Optogenetic Stimulation Increases Level of Antiapoptotic Protein Bcl-xL in Neurons
D. A. Lanshakov1, U. S. Drozd2, and N. N. Dygalo1,2*
1Institute of Cytology and Genetics, Siberian Division of the Russian Academy of Sciences, 630090 Novosibirsk, Russia2Novosibirsk National Research State University, 630090 Novosibirsk, Russia; E-mail: dygalo@bionet.nsc.ru
* To whom correspondence should be addressed.
Received September 9, 2016
The antiapoptotic protein Bcl-xL is associated with several neuroplastic processes such as formation of synapses, regulation of spontaneous and evoked synaptic responses, and release of neurotransmitters. Dependence of expression on activity of neurons is characteristic for many proteins participating in regulation of neuroplasticity. Whether such property is exhibited by the Bcl-xL protein was analyzed using in vivo optogenetic stimulation of hippocampal glutamatergic neurons expressing channelrhodopsin ChR2H134 under CAMKIIa promoter in the adeno-associated viral vector, followed by immunohistochemical determination of the level of Bcl-xL protein in these neurons and surrounding cells. Increase in the level of early response c-Fos protein following illumination with blue light was indicative of activation of these hippocampal neurons. The optogenetic activation of hippocampus resulted in a significant increase in the level of antiapoptotic protein Bcl-xL in the photosensitive neurons as well as in the surrounding cells. The dependence of the level of expression of Bcl-xL protein on the activity of neurons indicates that this protein possesses one more important property that is essential for participation in neuroplastic processes in the brain.
KEY WORDS: protein Bcl-xL, protein c-Fos, AAV vector, optogenetics, hippocampal glutamatergic neuron, activity-dependent protein expressionDOI: 10.1134/S0006297917030129
Abbreviations: AAV, adeno-associated viruses; ChR2 or ChR2H134, short and full designation of channelrhodopsin-2, respectively; PBS, phosphate buffered saline; PBST, PBS containing 0.1% Triton X-100; YFP and EGFP, yellow and enhanced green fluorescent proteins, respectively.
Neurons and their networks can change their morphological and functional
parameters in response to external stimuli, which is essential for the
neural regulation of adaptive reactions of an organism to changing
internal and external conditions. This most important property –
neuroplasticity – is realized depending on the activity of brain
cells and involves formation of new dendrites and spines, as well as
synaptic contacts. Furthermore, the level of neuronal activity affects
the efficiency of interneuron signal transmission via redistribution of
receptor proteins in synaptic membranes and change in recycling of
vesicles releasing neurotransmitters into the synaptic cleft. It was
found that the antiapoptotic protein Bcl-xL was involved in some of
these processes. It was established that in addition to its main
function – protection of cells of all types from programmed
cell death on the action of apoptogenic factors – it can
increase the number, size, and activity of synapses, increasing the
release of neurotransmitters, recycling of synaptic vesicles, as well
as enhancing spontaneous and evoked synaptic responses [1-4]. These functions of Bcl-xL
are characteristic for neuroplasticity-associated proteins that
participate in the change in properties of neurons depending on their
discharge activity. However, unlike the well-investigated products of
the early response genes, which comprise most of this type of proteins
[5-7], interrelation between
the level of synthesis of the Bcl-xL protein and neuronal activity
remains poorly explored. Investigation of such correlation is hindered
by the fact that the expression of this protein is also controlled by
many other stimuli associated, for example, with the protection of
cells from death [8] or providing of energy sources
under conditions of stress [9, 10]. One of the possible ways to solve this problem
could be direct neuronal stimulation and evaluation of the expression
of Bcl-xL protein in the stimulated neurons. The targeted activation of
only marked neurons and the selective assessment of the level of the
analyzed protein in them could be realized by the combined application
of optogenetics and immunohistochemistry. In this study, we
investigated the effect of optogenetic activation of hippocampal
glutamatergic neurons on the level of the Bcl-xL protein in the
neurons, assayed using immunohistochemical methods.
MATERIALS AND METHODS
Viral vectors were constructed based on plasmids designed by the K. Deisseroth group and provided by Addgen (USA). Adeno-associated viruses (AAV) of the mixed 1-2 serotype were obtained by transfection of HEK29 cells by the mixture of plasmids for the virus assembly (pDP1 and pDP2), as well as a pAAV-CAMKIIa-ChR2H134-YFP plasmid carrying the sequence of the channelrhodopsin ChR2H134 and yellow fluorescent protein (YFP) using polyethyleneimine [11]. An AAV-CAMKIIa-EGFP vector encoding the green fluorescent protein similarly to the opto-vector, but not the photochannel, was used as a control. Virus particles were purified on heparin-Sepharose columns and concentrated using Millipore centrifuge concentrators to the titer of 1011. The transgene expression in these vectors is under control of the CAMKIIa promoter, which is active only in glutamatergic neurons. Aliquots (5 µl) of viral vectors pAAV-CAMKIIa-ChR2H134-YFP and AAV-CAMKIIa-EGFP were injected into the brain lateral ventricles of 3-day-old rats from the control and test group under hypothermic anesthesia in a stereotaxic instrument as described previously [12, 13].
The effect of photostimulation on expression of the early response protein c-Fos and Bcl-xL protein in hippocampus was analyzed three weeks after injection of the vectors into the brain of the rats. For this purpose, animals from both groups that received injections with vector carrying the photochannel (ChR2) gene (test group) or only enhanced green fluorescent protein (EGFP) gene (control group) were anaesthetized with urethane and placed into a stereotaxic instrument. An optoprobe (A1 × 16–5mm–100–177–OA16LP; NeuroNexus Technologies, USA) was introduced into the CA1 field of the hippocampus through a small hole over the hippocampus, which allowed photostimulation of tissue (480 nm, 1.2 mV/mm2, 20 pulses/s, 5 min, blue light source – photodiode; Thorlabs, USA) near the end of the optical fiber. Immunoreactivity of the c-Fos and Bcl-xL proteins in hippocampus was investigated 30 min after the completion of optical stimulation. For this purpose, the animals were anaesthetized with avertin and perfused transcardially with PBS containing 4% paraformaldehyde. Then the brain was post-fixed for 4 h in 4% paraformaldehyde, and 300-µm-thick brain slices were prepared with a vibratome (Microm, Germany).
Immunohistochemical protein identification was conducted using common procedures [7]. Slices were washed twice in PBS containing 0.1% Triton X-100 (PBST) for 15 min. Nonspecific binding was blocked by incubation of slices in 1.5% solution of bovine serum albumin in PBST for 2 h. Then the slices were incubated with primary antibodies (Cell Signaling, USA) using a 1 : 500 dilution overnight at 4°C. After that, slices were washed three times for 15 min in PBST and incubated for 4 h with secondary antibodies conjugated with Alexa Fluor 568 (Jackson Immunoresearch, USA) at dilution 1 : 500. Next the slices were washed, mounted on microscope slides, and embedded into medium with nuclear stain DAPI (4′,6-diamidino-2-phenylindole). The preparations were imaged using a LSM510META confocal microscope (Carl Zeiss, Germany) equipped with lasers with wavelengths 405, 488, and 533 nm. An image-processing program ZEN (Carl Zeiss) was used for image analysis. The total number of immunopositive cells for the target proteins (c-Fos or Bcl-xL) per 1 mm2 was determined in 8-10 samples of each experimental group (test and control) both in the cells with fluorescent marker and without it. In addition, the number of cells expressing simultaneously one of the target proteins and the fluorescently labeled transgene ChR2 or EGFP was determined. The quantitative data were represented as mean value (M) ± standard deviation. The differences between the test and control groups in the total number of cells expressing the target protein as well and the number of cells expressing this protein simultaneously with the fluorescent expression marker of the AAV-vector were evaluated using Student’s t-test, assuming statistically significant difference at p < 0.05.
RESULTS
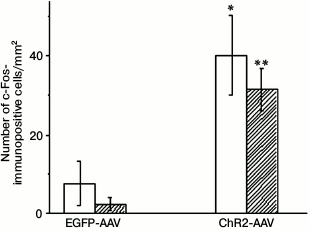
Injection of the AAV-vectors carrying sequences encoding fluorescent proteins into the brain of neonatal rats resulted in the emergence of 35-40, on the average, fluorescently labeled cells per 1 mm2 of hippocampus area illuminated with blue light 3 weeks after the procedure. The immunohistochemical staining of the early response protein c-Fos revealed its presence both in the photosensitive cells and in a small number of cells not containing the fluorescent label of the photosensitive channel ChR2 (Fig. 1). Illumination with blue light of the samples from the test group of animals, in which the vector encoding the ChR2 channel was injected, demonstrated that the total number of c-Fos-positive cells (p < 0.03), and especially the number of cells coexpressing c-Fos and the vector fluorescent marker (p < 0.0001) were significantly higher in comparison with similar preparations from the control group of animals that expressed only the EGFP fluorescent protein. The results of experiment presented in Fig. 1 indicates that the increase in the number of c-Fos-positive cells in the test group was achieved due to cells expressing this channel.
Fig. 1. Number of c-Fos-immunopositive cells on slides from animals injected with AAV-vectors encoding fluorescent protein (EGFP-AAV) or photosensitive ion channel (ChR2-AAV) after their illumination with blue light; empty bars – total number of c-Fos-positive cells; filled bars – number of c-Fos-positive cells coexpressing AAV-vector; * p < 0.03; ** p < 0.0001 {{anchor|GoBack}} in comparison with the respective indicators of the EGFP-AAV group.
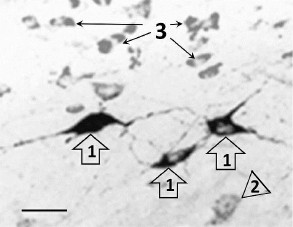
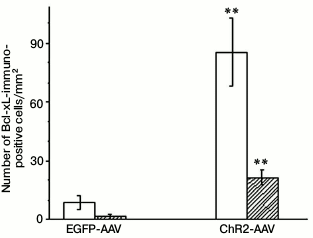
The immunohistochemical staining of the Bcl-xL protein also revealed its presence both in the photosensitive cells as well as in the cells not containing fluorescent label. The Bcl-xL protein determined via immunohistochemical staining in the preparations where some cells expressed vector with the ChR2 channel was also observed in many cells that were not labeled with the photosensitive vector (Fig. 2). In the samples from the test group of animals illuminated with blue light, the number of cells coexpressing the antiapoptotic protein and the vector fluorescent label (p < 0.001), as well as the total number of the Bcl-xL-positive cells (p < 0.001), were significantly higher than in the similar preparation from the control group of animals (Fig. 3).
Fig. 2. Typical microphotograph of the Bcl-xL-immunopositive cells in the preparation treated with AAV-vector encoding photosensitive ion channel (ChR2-AAV) after illumination with blue light. 1) Cells coexpressing ChR2-AAV and Bcl-xL protein; 2) cell synthesizing only ChR2-AAV; 3) cells producing only Bcl-xL protein. Scale bar – 20 µm.
Fig. 3. Number of Bcl-xL-immunopositive cells on slides from animals injected with AAV-vectors encoding fluorescent protein (EGFP-AAV) or photosensitive ion channel (ChR2-AAV) after their illumination with blue light. Empty bars – total number of Bcl-xL positive cells; filled bars – number of Bcl-xL-positive cells coexpressing AAV-vector; ** p < 0.001 in comparison with the respective indicators of the EGFP-AAV group.
DISCUSSION
The increase in expression of the early response protein c-Fos after stimulation of hippocampal glutamatergic neurons expressing the ChR2 photochannel under control of the CAMKIIa with blue light clearly indicates optogenetic activation of these neurons. The c-Fos protein is a transcription factor that is expressed in the neurons even without stimulation according to their spontaneous discharge activity [5, 6]. As shown in numerous studies, optogenetic stimulation of brain cells expressing channelrhodopsin increases the level of synthesis of this early response protein simultaneously with the increase in discharge activity of the neurons [14, 15].
In this work, we demonstrated that optogenetic stimulation of hippocampal glutamatergic neurons resulted in a significant increase in the level of production of the Bcl-xL antiapoptotic protein in the photosensitive neurons as well as in adjacent cells. This increase was observed less than one hour after the neuron stimulation onset and, thus, was in the time-frame for the induction of the early response proteins, for example c-Fos, which was found in many experiments including ours. The fast increase in the level of the Bcl-xL protein upon stimulation of neuronal activity together with its mentioned ability to increase the number, size, and activity of synapses, release of neurotransmitters, recycling of synaptic vesicles, as well as spontaneous and evoked synaptic responses [1-4] complement the spectrum of its properties characteristic for the proteins participating in neuroplasticity processes, changing the structure and properties of neurons during their activation. These properties can provide the basis of the observed earlier association of the increased expression of this protein with psychoemotional and neurochemical stability under conditions of the short-term stress as well as under the action of antidepressants [16-19].
The pathway from depolarization of cell membrane caused by the release of positively charged ions during activation of the channelrhodopsin ChR2 by blue light [20] to the change in gene expression in the neuron is under active investigation, but it is still a poorly understood complex of intracellular events. The influx of calcium ions due to the discharge activity of the neuron and initiation of the Ca2+-dependent kinase cascades followed by the stimulation of transcription factors by the kinases comprise the recognized main stages of this process [21]. For example, the transcription factor CREB participates in the regulation of c-Fos protein expression, which is dependent on neuronal activity [22]. Another participant of the rapid regulation of gene expression on the increase in the frequency of neuron firing is transcription factor NF-κB [23], which regulates the synthesis of the Bcl-xL protein together with c-Fos [24, 25]. However, it must be mentioned that participation of these transcription factors in the change in the expression level of Bcl-xL protein with increasing neuronal activity found in this work is only suggested and needs further experimental validation.
The fact that the light-mediated increase in Bcl-xL protein content in the non-light-sensitive cells was observed in preparations containing neurons expressing photosensitive channel is intriguing. This at first glance unexpected result can be explained by sensing of the increase in the light-sensitive neuronal activity induced by light by the adjacent cells. For example, astrocytes are sensitive to the activity of neurons located in their vicinity. Neuronal stimulation causes changes in the electrophysiological characteristics of astrocytes as well as of the concentration of Ca2+ in them during cultivation of these cells and brain slices in vitro [26]. The astrocytes in hippocampus and, in particular, in its CA1-field respond to the stimulation-induced changes of Ca2+ concentration in intracellular space by generation of the so-called “calcium wave”, which spreads fast and thus involves additional cells that do not have direct contact with the initial stimulus into the area of its effect [27]. Considering that the first stage of activity-dependent induction of gene expression in the cell is the increase in concentration of Ca2+ in cytoplasm [21], it is quite possible that the “calcium wave” is a cause of the increase of the level of Bcl-xL protein in the cells lacking the photochannel.
Overall, the results of this study indicate that the expression of the Bcl-xL protein increases in the photosensitive hippocampal glutamatergic neurons very fast, by one order of magnitude in less than 1 h, during their optogenetic stimulation. The dependence of the expression level of the Bcl-xL protein on neuronal activity, which is first demonstrated in this work, supplemented the set of its properties as a participant of brain neuroplasticity processes.
Acknowledgements
This work was financially supported by the Russian Science Foundation (project No. 14-15-00115).
REFERENCES
1.Jonas, E. (2006) Bcl-xL regulates synaptic
plasticity, Mol. Interv., 6, 208-222.
2.Gal, A., Pentelenyi, K., Remenyi, V., Wappler, E.
A., Safrany, G., Skopal, J., and Nagy, Z. (2009) Bcl-2 or Bcl-xL gene
therapy increases neural plasticity proteins nestin and c-fos
expression in PC12 cells, Neurochem. Int., 55,
349-353.
3.Li, H., Chen, Y., Jones, A. F., Sanger, R. H.,
Collis, L. P., Flannery, R., McNay, E. C., Yu, T., Schwarzenbacher, R.,
Bossy, B., Bossy-Wetzel, E., Bennett, M. V., Pypaert, M., Hickman, J.
A., Smith, P. J., Hardwick, J. M., and Jonas, E. A. (2008) Bcl-xL
induces Drp1-dependent synapse formation in cultured hippocampal
neurons, Proc. Natl. Acad. Sci. USA, 105, 2169-2174.
4.Li, H., Alavian, K. N., Lazrove, E., Mehta, N.,
Jones, A., Zhang, P., Licznerski, P., Graham, M., Uo, T., Guo, J.,
Rahner, C., Duman, R. S., Morrison, R. S., and Jonas, E. A. (2013) A
Bcl-xL–Drp1 complex regulates synaptic vesicle membrane dynamics
during endocytosis, Nat. Cell. Biol., 15, 773-785.
5.Yassin, L., Benedetti, B. L., Jouhanneau, J. S.,
Wen, J. A., Poulet, J. F., and Barth, A. L. (2010) An embedded
subnetwork of highly active neurons in the neocortex, Neuron,
68, 1043-1050.
6.Peter, M., Bathellier, B., Fontinha, B., Pliota,
P., Haubensak, W., and Rumpel, S. (2013) Transgenic mouse models
enabling photolabeling of individual neurons in vivo, PLoS
One, 8, e62132.
7.Lanshakov, D. A., Sukhareva, E. V., Kalinina, T.
S., and Dygalo, N. N. (2016) Dexamethasone-induced acute excitotoxic
cell death in the developing brain, Neurobiol. Dis., 91,
1-9.
8.Formentini, L., Pereira, M. P., Sanchez-Cenizo, L.,
Santacatterina, F., Lucas, J. J., Navarro, C., Martinez-Serrano, A.,
and Cuezva, J. M. (2014) In vivo inhibition of the mitochondrial
H+-ATP synthase in neurons promotes metabolic
preconditioning, EMBO J., 33, 762-778.
9.Du, J., McEwen, B., and Manji, H. K. (2009)
Glucocorticoid receptors modulate mitochondrial function: a novel
mechanism for neuroprotection, Commun. Integr. Biol., 2,
350-352.
10.Du, J., Wang, Y., Hunter, R., Wei, Y.,
Blumenthal, R., Falke, C., Khairova, R., Zhou, R., Yuan, P.,
Machado-Vieira, R., McEwen, B. S., and Manji, H. K. (2009) Dynamic
regulation of mitochondrial function by glucocorticoids, Proc. Natl.
Acad. Sci. USA, 106, 3543-3548.
11.McClure, C., Cole, K. L., Wulff, P., Klugmann,
M., and Murray, A. J. (2011) Production and titering of recombinant
adeno-associated viral vectors, J. Vis. Exp., 27,
e3348.
12.Shishkina, G. T., Kalinina, T. S., and Dygalo, N.
N. (2004) Attenuation of alpha2A-adrenergic receptor expression in
neonatal rat brain by RNA interference or antisense oligonucleotide
reduced anxiety in adulthood, Neuroscience, 129,
521-528.
13.Dygalo, N. N., Kalinina, T. S., and Shishkina, G.
T. (2008) Neonatal programming of rat behavior by downregulation of
alpha2A-adrenoreceptor gene expression in the brain, Ann. N. Y.
Acad. Sci., 1148, 409-414.
14.Ramirez, S., Liu, X., MacDonald, C. J., Moffa,
A., Zhou, J., Redondo, R. L., and Tonegawa, S. (2015) Activating
positive memory engrams suppresses depression-like behavior,
Nature, 522, 335-339.
15.Lanshakov, D. A., Drozd, U. S., Zapara, T. A.,
and Dygalo, N. N. (2016) Transfer of optogenetic vectors into the brain
of neonatal animals to study neuron functions during subsequent periods
of development, Vavilov J. Genet. Breed., 20,
255-261.
16.Shishkina, G. T., Kalinina, T. S., Berezova, I.
V., Bulygina, V. V., and Dygalo, N. N. (2010) Resistance to the
development of stress-induced behavioral despair in the forced swim
test associated with elevated hippocampal Bcl-xl expression, Behav.
Brain Res., 213, 218-224.
17.Dygalo, N. N., Kalinina, T. S., Bulygina, V. V.,
and Shishkina, G. T. (2012) Increased expression of the anti-apoptotic
protein Bcl-xL in the brain is associated with resilience to
stress-induced depression-like behavior, Cell. Mol. Neurobiol.,
32, 767-776.
18.Shishkina, G. T., Kalinina, T. S., Berezova, I.
V., and Dygalo, N. N. (2012) Stress-induced activation of the brainstem
Bcl-xL gene expression in rats treated with fluoxetine: correlations
with serotonin metabolism and depressive-like behavior,
Neuropharmacology, 62, 177-183.
19.Shishkina, G. T., Kalinina, T. S., Bulygina, V.
V., Lanshakov, D. A., Babluk, E. V., and Dygalo, N. N. (2015)
Anti-apoptotic protein Bcl-xL expression in the midbrain raphe region
is sensitive to stress and glucocorticoids, PLoS One, 10,
e0143978.
20.Tye, K. M., and Deisseroth, K. (2012) Optogenetic
investigation of neural circuits underlying brain disease in animal
models, Nat. Rev. Neurosci., 13, 251-266.
21.Kawashima, T., Okuno, H., and Bito, H. (2014) A
new era for functional labeling of neurons: activity-dependent
promoters have come of age, Front. Neural. Circuits, 23,
doi: 10.3389/fncir.2014.00037.
22.Cohen, S., and Greenberg, M. E. (2008)
Communication between the synapse and the nucleus in neuronal
development, plasticity, and disease, Annu. Rev. Cell. Dev.
Biol., 24, 183-209.
23.De la Fuente, V., Federman, N., Zalcman, G.,
Salles, A., Freudenthal, R., and Romano, A. (2015) NF-κB
transcription factor role in consolidation and reconsolidation of
persistent memories, Front. Mol. Neurosci., 9, doi:
10.3389/fnmol.2015.00050.
24.Grillot, D. A., Gonzalez-Garcia, M., Ekhterae,
D., Duan, L., Inohara, N., Ohta, S., Seldin, M. F., and Nunez, G.
(1997) Genomic organization, promoter region analysis, and chromosome
localization of the mouse bcl-x gene, J. Immunol., 158,
4750-4757.
25.Grad, J. M., Zeng, X. R., and Boise, L. H. (2000)
Regulation of Bcl-xL: a little bit of this and a little bit of STAT,
Curr. Opin. Oncol., 12, 543-549.
26.Schipke, C. G., and Kettenmann, H. (2004)
Astrocyte responses to neuronal activity, Glia, 47,
226-232.
27.Croft, W., Dobson, K. L., and Bellamy, T. C.
(2015) Plasticity of neuron-glial transmission: equipping glia for
long-term integration of network activity, Neural Plast., doi:
10.1155/2015/765792.