Toward Gene Therapy of Hypertension: Experimental Study on Hypertensive ISIAH Rats
M. N. Repkova1,2, A. S. Levina1,2, A. A. Seryapina1,3, N. V. Shikina1,4, E. V. Bessudnova1,4, V. F. Zarytova1,2, and A. L. Markel1,3*
1Novosibirsk State University, 630090 Novosibirsk, Russia; E-mail: markel@bionet.nsc.ru2Institute of Chemical Biology and Fundamental Medicine, Siberian Branch of the Russian Academy of Sciences, 630090 Novosibirsk, Russia; E-mail: repk@niboch.nsc.ru
3Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences, 630090 Novosibirsk, Russia; E-mail: icg-adm@bionet.nsc.ru
4Institute of Catalysis, Siberian Branch of the Russian Academy of Sciences, 630090 Novosibirsk, Russia; E-mail: bic@catalysis.ru
* To whom correspondence should be addressed.
Received September 27, 2016; Revision received November 7, 2016
TiO2-based nanocomposites were prepared to deliver oligonucleotides into cells. The nanocomposites were designed by the immobilization of polylysine-containing oligonucleotides on TiO2-nanoparticles (TiO2·PL-DNA). We showed for the first time the possibility of using the proposed nanocomposites for treatment of hypertensive disease by introducing them into hypertensive ISIAH rats developed as a model of stress-sensitive arterial hypertension. The mRNA of the gene encoding angiotensin I-converting enzyme (ACE1) involved in the synthesis of angiotensin II was chosen as a target. Administration (intraperitoneal injection and inhalation) of the nanocomposite showed a significant (by 20-30 mm Hg) decrease in systolic blood pressure when the nanocomposite contained the ACE1 gene-targeted oligonucleotide. When using the oligonucleotide with a random sequence, no effect was observed. Further development and improvement of the inhalation nanocomposite drug delivery to systemic hypertensive disease treatment promises new possibilities for clinical practice.
KEY WORDS: antisense oligonucleotides, hypertension, ISIAH rats, nanocompositesDOI: 10.1134/S000629791704006X
Abbreviations: ACE1, angiotensin I-converting enzyme; BP, arterial blood pressure; ISIAH, inherited stress-induced arterial hypertension; NA, nucleic acid; TiO2·PL-DNA, nanocomposites designed by the immobilization of polylysine-containing oligonucleotides on TiO2-nanoparticles.
Diseases of the cardiovascular system are still the leading cause of
death in humans in developed countries [1].
Hypertension plays a central role in the development of severe
complications, such as myocardial infarction and cerebral stroke.
Despite great medical advances in the creation of effective
antihypertensive drugs, the necessary control of blood pressure (BP) is
possible only in every third patient with essential hypertension [2]. The efficacy of the pharmacological treatment of
hypertension seems to reach their ceiling, and further progress depends
on the development of innovative approaches and drugs.
A new trend is gene therapy, which implies the use of nucleic acid (NA) fragments aimed at the key links of the disease. Gene therapy in a broad sense implies the introduction into the body for therapeutic purposes of specially designed NA fragments that have an impact on the target node links in the pathogenesis of the disease.
In this work, we studied the possibility of the treatment of hypertension by using TiO2·PL-DNA nanocomposites containing conjugates of antisense oligonucleotides with polylysine (PL-DNA) immobilized on TiO2 nanoparticles. Titanium dioxide nanoparticles provide delivery of oligonucleotides into cells, and the oligonucleotides can block the function of key genes for development of hypertension. We used the nanocomposite bearing an oligonucleotide that is targeted to the gene encoding the synthesis of angiotensin I-converting enzyme (ACE1) involved in pathogenesis of hypertension. The ability of the proposed nanocomposites to decrease BP was studied on ISIAH rats, which are an experimental model of inherited stress-induced arterial hypertension. Along with traditional intraperitoneal injection of the nanocomposites, we used the inhalation way of administration.
MATERIALS AND METHODS
TiO2 nanoparticles in the crystal form (anatase) were prepared by hydrolysis of titanium isopropoxide with additional treatment of the formed soles by glycidyl isopropyl ether [3]. Antisense oligodeoxyribonucleotide DNA1 (5′-GCCCCCATGGCGCGGTp) targeted to mRNA of the ACE1 gene and oligodeoxyribonucleotide DNA2 (5′-CCGTCGGTACCGGCCGp) with a random sequence were synthesized using an ASM-800 DNA synthesizer (Biosset, Russia) with phosphoroamidite monomers (Glen Research, USA). To synthesize polylysine-containing oligonucleotides, the 3′-terminal phosphate groups were activated by the triphenylphosphine/dipyridyldisulfide pair in the presence of nucleophilic catalyst 4-dimethylaminopyridine-1-oxide (DMAPO). The reaction of the resulting activated derivatives with polylysine led to the formation of PL-DNA conjugates with a yield of ~90% [4]. TiO2·PL-DNA nanocomposites were prepared by the immobilization of PL-DNA on TiO2 nanoparticles [3, 5] with a yield of 90-95%. The capacity of the nanocomposites for oligonucleotides was ~60 nmol/mg.
We used male hypertensive ISIAH rats aged five months as a model of stress-induced arterial hypertension. This rat line was developed by long-term selection for high blood pressure under emotional stress [6, 7]. The work was performed at the facilities of the vivarium of the Institute of Cytology and Genetics (Siberian Branch, Russian Academy of Sciences). The rats were kept under standard conditions; water and balanced food were provided without limitation. All experiments were conducted according to international rules for research using experimental animals (UFAW Handbook) and were approved by the Bioethics Committee of the Institute of Cytology and Genetics (Siberian Branch, Russian Academy of Sciences).
The rats were divided into three groups of five each. The first and the third group of rats received intraperitoneal injection of the TiO2·PL-DNA1 and TiO2·PL-DNA2 nanocomposites, respectively. The second group of rats was subjected to inhalation of the TiO2·PL-DNA1 nanocomposite. In the case of intraperitoneal injection, a single dose of the nanocomposite was 0.5 ml (1 mg/ml, 60 µM for oligonucleotide). In the case of inhalation, the rat was placed in a wire restrictor. A funnel-equipped nebulizer was placed in front of the rat’s nostrils to deliver the suspension of the nanocomposite to the respiratory tract. The dose of the preparation was the same as in the case of intraperitoneal injection. Systolic BP in the rats was measured by the indirect tail cuff method using a BioPac System (USA) instrument. The pressure was measured two days before the experiment, then the next day and one, two, and three weeks after administration.
The data were statistically processed using dispersion analysis. We calculated two-factor dispersion complex containing factors of the sample introduction (three grades) and measurement time (five grades). The post-hoc Tukey test was used to compare mean values.
RESULTS AND DISCUSSION
The development of the so-called antisense approach based on the use of the nucleic acid fragments as a new generation of pharmaceuticals is now underway. NA fragments (oligonucleotides and their analogs) due to their physicochemical and pharmacological properties affect genetic material through fundamentally different mechanisms compared to drugs currently used in medicine. RNA and DNA fragments, short interfering RNA (siRNA), ribozymes and deoxyribozymes, etc., which are used as nucleic acid fragments, can recognize certain sequences in genes or mRNA and selectively and efficiently inhibit their functions in cells and in the body with minimal side effects. Due to complementary interactions, nucleic acid fragments act on the root cause of the disease associated with the presence of particular genes, and this is a significant advantage of these compounds compared to conventional pharmaceutical preparations. In addition, the effects of NA-based drugs are generally more durable and permanent compared to traditional preparations that must be taken at least once a day.
Research in this area began long ago, and the results of using gene therapy in animal models of hypertensive disease are described in many publications [8]. However, one can note the presence of a temporary break in the number of these studies since the 2000s primarily because of the lack of effective vectors for transferring genetic material into cells. Increasing interest in the problem of gene therapy of hypertension has been revived in recent years due to the emergence of a relatively safe vector, adeno-associated viruses (AAV) [9-11], and to the success of nanomedicine.
The use of viral vectors has some limitations because they may cause immune or inflammatory response. Therefore, the prospects of gene therapy will likely be associated with nonviral vectors that might provide safe delivery of NA-based drugs for the regulation of gene expression inside cells and organisms [12]. Several reviews were published in 2015 on the delivery of NA-based drugs into cells (e.g. [13]). The possible use of nonviral vectors was demonstrated with nanoparticles capable of transferring drugs, including oligonucleotides [14].
We earlier created TiO2·PL-DNA nanocomposites consisting of titanium dioxide nanoparticles and polylysine-containing DNA fragments to deliver them into cells without transfection agents or physical impacts on cells [3, 5]. It was shown that the proposed nanocomposites exhibited low toxicity (TC50/ml ≈ 1.8 mg/ml) and very high activity against influenza A virus in cell culture [15-17].
Here we studied the ability of the proposed nanocomposites to affect specific genes that modify functions of the blood pressure-regulating systems and prevent or correct stress-induced arterial hypertension. For this study, we used ISIAH rats, whose hypertension is the result of a severe hereditary predisposition to the development of hypertensive reactions under emotional stress. The ISIAH rats also show other characteristic features of arterial hypertension: hypertrophy of the left ventricle, increase in the wall thickness of the small arteries, alterations in kidney functions, signs of glomerulosclerosis, and changed reactivity of sympathetic adrenal and other neuroendocrine systems [18-21].
To date, several genetic targets were found that might be used for gene-targeted therapy of hypertension with the use of antisense oligonucleotides. The gene encoding angiotensin I-converting enzyme (ACE1) involved in the synthesis of angiotensin II was chosen as a target because ACE1 blockade is a common and effective way to treat human hypertension disease using pharmaceutical drugs (captopril, monopril, etc.).
We prepared TiO2·PL-DNA1 and TiO2·PL-DNA2 nanocomposites containing oligonucleotide DNA1 complementary to the conservative region of the chosen target and oligonucleotide DNA2 with a random sequence. Intraperitoneal injection and inhalation were used to introduce the nanocomposites into ISIAH rats. In the case of inhalation, the dose of the preparation was the same as in the intraperitoneal injection (0.5 ml, 1 mg/ml, 60 µM for the oligonucleotide). However, the rats received a lower amount of the nanocomposite during inhalation because a part of the sprayed substance does not enter the rats’ lungs. Nevertheless, it was reasonable to test this route of administration of the nanocomposite intended to block ACE1 because ~80% of ACE1 is known to be synthesized in the walls of pulmonary vessels. The approach of the drug to the target could have a significant effect even if its dose is insufficient.
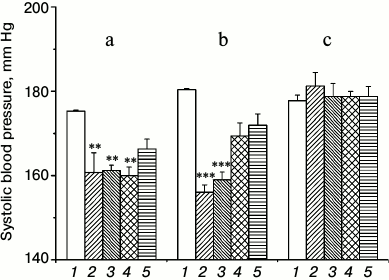
The results of the measurement of systolic BP in rats after the injection of the preparation showed that both routes of administration led to a reliable decrease in BP (figure, panels (a) and (b)). Nevertheless, there is a difference in the effects when using the different routes of administration, which is confirmed by dispersion analysis. Significant effects of the route of drug administration (F2,50 = 49.47, p < 0.0000001), measurement time (F4,50 = 12.79, p < 0.0000001), and the interaction between these parameters (F8,50 = 6.36, p < 0.000011) were revealed. The correlation between the parameters is due to a difference in the dynamics of the BP changes in response to the introduction of the sample. With intraperitoneal injection, the BP was significantly reduced the next day after injection and remained virtually unchanged for two weeks and raised by the end of the third week, whereas after inhalation, the return to the original level began at the end of the second week. Another feature was that the total BP reduction after the next two measurements (next day and at the end of the first week) was slightly lower after the injection than after inhalation: 14.25 ± 2.3 vs. 22.9 ± 1.32 mm Hg (p < 0.004). Thus, inhalation gives a more pronounced effect during the first week, but BP starts rising and by the end of the second week almost achieves the initial level (although this value was insignificantly lower than the initial BP). The intraperitoneal injection provided a lower but more prolonged effect (a slight increase in BP was observed only by the end of the third week) compared to the inhalation route of administration. The exact cause of this phenomenon remains unclear. This is probably due to the difference in the dose of the preparation or/and to the feature of pharmacokinetics.
Systolic blood pressure in hypertensive ISIAH rats after the introduction of TiO2·PL-DNA nanocomposites. Intraperitoneal injection (a) and inhalation (b) of TiO2·PL-DNA1 containing the oligonucleotide targeted to mRNA-ACE1; intraperitoneal injection of TiO2·PL-DNA2 containing oligonucleotide with a random sequence (c). Pressure: basal level (1), next day (2), in one week (3), in two weeks (4), and in three weeks (5); ** p < 0.01, *** p < 0.001
It is important to emphasize that in both cases the introduction of the TiO2·PL-DNA1 nanocomposite containing the antisense oligonucleotide targeted to mRNA-ACE1 was accompanied by significant and prolonged reduction in BP compared to traditional preparations, which must be taken at least once a day. TiO2·PL-DNA2 nanocomposite bearing oligonucleotide with random sequence had no effect (figure, panel (c)), which indicates the selective effect of the proposed nanocomposites.
Thus, we have shown for the first time that the proposed TiO2·PL-DNA nanocomposites are promising preparations for the treatment of hypertension. Moreover, it has been demonstrated that efficient therapy of hypertension can be achieved using the inhalation route of drug administration, which in this case is motivated by the fact that the main amount of ACE1 is synthesized in the pulmonary vessels endothelium. Moreover, this method of NA administration is preferable for clinical use.
It is important to emphasize that the studied nanocomposites can be prepared with unmodified phosphodiester oligodeoxynucleotides, which are less expensive compared to oligonucleotide derivatives or analogs mostly used in the antisense approach.
The use of antisense oligonucleotides in the composition with various nanocarriers opens new opportunities for the development of alternative methods of the struggle not only with hypertension, but also with other pathologies. The advantage of the proposed approach is primarily the fact that an antisense oligonucleotide delivered into cells in the nanocomposite is strongly aimed at a certain target, which provides high specificity and eliminates many adverse effects typical of traditional pharmaceuticals.
Acknowledgements
This work was supported by the Russian Science Foundation (project No. 16-15-10073).
REFERENCES
1.Kumar, J. (2013) Epidemiology of hypertension,
Clin. Queries Nephrol., 2, 56-61.
2.Chobanian, A. V., Bakris, G. L., Black, H. R.,
Cushman, W. C., Green, L. A., Izzo, J. L., Jones, D. W., Materson B.
J., Oparil, S., Wright, J. T., and Roccella, E. J. (2003) The seventh
report of the Joint National Committee on prevention, detection,
evaluation, and treatment of high blood pressure: the JNC 7 report,
JAMA, 289, 2560-2572.
3.Levina, A., Ismagilov, Z., Repkova, M., Shatskaya,
N., Shikina, N., Tusikov, F., and Zarytova, V. (2012) Nanocomposites
consisting of titanium dioxide nanoparticles and oligonucleotides,
J. Nanosci. Nanotechnol., 12, 1812-1820.
4.Levina, A. S., Mikhaleva, E. A., Repkova, M. N.,
and Zarytova, V. F. (2008) Synthesis of polyamine-containing
oligonucleotides, Bioorg. Khim., 34, 89-95.
5.Levina, A. S., Ismagilov, Z. R., Repkova, M. N.,
Shikina, N. V., Baiborodin, S. I., Shatskaya, N. V., Zagrebelniy, S.
N., and Zarytova, V. F. (2013) The creation of
TiO2~DNA-nanocomposites, able to enter the cells, Bioorg.
Khim., 39, 87-98.
6.Markel, A. L. (1992) Development of a new strain of
rats with inherited stress-induced arterial hypertension, in:
Genetic Hypertension Colloque INSERM (Sassard, J., ed.) Vol.
218, John Libbey Eurotext Ltd., London, pp. 405-407.
7.Markel, A. L., Maslova, L. N., Shishkina, G. T.,
Mahanova, N. A., and Jacobson, G. S. (1999) Developmental influences on
blood pressure regulation in ISIAH rats, in: Development of the
Hypertensive Phenotype, Basic and Clinical Studies. In the series
Handbook of Hypertension (McCarty, R., Blizard, D. A., and
Chevalier, R. L., eds.) Elsevier, Amsterdam, pp. 93-526.
8.Phillips, M. I. (2001) Gene therapy for
hypertension: the preclinical data, Hypertension, 38,
543-548.
9.Daya, S., and Berns, K. I. (2008) Gene therapy
using adeno-associated virus vectors, Clin. Microbiol. Rev.,
21, 583-593.
10.Cataliotti, A., Tonne, J. M., Bellavia, D.,
Martin, F. L., Oehler, E. A., Harders, G. E., Campbell, J. M., Peng,
K.-W., Russell, S. J., Malatino, L. S., Burnett, J. C., and Ikeda, Y.
(2011) Long-term cardiac pro-B-type natriuretic peptide gene delivery
prevents the development of hypertensive heart disease in spontaneously
hypertensive rats, Circulation, 123, 1297-1305.
11.Aguero, J., Ishikawa, K., Hadri, L.,
Santos-Gallego, C. G., Fish, K. M., Kohlbrenner, E., Hammoudi, N., Kho,
C., Lee, A., Ibanez, B., Garcia-Alvarez, A., Zsebo, K., Maron, B. A.,
Plataki, M., Fuster, V., Leopold, J. A., and Hajjar, R. J. (2016)
Intratracheal gene delivery of SERCA2a ameliorates chronic
post-capillary pulmonary hypertension. A large animal model, J. Am.
Coll. Cardiol., 67, 2032-2046.
12.Turnbull, I. C., Eltoukhy, A. A., Fish, K. M.,
Nonnenmacher, M., Ishikawa, K., Chen, J., Hajjar, R. J., Anderson, D.
G., and Costa, K. D. (2016) Myocardial delivery of lipidoid
nanoparticle carrying modRNA induces rapid and transient expression,
Mol. Ther., 24, 66-75.
13.Juliano, R. L., Ming, X., and Nakagawa, O. (2012)
Cellular uptake and intracellular trafficking of antisense and siRNA
oligonucleotides, Bioconjug. Chem., 23, 147-157.
14.Petros, R. A., and DeSimone, J. M. (2010)
Strategies in the design of nanoparticles for therapeutic applications,
Nat. Rev. Drug Discov., 9, 615-627.
15.Levina, A., Repkova, M. N., Ismagilov, Z. R.,
Shikina, N. V., Malygin, E. G., Mazurkova, N. A., Zinov’ev, V.
V., Evdokimov, A., Baiborodin, S. I., and Zarytova, V. F. (2012)
High-performance method for specific effect on nucleic acids in cells
using TiO2~DNA nanocomposites, Sci. Rep., 2,
756.
16.Levina, A. S., Repkova, M. N., Mazurkova, N. A.,
Makarevich, E. V., Ismagilov, Z. R., and Zarytova, V. F. (2015)
Knockdown of different influenza A virus subtypes in cell culture by a
single antisense oligodeoxyribonucleotide, Int. J. Antimicrob.
Agents, 46, 125-128.
17.Levina, A. S., Repkova, M. N., Bessudnova, E. V.,
Filippova, E. I., Mazurkova, N. A., and Zarytova, V. F. (2016) High
antiviral effect of DNA fragments in TiO2•PL-DNA
nanocomposites targeted to conservative regions of (–)RNA and
(+)RNA of influenza A virus, Beilstein J. Nanotechnol.,
7, 1166-1173.
18.Dubinina, A. D., Nizomov, S. A., Markel, A. L.,
and Ivanova, L. N. (2016) The reaction of the kidney in rats with
stress-induced hypertension (ISIAH line) on the osmotic load of sodium
chloride, Ros. Fiziol. Zh., 102, 56-66.
19.Antonov, Y. V., Alexandrovich, Y. V., Redina, O.
E., Gilinsky, M. A., and Markel, A. L. (2016) Stress and hypertensive
disease: adrenals as a link. Experimental study on hypertensive ISIAH
rat strain, Clin. Exp. Hypertens., 38, 415-423.
20.Fedoseeva, L. A., Ryazanova, M. A., Ershov, N.
I., Markel, A. L., and Redina, O. E. (2016) Comparative transcriptional
profiling of renal cortex in rats with inherited stress-induced
arterial hypertension and normotensive Wistar Albino Glaxo rats, BMC
Genet., 17, S1:12, 5-18.
21.Redina, O. E., Smolenskaya, S. E., Maslova, L.
N., and Markel, A. L. (2013) The genetic control of blood pressure and
body composition in rats with stress-sensitive hypertension, Clin.
Exp. Hypertens., 35, 484-495.