient’s organism without decreasing the treatment effect [67]. Normally, ecDNA is eliminated from the blood circulation by blood endonucleases and is removed from the organism as immune complexes with antibodies specific to CpG-DNA [118]. In many diseases, there is a pathologically high level of ecDNA in blood, and elimination of its excess would be useful. Approaches for stimulating the mechanism of ecDNA elimination from the organism are needed, especially those approaches that can increase the activity of endonucleases by injection of DNase I preparations into the blood flow and change the expression levels of endonucleases or of their protein inhibitors. Long-term periodic exposure to low doses of ionizing radiation is accompanied by a steady increase in the activities of blood endonucleases [119], which suggests the possibility of modulating the activities of endogenous DNases, in particular by external physical agents. It seems that artificial manipulation of ecDNA oxidation degree, its concentration, and the content in it of CpG-dinucleotides will allow us to positively influence the clinical course and outcome of chronic diseases that are accompanied by massive death of cells and DNA release from them [117].
Due to its low redox-potential, G within DNA is easily oxidized, producing several derivatives with 8-oxo-G as the major product. The genotoxicity of 8-oxo-G and mechanisms realizing its mutagenic potential in the cell are well-studied, but only recently a more complete picture of biological effects of 8-oxo-G formation in DNA appears. It became clear that 8-oxo-G cannot be considered only as undesired byproduct of oxidative metabolism, but it is necessary to consider its participation in the provision of important physiological processes in cells: chromatin relaxation, initiation of transcription, regulation of gene expression, photorepair of pyrimidine dimers, etc. [25, 26, 120, 121].
It has been experimentally demonstrated in recent years that in cells free 8-oxo-G and 8-oxo-G-containing molecules are not simply inert products of DNA repair or degradation, but are active participants of intracellular signalization capable of both initiating and inhibiting such processes. Thus, free 8-oxo-G and oxidized ecDNA act as alarmins and mediators of stress signalization in the cell, triggering and potentiating inflammatory and immune reactions, whereas 8-oxo-dGuo displays pronounced antiinflammatory and antioxidant properties, possibly balancing the effects of 8-oxo-G. Oxidized guanine and molecules containing it could be an important therapeutic target in the treatment of inflammatory, allergic, and autoimmune diseases whose pathogenesis is significantly influenced by oxidative stress.
Acknowledgments
This work was supported by the Russian Foundation for Basic Research (project No. 17-44-500476-p_a).
REFERENCES
1.Men’shchikova, E. B., Lankin, V. Z., Zenkov,
N. K., Bondar, I. A., Krugovykh, N. F., and Trufakin, V. A. (2006)
Oxidative Stress. Prooxidants and Antioxidants [in Russian],
Slovo, Moscow, pp. 136-140.
2.Nishimura, S. (2011) 8-Hydroxyguanine: a base for
discovery, DNA Repair, 10, 1078-1083.
3.Kasai, H., and Nishimura, S. (1984) Hydroxylation
of deoxyguanosine at the C-8 position by ascorbic acid and other
reducing agents, Nucleic Acids Res., 12, 2137-2145.
4.Steenken, S., and Jovanovic, S. V. (1997) How
easily oxidizable is DNA? One-electron reduction potentials of
adenosine and guanosine radicals in aqueous solution, J. Am. Chem.
Soc., 119, 617-618.
5.Burrows, C. J., and Muller, J. G. (1998) Oxidative
nucleobase modifications leading to strand scission, Chem.
Rev., 98, 1109-1152.
6.Luo, W., Muller, J. G., Rachlin, E. M., and
Burrows, C. J. (2001) Characterization of hydantoin products from
one-electron oxidation of 8-oxo-7,8-dihydroguanosine in a nucleoside
model, Chem. Res. Toxicol., 14, 927-938.
7.Cooke, M. S., Evans, M. D., Dizdaroglu, M., and
Lunec, J. (2003) Oxidative DNA damage: mechanisms, mutation, and
disease, FASEB J., 17, 1195-1214.
8.Kasai, H. (1997) Analysis of a form of oxidative
DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular
oxidative stress during carcinogenesis, Mutat. Res., 387,
147-163.
9.Gedik, C. M., and Collins, A.; ESCODD (2005)
Establishing the background level of base oxidation in human lymphocyte
DNA: results of an interlaboratory validation study, FASEB J.,
19, 82-84.
10.Nakamoto, H., Kaneko, T., Tahara, S., Hayashi,
E., Naito, H., Radak, Z., and Goto, S. (2007) Regular exercise reduces
8-oxo-dG in the nuclear and mitochondrial DNA and modulates the DNA
repair activity in the liver of old rats, Exp. Gerontol.,
42, 287-295.
11.Lindahl, T. (1993) Instability and decay of the
primary structure of DNA, Nature, 362, 709-715.
12.Grollman, A. P., and Moriya, M. (1993)
Mutagenesis by 8-oxoguanine: an enemy within, Trends Genet.,
9, 246-249.
13.Chernikov, A. V., Usacheva, A. M., and Bruskov,
V. I. (1996) Depurination of 8-oxo-7,8-dihydroguanine
(8-hydroxyguanine) nucleosides, Biochemistry (Moscow),
61, 35-38.
14.Evans, M. D., Mistry, V., Singh, R., Gackowski,
D., Rozalski, R., Siomek-Gorecka, A., Phillips, D. H., Zuo, J.,
Mullenders, L., Pines, A., Nakabeppu, Y., Sakumi, K., Sekiguchi, M.,
Tsuzuki, T., Bignami, M., Olinski, R., and Cooke, M. S. (2016)
Nucleotide excision repair of oxidized genomic DNA is not a source of
urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine, Free Radic. Biol.
Med., 99, 385-391.
15.Shinmura, K., Yamaguchi, S., Saitoh, T.,
Takeuchi-Sasaki, M., Kim, S. R., Nohmi, T., and Yokota, J. (2000)
Adenine excisional repair function of MYH protein on the
adenine:8-hydroxyguanine base pair in double-stranded DNA, Nucleic
Acids Res., 28, 4912-4918.
16.Cooke, M. S., Evans, M. D., Dove, R., Rozalski,
R., Gackowski, D., Siomek, A., Lunec, J., and Olinski, R. (2005) DNA
repair is responsible for the presence of oxidatively damaged DNA
lesions in urine, Mutat. Res., 574, 58-66.
17.Kim, J. E., Hyun, J. W., Hayakawa, H., Choi, S.,
and Chung, M. H. (2006) Exogenous 8-oxo-dG is not utilized for
nucleotide synthesis but enhances the accumulation of 8-oxo-Gua in DNA
through error-prone DNA synthesis, Mutat. Res., 596,
128-136.
18.Kim, J. E., and Chung, M. H. (2006)
8-Oxo-7,8-dihydro-2′-deoxyguanosine is not salvaged for DNA
synthesis in human leukemic U937 cells, Free Radic. Res.,
40, 461-466.
19.Choi, S., Choi, H.-H., Lee, S.-H., Ko, S.-H.,
You, H.-J., Ye, S.-K., and Chung, M.-H. (2007) Anti-inflammatory
effects of 8-hydroxy-2′-deoxyguanosine on
lipopolysaccharide-induced inflammation via Rac suppression in Balb/c
mice, Free Radic. Biol. Med., 43, 1594-1603.
20.Hu, C. W., Cooke, M. S., Tsai, Y. H., and Chao,
M. R. (2015) 8-Oxo-7,8-dihydroguanine and
8-oxo-7,8-dihydro-2′-deoxyguanosine concentrations in various
human body fluids: implications for their measurement and
interpretation, Arch. Toxicol., 89, 201-210.
21.Hyun, J. W., Jung, Y. C., Kim, H. S., Choi, E.
Y., Kim, J. E., Yoon, B. H., Yoon, S. H., Lee, Y. S., Choi, J., You, H.
J., and Chung, M. H. (2003) 8-Hydroxydeoxyguanosine causes death of
human leukemia cells deficient in 8-oxoguanine glycosylase 1
activity by inducing apoptosis, Mol. Cancer Res., 1,
290-299.
22.Lu, A. L., Li, X., Gu, Y., Wright, P. M., and
Chang, D. Y. (2001) Repair of oxidative DNA damage: mechanisms and
functions, Cell Biochem. Biophys., 35, 141-170.
23.Dizdaroglu, M. (2012) Oxidatively induced DNA
damage: mechanisms, repair and disease, Cancer Lett.,
327, 26-47.
24.Cadet, J., Davies, K. J. A., Medeiros, M. H., Di
Mascio, P., and Wagner, J. R. (2017) Formation and repair of
oxidatively generated damage in cellular DNA, Free Radic. Biol.
Med., 107, 13-34.
25.Radak, Z., and Boldogh, I. (2010)
8-Oxo-7,8-dihydroguanine: links to gene expression, aging, and defense
against oxidative stress, Free Radic. Biol. Med., 49,
587-596.
26.Marmii, N. V., and Esipov, D. S. (2015)
Biological role of 8-oxo-2′-deoxyguanosine, Vestnik Mosk.
Univ. Ser. Biol., 4, 19-23.
27.Kong, Q., and Lin, C. L. (2010) Oxidative damage
to RNA: mechanisms, consequences, and diseases, Cell. Mol. Life
Sci., 67, 1817-1829.
28.Stuart, J. A., Bourque, B. M., De Souza-Pinto, N.
C., and Bohr, V. A. (2005) No evidence of mitochondrial respiratory
dysfunction in OGG1-null mice deficient in removal of
8-oxodeoxyguanine from mitochondrial DNA, Free Radic. Biol.
Med., 38, 737-745.
29.Mabley, J. G., Pacher, P., Deb, A., Wallace, R.,
Elder, R. H., and Szabo, C. (2005) Potential role for 8-oxoguanine DNA
glycosylase in regulating inflammation, FASEB J.,
19, 290-292.
30.Perillo, B., Ombra, M. N., Bertoni, A., Cuozzo,
C., Sacchetti, S., Sasso, A., Chiariotti, L., Malorni, A., Abbondanza,
C., and Avvedimento, E. V. (2008) DNA oxidation as triggered by H3K9me2
demethylation drives estrogen-induced gene expression, Science,
319, 202-206.
31.Kuraoka, I., Suzuki, K., Ito, S., Hayashida, M.,
Kwei, J. S., Ikegami, T., Handa, H., Nakabeppu, Y., and Tanaka, K.
(2007) RNA polymerase II bypasses 8-oxoguanine in the presence of
transcription elongation factor TFIIS, DNA Repair, 6,
841-851.
32.Kim, J.-E., Choi, S., Yoo, J.-A., and Chung,
M.-H. (2004) 8-Oxoguanine induces intramolecular DNA damage but free
8-oxoguanine protects intermolecular DNA from oxidative stress, FEBS
Lett., 556, 104-110.
33.Ock, C. Y., Hong, K. S., Choi, K.-S., Chung,
M.-H., Kim, Y. S., Kim, J. H., and Hahm, K.-B. (2011) A novel approach
for stress-induced gastritis based on paradoxical anti-oxidative and
anti-inflammatory action of exogenous 8-hydroxydeoxyguanosine,
Biochem. Pharmacol., 81, 111-122.
34.Lee, J.-K., Ko, S.-H., Ye, S.-K., and Chung,
M.-H. (2013) 8-Oxo-2′-deoxyguanosine ameliorates UVB-induced skin
damage in hairless mice by scavenging reactive oxygen species and
inhibiting MMP expression, J. Dermatol. Sci., 70,
49-57.
35.Gudkov, S. V., Shtarkman, I. N., Smirnova, V. S.,
Chernikov, A. V., and Bruskov, V. I. (2006) Guanosine and inosine
display antioxidant activity, protect DNA in vitro from
oxidative damage induced by reactive oxygen species, and serve as
radioprotectors in mice, Radiat. Res., 165, 538-545.
36.Kim, D., Quan, Y., Park, B., Chung, M., and Ro,
J. (2009) Inhibitory effects of 8-oxo-dG on the cellular damage in the
mouse whole body irradiation, Allergy, 64, Suppl. s90,
11.
37.Geronikaki, A. A., and Gavalas, A. M. (2006)
Antioxidants and inflammatory disease: synthetic and natural
antioxidants with anti-inflammatory activity, Comb. Chem. High
Throughput Screen., 9, 425-442.
38.Ro, J. Y., Kim, D. Y., Lee, S.-H., Park, J. W.,
and Chung, M.-H. (2009) Effects of 7,8-dihydro-8-oxo-deoxyguanosine on
antigen challenge in ovalbumin-sensitized mice may be mediated by
suppression of Rac, Br. J. Pharmacol., 158,
1743-1752.
39.Kim, J.-S., Kim, D.-Y., Lee, J.-K., Ro, J.-Y.,
and Chung, M.-H. (2011) 8-Oxo-2′-deoxyguanosine suppresses
allergy-induced lung tissue remodeling in mice, Eur. J.
Pharmacol., 651, 218-226.
40.Hong, G. U., Kim, N. G., and Ro, J. Y. (2014)
Expression of airway remodeling proteins in mast cell activated by
TGF-β released in OVA-induced allergic responses and their
inhibition by low-dose irradiation or 8-oxo-dG, Radiat. Res.,
181, 425-438.
41.Kim, H. S., Ye, S.-K., Cho, I. H., Jung, J. E.,
Kim, D.-H., Choi, S., Kim, Y.-S., Park, C.-G., Kim, T.-Y., Lee, J. W.,
and Chung, M.-H. (2006) 8-Hydroxydeoxyguanosine suppresses NO
production and COX-2 activity via Rac1/STATs signaling in LPS-induced
brain microglia, Free Radic. Biol. Med., 41,
1392-1403.
42.Kim, D. H., Cho, I. H., Kim, H. S., Jung, J. E.,
Kim, J. E., Lee, K. H., Park, T., Yang, Y. M., Seong, S.-Y., Ye, S.-K.,
and Chung, M.-H. (2006) Anti-inflammatory effects of
8-hydroxydeoxyguanosine in LPS-induced microglia activation:
suppression of STAT3-mediated intercellular adhesion molecule-1
expression, Exp. Mol. Med., 38, 417-427.
43.Hong, G. U., Kim, N. G., Jeoung, D., and Ro, J.
Y. (2013) Anti-CD40 Ab- or 8-oxo-dG-enhanced Treg cells reduce
development of experimental autoimmune encephalomyelitis via
down-regulating migration and activation of mast cells, J.
Neuroimmunol., 260, 60-73.
44.Clempus, R. E., and Griendling, K. K. (2006)
Reactive oxygen species signaling in vascular smooth muscle cells,
Cardiovasc. Res., 71, 216-225.
45.Martinet, W., Knaapen, M. W., De Meyer, G. R.,
Herman, A. G., and Kockx, M. M. (2002) Elevated levels of oxidative DNA
damage and DNA repair enzymes in human atherosclerotic plaques,
Circulation, 106, 927-932.
46.Huh, J. Y., Son, D. J., Lee, Y., Lee, J., Kim,
B., Lee, H. M., Jo, H., Choi, S., Ha, H., and Chung, M.-H. (2012)
8-Hydroxy-2-deoxyguanosine prevents plaque formation and inhibits
vascular smooth muscle cell activation through Rac1 inactivation,
Free Radic. Biol. Med., 53, 109-121.
47.Ock, C.-Y., Kim, E.-H., Choi, D. J., Lee, H. J.,
Hahm, K.-B., and Chung, M. H. (2012) 8-Hydroxydeoxyguanosine: not mere
biomarker for oxidative stress, but remedy for oxidative
stress-implicated gastrointestinal diseases, World J.
Gastroenterol., 18, 302-308.
48.Ock, C.-Y., Kim, E.-H., Hong, H., Hong, K. S.,
Han, Y.-M., Choi, K.-S., Hahm, K.-B., and Chung, M.-H. (2011)
Prevention of colitis-associated colorectal cancer with
8-hydroxydeoxyguanosine, Cancer Prev. Res., 4,
1507-1521.
49.Hanson, R. L., Imperatore, G., Bennett, P. H.,
and Knowler, W. C. (2002) Components of the “metabolic
syndrome” and incidence of type 2 diabetes, Diabetes,
51, 3120-3127.
50.Ko, S.-H., Lee, J.-K., Lee, H. J., Ye, S.-K.,
Kim, H.-S., and Chung, M.-H. (2014) 8-Oxo-2′-deoxyguanosine
ameliorates features of metabolic syndrome in obese mice, Biochem.
Biophys. Res. Commun., 443, 610-616.
51.Grivennikov, S. I., Greten, F. R., and Karin, M.
(2010) Immunity, inflammation, and cancer, Cell,
140, 883-899.
52.Hyun, J.-W., Jung, Y.-Ch., Kim, H.-S., Choi,
E.-Y., Kim, J.-E., Yoon, B.-H., Yoon, S.-H., Lee, Y.-S., Choi, J., You,
H.-J., and Chung, M.-H. (2003) 8-Hydroxydeoxyguanosine causes death of
human leukemia cells deficient in 8-oxoguanine glycosylase 1 activity
by inducing apoptosis, Mol. Cancer Res., 1, 290-299.
53.Hyun, J.-W., Choi, J.-Y., Zeng, H.-H., Lee,
Y.-S., Kim, H.-S., Yoon, S.-H., and Chung, M.-H. (2000) Leukemic cell
line KG-1 has a functional loss of hOGG1 enzyme due to a point mutation
and 8-hydroxydeoxyguanosine can kill KG-1, Oncogene, 19,
4476-4479.
54.Hyun, J. W., Yoon, S. H., Yu, Y., Han, C. S.,
Park, J. S., Kim, H. S., Lee, S. J., Lee, Y. S., You, H. J., and Chung,
M. H. (2006) oh8dG induces G1 arrest in a human
acute leukemia cell line by upregulating P21 and blocking the RAS to
ERK signaling pathway, Int. J. Cancer, 118, 302-309.
55.Choi, S., Choi, H. H., Choi, J.-H., Yoon, B.-H.,
You, H. J., Hyun, J.-W., Kim, J.-E., Ye, S.-K., and Chung, M.-H. (2006)
Inhibitory effect of 8-oxo-7,8-dihydro-2′-deoxyguanosine on the
growth of KG-1 myelosarcoma in Balb/c nude mice, Leuk. Res.,
30, 1425-1436.
56.Ba, X., Aguilera-Aguirre, L., Rashid, Q. T.,
Bacsi, A., Radak, Z., Sur, S., Hosoki, K., Hegde, M. L., and Boldogh,
I. (2014) The role of 8-oxoguanine DNA glycosylase-1 in inflammation,
Int. J. Mol. Sci., 15, 16975-16997.
57.Aguilera-Aguirre, L., Hosoki, K., Bacsi, A.,
Radak, Z., Wood, T. G., Widen, S. G., Sur, S., Ameredes, B. T.,
Saavedra-Molina, A., Brasier, A. R., Ba, X., and Boldogh, I. (2015)
Whole transcriptome analysis reveals an 8-oxoguanine DNA
glycosylase-1-driven DNA repair-dependent gene expression linked to
essential biological processes, Free Radic. Biol. Med.,
81, 107-118.
58.Aguilera-Aguirre, L., Hosoki, K., Bacsi, A.,
Radak, Z., Sur, S., Hegde, M. L., Tian, B., Saavedra-Molina, A.,
Brasier, A. R., Ba, X., and Boldogh, I. (2015) Whole transcriptome
analysis reveals a role for OGG1-initiated DNA repair signaling in
airway remodeling, Free Radic. Biol. Med., 89, 20-33.
59.Marmii, N. V., Morgunova, G. V., Esipov, D. S.,
and Khokhlov, A. N. (2016) 8-Oxo-2′-deoxyguanosine: biomarker of
cell aging and oxidative stress or a potential drug against age-related
diseases? Klin. Gerontol., 22, 46-47.
60.German, P., Saenz, D., Szaniszlo, P.,
Aguilera-Aguirre, L., Pan, L., Hegde, M. L., Bacsi, A., Hajas, G.,
Radak, Z., Ba, X., Mitra, S., Papaconstantinou, J., and Boldogh, I.
(2017) 8-Oxoguanine DNA glycosylase 1-driven DNA repair – a
paradoxical role in lung aging, Mech. Ageing Dev., 161,
51-65.
61.Gahan, P. B., and Swaminathan, R. (2008)
Circulating nucleic acids in plasma and serum, Ann. N. Y. Acad.
Sci., 1137, 1-6.
62.Vlassov, V. V., Laktionov, P. P., and Rykova, E.
Y. (2007) Extracellular nucleic acids, BioEssays, 29,
654-667.
63.Vasil’eva, I. N., Podgornaya, O. I., and
Bespalov, V. G. (2015) Nucleosomal fraction of extracellular DNA as a
marker of apoptosis, Tsitologiya, 57, 87-94.
64.Kostyuk, S., Tabakov, V. J., Chestkov, V. V.,
Konkova, M. S., Glebova, K. V., Baydakova, G. V., Ershova, E. S.,
Izhevskaya, V. L., Baranova, A., and Veiko, N. N. (2013) Oxidized DNA
induces an adaptive response in human fibroblasts, Mutat.
Res., 747-748, 6-18.
65.Glebova, K., Veiko, N., Kostyuk, S., Izhevskaya,
V., and Baranova, A. (2015) Oxidized extracellular DNA as a stress
signal that may modify response to anticancer therapy, Cancer
Lett., 356, 22-33.
66.Loseva, P., Kostyuk, S., Malinovskaya, E.,
Clement, N., Dechesne, C. A., Dani, C., Smirnova, T., Glebova, K.,
Baidakova, G., Baranova, A., Izhevskaia, V., Ginter, E., and Veiko, N.
(2012) Extracellular DNA oxidation stimulates activation of Nrf2 and
reduces the production of ROS in human mesenchymal stem cells,
Expert Opin. Biol. Ther., 12, Suppl. 1, S85-S97.
67.Kostyuk, S. V., Ermakov, A. V., Alekseeva, A. Y.,
Smirnova, T. D., Glebova, K. V., Efremova, L. V., Baranova, A., and
Veiko, N. N. (2012) Role of extracellular DNA oxidative
modification in radiation induced bystander effects in human
endotheliocytes, Mutat. Res., 729, 52-60.
68.Kostyuk, S. V., Konkova, M. S., Ershova, E. S.,
Alekseeva, A. J., Smirnova, T. D., Stukalov, S. V., Kozhina, E. A.,
Shilova, N. V., Zolotukhina, T. V., Markova, Z. G., Izhevskaya, V. L.,
Baranova, A., and Veiko, N. N. (2013) An exposure to the oxidized DNA
enhances both instability of genome and survival in cancer cells,
PLoS One, 8, e77469.
69.Ermakov, A. V., Konkova, M. S., Kostyuk, S. V.,
Smirnova, T. D., Malinovskaya, E. M., Efremova, L. V., and Veiko, N. N.
(2011) An extracellular DNA mediated bystander effect produced from low
dose irradiated endothelial cells, Mutat. Res., 712,
1-10.
70.Kostyuk, S. V., Alekseeva, A. Y., Konkova, M. S.,
Glebova, K. V., Smirnova, T. D., Kameneva, L. A., Izhevskaya, V. L.,
and Veiko, N. N. (2014) Oxidized extracellular DNA decreases the
production of nitric oxide by endothelial NO-synthase (eNOS) in human
endothelial cells (HUVEC), Bull. Eksp. Biol. Med., 157,
163-168.
71.Yoshida, H., Nishikawa, M., Kiyota, T., Toyota,
H., and Takakura, Y. (2011) Increase in CpG DNA-induced
inflammatory responses by DNA oxidation in macrophages and mice,
Free Radic. Biol. Med., 51, 424-431.
72.Jurk, M., Kritzler, A., Debelak, H., Vollmer, J.,
Krieg, A. M., and Uhlmann, E. (2006) Structure–activity
relationship studies on the immune stimulatory effects of base-modified
CpG Toll-like receptor 9 agonists, ChemMedChem, 1,
1007-1014.
73.Collins, L. V., Hajizadeh, S., Holme, E.,
Jonsson, I.-M., and Tarkowski, A. (2004) Endogenously oxidized
mitochondrial DNA induces in vivo and in vitro
inflammatory responses, J. Leukoc. Biol., 75,
995-1000.
74.Hudson, E. K., Hogue, B. A., Souza-Pinto, N. C.,
Croteau, D. L., Anson, R. M., Bohr, V. A., and Hansford, R. G. (1998)
Age-associated change in mitochondrial DNA damage, Free Radic.
Res., 29, 573-579.
75.Shimada, K., Crother, T. R., Karlin, J.,
Dagvadorj, J., Chiba, N., Chen, S., Ramanujan, V. K., Wolf, A. J.,
Vergnes, L., Ojcius, D. M., Rentsendorj, A., Vargas, M., Guerrero, C.,
Wang, Y., Fitzgerald, K. A., Underhill, D. M., Town, T., and Arditi, M.
(2012) Oxidized mitochondrial DNA activates the NLRP3
inflammasome during apoptosis, Immunity, 36,
401-414.
76.Pazmandi, K., Agod, Z., Kumar, B. V., Szabo, A.,
Fekete, T., Sogor, V., Veres, A., Boldogh, I., Rajnavolgyi, E., Lanyi,
A., and Bacsi, A. (2014) Oxidative modification enhances the
immunostimulatory effects of extracellular mitochondrial DNA on
plasmacytoid dendritic cells, Free Radic. Biol. Med., 77,
281-290.
77.Lood, C., Blanco, L. P., Purmalek, M. M.,
Garmona-Rivera, C., De Ravin, S. S., Smith, C. K., Malech, H. L.,
Ledbetter, J. A., Elkon, K. B., and Kaplan, M. J. (2016) Neutrophil
extracellular traps enriched in oxidized mitochondrial DNA are
interferogenic and contribute to lupus-like disease, Nat. Med.,
22, 146-153.
78.Prikhodko, A. S., Shabanov, A. K., Zinovkina, L.
A., Popova, E. N., Aznauryan, M. A., Lanina, N. O., Vitushkina, M. V.,
and Zinovkin, R. A. (2015) Pure mitochondrial DNA does not activate
human neutrophils in vitro, Biochemistry (Moscow), 80, 629-635.
79.Hajizadeh, S., DeGroot, J., TeKoppele, J. M.,
Tarkowski, A., and Collins, L. V. (2003) Extracellular mitochondrial
DNA and oxidatively damaged DNA in synovial fluid of patients with
rheumatoid arthritis, Arthritis Res. Ther., 5,
R234-R240.
80.Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S.
P., and Malik, A. B. (2014) Reactive oxygen species in
inflammation and tissue injury, Antioxid. Redox Signal.,
20, 1126-1167.
81.Finkel, T. (2003) Oxidant signals and oxidative
stress, Curr. Opin. Cell Biol., 15, 247-254.
82.Gupta, S., Campbell, D., Derijard, B., and Davis,
R. J. (1995) Transcription factor ATF2 regulation by the JNK signal
transduction pathway, Science, 267, 389-393.
83.Bustelo, X. R., Sauzeau, V., and Berenjeno, I. M.
(2007) GTP-binding proteins of the Rho/Rac family: regulation,
effectors and functions in vivo, BioEssays, 29,
356-370.
84.Bishop, A. L., and Hall, A. (2000) Rho GTPases
and their effector proteins, Biochem. J., 348, Pt. 2,
241-255.
85.Turkson, J., Bowman, J., Adnane, J., Zhang, Y.,
Djeu, J. Y., Sekharam, M., Frank, D. A., Holzman, L. B., Wu, J., Sebti,
S., and Jove, R. (1999) Requirement for Ras/Rac1-mediated p38 and c-Jun
N-terminal kinase signaling in Stat3 transcriptional activity induced
by the Src oncoprotein, Mol. Cell. Biol., 19,
7519-7528.
86.Simon, A. R., Vikis, H. G., Stewart, S., Fanburg,
B. L., Cochran, B. H., and Guan, K.-L. (2000) Regulation of STAT3 by
direct binding to the Rac1 GTPase, Science, 290,
144-147.
87.Levy, D. E., and Lee, C. (2002) What does STAT3
do? J. Clin. Invest., 109, 1143-1148.
88.Lee, S. H., Han, S. T., Choi, S. W., Sung, S. Y.,
You, H. J., Ye, S. K., and Chung, M. H. (2009) Inhibition of Rac and
Rac-linked functions by 8-oxo-2′-deoxyguanosine in murine
macrophages, Free Radic. Res., 43, 78-84.
89.Yoon, S. H., Hyun, J. W., Choi, J., Choi, E. Y.,
Kim, H. J., Lee, S. J., and Chung, M. H. (2005) In vitro
evidence for the recognition of 8-oxoGTP by Ras, a small GTP-binding
protein, Biochem. Biophys. Res. Commun., 327,
342-348.
90.Boldogh, I., Hajas, G., Aguilera-Aguirre, L.,
Hegde, M. L., Radak, Z., Bacsi, A., Sur, S., Hazra, T. K., and Mitra,
S. (2012) Activation of Ras signaling pathway by 8-oxoguanine DNA
glycosylase bound to its excision product, 8-oxoguanine, J. Biol.
Chem., 287, 20769-20773.
91.German, P., Szaniszlo, P., Hajas, G., Radak, Z.,
Bacsi, A., Hazra, T. K., Hegde, M. L., Ba, X., and Boldogh, I. (2013)
Activation of cellular signaling by 8-oxoguanine DNA
glycosylase-1-initiated DNA base excision repair, DNA Repair,
12, 856-863.
92.Hajas, G., Bacsi, A., Aguilera-Aguirre, L.,
Hegde, M. L., Hazra, T. K., Sur, S., Radak, Z., Ba, X., and Boldogh, I.
(2013) 8-Oxoguanine DNA glycosylase-1 links DNA repair to cellular
signaling via the activation of the small GTPase Rac1, Free Radic.
Biol. Med., 61, 384-394.
93.Fromme, J. C., Bruner, S. D., Yang, W., Karplus,
M., and Verdine, G. L. (2003) Product-assisted catalysis in
base-excision DNA repair, Nat. Struct. Biol., 10,
204-211.
94.Aguilera-Aguirre, L., Bacsi, A., Radak, Z.,
Hazra, T. K., Mitra, S., Sur, S., Brasier, A. R., Ba, X., and Boldogh,
I. (2014) Innate inflammation induced by the 8-oxoguanine DNA
glycosylase-1-KRAS-NF-κB pathway, J. Immunol., 193,
4643-4653.
95.Luo, J., Hosoki, K., Bacsi, A., Radak, Z., Hegde,
M. L., Sur, S., Hazra, T. K., Brasier, A. R., Ba, X., and Boldogh, I.
(2014) 8-Oxoguanine DNA glycosylase-1-mediated DNA repair is associated
with Rho GTPase activation and α-smooth muscle actin
polymerization, Free Radic. Biol. Med., 73, 430-438.
96.Ba, X., Aguilera-Aguirre, L., Sur, S., and
Boldogh, I. (2015) 8-Oxoguanine DNA glycosylase-1-driven DNA base
excision repair: role in asthma pathogenesis, Curr. Opin. Allergy
Clin. Immunol., 15, 89-97.
97.Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S.
P., and Malik, A. B. (2014) Reactive oxygen species in
inflammation and tissue injury, Antioxid. Redox Signal.,
20, 1126-1167.
98.Pandita, T. K. (2014) Unraveling the novel
function of the DNA repair enzyme 8-oxoguanine-DNA glycosylase in
activating key signaling pathways, Free Radic. Biol. Med.,
73, 439-440.
99.Choudhary, S., Boldogh, I., and Brasier, A. R.
(2016) Inside-out signaling pathways from nuclear reactive oxygen
species control pulmonary innate immunity, J. Innate Immun.,
8, 143-155.
100.Mitchell, L., Hobbs, G. A., Aghajanian, A., and
Campbell, S. L. (2013) Redox regulation of Ras and Rho GTPases:
mechanism and function, Antioxid. Redox Signal., 18,
250-258.
101.Kim, H. S., Kim, B.-H., Jung, J. E., Lee, C.
S., Lee, H. G., Lee, J. W., Lee, K. H., You, H. J., Chung, M.-H., and
Ye, S.-K. (2016) Potential role of 8-oxoguanine DNA glycosylase 1 as a
STAT1 coactivator in endotoxin-induced inflammatory response,
Free Radic. Biol. Med., 93, 12-22.
102.Chakraborty, A., Wakamiya, M., Venkova-Canova,
T., Pandita, R. K., Aguilera-Aguirre, L., Sarker, A. H., Singh, D. K.,
Hosoki, K., Wood, T. G., Sharma, G., Cardenas, V., Sarkar, P. S., Sur,
S., Pandita, T. K., Boldogh, I., and Hazra, T. K. (2015) Neil2-null
mice accumulate oxidized DNA bases in the transcriptionally active
sequences of the genome and are susceptible to innate inflammation,
J. Biol. Chem., 290, 24636-24648.
103.Wagner, H., and Bauer, S. (2006) All is not
Toll: new pathways in DNA recognition, J. Exp. Med., 203,
265-268.
104.Hartmann, G., and Krieg, A. M. (2000) Mechanism
and function of a newly identified CpG DNA motif in human primary
B cells, J. Immunol., 164, 944-953.
105.Barber, G. N. (2011) Cytoplasmic DNA innate
immune pathways, Immunol. Rev., 243, 99-108.
106.Blasius, A. L., and Beutler, B. (2010)
Intracellular toll-like receptors, Immunity, 32,
305-315.
107.Alekseeva, A. Y., Kameneva, L. V., Kostyuk, S.
V., and Veiko, N. N. (2016) Multiple ways of cfDNA reception and
following ROS production in endothelial cells, Adv. Exp. Med.
Biol., 924, 127-131.
108.Zhang, Q., Raoof, M., Chen, Y., Sumi, Y.,
Sursal, T., Junger, W., Brohi, K., Itagaki, K., and Hauser, C. J.
(2010) Circulating mitochondrial DAMPs cause inflammatory
responses to injury, Nature, 464, 104-107.
109.Gu, X., Wu, G., Yao, Y., Zeng, J., Shi, D., Lv,
T., Luo, L., and Song, Y. (2015) Intratracheal administration of
mitochondrial DNA directly provokes lung inflammation through the
TLR9–p38 MAPK pathway, Free Radic. Biol. Med., 83,
149-158.
110.Oka, T., Hikoso, S., Yamaguchi, O., Taneike,
M., Takeda, T., Tamai, T., Oyabu, J., Murakawa, T., Nakayama, H.,
Nishida, K., Akira, S., Yamamoto, A., Komuro, I., and Otsu, K. (2012)
Mitochondrial DNA that escapes from autophagy causes inflammation and
heart failure, Nature, 485, 251-255.
111.Muruve, D. A., Petrilli, V., Zaiss, A. K.,
White, L. R., Clark, S. A., Ross, P. J., Parks, R. J., and Tschopp, J.
(2008) The inflammasome recognizes cytosolic microbial and host
DNA and triggers an innate immune response, Nature, 452,
103-107.
112.Barber, G. N. (2014) STING-dependent cytosolic
DNA sensing pathways, Trends Immunol., 35, 88-93.
113.White, M. J., McArthur, K., Metcalf, D., Lane,
R. M., Cambier, J. C., Herold, M. J., Van Delft, M. F., Bedoui, S.,
Lessene, G., Ritchie, M. E., Huang, D. C. S., and Kile, B. T. (2014)
Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN
production, Cell, 159, 1549-1562.
114.Bruskov, V. I., Malakhova, L. V., Masalimov, Z.
K., and Chernikov, A. V. (2002) Heat-induced formation of reactive
oxygen species and 8-oxoguanine, a biomarker of damage to DNA,
Nucleic Acids Res., 30, 1354-1363.
115.Van Loon, B., Markkanen, E., and Hubscher, U.
(2010) Oxygen as a friend and enemy: how to combat the mutational
potential of 8-oxo-guanine, DNA Repair, 9, 604-616.
116.Bianchi, M. E. (2007) DAMPs, PAMPs and
alarmins: all we need to know about danger, J. Leukoc. Biol.,
81, 1-5.
117.Ermakov, A. V., Konkova, M. S., Kostyuk, S. V.,
Izevskaya, V. L., Baranova, A., and Veiko, N. N. (2013) Oxidized
extracellular DNA as a stress signal in human cells, Oxid. Med.
Cell. Longev., 649747.
118.Veiko, N. N., Bulycheva, N. V., Roginko, O. A.,
Veiko, R. V., Ershova, E. S., Kozdoba, O. A., Kuzmin, V. A.,
Vinogradov, A. M., Yudin, A. A., and Speransky, A. I. (2008) Fragments
of the transcribed region of the ribosomal repeat in extracellular DNA
is a marker of the organism cell death, Biomed. Khim.,
54, 78-93.
119.Korzeneva, I. B., Kostuyk, S. V., Ershova, L.
S., Osipov, A. N., Zhuravleva, V. F., Pankratova, G. V., Porokhovnik,
L. N., and Veiko, N. N. (2015) Human circulating plasma DNA
significantly decreases while lymphocyte DNA damage increases
under chronic occupational exposure to low-dose gamma-neutron and
tritium β-radiation, Mutat. Res., 779, 1-15.
120.Delaney, S., Jarem, D. A., Volle, C. B., and
Yennie, C. J. (2012) Chemical and biological consequences of
oxidatively damaged guanine in DNA, Free Radic. Res., 46,
420-441.
121.Fleming, A. M., and Burrows, C. J. (2017)
8-Oxo-7,8-dihydroguanine, friend and foe: epigenetic-like regulator
versus initiator of mutagenesis, DNA Repair, 56,
75-83.