REVIEW: Evolvability, Population Benefit, and the Evolution of Programmed Aging in Mammals
T. C. Goldsmith
Azinet LLC, Box 239 Crownsville, MD 21032 USA; E-mail: tgoldsmith@azinet.com
Received April 6, 2017; Revision received August 1, 2017
Programmed aging theories contend that evolved biological mechanisms purposely limit internally determined lifespans in mammals and are ultimately responsible for most instances of highly age-related diseases and conditions. Until recently, the existence of programmed aging mechanisms was considered theoretically impossible because it directly conflicted with Darwin’s survival-of-the-fittest evolutionary mechanics concept as widely taught and generally understood. However, subsequent discoveries, especially in genetics, have exposed issues with some details of Darwin’s theory that affect the mechanics of the evolution process and strongly suggest that programmed aging mechanisms in humans and other mammals can and did evolve, and more generally, that a trait that benefits a population can evolve even if, like senescence, it is adverse to individual members of the population. Evolvability theories contend that organisms can possess evolved design characteristics (traits) that affect their ability to evolve, and further, that a trait that increases a population’s ability to evolve (increases evolvability) can be acquired and retained even if it is adverse in traditional individual fitness terms. Programmed aging theories based on evolvability contend that internally limiting lifespan in a species-specific manner creates an evolvability advantage that results in the evolution and retention of senescence. This issue is critical to medical research because the different theories lead to dramatically different concepts regarding the nature of biological mechanisms behind highly age-related diseases and conditions.
KEY WORDS: aging theory, senescence, medicine, gerontology, evolutionary mechanics theoriesDOI: 10.1134/S0006297917120021
Abbreviations: SNPs, single nucleotide polymorphisms.
There is very strong scientific agreement regarding most aspects of
Darwin’s evolution theory [1]: evolution of
Earth-life has taken place; current species have descended from earlier
different species; evolution is a slow incremental process that has
operated for billions of years; and in some form evolution is driven by
natural selection or survival of the fittest. Darwin’s
evolutionary mechanics concepts regarding the nature of the evolution
process explain the vast majority of biological observations concerning
organism designs. However, despite many decades of argument, no
consensus has been reached for an aspect that concerns the details of
the evolutionary relationship between individual members of a species
and populations of those individuals. In the vast majority of cases, a
trait that produces an advantage for an individual would also produce
an advantage for a population of those individuals. However, exceptions
have been identified, specifically for mammal senescence. Can an
organism evolve an inherited design characteristic or trait that
benefits a population at the expense of individual members of this
population? Could organisms evolve aging programs, essentially suicide
mechanisms, which purposely limit internally determined individual
lifespan in order to obtain a population advantage? Darwin’s
evolutionary mechanics theory, as currently widely taught, is extremely
individual-oriented and contends that the evolution process causes
organisms to evolve traits that cause possessing individuals to have a
higher probability of producing adult descendants.
However, since 1952 a series of more population-oriented concepts have appeared. Discoveries, especially in genetics, have exposed issues with details of Darwinian mechanics and strongly support the newer concepts.
This individual vs. population issue might appear to be a semi-trivial, arcane, and academic matter but has immense practical consequences for medical research, because the two concepts logically lead to very different conclusions regarding the nature of human senescence and therefore, the nature of the many highly age-related diseases and conditions, such as cancer and heart disease.
Despite more than 150 years of efforts, theorists have been unable to produce an aging theory that plausibly explains mammal senescence while fully complying with Darwin’s evolutionary mechanics concepts. In particular, why do biochemically similar species (e.g., mammals) have such different internally determined lifespans? Mammal lifespans vary over a range of more than 200 to 1, and fish lifespans vary over a range of more than 1300 to 1 [2]. Because a longer-lived species “A” has a longer lifespan, proving that it is possible, why did not a very similar shorter-lived mammal “B” also evolve a longer reproductive lifespan, given that it would obviously convey a Darwinian advantage? These questions surfaced immediately after publication of Origin in 1859. Consequently, as summarized here, all modern theories of aging that provide plausible multi-species senescence explanations involve modifications to Darwin’s mechanics concept that increase the importance of populations relative to individuals. This article describes how the need for evolvability, a population property, has caused the evolution of programmed aging in mammals and other organisms.
EVOLVABILITY AND THE EVOLUTIONARY MECHANICS OF AGING
Darwin’s evolutionary mechanics concept assumes that the ability to evolve is a fixed inherent property of all living organisms. All wild organisms are capable of passing information on their designs to descendants (biological inheritance), are susceptible to mutations that would change that information and these designs, and are subject to competition and natural selection.
Evolvability theories (e.g. [3, 4]) suggest that populations of a species can differ in their ability to evolve (genetically adapt to changes in the environment) and that traits that increase the rate or comprehensiveness of such adaptation (increase evolvability) can be selected by the evolution process despite being adverse from the point of view of an individual organism. Aging theories based on evolvability [5-7] contend that a purposely limited lifespan increases evolvability in multiple ways and that the mechanisms that cause such limitation have therefore been selected. In addition to providing explanations for observations concerning senescence, evolvability theories also explain other observations that are troublesome with regard to traditional Darwinian evolutionary mechanics, e.g., sexual reproduction, apparently unnecessarily delayed reproductive maturity (especially in males), and certain animal behavioral traits, such as animal altruism and individually adverse mating behavior [3]. Evolvability theories are among a family of post-1952 theories to the effect that the evolution process is directed at the survival and success of a population as opposed to individual survival and reproduction, as emphasized by traditional Darwinian theory.
The evolution process is clearly population oriented. Whether a particular individual having a certain inherited phenotypic design lives longer and breeds more than another individual having a slightly different design is essentially a matter of luck or chance. What we can say is that individuals having a particular inherited design have a greater probability of surviving and reproducing than some other individuals possessing a different design. We can therefore consider that in evolutionary terms, the life of an individual is a trial in the probability sense of a particular inherited design possessed by that individual. Does this design have a greater probability of producing adult descendants under wild conditions than some other designs? Extending this concept, the rate at which the evolution process proceeds and the precision with which it can determine the answer to the above question depend on the rate at which the trials are conducted or the rate at which lives are lived, i.e., death rate.
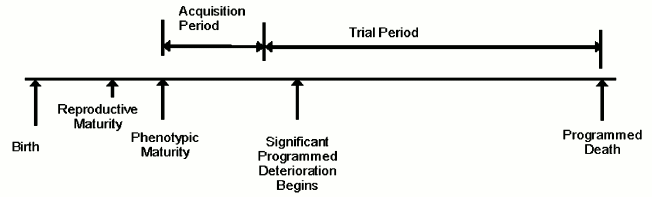
The evolution process is also performance-oriented and measures how well a particular design performs in living longer and breeding more relative to some other design. Latent characteristics that do not affect performance cannot influence this aspect of the evolution process. Adult traits are not fully expressed in juveniles. Therefore deaths that occur in juveniles generally do not contribute to the evolution of adult traits. Consequently, we can extend the previous paragraph to read that the evolution process is a function of adult death rate. Figure describes the life of an organism in these terms.
Organism lifetime – evolvability concept
Adult death rate in its turn is directly proportional to the size of the population and inversely proportional to average lifetime, in addition to other species-specific factors. In order to optimize the evolution process, organisms must live long enough to become mature adults and participate in a trial, but not too much longer! One might say that external causes of mortality in the wild would naturally limit lifetime and, therefore, remove the need for an internal senescence mechanism. However, in such a non-senescing population, some individuals would live very long lives and, assuming population size is determined by external limitations, many others would necessarily die as juveniles, therefore reducing adult death rate. This problem would be more severe in more complex animals possessing a social structure, or “pecking order”. The “king of the hill” is less likely to die in combat or from starvation than other animals and, if lacking internal limitations on lifespan, could live a very long life and produce a very large number of descendants, thereby reducing adult death rate, genetic diversity, variation, and evolvability.
Many other evolvability advantages of an internally limited lifespan have been identified, especially in more complex organisms, such as mammals [3]. For example, internally limited lifespan aids the evolution of traits such as intelligence or immunity that depend for their evolutionary value on the acquisition of something that accumulates during an organism’s lifetime. Where intelligence is the genetically determined trait, the selectable (fitness) trait is wisdom, essentially the product of intelligence and non-genetically acquired experience. In a non-senescent population, less intelligent but older and more experienced animals would have a greater advantage than in a senescing population, therefore detracting from the evolution of intelligence. The evolution of acquired traits such as these would require a period of time to allow accumulation of the acquired benefit to occur (figure). Some of us [3, 6] have further suggested that gradual senescence and multiparous reproduction, such as seen in mammals, enhance the evolution of acquired traits (relatively to acute suicide and semelparity) by gradually compensating for the age advantage that would otherwise exist, and by creating a “challenge effect”, which increases the degree to which a life contributes to the evolution process.
Traits that contribute to evolvability (e.g., senescence) are neutral or adverse in traditional fitness terms. Genetics discoveries have revealed many aspects of organism genomic design that have no known phenotypic effect but clearly do constrain the evolution process and affect evolvability [3].
Darwin suggested [1] that the evolution process is extremely incremental and occurs in “tiny steps”, an idea that has been since substantially confirmed (see [3]). This suggests that the evolution process must be capable of distinguishing “tiny” differences in benefit, which in turn suggests that evolvability and statistics (precision) qualities associated with populations are essential to the evolution process.
All the aforementioned suggests that larger organisms, necessarily forming smaller populations and requiring a longer period to achieve maturity and, therefore, having longer lifespans, would evolve much more slowly than smaller organisms, because of lower adult death rates. However, a large number of evolvability traits, such as described here, greatly contribute to evolvability in complex organisms [3]. It is reasonable to conclude that in complex organisms, virtually all of an organism’s ability to evolve is itself the result of evolved traits, such as senescence, diploid reproductive schemes, and sexual reproduction.
MEDAWAR’S MODIFICATION
Darwin’s evolutionary mechanics concept does not assume that the evolutionary value of living longer and breeding more varies with age and, therefore, suggests that the force of evolution is directed toward a non-senescent state. This concept, when coupled with the observation that senescence exists, inevitably leads to the idea that aging is the result of fundamental limitations, such as laws of physics or chemistry, that cannot be overcome by the evolution process. Entropy, oxidation, wear and tear, and other natural and universal causes of damage and dysfunction are often mentioned as the causes of senescence in aging theories based on unmodified Darwinian mechanics [8]. Because nearly everyone receives training in Darwin’s theory, this idea is still popular, especially with those only concerned with human aging. However, as suggested earlier, multi-species observations immediately exposed several major issues. Why would a 50-kg dog be affected by some law of physics seven times as severely as a 50-kg human? What law of chemistry would cause a parrot to live six times longer than a crow? Eventually, species with no measurable senescence have been discovered [9], somehow undeterred by the laws of physics and chemistry! These issues have ultimately led to modern aging theories based on modifications to Darwin’s mechanics.
In 1952 Medawar proposed a modification [10] to Darwin’s evolutionary mechanics concept to the effect that senescence, although adverse and ultimately catastrophic from an individual’s viewpoint, has little effect on a wild population because of attrition due to external causes of mortality, such as predators, severe environmental conditions, starvation, and infectious diseases. For any given-size wild population (even if non-senescent and not possessing internal limitations on lifetime), the size of an age cohort decreases with age at a rate proportional to the population-specific severity of external attrition, and consequently, the effectiveness of the evolution process decreases with age at a rate determined by the size of the age cohort. Trivial example: we can imagine that in a wild mouse population (even if non-senescent), few individuals would survive beyond 3 years of age. Therefore, there would be little population advantage from the individuals having an internal capacity for living longer and little population-based evolutionary force toward evolving a longer lifespan. Modern programmed (adaptive) aging theories and modern non-programmed (non-adaptive) aging theories (e.g., mutation accumulation theory [10], antagonistic pleiotropy theory [11], and disposable soma theory [12]) are all based on Medawar’s modification, because these theories provide a much better match to the huge variety of internally determined lifespans seen in biochemically similar species than earlier theories based on unmodified Darwinian theory.
The reader will notice that Medawar’s idea is a population concept based on logic that is very similar to the evolvability concepts discussed earlier. The effectiveness of the evolution process is influenced by the size of an age cohort, which is of course affected by the overall size of the population. For example, it is logically inconsistent to believe that the evolution of increased longevity is limited by the size of the cohort (that would benefit), but simultaneously assume that a mutation in a single individual would immediately affect the evolution process. Wouldn’t the force of evolution toward selection of a mutation be proportional to the size of the cohort possessing this mutation? If you accept Medawar’s concept, you also logically assume that organism populations can possess characteristics, like population size and adult death rate from external causes, that alter their ability to evolve. Similarly, species-specific internal characteristics, such as age at puberty and other reproductive traits, would clearly alter adult death rate and species’ need for a particular lifespan.
Medawar’s modification has led to the origin of several modern non-programmed aging theories [10-12] to the effect that the evolution process causes each species to evolve a particular minimum lifespan, i.e., the internal capacity for living and reproducing for a certain species-specific period. Following that period, natural deteriorative processes, such as wear and tear, random (stochastic) mutations, and the ever-popular entropy (now unopposed by the evolution process), might cause senescence. No one denies the existence of natural deteriorative processes, and these theories provide a better match to the multi-species observations.
Following Medawar’s modification, in 1957 Williams introduced a now widely accepted objection [11] that observed fitness deterioration from mammal senescence occurs at too early age to have a negligible effect on a wild mammal population and, therefore, senescence must convey a compensating population benefit that acts to prevent the evolution of a longer lifespan and later appearance of significant fitness deterioration in any particular species. Studies of wild mammal populations [13] confirmed this idea by showing that adult death rates increase with age and that, therefore, senescence causes at least some population disadvantage. Evolvability-based theories of aging contend that increased evolvability is the compensating benefit of senescence! Note also that because of the acquired traits in animals, we would expect adult death rates to decline with age if senescence were not causing a population disadvantage. Modern non-programmed theories [10-12] have difficulties in providing plausible explanations for the evolutionary benefit of senescence [3, 14]. This has resulted in multiple non-programmed theories and no strong consensus supporting any particular theory.
DIGITAL GENETICS AND THE EVOLUTION OF LOCAL VARIATION
Darwin specified that “natural” variation in inherited characteristics was essential to the evolution process. Without variation there would be no way for an organism to have differences in inherited characteristics from other members of its species and, therefore, nothing for the natural selection process to select. “Local” means that the variation would need to exist between individuals that could plausibly compete or interact otherwise with each other. Darwin assumed that variation was an inherent “natural” property of wild organisms: All organisms have the ability to transfer information on their designs to descendants, and all of them are susceptible to mutations that would change that information in their descendants.
In addition, Darwin reasonably assumed that the information transfer process is analog in nature, and such information transfer schemes inherently produce variation. In analog schemes, variation always occurs and the amplitude of this variation is inversely proportional to the frequency of its occurrence. Larger deviations are progressively less likely than smaller deviations, which at least superficially matches observed variations, such as those between mammal parents and their immediate progeny.
However, subsequent genetics discoveries [15] have revealed that the biological information transfer process is actually digital in nature and is accomplished by the combinations of four nucleotides in long DNA molecules. Digital information transfer does not inherently cause structured variations as described above, but most often produces either exact duplicates or gross unstructured “errors” [3]. In complex organisms, the observed variation in a population is actually almost entirely the result of very complex and obviously evolved mechanisms that handle the digital data, such as genes, chromosomes, meiosis, unequal crossover, diploid genomic organization, and sexual reproduction.
Because variation is produced by evolved traits, and variation is essential to the evolution process, we can consider traits that produce variation to be evolvability traits. Like other evolvability traits, they are adverse in terms of traditional Darwinian fitness. For example, if we consider a population that is well adapted to its environment, there would exist an optimal organism design for this population. Any deviation from this design would represent a reduction in fitness. Therefore, the fittest population with the largest probability of avoiding extinction under given external conditions would be the one in which there exists no variation and, therefore, no evolvability and no capability for adapting to changes in its environment. More variation would result in more evolvability but less average fitness. Variation is a property of a population.
Note that variation can be affected by evolved behavioral traits in animals. An animal that has a behavioral trait that causes it to seek mates different from itself or remotely located would produce more variation. A trait that caused an animal to prefer mating locally or with close relatives would decrease variation.
The digital nature of inheritance imposes other attributes and limitations that are common to any digital information scheme [3]. For example, quantizing. The precision with which an organism design parameter can be uniquely specified depends on the number of symbols (in this case, in the sequences composed of genomic symbols A, C, G, and T) that are used to communicate this parameter. Genomic designs required for the precise genetic specification of complex organs (eye, ear) are therefore different from genomic designs required for specifying simpler anatomical structures. Language – the digital scheme requires that both ends of the data communication path possess, in advance, information on the meaning that is arbitrarily assigned to particular digital sequences. For example, the CAT codon encodes histidine and not lysine.
These and other aspects of digital genomic design greatly complicate the evolution process and also suggest that, overall, the evolution process is much more time-consuming than previously thought. In addition to mutations and natural selection, many other evolutionary processes are involved that operate over vastly different time scales [3].
RELATIONSHIP BETWEEN MUTATIONS AND NATURAL SELECTION
Darwin’s mechanics, as generally understood, can be summarized as follows. A mutation occasionally occurs that changes the inheritable phenotypic design of a single organism. If this mutation subsequently causes the possessing individuals to produce more adult descendants than competing non-possessing individuals, it propagates in a population. Natural selection individually evaluates each mutation. This “one mutation at a time” concept essentially precludes the existence of programmed aging or any other evolved trait that limits an individual’s ability to produce adult descendants (e.g., animal altruism, delayed puberty, mating behaviors that limit individual reproduction, or even sexual reproduction). Darwin knew that a single mutation could cause major adverse effects but considered that only minor changes could potentially cause a beneficial effect.
However, recent genetics discoveries have revealed that the medically normal (healthy) human population contains at least ninety-seven million individual genetic differences (the so-called single nucleotide polymorphisms, or SNPs), most of which originally occurred in a different individual at a different time and place [16]. Based on the SNP definition, each SNP allele appears in at least 1% of the population. Furthermore, the phenotypic effect of any individual SNP is generally minor. The observed phenotypic differences between diploid individuals result from the combination of SNP alleles that produce particular genetic sets. A tall individual could be a result of combining of hundreds or even thousands of preexisting SNP alleles, each of them having a minor positive effect on height. An individual diploid organism can possess substantial genetic diversity resulting from differences between its two sets of genetic data. This is a major evolvability advantage over haploid reproduction: If some event decimates a population, it can recover into one having substantial variation. In a case of diploid organisms, a single pair of parents can produce descendants having diverse phenotypes obviously increasing local variation.
It is unlikely that any single change in a complex organism’s phenotypic design would be beneficial. Suppose we assume that more speed would help an antelope. Longer legs might help with speed. However, longer leg bones would actually be adverse unless accompanied by bigger leg muscles, changes in other bones, better joints, and a long list of other complementing changes, each of which would be adverse by itself. Darwin assumed that, therefore, evolution is extremely incremental in nature.
THE EVOLUTION OF DIPLOID ORGANISMS AND EVOLVABILITY
It is widely accepted that sexually reproducing diploid organisms evolved from haploid organisms despite the fact that sexual reproduction is massively adverse in traditional fitness terms as compared to asexual reproduction and haploid inheritance [3]. Thus, in sexual reproduction, reproductive effectiveness is nominally reduced by a factor of two because of the relative reproductive uselessness of males. This is one of the proofs that evolvability can trade off against traditional individual fitness.
In addition, it is common for a mutation (i.e., the origin of a new SNP) to have little or no phenotypic effect unless the organism possesses this new SNP allele in both genomes. This has the effect of substantially aiding the propagation of mutations that have minor adverse effects on fitness while suppressing propagation of mutations that have a beneficial effect on fitness – another proof that diploid reproduction would have been unlikely to evolve if the Darwinian concepts were correct. Note that this effect aids the propagation of slightly adverse mutations that can later be recombined to produce a net beneficial effect, as described in the antelope example.
GENETIC LINKAGE
Genetics discoveries have revealed many ways in which a trait could be genetically linked to other traits a manner that would affect the evolution process [3]. Mutational changes are not random but severely restrained by aspects of the genomic design of a diploid species. In 1957 Williams suggested [11] that a particular form of genetic linkage, antagonistic pleiotropy, could link a beneficial trait to an adverse trait (in our case, senescence) in such a way as to impede the evolutionary rejection of senescence, because doing so would also eliminate the beneficial effect and cause a net disadvantage. He suggested that this effect would explain the existence of senescence despite his simultaneous belief that senescence per se was somewhat adverse to populations. Many other sources of genomic linkage have been identified since then [3] that occur on vastly different time scales. That is, the difficulty and, therefore, the evolutionary time required to produce genomic changes necessary to accomplish the needed beneficial change without causing linked adverse changes varies greatly depending on a particular linkage mechanism.
Thus, it is now known [17] that because of the nature of meiosis and unequal crossover, the traits that are affected by genes on the same chromosome are genetically linked, and the strength of the linkage is determined by the genomic distance between the genes.
Another example – the evolution of a new hormone with an evolutionary value requires the existence of a complex mechanism that includes new genes for producing the hormone and determining when to produce the hormone. However, hormone biosynthesis in an organism creates no value unless there are also receptors for this hormone in proper tissues and systems that mediate the beneficial effect. Therefore, genetic changes required to change the amount of the hormone are essentially trivial compared to those required for the appearance of a functionally new protein. This sort of the “chicken and egg” problem can plausibly take longer to solve than the time a mammal species has existed. Indeed, interspecific genomic comparisons suggest that genes are widely conserved between mammal species [17]. One famous consequence is that humans can and did use porcine insulin.
OTHER POPULATION-ORIENTED EVOLUTIONARY MECHANICS THEORIES
Beginning in 1962, more evolutionary mechanics theories have appeared (e.g., group selection [18], kin selection [19], and small group selection [20]) that emphasize that a population benefit (one that increases the probability of a population to avoid extinction or produce descendant species) could offset individual disadvantage and allow the evolution of a trait (e.g., senescence) that produces disadvantage for an individual. These theories were originally developed in efforts to explain phenomena other than senescence (e.g., animal altruism) that conflicted with the traditional theory. Eventually, they have extended to include programmed aging theories based on population benefits other than evolvability [21, 22]. Note that genetic linkage (see above) explains how an individually adverse trait could be retained long enough to provide a benefit for the population and thus gives a solution for the future vs. present problem (below).
OBJECTIONS TO EVOLVABILITY THEORIES AND PROGRAMMED AGING
THEORIES
Many bioscientists have taken the position that programmed aging obviously and grossly violates Darwin’s individual-oriented survival-of-the-fittest concept and can, therefore, be dismissed as ridiculous and “impossible” without any further investigation, review of current literature, scientific rationale, or counter-arguments [23]. Some have equated programmed aging to popular but scientifically ridiculous evolution concepts, such as creationism and intelligent design. Indeed, Darwin’s theory is widely taught as the only science-based evolutionary mechanics concept. Theories of aging based on fundamental limitations are still popular despite gross conflicts with multi-species observations. Many social and academic forces act to perpetuate this situation [3].
In 1882 Weismann proposed [7] what was essentially an evolvability-based programmed aging theory. This idea (like Darwin’s) predated the entire science of genetics and was almost universally dismissed because of the conflict with the Darwinian mechanics. Today it is cited by some critics as an evidence that programmed aging is an early but long-discredited and obsolete idea.
These critics are taking what is essentially a philosophical (as opposed to science-based) position to the effect that any deviation from Darwin’s mechanics is by definition incorrect, regardless of current evidence or logic. This position can be expected from people who are very aware of Darwin’s ideas but not as familiar with modern genetics discoveries or their impact on the theories of evolutionary mechanics and aging. However, as summarized here, genetics discoveries and other developments, some of them quite recent, have disproved multiple details of Darwin’s mechanics that are key to the evolution of senescence. These include Darwinian concepts regarding the nature of evolvability, the nature of variation, the analog vs. digital nature of biological inheritance, the random nature of mutations, the “one mutation at a time” concept, and the individual vs. population nature of evolution. In general, it is now obvious that, as it so often happens in science, the evolution process is significantly more complex than originally thought. Darwin’s theory was based on very detailed phenotypic comparisons between individuals and species. Our ability to perform similarly detailed genomic comparisons is still in its infancy. Details of biological inheritance mechanisms clearly affect evolutionary mechanics theories; and there are few scientists that believe that we are close to completely understanding biological inheritance.
With regard to modern science-based opposition, there have been no scientific discussions on the idea that a hypothetical trait could benefit populations at the expense of individuals, nor on the idea that limiting individual lifespan could benefit a population, nor on the idea that limiting lifespan benefits evolvability, nor on the idea that increasing evolvability benefits populations. Neither there have been objections to any specific proposed evolvability benefit of a limited lifespan such as summarized here. Furthermore, even fierce proponents of modern non-programmed aging theories who have attempted science-based counter-arguments [24, 25] no longer claim that programmed aging is “impossible”, but only that it is less likely than their particular non-programmed theory.
Since the more recent population benefit theories first appeared in 1962, the primary objection has been what might be termed the “present vs. future” or the “short-term vs. long-term” issue. This sort of analysis attempts to show that a mutational change that produces a long-term benefit (e.g., reduces the probability of a population extinction) cannot propagate if it also produces a short-term disadvantage (e.g., reduces the probability that a possessing individual will produce adult descendants). In brief, they accept Medawar’s modification but reject all of the later population-oriented theories. This sort of logic is a version of the individual-oriented “one mutation at a time” concept that has been already disproved (for diploid organisms); it is also inconsistent with Medawar’s modification, as described earlier, and ignores the evolutionary effects of genetics discoveries. This issue has resulted in multiple versions of “group selection” theories that differ regarding the size of the group and, therefore, the magnitude of the short-term vs. long-term issue. There are now kin selection theories [19], small-group theories [20], etc. In this connection, some critics have suggested that evolvability benefits a species and, therefore, evolvability theories can be dismissed as versions of the “species-level” group selection concept that is widely believed to be the most unlikely. However, evolvability benefits the evolution process [3] and, hence, is essential for it regardless of what size group or what time scale are considered. In addition, genetic linkage arguments suggest that even the species-level group selection concept is feasible [3]. The short-term vs. long-term issue depends on one’s concept regarding the “term” associated with the evolution process itself. Genetics discoveries have revealed that various evolutionary processes operate on a time scale that is long even by comparison to the time any mammal species has existed [3].
REFERENCES
1.Darwin, C. (1859) On the Origin of Species by
Means of Natural Selection, (John Murray, ed.) 6th Edn., Chap. 7.
Miscellaneous Objections to the Theory of Natural Selection,
London.
2.Max Planck Institute (2002) Life Spans of
Mammals, Birds, Amphibians, Reptiles, and Fish.
3.Goldsmith, T. (2014) The Evolution of Aging,
3rd Edn., Annapolis Azinet Press.
4.Wagner, G., and Altenberg, L. (1996) Perspective:
complex adaptations and the evolution of evolvability,
Evolution, 50, 3.
5.Goldsmith, T. (2008) Aging, evolvability, and the
individual benefit requirement; medical implications of aging theory
controversies, J. Theor. Biol., 252, 764-768.
6.Skulachev, V. (2011) Aging as a particular case of
phenoptosis, the programmed death of an organism (A response to
Kirkwood–Melov “On the programmed/non-programmed nature of
aging within the life history”), Aging (Albany, NY),
3, 1120-1123.
7.Weismann, A. (1892) Uber die Dauer des
Lebens, Fischer, Jena.
8.Harman, D. (1956) Aging: a theory based on free
radical and radiation chemistry, J. Gerontol., 11,
298-300.
9.Bennett, J. T., Boehlert, G. W., and Turekian, K.
K. (1982) Confirmation on longevity in Sebastes diploproa
(Pisces: Scorpaenidae) from 210Pb/226Ra measurements in otoliths,
Marit. Biol., 71, 209-215.
10.Medawar, P. (1952) An Unsolved Problem of
Biology, H. K. Lewis, London.
11.Williams, G. (1957) Pleiotropy, natural selection
and the evolution of senescence, Evolution, 11,
398-411.
12.Kirkwood, T., and Holliday, F. (1979) The
evolution of ageing and longevity, Proc. R. Soc. Lond. B,
205, 531-546.
13.Loison, A., Festa-Bianchet, M., Gaillard, J.-M.,
Jorgenson, J. T., and Jullien, J.-M. (1999) Age-specific survival in
five populations of ungulates: evidence of senescence, Ecology,
80, 2539-2554.
14.Goldsmith, T. (2013) Arguments against
non-programmed aging theories, Biochemistry (Moscow), 78,
971-978.
15.Watson, J., and Crick, F. A. (1953) Structure for
deoxyribose nucleic acid, Nature, 4356.
16.National Center for Biotechnology Information.
dbSNP 8 June 2015.
17.Lewin, B. (2004) Genes VIII, Pearson
Prentice Hall.
18.Wayne-Edwards, V. (1962) Animal Dispersion in
Relation to Social Behaviour, Oliver & Boyd, Edinburgh.
19.Hamilton, W. (1963) The evolution of altruistic
behavior, Am. Naturalist, 97, 354-356.
20.Travis, J. (2004) The evolution of programmed
death in a spatially structured population, J. Gerontol.,
59, 301-305.
21.Mitteldorf, J. (2006) Chaotic population dynamics
and the evolution of ageing, Evol. Ecol. Res., 8,
561-574.
22.Libertini, G. (1988) An adaptive theory of
increasing mortality with increasing chronological age in populations
in the wild, J. Theor. Biol., 132, 145-162.
23.Olshansky, S., Hayflick, L., and Carnes, B.
(2002) No truth to the fountain of youth, Sci. Am., 286,
92-95.
24.Kowald, A., and Kirkwood, T. (2016) Can aging be
programmed? A critical literature review, Aging Cell, doi:
10.1111/acel.12510.
25.Kirkwood, T., and Melov, S. (2011) On the
programmed/non-programmed nature of ageing within the life history,
Curr. Biol., 21, R701-R707.