Loss of Melanin by Eye Retinal Pigment Epithelium Cells Is Associated with Its Oxidative Destruction in Melanolipofuscin Granules
A. E. Dontsov, N. L. Sakina, and M. A. Ostrovsky*
Emanuel Institute of Biochemical Physics, Russian Academy of Sciences, 119334 Moscow, Russia; E-mail: ostrovsky3535@mail.ru* To whom correspondence should be addressed.
Received April 6, 2017; Revision received May 15, 2017
The effect of superoxide radicals on melanin destruction and degradation of melanosomes isolated from cells of retinal pigment epithelium (RPE) of the human eye was studied. We found that potassium superoxide causes destruction of melanin in melanosomes of human and bovine RPE, as well as destruction of melanin from the ink bag of squid, with the formation of fluorescent decay products having an emission maximum at 520-525 nm. The initial kinetics of the accumulation of the fluorescent decay products is linear. Superoxide radicals lead simultaneously to a decrease in the number of melanosomes and to a decrease in concentration of paramagnetic centers in them. Complete degradation of melanosomes leads to the formation of a transparent solution containing dissolved proteins and melanin degradation products that do not exhibit paramagnetic properties. To completely degrade one melanosome of human RPE, 650 ± 100 fmol of superoxide are sufficient. The concentration of paramagnetic centers in a melanolipofuscin granule of human RPE is on average 32.5 ± 10.4% (p < 0.05, 150 eyes) lower than in a melanosome, which indicates melanin undergoing a destruction process in these granules. RPE cells also contain intermediate granules that have an EPR signal with a lower intensity than that of melanolipofuscin granules, but higher than that of lipofuscin granules. This signal is due to the presence of residual melanin in these granules. Irradiation of a mixture of melanosomes with lipofuscin granules with blue light (450 nm), in contrast to irradiation of only melanosomes, results in the appearance of fluorescent melanin degradation products. We suggest that one of the main mechanisms of age-related decrease in melanin concentration in human RPE cells is its destruction in melanolipofuscin granules under the action of superoxide radicals formed during photoinduced oxygen reduction by lipofuscin fluorophores.
KEY WORDS: retinal pigment epithelium, melanosomes, superoxide, melanolipofuscin, lipofuscin, destruction, electron paramagnetic resonanceDOI: 10.1134/S0006297917080065
Abbreviations: RPE, retinal pigment epithelium.
Aging causes significant changes in human retinal pigment epithelium
(RPE) cells [1]. In addition to different
biochemical and morphological changes in these nondividing, postmitotic
cells, an “aging pigment” is accumulated with
age – lipofuscin granules and complex pigment granules, such
as melanolipofuscin; at the same time, the number of melanin-containing
melanosomes decreases [2].
Melanosomes located in the body and processes of the eye RPE cell play a double role: they reduce chromatic aberration, increasing visual acuity, and protect cells from oxidative stress, acting as intracellular antioxidants [3-6].
Melanosomes, as specialized organelles of RPE, contain a protein part and melanins themselves (eumelanin). RPE melanosomes are unique in that they are formed already during embryonic development and carry out their biological functions during the entire life of an individual, since melanin is practically not renewed in these postmitotic cells [1].
However, a significant decrease in melanin concentration occurs in RPE cells with age (generally, after 40 years) [7, 8]. Morphological and EPR measurements have shown that human RPE from the age group of 20-30 years contain 36% more melanin that those in the age group of 60-90 years [9]. At the same time, a significant decrease in the number of melanosomes is observed in RPE cells. While in the age group under 20 years melanosomes occupy ~8% RPE cell volume, in the age group of 41-90 years their volume gradually decreases to 3.5% [7]. In the age group of 90-101 years, melanosomes are almost completely replaced by mixed melanolipofuscin granules [10].
Nevertheless, specific mechanisms of melanosome degradation in RPE cells are still unknown. At least three different mechanisms are currently being discussed that could explain the age-related decrease in melanin content in cells. They are melanosome exocytosis from RPE cells, melanosome degradation by lysosomal system enzymes, and chemical degradation of melanin in melanosomes by oxidative and photoinduced reactions. The first mechanism is not yet confirmed by experimental facts, since melanosomes were found neither in Bruch’s membrane nor in interphotoreceptor matrix. The second mechanism is supported by the increase in the number of melano-lysosomal granules in an RPE cell with age [11]. However, this fusion of melanosomes and lysosomes apparently results only in degradation of the protein part of melanosomes. Melanin itself remains intact, since lysosomes do not contain enzymes capable of its degradation. It was also suggested [12] that melanin may be degraded by oxidative destruction in melanolipofuscin granules formed during autophagic digestion of melanosomes and formation of non-lysing residues. However, neither the data obtained in that work [12] nor the suggestion of the authors on the origin of melanolipofuscin granules in RPE cells were confirmed in subsequent publications.
The third mechanism appears most probable, which is chemical degradation of melanosomes in oxidative and photooxidative processes that may develop in melanosomes or melanolipofuscin granules. Relatively recently, it has been shown that dihydroxyindole-carboxylic monomeric units of eumelanin undergo oxidative decay under the action of ultraviolet (UV) light and/or hydrogen peroxide with the formation of free pyrrole-3-carboxylic acids [13]. During melanin destruction, fluorescent decay products are formed [14-17]. The destruction process may be caused by either intensive illumination with UV and/or visible light, or by treatment with strong oxidants. For instance, prolonged illumination (for ~984 h) of melanosomes from bovine RPE in the presence of oxygen by intensive light in the range 348-591 nm (UVA-visible light) was shown to result in a twofold decrease in melanin concentration as determined by EPR signal intensity [18]. However, illumination of melanosomes under the same conditions for a shorter but still considerable time (500 h) does not result in a decrease in melanin concentration in them [18]. This indicates high resistance of melanosomes to intensive UVA-visible irradiation. We have also shown recently that both DOPA-melanin and bovine eye RPE melanosomes are, on one hand, extremely resistant to UV and intensive visible light, and, on the other hand, are relatively easily destroyed in the dark in the presence of millimolar quantities of hydrogen peroxide [19]. Since UV does not reach RPE tissue located at the very bottom of the eyeball, and intensive irradiation of this tissue by visible light is possible only under extreme conditions, it can be assumed that destruction of melanosome melanin during aging is due to a certain oxidant that is regularly formed in these cells during normal vital activities. Moreover, the concentration of this oxidant in RPE cells should increase with age, and it should be able to induce complete melanosome degradation. Such active molecules capable of melanin destruction may be superoxide radicals. The latter can be generated in significant quantities both during dark processes, for example, in the mitochondrial respiratory chain, and under the action of visible light. The source of light-induced superoxide radical generation in an RPE cell has long been known. These are lipofuscin granules, which, when exposed to visible light, especially in the blue range, reduce oxygen to superoxide radicals [20, 21]. In this case, the intensity of superoxide generation by lipofuscin granules should increase with age, since the number of these granules rises sharply with age.
It can be assumed that age-related melanin destruction by superoxide radicals occurs according to two main scenarios. In the first case, superoxide radicals attack melanosomes that are in the form of separate granules loosely located in the RPE cell cytoplasm. In the second case, destruction of melanosomes occurs after their fusion with the source of superoxide radicals – lipofuscin granules. In the first case, the age-related decrease in melanin concentration in an RPE cell occurs due to an increase in the number of free melanosomes with decreased melanin content. However, as we showed earlier, the average melanin concentration in melanosomes is practically unchanged with age [22]. In the second case, age-related decrease in melanin concentration in a RPE cell occurs due to a decrease in the number of melanosomes caused by their fusion with lipofuscin granules and subsequent melanin destruction already inside these complex granules. Then, if melanin destruction occurs within melanolipofuscin granules, a decrease in melanin concentration may be expected in them. Gradual melanin destruction in melanolipofuscin granules would lead to accumulation of melanolipofuscin granules with minor inclusions of melanin that has not yet been destroyed by superoxide radicals.
The aim of this study was, first, to experimentally test the ability of superoxide radicals to cause complete melanin destruction in melanosomes under physiological conditions, and second, to compare melanin concentration in melanosomes and melanolipofuscin granules.
MATERIALS AND METHODS
Reagents from Sigma, Sigma-Aldrich, and Fluka (all from USA) were used in this study. All experiments were carried out at room temperature.
Isolation of melanosomes, melanolipofuscin, and lipofuscin granules from human eye retinal pigment epithelium cells. Cadaver human eyes were obtained from the Eye Tissue Bank of S. N. Fyodorov Eye Microsurgery Complex. A total of 150 cadaver eyes from donors 45-70 years of age were analyzed. Pigment granules were isolated from retinal pigment epithelium cells by the modified procedure proposed in [23]. RPE cells in 0.1 M potassium phosphate buffer, pH 7.4, were treated with ultrasound two times for 40 s, at 4°C and maximum resonance. Then intact cell membranes were removed by centrifugation at 60g for 15 min. The supernatant was centrifuged at 6000g for 15 min, and the residue was resuspended in 0.3 M sucrose and centrifuged in sucrose density gradient (2.0, 1.8, 1.6, 1.5, 1.4, 1.2, 1.0, 0.3 M) at 103,000g for 1 h. During centrifugation, pigment granules were separated into fractions. Melanosomes formed a dark brown residue. Between the layers with sucrose density from 1.0 to 1.4 M, layers of lipofuscin and intermediate granules were concentrated, and in the gradient region with sucrose density of 1.6-1.8 M, melanolipofuscin granules were concentrated. Melanosome residue, layers of melanolipofuscin, and lipofuscin granules were washed free from sucrose using potassium phosphate buffer, resuspended in phosphate buffer, and stored in a freezer at –20°C. The concentration of granules was determined in a Goryaev chamber using a MikMed-2 (LOMO, Russia) fluorescent microscope equipped with a camera.
Bovine RPE melanosomes were obtained similarly to human RPE melanosomes.
Squid melanin. Sepia melanin was obtained by purifying squid ink bag melanin. For this, ink bag melanin was diluted in 2 M KOH, the undissolved residue was separated, and then the resulting solution was acidified with hydrochloric acid to pH 2.0, melanin residue was separated, and this procedure was repeated once more. The residue of sepia melanin was washed with a weak solution of hydrochloric acid (10 mM), dried, and used as a uniform homogeneous suspension in potassium phosphate buffer.
Degradation products of melanins. Degradation products of melanins under the action of light and superoxide radicals were registered by accumulation of low molecular weight fluorescent compounds, which were measured at the excitation light wavelength of 440-470 nm [15]. For that, the low molecular weight degradation products were separated from the intact part of the polymer centrifugation (6000g, 20 min) in tubes containing an Amicon Ultra-0.5 ml 3K (Millipore, USA) filter element. Emission intensity was measured on a Shimadzu RF5301PC spectrofluorophotometer (Japan). The concentration of melanin destruction products was estimated by comparing fluorescence spectra of irradiated samples containing fluorophores with a dark control. As a second control, irradiated samples were used that contained melanin but not the fluorophore.
Kinetics of melanin destruction by superoxide radicals. Reaction of melanins with superoxide radicals was carried out in 0.1 M potassium phosphate buffer with intensive stirring. Potassium superoxide (Sigma) was used as a source of superoxide, either as powder or as a uniform suspension in dimethyl sulfoxide (50 mg/ml). Concentrations of 10-100 mM were used for potassium superoxide in the experiments. After the end of the reaction, degradation products were separated from the remaining melanin by centrifugation.
Irradiation of samples with blue light. For sample irradiation, a blue LED lamp was used with irradiation energy of 4 W/cm2 and irradiation wavelength of 450 nm at the distance of 5 cm from the object with constant stirring.
Measuring melanin EPR signal. It is known that melanin pigments, including DOPA-melanin, emit a stable singlet EPR signal with a high spin concentration. It is a characteristic EPR spectrum – a stable narrow signal with g-factor of 2.003 ± 0.002. Samples of melanosomes and melanolipofuscin granules for EPR analysis were prepared with a nozzle made from a 10-15 mm polyethylene tube with inner diameter of 0.45 mm. For each sample, a suspension of melanosomes and melanofuscin granules was taken with a volume of 0.3 ml and quickly frozen in a polyethylene tube in liquid nitrogen (–196°C). The samples were stored in the frozen state until the time of measurements. To measure EPR spectra, a frozen column of a sample was squeezed out of a tube with a piston. EPR spectra were registered at 77°K on a Bruker EMX EPR radiospectrometer (Germany) in a cylindrical resonator. Conditions for recording EPR spectra: ΔH – 50 G; H center – 3440 G; modulation amplitude – 3 G; SHF power – 20 µW. The standard for determining spin concentration – UDD No. 5, calibration certificate No. 905/910-2012.
Statistical analysis of the results was carried out using Student’s t-test.
RESULTS
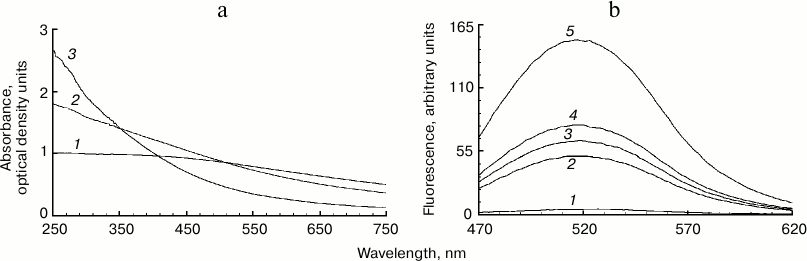
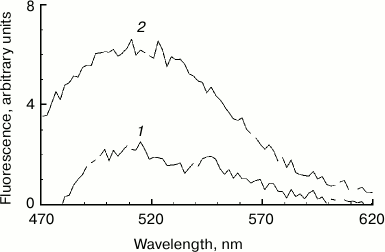
Increased fluorescence of melanosome suspension after reaction with superoxide. Incubation of human RPE melanosome suspension with relatively low concentration of potassium superoxide results in clearing of the suspension and a significant decrease in the absorption in the long-wavelength range of the spectrum (Fig. 1a). Under these circumstances, absorption in the short-wavelength spectral range is increased (below 400 nm), which is probably linked to accumulation of water-soluble products of melanin destruction in the incubation medium. Indeed, as seen on Fig. 1b, addition of potassium superoxide to a suspension of human RPE melanosomes results in the appearance of water-soluble products (with emission maximum at 520 nm) that can be easily isolated from the intact share of melanosomes by centrifugation or filtration. These water-soluble products have emission maximum in the 520-525 nm range (with excitation wavelength of 450-470 nm) depending on the nature of the original melanin pigment. Incubation of melanosomes or melanins of various origins with potassium superoxide also results in the appearance of water-soluble fluorescent products with the same emission maximum as in the case of human RPE melanosomes. Figure 2 demonstrates emission spectra of destruction products of melanosome melanin from bovine eye RPE (Fig. 2a) and of melanin isolated from squid ink bag sepia melanin (Fig. 2b). It is clearly seen that 30-min incubation of a melanin-containing sample with potassium superoxide results in an increase in fluorescence intensity of the water-soluble fraction; the higher the concentration of the added superoxide, the more pronounced is an increase. It is important to note that the process of melanin destruction by superoxide radicals, accompanied by appearance of fluorescent products, occurs under normal conditions that are close to physiological – water medium without the addition of organic solvents, physiological pH (7.4), room temperature.
Fig. 1. Destruction of melanin in human RPE melanosomes by potassium superoxide. a) Bleaching of human RPE melanosome suspension depending on superoxide concentration. The reaction medium contained 0.1 M potassium phosphate buffer, pH 7.4, and 4·108 granules/ml of melanosomes. Curves: 1) initial spectrum; 2) after addition of 35 mM superoxide; 3) after addition of 105 mM superoxide. Spectra were registered after a 2-h incubation of samples after their 10-fold dilution. b) Accumulation of fluorescent products of melanin destruction depending on duration of incubation in the presence of superoxide radicals. The reaction medium contained 0.1 M potassium phosphate buffer, pH 7.4, 1.4·108 granules/ml of melanosomes, and 60 mM KO2. Curves: 1-5) 0, 30, 60, 90, and 180 min of incubation, respectively. Excitation wavelength – 450 nm.
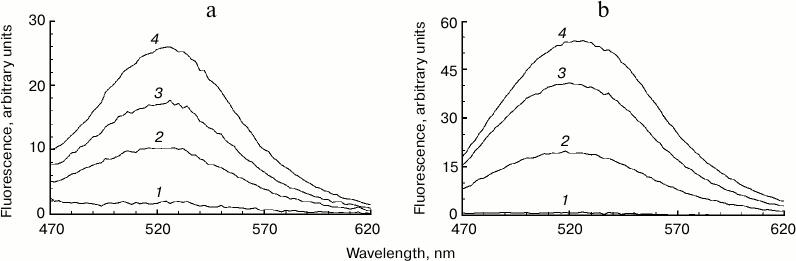
Fig. 2. Accumulation of fluorescent products of melanin destruction depending on superoxide concentration. a) Destruction of bovine eye RPE melanosomes. The reaction medium contained 0.1 M potassium phosphate buffer, pH 7.4, 108 granules/ml of bovine melanosomes, and various superoxide concentrations. Curves: 1) 0; 2) 35 mM; 3) 70 mM; 4) 210 mM. Time for incubation of melanosomes with superoxide – 30 min, excitation wavelength – 450 nm. b) Destruction of squid ink bag melanin. The reaction conditions were the same as for Fig. 2a, sepia melanin concentration – 0.5 mg/ml.
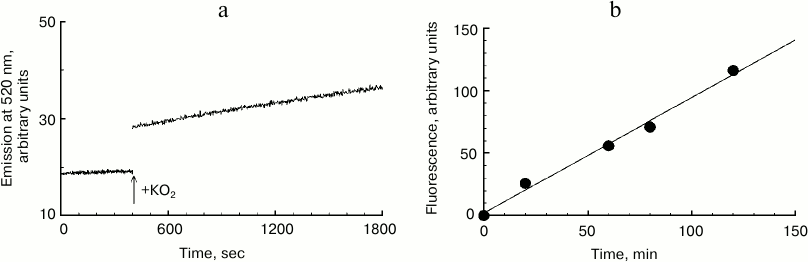
Kinetics of melanosome melanin destruction. In this series of experiments, the effect of potassium superoxide on formation kinetics of fluorescent destruction products of human RPE melanosome melanin was studied (Fig. 3). Figure 3a shows a record of the increase in emission intensity at 520 nm (excitation wavelength 450 nm) for human RPE melanosome suspension upon the addition of 37 mM potassium superoxide. In these experiments, fluorescent products of melanin destruction were not isolated from the intact share of the pigment. Therefore, for minimizing the effect of degradation product fluorescence quenching with black melanin, suspensions of melanosomes were used that had small absorption values at 450 nm. It can be seen that immediately after the addition of KO2 to a melanosome suspension, a gradual increase in fluorescence intensity is observed that is strictly linear. The accumulation of melanosome destruction products during their interaction with potassium superoxide remains practically linear even with long reaction times. In Fig. 3b, accumulation kinetics for human RPE melanosome destruction products is demonstrated under the action of 60 mM superoxide. It can be clearly seen that this process is linear, and the rate of accumulation of destruction products in this case is one relative unit per min of incubation.
Fig. 3. Destruction kinetics for human RPE melanosomes. a) Initial kinetics of emission increase at 520 nm after addition of potassium superoxide to a melanosome suspension. The reaction medium contained 0.1 M potassium phosphate buffer, 7·107 granules/ml of melanosomes and 37 mM KO2. b) Accumulation kinetics of fluorescent products of human melanosome destruction. The reaction medium contained 0.1 M potassium phosphate buffer, pH 7.4, 1.3·108 granules/ml of melanosomes, and 60 mM KO2.
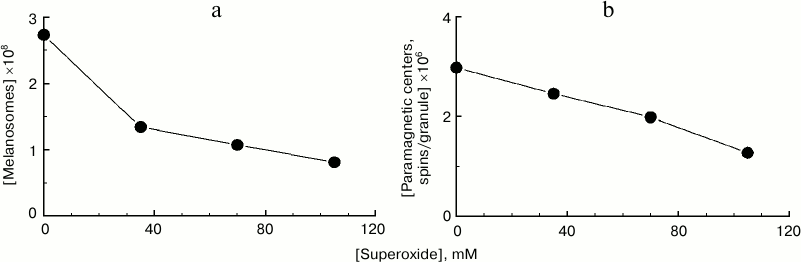
Degradation of melanosomes during their interaction with superoxide radicals. The reaction of melanosomes with superoxide results not only in melanosome melanin destruction accompanied by accumulation of fluorescent products, but also to degradation and disappearance of melanosomes as organelles themselves. This can be clearly seen in Fig. 4a, demonstrating the dependence of the number of melanosomes in a sample on potassium superoxide concentration. Potassium superoxide was added at various concentrations to the same initial concentration of melanosome suspension, and after 2-h incubation, the number of melanosomes was again determined microscopically in the sample. With superoxide concentration of 100 mM, an almost 3-fold decrease in the number of melanosomes in the sample is observed after 2 h of incubation. By increasing superoxide concentration to 170-180 mM, complete degradation of all melanosomes initially present in the suspension was achieved. Under these conditions, the suspension becomes transparent, and microscopic control shows total absence of visible granules. Under these conditions, disappearance of the initial EPR melanin signal is observed, indicating complete polymer destruction. Figure 4b demonstrates the dependence of concentration of paramagnetic centers per granule on superoxide concentration. Incubation of human RPE melanosomes with various concentrations of superoxide for 2 h results in a significant decrease in the concentration of paramagnetic centers per granule, indicating a decrease in melanin content in melanosomes.
Fig. 4. Degradation of human RPE melanosomes by potassium superoxide. a) Superoxide causes a decrease in concentration of melanosomes. The initial concentration of melanosomes was 2.7·108 granules/ml; incubation time – 2 h. b) Superoxide causes a decrease in concentration of paramagnetic centers in melanosome granules. The initial concentration of paramagnetic centers was 3·106 spins/granule; incubation time – 2 h.
Thus, superoxide causes both a decrease in melanin content within a single melanosome and a general decrease in the concentration of melanosomes themselves.
Photodegradation of melanosome melanin during illumination of a mixture of melanosomes and lipofuscin granules by blue light. In the previous experimental sections, we demonstrated that superoxide causes melanosome melanin destruction at high concentrations. It was necessary to attempt observing such melanosome melanin destruction with a relatively low generation of superoxide by lipofuscin granules. We observed the appearance of fluorescent products of melanin decay upon irradiation of a mixture of melanosomes with lipofuscin granules by blue light (Fig. 5). In this experiment, a mixture of granules was used with the ratio of one melanosome to five lipofuscin granules. The suspension containing this mixture of granules was treated with ultrasound for 15 s to obtain a uniform suspension. Irradiation of this mixture for 90 min with blue light of a LED lamp with energy of 30 J/cm2 resulted in the appearance of the characteristic fluorescent products of melanin destruction (Fig. 5, curve 1). This process intensifies with longer irradiation (Fig. 5, curve 2). In control experiments, blue light irradiation of a suspension containing only melanosomes did not result in accumulation of fluorescent products of melanin destruction. These results show that superoxide radicals generated by lipofuscin granules under the action of light also cause destruction of melanosome melanin.
Fig. 5. Appearance of fluorescent products of melanin destruction during illumination of a mixture of melanosomes and lipofuscin granules. The reaction medium contained 0.1 M potassium phosphate buffer, pH 7.4, 2·107 granules/ml for melanosomes, 108 granules/ml for lipofuscin granules. Curves: 1) 90 min of illumination; 2) 180 min of illumination with blue light.
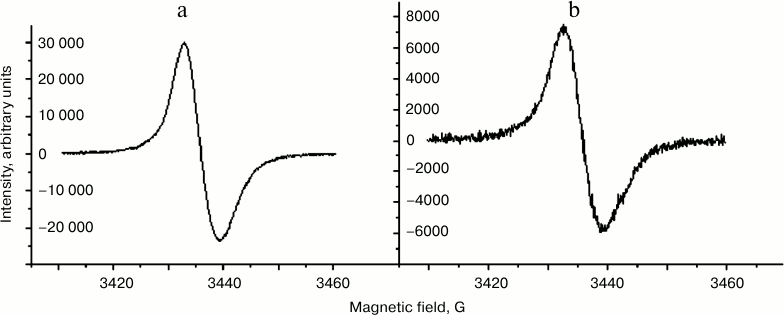
Comparison of EPR characteristics of melanosomes and melanolipofuscin granules. It is known that human eye RPE melanosomes emit a stable singlet EPR signal with very high spin concentration, and that the nature of this signal is due to the presence of melanin in melanosomes [24-26]. Here we show that melanolipofuscin granules isolated from human eye RPE emit an EPR signal nearly identical to that of melanosomes in its form (Fig. 6) and characteristics. However, the concentration of paramagnetic centers attributed to a single organelle is usually significantly higher for melanosomes than for melanolipofuscin granules. In our experiments, we compared mean concentration of melanin in melanosomes and melanolipofuscin granules. A total of 150 human cadaver eyes was used in this study. Melanin concentration in human RPE melanosomes determined by EPR signal intensity was 32.5 ± 10.4% (p < 0.05) higher than in a melanolipofuscin granule. We assume that this is due to melanin destruction in the melanolipofuscin granule.
Fig. 6. EPR spectra of melanosomes (a) and melanolipofuscin granules (b) from human RPE cells.
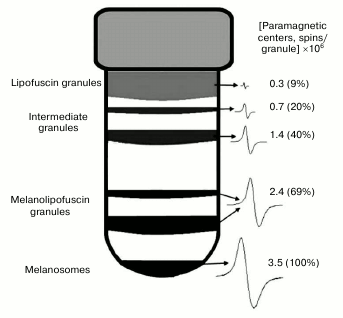
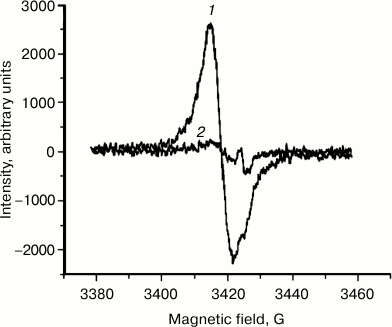
EPR signal distribution in fractions of granules separated in sucrose density gradient. We found that an EPR signal characteristic of melanin is observed in practically all fractions of granules separated in a sucrose density gradient. In these experiments, EPR signal intensity was measured for various fractions of granules separated in a sucrose density gradient (Fig. 7). The concentration of paramagnetic centers was attributed to the number of granules present in each fraction. The maximum signal attributed to a single granule was observed in melanosomes, the minimum signal in the layer of lipofuscin granules. The EPR signal of lipofuscin and intermediate granules corresponded to the EPR signal of melanin in its characteristics, but it was significantly lower in intensity (Fig. 8, curve 1). The EPR signal of lipofuscin granules disappeared almost completely when these granules were treated with potassium superoxide (Fig. 8, curve 2).
Fig. 7. Distribution of paramagnetic centers in pigment granules of human RPE separated in a sucrose density gradient.
Fig. 8. Loss of paramagnetic properties of lipofuscin granules after their incubation in the presence of potassium superoxide. Samples containing 2·108 granules/ml of lipofuscin granules were separated into two identical samples. The first sample was used as control, and KO2 was added to the second sample. Both samples were incubated for 2 h and then were frozen in liquid nitrogen, and the intensity of the EPR signal was measured. Curves: 1) EPR spectrum of the first sample; 2) EPR spectrum of the second sample.
DISCUSSION
The results of our study indicate that regardless of the nature of melanin, i.e. whether melanin from human or bovine eye RPE melanosomes, or melanin from squid ink bag, or synthetic DOPA-melanin (not shown in this work), superoxide radicals at physiological pH and in the absence of organic solvents cause chemical destruction of melanin structure with the formation of fluorescent metabolites with emission maximum at 520-525 nm, resulting in complete degradation of the melanosome as an intracellular organelle. The identity of fluorescence spectra for decay products of melanin of various origins apparently indicates a similar structure of these molecules and allows qualitative and quantitative identification of melanins by their fluorescence spectra.
Melanin was shown earlier to interact with superoxide radicals, causing both the oxidation of the latter to molecular oxygen and reduction of superoxide to hydrogen peroxide [27-30]. However, no data on structural destruction of melanin itself and on formation of fluorescent destruction products upon the interaction of melanosome melanin with superoxide radicals was given in those studies.
The mechanism of melanin destruction by superoxide is not clear. It may be linked not only to a direct interaction of a superoxide molecule with monomeric units of the melanin polymer, but also to formation of other active molecules, for example, hydroxyl radicals, capable of breaking bonds in the polymer. It is known that superoxide radicals can form both hydrogen peroxide and highly active hydroxyl radicals under certain conditions [31]. The question of whether hydroxyl radicals and/or hydrogen peroxide take part in the process of melanosome melanin destruction in our experiments remains open and requires additional experiments that are planned for the near future. However, the superoxide that may be formed in RPE cells mainly by lipofuscin granules under the action of light in large quantities is apparently the key molecule initiating the whole process of melanin degradation.
The kinetics of melanin destruction by potassium superoxide is linear, and with a sufficient amount of superoxide molecules complete melanosome degradation is observed. This is evidenced by data on a decrease in intensity of EPR signal and a decrease in the number of melanosome granules during their interaction with superoxide (Fig. 4, a and b). Destruction of melanin in a melanosome results in a decrease in the number of paramagnetic centers per granule. After destruction of melanin, disappearance of a melanosome as a separate granule and formation of transparent solution with absorption maximum at 280 nm is observed microscopically. Treatment of this solution with 10% trichloroacetic acid results in residue settling and disappearance of the absorption maximum at 280 nm in the supernatant. This apparently indicates that the protein part of a melanosome, which was earlier bound to melanin in a stable complex, is released and transits into a dissolved state.
Our data regarding the dependence of the decrease in the number of human RPE melanosomes on superoxide concentration allows estimation of the number of superoxide molecules required for complete degradation of a single melanosome. In our experiments, 175 ± 25 µmol of superoxide on average were required for complete destruction of 2.7·108 melanosomes. Therefore, 650 ± 100 fmol of superoxide are required for complete degradation of one melanosome. It is known [21] than in a human RPE cell containing ~700 lipofuscin granules, under relatively low blue light irradiation (1.5 mW/cm2), 0.56 fmol of superoxide is formed in 1 min. It follows that with this activity, less than a day (approximately 19 h) is needed for generation of 650 fmol of superoxide required for destruction of one melanosome.
It is obvious that free superoxide radicals formed in a cell may react not only with a single melanosome, but also with a few of them or, probably, with other cellular components as well. This will result in accumulation of melanosomes with different quantitative contents of melanin in a RPE cell. However, as we showed earlier [22], melanin concentration decrease observed during aging in RPE cells is linked not to the overall drop in its content in melanosomes, but to the decrease in their number, since melanin concentration per melanosome does not change with age. It follows that either melanosomes with a lower content of melanin pigment in a granule (if they actually exist) do not enter the major melanosome fraction during their isolation from RPE cells, or they do not exist, and targeted melanin destruction occurs directly in individual melanosomes. Melanin destruction individual melanosomes may occur in the case of fusion of a melanosome and a lipofuscin granule with the formation of an intermediate melanolipofuscin granule [7]. Destruction of melanin by superoxide radicals formed by the action of light on fluorophores of a lipofuscin granule may occur in these intermediate organelles. This is confirmed by our experiments with blue light irradiation of a mixture of lipofuscin granules and melanosomes, in which formation of melanin destruction products was observed (Fig. 5). In melanolipofuscin granules, the superoxide radicals generated by lipofuscin fluorophores will directly react with melanin, resulting in its destruction and, therefore, in a decrease in its concentration. In this case, the amount of melanin per granule should be lower in a melanolipofuscin granule than in a melanosome. This is confirmed by our data on the intensity of EPR signal in melanosomes and melanolipofuscin granules (Fig. 6). On average in all experiments, melanin concentration per granule was 32.5 ± 10.4% lower in melanolipofuscin than in a melanosome. This indicates that a possible reason for the age-related decrease in melanin content of human RPE cells is a decrease in the number of melanosomes due to their fusion with lipofuscin granules and the following photoinduced destruction of the pigment by superoxide radicals formed upon absorption of light by lipofuscin fluorophores. Gradual degradation of melanosomes inside lipofuscin granules will lead to the appearance of intermediate granules containing various amounts of intact melanin. Such granules will have different density and may be separated by centrifugation in a sucrose density gradient. Our experiments have indeed shown the presence of intermediate granules which differ by their melanin content measured by the intensity of the EPR signal (Fig. 7). The upper layer, containing predominantly lipofuscin granules, has the lowest intensity EPR signal. This signal disappears after the treatment of the fraction of granules by superoxide radicals (Fig. 8). We observed a similar effect when treating melanosomes or melanin with superoxide, which apparently indicates destruction of residual melanin in these granules. Lipofuscin granules, which do not contain melanin, do not exhibit paramagnetism.
Thus, our results indicate that age-related melanin disappearance in a RPE cell is due to a significant extent to destruction of pigment located in melanolipofuscin granules by superoxide radicals generated by lipofuscin fluorophores under the action of visible light. The age-related decrease in the number of melanosomes in RPE cells, where they carry out shielding and antioxidant functions, and the simultaneous accumulation of melanolipofuscin and lipofuscin granules in these cells may result in photooxidative stress, which is directly linked to the development of such major pathologies as age-related macular degeneration of the retina and some forms of pigmented retinitis.
Acknowledgments
This study was supported by the Russian Foundation for Basic Research (project No. 15-29-03831).
REFERENCES
1.Boulton, M. (1998) Melanin and the retinal pigment
epithelium, in The Retinal Pigment Epithelium: Function and
Disease (Marmor, M. F., and Wolfensberger, T. J., eds.) Oxford
University Press, N. Y., pp. 65-85.
2.Feeney-Burns, L. (1980) The pigments of the retinal
pigment epithelium, Curr. Top. Eye Res., 2, 119-178.
3.Ostrovsky, M. A., and Dontsov, A. E. (1985)
Physiological functions of melanin in body, Hum. Physiol.,
11, 670-678.
4.Dontsov, A. E., and Ostrovsky, M. A. (2005)
Antioxidant role of screening pigments of eye – melanins and
ommochromes and physical-chemical mechanisms of their action, in
Chemical and Biological Kinetics. New Horizons [in Russian],
Vol. 2, Nauka, Moscow, pp. 155-174.
5.Wang, Z., Dillon, J., and Gaillard, E. R. (2006)
Antioxidant properties of melanin in RPE cells, Photochem.
Photobiol., 82, 474-479.
6.Dontsov, A. (2014) Protective Action of Melanins
under Oxidative Stress, Lambert Academic Publishing,
Saarbrucken, p. 165.
7.Feeney-Burns, L., Hilderbrand, E. S., and Eldridge,
S. (1984) Aging human RPE: morphometric analysis of macular,
equatorial, and peripheral cells, Invest. Ophthalmol. Vis. Sci.,
25, 195-200.
8.Weiter, J. J., Delori, F. C., Wing, G. L., and
Fitch, K. A. (1986) Retinal pigment epithelial lipofuscin and melanin
and choroidal melanin in human eyes, Invest. Ophthalmol. Vis.
Sci., 27, 145-152.
9.Rozanowski, B., Cuenco, J., Davies, S., Shamsi, F.
A., Zadlo, A., Dayhaw-Barker, P., Rozanowska, M., Sarna, T., and
Boulton, M. (2008) The phototoxicity of aged human retinal melanosomes,
Photochem. Photobiol., 84, 650-657.
10.Feeney-Burns, L., Burns, R. P., and Gao, C. L.
(1990) Age-related macular changes in humans over 90 years old, Am.
J. Ophthalmol., 109, 265-278.
11.Schraermeyer, U., Peters, S., Thumann, G.,
Kociok, N., and Heimann, K. (1999) Melanin granules of retinal pigment
epithelium are connected with the lysosomal degradation pathway,
Exp. Eye Res., 68, 237-245.
12.Warburton, S., Davis, W. E., Southwick, K., Xin,
H., Woolley, A. T., Burton, G. F., and Thulin, C. D. (2007) Proteomic
and phototoxic characterization of melanolipofuscin: correlation to
disease and model for its origin, Mol. Vis., 13,
318-329.
13.Wakamatsu, K., Nakanishi, Y., Miyazaki, N.,
Kolbe, L., and Ito, S. (2012) UVA-induced oxidative degradation of
melanins: fission of indole moiety in eumelanin and conversion to
benzothiazole moiety in pheomelanin, Pigment Cell Melanoma Res.,
25, 434-445.
14.Korzhova, L. P., Frolova, E. V., Romanov, Yu. A.,
and Kuznetsova, I. A. (1989) Photo-induced destruction of DOPA-melanin,
Biochemistry (Moscow), 54, 992-998.
15.Kayatz, P., Thumann, G., Luther, T. T., Jordan,
J. F., Bartz-Schmidt, K. V., Esser, P. J., and Schraermeyer, U.
(2001) Oxidation causes melanin fluorescence, Invest. Ophthalmol.
Vis. Sci., 42, 241-246.
16.Elleder, M., and Borovansky, J. (2001)
Autofluorescence of melanin induced by ultraviolet radiation and near
ultraviolet light. A histochemical and biochemical study, Histochem.
J., 33, 273-281.
17.Borovansky, J., and Elleder, M. (2003)
Melanosomes degradation: fact or fiction, Pigment Cell Res.,
16, 280-286.
18.Zadlo, A., Rozanowska, M. B., Burke, J. M., and
Sarna, T. J. (2006) Photobleaching of retinal pigment epithelium
melanosomes reduces their ability to inhibit iron-induced peroxidation
of lipids, Pigment Cell Res., 20, 52-60.
19.Dontsov, A. E., Sakina, N. L., Koromyslova, A.
D., and Ostrovsky, M. A. (2015) Influence of UV irradiation and
hydrogen peroxide on the antiradical and antioxidant activity of
DOPA-melanin and melanosomes from cells of retinal pigment epithelium,
Dokl. Acad. Nauk, 7, 1623-1628.
20.Ostrovsky, M. A., Dontsov, A. E., Sakina, N. L.,
Boulton, M., and Jarvis-Evans, J. (1992) Ability of lipofuscin granules
from human retinal pigment eye epithelium to photosensibilize lipids
oxidation under visible light, Sensor Syst., 6,
51-54.
21.Boulton, M., Dontsov, A., Jarvis-Evans, J.,
Ostrovsky, M., and Svistunenko, D. (1993) Lipofuscin is a
photo-inducible free radical generator, J. Photochem. Photobiol. B.
Biol., 19, 201-204.
22.Gulyaev, A. B., Dontsov, A. E., Ilyasova, V. B.,
and Ostrovsky, M. A. (1993) Melanin content determination in
melanosomes of eye pigment epithelium depending on human age, Rep.
Acad. Sci., 333, 257-259.
23.Boulton, M., and Marshall, J. (1985)
Re-pigmentation of human retinal pigment epithelial cell in
vitro, Exp. Eye Res., 41, 209-218.
24.Ostrovsky, M. A., and Kayushin, L. P. (1963)
Study of electron paramagnetic resonant in retina under light action,
Dokl. Acad. Nauk, 151, 986-988.
25.Ostrovsky, M. A. (1966) Reversible changes in EPR
signals of eye pigment epithelium under visible light, in Free
Radical Processes in Biological Systems, Proc. Moscow Soc.
Naturalists [in Russian], Nauka, Moscow, pp. 275-279.
26.Bilinska, B., Pilawa, B., Zawada, Z., Wylegala,
E., Wilczok, T., Dontsov, A. E., Sakina, N. L., Ostrovsky, M. A., and
Ilyasova, V. B. (2002) Electron spin resonance investigations of human
retinal pigment epithelium melanosomes from young and old donors,
Spectrochim. Acta, A Mol. Biomol. Spectroscopy, 58,
2257-2264.
27.Goodchild, N. T., Kwock, K., and Lin, P. S.
(1981) Effect of superoxide anion on the survival of Chinese hamster
cells, in Oxygen and Oxyradicals in Chemistry and Biology
(Rodgers, M. A. J., and Powers, E. L., eds.) Academic Press, N. Y., p.
648.
28.Lapina, V. A., Dontsov, A. E., Ostrovsky, M. A.,
and Emanuel, N. M. (1985) Interaction of oxygen anion-radicals with eye
melanins and ommochromes, Rep. Acad. Sci., 280,
1463-1465.
29.Korytowski, W., Kalyanaraman, B., Menon, T. A.,
Sarna, T., and Sealy, R. C. (1986) Reaction of superoxide anions with
melanins: electron spin resonance and spin trapping studies,
Biochim. Biophys. Acta, 882, 145-153.
30.Ostrovsky, M. A., Sakina, N. L., and Dontsov, A.
E. (1987) An antioxidative role of ocular screening pigments, Vis.
Res., 27, 893-899.
31.Chedekel, M. R., Smith, S. K., Post, P. W.,
Pokora, A., and Vessell, D. L. (1978) Photodestruction of pheomelanin:
role of oxygen, Proc. Natl. Acad. Sci. USA, 75,
5395-5399.