REVIEW: Externally Regulated Programmed Aging and Effects of Population Stress on Mammal Lifespan
T. C. Goldsmith
Azinet LLC, Box 239 Crownsville, MD 21032 USA; E-mail: tgoldsmith@azinet.com
Received June 8, 2017; Revision received August 10, 2017
Programmed (adaptive) aging refers to the idea that mammals, including humans and other complex organisms, have evolved mechanisms that purposely cause or allow senescence or otherwise internally limit their lifespans in order to obtain an evolutionary advantage. Until recently, programmed aging had been thought to be theoretically impossible because of the mechanics of the evolution process. However, there is now substantial theoretical and empirical support for the existence of programmed aging in mammals. Therefore, a comprehensive approach to medical research on aging and age-related diseases must consider programmed aging mechanisms and the detailed nature of such mechanisms is of major importance. Theories of externally regulated programmed aging suggest that in mammals and other complex organisms, genetically specified senescence mechanisms detect local or temporary external conditions that affect the optimal lifespan for a species population and can adjust the lifespans of individual members in response. This article describes why lifespan regulation in response to external conditions adds to the evolutionary advantage produced by programmed aging and why a specific externally regulated programmed aging mechanism provides the best match to empirical evidence on mammal senescence.
KEY WORDS: senescence, evolution, gerontology, health policy, medical research, evolvabilityDOI: 10.1134/S0006297917120033
In 1882, Weismann first proposed the idea that aging in humans and other organisms is genetically programmed in order to obtain an evolutionary advantage [1]. This idea is now gaining wider acceptance because of multiple theories [2-5] stating that an internal mechanism or program that purposely limits lifespan would benefit the survival (non-extinction) of a population of individual members of a species despite being adverse from an individual organism’s point of view.
Darwin’s evolutionary mechanics concept, as widely taught and generally understood, does not support the idea that such population benefit can offset the obvious individual disadvantage of senescence, and until recently, programmed aging had been widely thought theoretically impossible. However, several developments described in detail elsewhere now support multiple population-oriented evolutionary mechanics concepts that allow programmed aging based on the population benefit of limiting individual lifespan [2, 6]. These developments can be summarized as follows:
– efforts spanning more than 150 years have failed to produce theories that plausibly explain multi-species senescence observations while fully complying with Darwin’s individual-oriented evolutionary mechanics concept;
– genetics discoveries have exposed multiple issues with traditional evolutionary mechanics and support population benefit theories;
– empirical evidence of programmed lifespan limitation in various species continues to accumulate;
– many population benefits of an internally limited lifespan have been proposed and there has been no scientific effort toward showing that any of the suggested population benefits of senescence is invalid;
– population-oriented programmed aging theories provide an excellent match to multi-species observations related to senescence and lifespan;
– current scientific literature no longer supports the idea that programmed aging is theoretically impossible;
– substantial financial investment in programmed-aging-based medical research has begun.
Multiple post-1952 population-based evolutionary mechanics theories, including Medawar’s modification [7], group selection [8], kin selection [9], and small group selection [10], suggest that traits that benefit a population can evolve despite producing a disadvantage for an individual organism. Programmed aging theories based on these concepts include [3] and [5].
Recent evolutionary mechanics theories based on evolvability [2, 11] suggest that organisms can evolve and retain traits that benefit a population by increasing its ability to evolve (adapt by means of genomic change) even if these traits produce disadvantage for the individuals. Evolvability theories of aging [1, 4, 6] suggest that senescence increases evolvability in multiple ways and, therefore, organisms would logically develop biological programs that operate to limit individual lifespan in a species-specific manner.
There is wide agreement that many internal and external factors affect the lifespan needed by the population members. Age at reproductive maturity and many other programmed aspects of the organism’s reproductive design clearly affect needed lifespan. External factors, such as the extent of population stress from mortality due to predation, environmental conditions, and starvation, also affect the needed lifespan as described below.
NATURE OF THE MAMMAL SENESCENCE PROGRAM
For a growing group of scientists who believe in programmed mammal aging, the next logical step would be prediction of the nature of the programmed senescence mechanism. There are several possibilities.
1) Cells could be equipped with genetically defined clock mechanisms that determine at what age and to what degree senescence processes would be initiated in each individual cell.
2) A functionally common genetically defined biological clock mechanism could determine when and at what rate the senescence mechanism would be initiated and could send signals to tissues to activate or inhibit senescence mechanisms at the cell level in the organism’s tissues. Such signals could be assertive (initiate senescence), inhibitive (suppress senescence), or both. Multiple signals could be involved, e.g., neuronal or hormonal (chemical) signaling, or both.
3) A mechanism as described in (2), except that the biological clock mechanism could possess the capability for sensing external temporary or local conditions that affect the optimal operation of the aging mechanism and adjust the process of senescence accordingly, i.e., externally regulated programmed aging.
4) A mechanism such as described in (3), except that it primarily operates through downregulation of the maintenance and repair mechanisms that act to prevent the symptoms of senescence.
5) A mechanism as described in (4), except that it provides for coordinated control of senescence and reproductive parameters that affect the particular lifespan needed by an organism.
This article argues that (5) has the best theoretical basis and is also best supported by empirical evidence. Mammal senescence is controlled by an evolved biological program that coordinates senescence with reproductive parameters and external conditions in order to provide the best outcome for a population.
REGULATED MAINTENANCE AND REPAIR MECHANISMS CONTROL
LIFESPAN
There are many different deteriorative processes that affect living organisms: cells die, hair and nails wear away, wounds occur, infectious agents attack. It is also obvious that they are counteracted by diverse and complex maintenance and repair (M&R) mechanisms: cells, hair, and nails are replaced, wounds heal, and infections are combatted.
We can easily imagine different M&R mechanisms that act to prevent different symptoms of senescence. An anti-cancer mechanism could deter cancer or even a particular type of cancer. A very different M&R mechanism could prevent heart disease, and so forth. As suggested here, an aging program could act by downregulating multiple M&R mechanisms at a species-specific age and rate, thus allowing senescence symptoms to appear on a species-specific schedule. This model provides a good fit to the observation that different mammals have very different internally determined lifespans (more than a factor of 200 difference between some mice and some whales) but display rather similar symptoms of senescence, such as cancer, heart disease, sensory deficits, and mobility deterioration. In this model, an early case of cancer could be caused by carcinogens that add to the damaging cell processes or by a flaw in a cancer-specific M&R mechanism, while cancer and other highly age-related diseases and conditions at a later age would be largely the result of the senescence program. In general, accelerated senescence (e.g., Hutchinson–Gilford progeria or Werner syndrome [12]) would result from a flaw in the common part of the senescence mechanism, which would cause the multiple senescence symptoms to display themselves at an earlier than typical age. Similarly, a flaw that prevents activation of senescence at the optimum age could result in a population that does not exhibit measurable senescence, as is seen in some species [13]. Such a population would lack the many population benefits of senescence and would, therefore, be more likely to become extinct.
Lifespan control by a mechanism that directly causes senescence symptoms (as opposed to or in addition to one that does it indirectly by downregulating M&R mechanisms) is certainly possible. Indeed, the octopus suicide mechanism operates by causing the animal to stop eating [14]. However, the model suggested here has advantages in that the M&R mechanisms are required in any case, so senescence based on the M&R mechanisms is arguably simpler. It has also been suggested that gradual multi-symptom senescence has substantial evolvability advantages over acute single-symptom lifespan limitation [2, 4]. It is also possible that M&R processes can consume significant material and energy resources that must be obtained from food. Therefore, senescence by downregulation of M&R mechanisms could reduce a population’s food requirements in comparison to the gradual direct damage scheme, thereby creating an evolutionary advantage for the population.
REGULATION IN RESPONSE TO LOCAL OR TEMPORARY CONDITIONS
It is common for organisms to possess mechanisms by which a genetically specified inherited design parameter (trait) can be adjusted (within some range) during the organism’s lifetime in order to accommodate local or temporary external conditions that affect the optimal value of this parameter. For example, mammals have a large number of genetically specified skeletal muscles. However, the size, strength, and associated blood supply of a muscle can be adjusted to accommodate local or temporary conditions much more rapidly than via evolutionary adaptation that modifies the genome. An animal that happens to live in a mountainous area can acquire larger and stronger leg muscles. A genetically identical animal living in a flatland could acquire relatively smaller muscles and, therefore, lower body mass, higher maneuverability, and reduced energy needs – an obvious advantage for a population. Similarly, many mammals seasonally alter their fur coats. The capacity for such regulation, or “real-time adaptation”, has a clear evolutionary value for increasing the probability that a population will survive and/or produce descendant species.
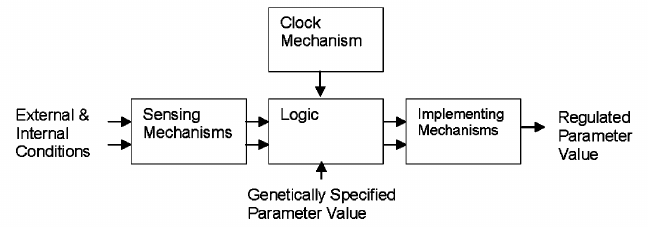
Any such regulatory mechanism requires four elements (figure): capability for detecting or sensing the relevant condition(s); logical processes for determining what action to take as a result of the detected condition(s); means for altering genetically specified parameters (e.g., muscle size, fur coat, or lifespan), generally proportionally to the magnitude of the detected condition(s). Because the part of an organism performing the sensing function is likely to not be the part requiring modification, signaling would typically be required as well. Time is a factor in lifespan and many other biological regulatory schemes thus requiring a biological clock mechanism.
Functional diagram of typical biological regulatory scheme
It is clear that many internal or external conditions, which can be temporary or local, can alter the optimal lifespan for an organism. More specifically, programmed and regulated internal reproductive parameters like age at reproductive maturity, gestation time, litter size, seasonal timing and duration of mating activities, and strength of reproductive urges, affect needed lifespan. A simple example – we can agree that a population that exhibits significant senescence prior to reaching puberty would not be a viable population.
LIFESPAN AND REPRODUCTION REGULATION SCENARIOS AND
STRATEGIES
How would we expect lifespans and reproductive parameters to be regulated in response to various conditions that vary on a time scale that is relatively short compared to the time required for genomic adaptation? External conditions that affect needed lifespan and reproductive parameters include the population stress caused by predation, infectious diseases, severe environmental conditions, famines, and overcrowding. We can discuss several strategies that might be employed by a regulated senescence program in response to these conditions: First, it is clear that evolvability is less urgent than a more immediate threat to a population’s survival, because a species might exist for a long period without evolving. During a famine, it would be logical for an organism program to increase individual lifespan while simultaneously reducing reproduction effort with respect to the genetically specified values. Because reproduction requires more food resources than mere survival, this strategy would allow a population to survive with less food at the expense of a temporary reduction in evolvability. Second, a temporary increase in predation would increase mortality. In this case, a population could respond by temporarily increasing lifespan while possibly increasing reproductive effort. Third, overpopulation would tend to reduce evolvability [2] and otherwise threaten a population [5]. Here, a logical response would be to reduce reproductive effort and/or reduce lifespan. Some mating behaviors (such as seen in Bighorn sheep) clearly act to suppress reproduction in a population-sensitive manner [2].
SENSING OF CONDITIONS AFFECTING OPTIMAL LIFESPAN
Altering lifespan and reproductive parameters in response to external conditions requires appropriate sensing mechanisms. We can imagine that there would be many internal consequences of famine and the consequent caloric restriction that could be sensed. Detection of overpopulation could involve sensing pheromones.
Detection of predators could involve recognition of physiological conditions likely present in survivors of predator attacks. The predation stress hypothesis suggests that lives of typical mammals consist of hours of relatively relaxed boredom interrupted by moments of sheer terror and intense physical activity, and that the frequency of terror episodes would be a measure of predation. Predation could therefore be sensed by detecting adrenal hormones or other internal indicators of terror. Similarly, typically brief but intense moments of physical activity experienced by the survivors could be detected by a senescence control mechanism. This model suggests that exercise (even periodic brief intense exercise) would act to generally delay mammal senescence.
BIOLOGICAL CLOCKS
The nature of biological clocks has historically been a rather academic question, but emergence of modern programmed aging theories has caused a situation in which understanding the aging clock could be critical for understanding senescence and age-related diseases. Many biological clocks, such as those involved in mating seasons and circadian rhythms, are obviously synchronized to external cues and, therefore, involve detection of external conditions. The aging clock could similarly be derived from or synchronized to external cues such as day/night cycle, or in longer-lived organisms, a seasonal cycle.
EMPIRICAL EVIDENCE FAVORING EXTERNALLY REGULATED PROGRAMMED
AGING
There is already a considerable body of empirical evidence supporting these scenarios: Exercise and some other forms of physical stress are widely thought to increase lifespan. Recent experiments [15] suggest that high-intensity interval training (HIIT) or an exercise regime calling for periodic brief, but intense aerobic exercise has a greater anti-aging effect than other forms of exercise. This finding supports the predation stress hypothesis. The caloric restriction effect seen in some mammals [16] suggests that lifespan could be increased by dietary limitations.
Regulation of the life-cycle processes is common. Internal and even external (pheromone) signaling and detection of external conditions are involved in reproduction. Neuronal or chemical (hormonal) signaling mechanisms are ubiquitous in coordinating the functions of diverse tissues and biological activities.
Heterochronic experiments, in which aged cells are exposed to blood components from young subjects, have demonstrated that blood signals can change cell senescence indicators [17]. Heterochronic plasma exchange (HPE) has been proposed as a method for studying the effects of blood plasma components on senescence regulation [18]. A human clinical trial is underway to study the effect of infusion of young person plasma on aging biomarkers [19].
Other experiments have shown that sensing pheromones is involved in the lifespan regulation in simple organisms [20].
The presence of many human hormones varies with age [21]. The concentrations of calcitonin, aldosterone, growth hormone, renin, estrogen, prolactin, and testosterone typically decrease with age, while the concentrations of follicle-stimulating hormone, luteinizing hormone, norepinephrine, and parathyroid hormones increase with age. Many others are unaffected by age. If the model suggested here is correct, it is essentially inescapable that age-related hormonal changes in later life are signaling manifestations of the aging program. Therefore, replacement of hormones that decline with age and/or suppression of hormones that increase with age represent an obvious research avenue. Hormone replacement therapy and HPE have been proposed as treatments for senescence [22].
REFERENCES
1.Weismann, A. (1882) Uber die Dauer des
Lebens, Fischer, Jena.
2.Goldsmith, T. (2014) The Evolution of Aging,
3rd Edn., Azinet, Annapolis.
3.Libertini, G. (1988) An adaptive theory of
increasing mortality with increasing chronological age in populations
in the wild, J. Theor. Biol., 132, 145-162.
4.Skulachev, V. (1997) Aging is a specific biological
function rather than the result of a disorder in complex living
systems: biochemical evidence in support of Weismann’s
hypothesis, Biochemistry (Moscow), 62, 1191.
5.Mitteldorf, J. (2006) Chaotic population dynamics
and the evolution of ageing, Evol. Ecol. Res., 8,
561-574.
6.Goldsmith, T. (2017) Evolvability, population
benefit, and the evolution of programmed aging in mammals,
Biochemistry (Moscow), 12, 1423-1429.
7.Medawar, P. (1952) An Unsolved Problem of
Biology, H. K. Lewis & Co., London.
8.Wayne-Edwards, V. (1962) Animal Dispersion in
Relation to Social Behaviour, Oliver & Boyd, Edinburgh.
9.Hamilton, W. (1963) The evolution of altruistic
behavior, Am. Naturalist, 97, 354-356.
10.Travis, J. (2004) The evolution of programmed
death in a spatially structured population, J. Gerontol. A Biol.
Sci. Med. Sci., 59, 301-305.
11.Wagner, G., and Altenberg, L. (1996) Perspective:
complex adaptations and the evolution of evolvability,
Evolution, 50, 267-276.
12.Eriksson, M., Brown, W. T., Gordon, L. B., Glynn,
M. W., Singer, J., Scott, L., Erdos, M. R., Robbins, C. M., Moses, T.
Y., Berglund, P., Dutra, A., Pak, E., Durkin, S., Csoka, A. B.,
Boehnke, M., Glover, T. W., and Collins, F. S. (2003) Recurrent
de novo point mutations in lamin A cause
Hutchinson–Gilford progeria syndrome, Nature, 423,
293-298.
13.Bennett, J., Boehlert, G. W., and Turekian, K. K.
(1982) Confirmation on longevity in Sebastes diploproa (Pisces:
Scorpaenidae) from 210Pb/226Ra measurements in otoliths, Maritime
Biol., 71, 209-215.
14.Wodinsky, J. (1977) Hormonal inhibition of
feeding and death in octopus: control by optic gland secretion,
Science, 198, 948-951.
15.Robinson, M., Dasari, S., Konopka, A. R.,
Johnson, M. L., Manjunatha, S., Esponda, R. R., Carter, R. E., Lanza,
I. R., and Nair, K. S. (2017) Enhanced protein translation underlies
improved metabolic and physical adaptations to different exercise
training modes in young and old humans, Cell Metab., 25,
581-592.
16.Spindler, S., Dasari, S., Konopka, A. R.,
Johnson, M. L., Manjunatha, S., Esponda, R. R., Carter, R. E., Lanza,
I. R., and Nair, K. S. (2005) Rapid and reversible induction of the
longevity, anticancer and genomic effects of caloric restriction,
Mech. Ageing Dev., 126, 960-966.
17.Conboy, I., Conboy, M. J., Wagers, A. J., Girma,
E. R., Weissman, I. L., and Rando, T. A. (2005) Rejuvenation of aged
progenitor cells by exposure to a young systemic environment,
Nature, 433, 760-764.
18.Katcher, H. (2013) Studies that shed new light on
aging, Biochemistry (Moscow), 78, 9.
19.Ambrosia LLC (2016) Clinical Trial: Young
Donor Plasma Transfusion and Age-Related Biomarkers, NIH
identifier: NCT02803554.
20.Apfeld, J., and Kenyon, C. (1999) Regulation of
lifespan by sensory perception in Caenorhabditis elegans,
Nature, 402, 804-809.
21.Skaznik-Wikiel, M. E., Traub, M. L., and Santoro,
N. (2016) Menopause, in Endocrinology: Adult and
Pediatric (Jameson, J. L., and De Groot, L. J., eds.) 7th Edn.,
Chap. 135, Elsevier Saunders, Philadelphia, PA, pp. 2310-2322.
22.Lobo, R. (2016) Hormone-replacement therapy:
current thinking, Nat. Rev. Endocrinol., 13, 220-231.