REVIEW: Role of Nerve Growth Factor in Plasticity of Forebrain Cholinergic Neurons
N. K. Isaev1,2*, E. V. Stelmashook2, and E. E. Genrikhs2
1Lomonosov Moscow State University, Belozersky Institute of Physico-Chemical Biology, 119991 Moscow, Russia; E-mail: isaev@genebee.msu.ru2Research Center of Neurology, 125367 Moscow, Russia; E-mail: estelmash@mail.ru
* To whom correspondence should be addressed.
Received October 29, 2016; Revision received November 11, 2016
Neuronal plastic rearrangements during the development and functioning of neurons are largely regulated by trophic factors, including nerve growth factor (NGF). NGF is also involved in the pathogenesis of Alzheimer’s disease. In the brain, NGF is produced in structures innervated by basal forebrain cholinergic neurons and retrogradely transported along the axons to the bodies of cholinergic neurons. NGF is essential for normal development and functioning of the basal forebrain; it affects formation of the dendritic tree and modulates the activities of choline acetyltransferase and acetylcholinesterase in basal forebrain neurons. The trophic effect of NGF is mediated through its interactions with TrkA and p75 receptors. Experimental and clinical studies have shown that brain levels of NGF are altered in various pathologies. However, the therapeutic use of NGF is limited by its poor ability to penetrate the blood–brain barrier, adverse side effects that are due to the pleiotropic action of this factor, and the possibility of immune response to NGF. For this reason, the development of gene therapy methods for treating NGF deficit-associated pathologies is of particular interest. Another approach is creation of low molecular weight NGF mimetics that would interact with the corresponding receptors and display high biological activity but be free of the unfavorable effects of NGF.
KEY WORDS: nerve growth factor, cholinergic neurons, plasticity, Alzheimer’s diseaseDOI: 10.1134/S0006297917030075
Abbreviations: AChE, acetylcholinesterase; AD, Alzheimer’s disease; ChAT, choline acetyltransferase; GK-2, bis-(N-succinyl-glutamyl-lysine)hexamethylenediamide; NGF, nerve growth factor.
Nerve growth factor (NGF) was discovered in the 1950s by Levi-Montalcini
and Hamburger, who described it as a trophic factor for sympathetic
adrenergic and some sensory neurons. NGF was found to affect the
survival and development of these neurons, as well as neurite growth
(Fig. 1) [1-3]. NGF deprivation of sympathetic ganglia leads to
their degeneration and massive death of neurons [4]. NGF belongs to the neurotrophin family that also
includes brain-derived neurotrophic factor (BDNF), neurotrophin-3
(NT3), and neurotrophin-4/5 (NT4/5). The NGF molecule is composed of
two identical 13-kDa polypeptide chains (118 amino acids each) with
N-terminal serine and C-terminal arginine residues. The nucleotide
sequences of mammalian NGF genes are highly homologous [5], which characterizes NGF as an evolutionarily
conserved protein.
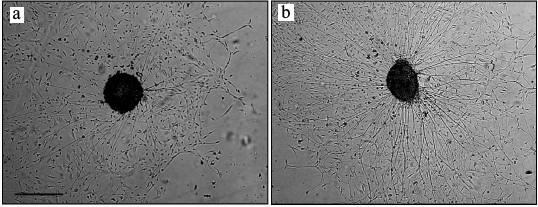
Fig. 1. NGF stimulated nerve fiber growth in cultured spinal ganglion from an 18-day-old rat embryo (phase-contrast microscopy): a) control; b) NGF. Scale, 500 µm.
The trophic activity of NGF is mediated through its interactions with TrkA and p75 receptors [6, 7]. In humans, the gene coding for the NGF precursor is located on chromosome 1. Mature NGF is formed from its precursor by proteolytic cleavage [8]. The precursor (proNGF) is synthesized as a monomer that can be either secreted into the extracellular space or processed into mature NGF inside the cell [9]. It is now believed that dysfunctions in NGF extracellular metabolism can lead to accelerated degradation of the mature NGF molecule in Alzheimer’s disease (AD) [10].
The highest levels of NGF were found in the submandibular glands of Swiss-Webster male mice, where it is synthesized as a complex composed of three types of subunits. The β-subunits are solely responsible for the biological activity of NGF. The γ-subunit is an active serine proteinase capable of processing the precursor form of β-NGF, whereas α-NGF is an inactive proteinase. In the brain, relatively high NGF levels were found in the basal forebrain [11] and regions innervated by the basal forebrain cholinergic neurons (hippocampus, cortex) [11, 12]. However, NGF mRNA was found only in the cortex and the hippocampus [13]. Together with the fact that after injection into the cortex labeled NGF is transported to the cholinergic neurons in the nucleus basalis region [14], these data confirm that NGF is synthesized in the structures innervated by cholinergic neurons and then retrogradely transported via axons to the neurons’ bodies of the septum, nuclei of the vertical and horizontal limbs of the diagonal band of Broca, and nucleus basalis of Meynert [15, 16], i.e. structures involved in cognition, learning, and memory [17]. It was found that the age-associated decline in cognitive abilities is accompanied by a decrease in the levels of choline acetyltransferase (ChAT), an enzyme that synthesizes acetylcholine in the cortex and hippocampus, and by a loss of cholinergic neurons in basal nuclei [18]. These changes are especially pronounced in AD patients, who display progressive neurodegeneration resulting in the loss of memory and cognitive functions.
NGF IN THE BRAIN
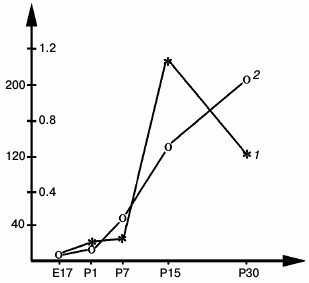
During postnatal development, the time courses of NGF and ChAT in rat hippocampus correlate, including the most rapid increase between P12 and P14 paralleled by a rapid increase in the levels of ChAT and acetylcholinesterase (AChE) in the septum (Fig. 2) [19]. According to Auburger et al. [20], these changes are also closely reflected by a peak increase in the NGF concentration in the septum [20]. The concentrations of NGF and ChAT in this structure then decrease during the following days [19, 20].
Fig. 2. Activities of ChAT (1) (right y-axis) and AChE (2) (left y-axis) in the developing rat septum; x-axis, days of embryonic (E) and postnatal (P) development; y-axis, enzyme activity, nmol acetylcholine/mg protein per minute [19].
The distribution of NGF-binding neurons resembles the distribution of cholinergic neurons in the basal forebrain [21], where NGF receptors and ChAT colocalize by 92% [22]. This confirms the importance of NGF for the functioning of cholinergic neurons in the basal forebrain. As mentioned above, the trophic activity of NGF is mediated by its interaction with TrkA and p75 receptors. Coexpression of these two receptors could potentially increase cell response to NGF. Each of the receptors binds NGF independently and with predominately low affinity (Kd = 10–9 M); however, when coexpressed, they produce a high-affinity NGF-binding site (Kd = 10–11 M). It was suggested that the transmembrane and cytoplasmic domains of TrkA and p75 participate in the formation of the high-affinity site via p75-induced changes in TrkA conformation [7].
NGF REGULATES ACTIVITY AND SURVIVAL OF BASAL FOREBRAIN
CHOLINERGIC NEURONS
Numerous experiments with animals have shown that NGF is essential for the normal development and functioning of the cholinergic nuclei of the basal forebrain. According to the commonly accepted classification based on human brain studies, the basal forebrain includes substantia innominata, vertical diagonal band nucleus, medial septal nucleus, horizontal diagonal band nucleus, and nucleus basalis of Meynert. Note that in rats the nucleus basalis is less developed than in humans and consists of a small group of large multipolar cholinergic neurons. Hippocampus, olfactory bulbs, and neocortex are innervated targets for neurons of the basal forebrain [23].
In normally developing postnatal rat brain, basal forebrain neurons undergo considerable plastic rearrangements that include progressive increase in the cross-sectional cell body area and the number and length of primary dendrites that peak at P18 and thereafter decrease to smaller adult values [24]. NGF that is retrogradely transported from the innervated targets is extremely important for the survival of forebrain cholinergic neurons. NGF injection into brain ventricles of newborn rats within the first postnatal week elevates the ChAT activity in the septum, hippocampus, and neocortex by 78, 30, and 70%, respectively [25]. Disruption of a single allele of the NGF gene in transgenic animals (NGF+/–) results in atrophy of septal cholinergic neurons accompanied by memory and learning deficits [26]. The survival and functioning of basal forebrain cholinergic neurons in adults also depend on NGF. Deterioration of the septo-hippocampal connections disturbs NGF transport to septal neurons, which in turn reduces the number of cholinergic neurons [27]. NGF injection into brain ventricles of rats with septo-hippocampal pathway lesions prevents both the lesion-induced loss of cholinergic neurons [28] and the decrease in the ChAT and AChT activities in the septum and the hippocampus [27]. Discontinuation of chronic NGF treatment results in the restoration of degenerative processes in the basal forebrain [29]. However, these negative changes are reversible. Thus, delayed treatment with NGF three weeks after lesion induction induced a dramatic reappearance of the apparently lost ChAT-expressing neurons [30]. Blockade of endogenous NGF in the hippocampus of adult rats with antibodies significantly reduces hippocampal long-term potentiation and impaired retention of spatial memory [31].
Neurodegenerative changes in the basal forebrain accumulate in the course of normal biological aging. Thus, the number [32] and size [32, 33] of cholinergic neurons decrease in aging rats, as well as the levels of key enzymes of acetylcholine synthesis and degradation (ChAT and AChT) in these neurons [34]. In human brain, aging is accompanied by the loss of cholinergic neurons in the nucleus basalis of Meynert [35]. In contrast to “healthy aging”, AD causes progressive cognitive impairments, degradation of synapses, and considerable loss of neurons in the cholinergic basal nuclei, cortical areas innervated by these neurons, and in the cerebellum [36, 37]. Although degenerative changes of basal forebrain cholinergic nuclei are observed during normal aging as well, basal forebrain atrophy is much more pronounced in AD patients [38]. The massive death of neurons in AD brain causes disintegration of connections between various brain regions and deterioration of brain functions, while in “healthy aging” brain, the loss of brain connections and the associated cognitive decline are partially compensated by delocalization of brain activities, i.e. involvement of additional brain structures in cognitive processes [39, 40].
The hypothesis of deterioration of NGF trophic support of basal forebrain cholinergic neurons in AD, as well as the possibility of therapeutic use of NGF, are supported by data obtained for transgenic AD11 mice expressing neutralizing anti-NGF antibodies after birth [41]. Chronic NGF deprivation results in cholinergic deficit, loss of neurons and synapses, accumulation of extra- and intracellular β-amyloid peptide, formation of neurofibrillary tangles in hippocampus, synaptic plasticity decline, memory loss, and long-term potentiation impairments in the neocortex [42-44]. Intranasal application of NGF in AD11 mice prevents memory loss, cholinergic signaling deficit, β-amyloid accumulation, and tau-protein hyperphosphorylation [45].
According to current concepts on NGF neurotrophic activities, NGF prevents AD by suppressing hyperproduction of β-amyloid peptide [46].
NGF-INDUCED PLASTIC REARRANGEMENTS OF BASAL FOREBRAIN CHOLINERGIC
NEURONS in vitro
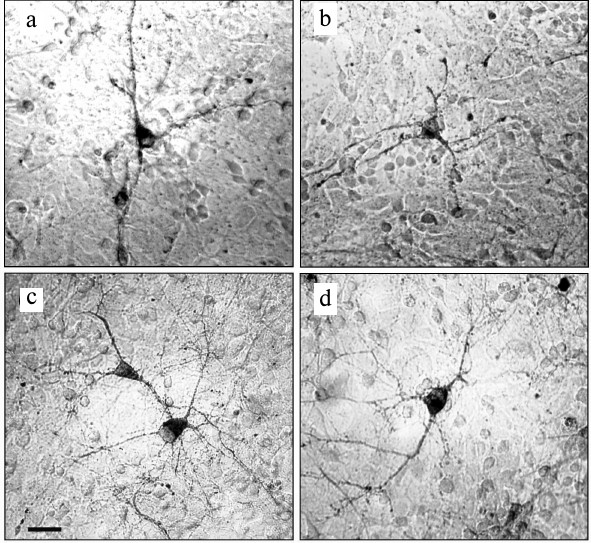
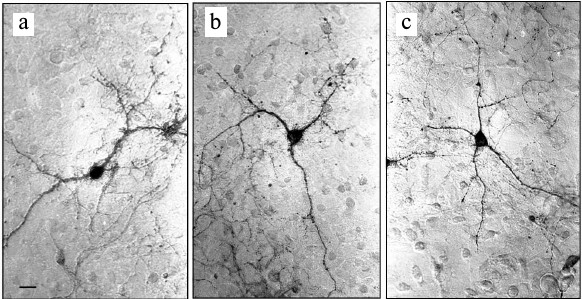
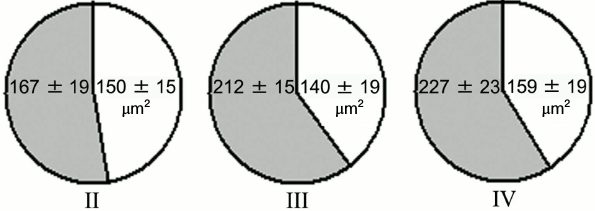
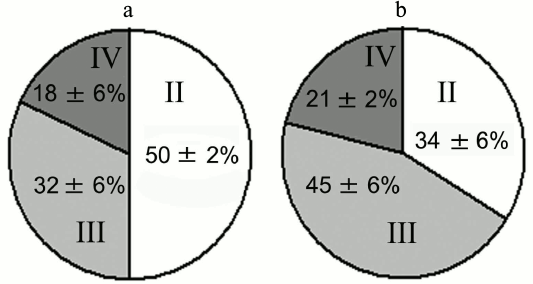
Experiments with nerve tissue cultures convincingly demonstrated that NGF is essential for plastic rearrangements of cultured basal forebrain cholinergic neurons during their differentiation. Organotypic septal cultures treated with NGF displayed elevated ChAT activity [47]. NGF also increased 2.5-fold the number of ChAT-positive septo-hippocampal projections in cocultures of rat septal and hippocampal explants [47, 48]. Similar results were obtained in dissociated septal cell cultures in which the presence of NGF in the culture medium caused dose-dependent elevation in ChAT and AChE levels [27, 49]. Interestingly, the increase in activity of these enzymes was more pronounced when the neurons were cocultured with glial cells. When the content of glial cells in the neuronal cultures was low, the effect of NGF was less noticeable. Computer image analysis revealed that NGF stimulates the growth of cell bodies of cultured rat septal cholinergic neurons, especially at the early stages of culturing (week 1) (Fig. 3). The cell body cross-area of control neurons averaged 167 ± 6 µm2, while in the NGF-treated neurons, this parameter reached 230 ± 10 µm2 (Fig. 3) [50]. The NGF-induced increase in the body size was also observed upon longer in vitro neuron culturing (up to 14 days) [51]. By this time, the population of septal cholinergic neurons formed a well-developed dendritic network and contained easily distinguished two-, three-, and four-dendrite neurons (Fig. 4). The effect of NGF on neuron body size was more pronounced in multipolar AChE-positive neurons than in bipolar neurons (Fig. 5) [51]. In addition, NGF significantly increased the number of AChE- and ChAT-positive cells in dissociated cultures of septal cholinergic neurons [52-54]. Brain ontogenesis results in the formation of a complex dendritic network of septal cholinergic neurons [24]. It is possible that the number of dendrites and their arborization are regulated by NGF, because an increase in the number of septal cholinergic neurons correlates with elevation in the NGF content in the hippocampus, which is a target for septal neuron innervation [20, 24]. This suggestion is confirmed by the fact that in chick embryo septal cell cultures, NGF increases the percentage content of multipolar AChE-positive neurons and decreases the percentage content of bipolar neurons compared to the control (Fig. 6) [54]. Also, NGF increases the total length of dendrites, dendritic territory (area occupied by dendrites), and dendrite arborization, i.e. affects parameters that characterize the extent of dendritic network development [55]. NGF also increases the total length of neurites (both axons and dendrites) of septal cholinergic neurons [53]. These data demonstrate that NGF shifts the population of cholinergic neurons in the basal forebrain septal dissociated cultures toward the formation of neurons with a developed dendritic network. This might occur due to NGF-induced increase in the number and length of primary dendrites of cholinergic neurons or to better survival of multipolar neurons, because these neurons are extremely dependent on NGF. Septal cholinergic neurons undergo similar plastic rearrangements in vivo in the course of brain ontogenesis [24].
Fig. 3. NGF increases cell body size of neurons in septal cell cultures from 18-day-old rat embryos after 7 days of culturing: a, b) controls; c, d) NGF. Cells were fixed and stained histochemically for acetylcholinesterase. Scale, 20 µm.
Fig. 4. Neurons in septal cell cultures obtained from 18-day-old rat embryos after 14 days of culturing: a) bipolar two-dendrite neuron; b) multipolar three-dendrite neuron; c) multipolar four-dendrite neuron. The cells were fixed and stained histochemically for acetylcholinesterase. Scale, 20 µm.
Fig. 5. Effect of NGF on cell body size of AChE-positive neurons in cell cultures obtained from 18-19-day-old rat embryos after 14 days of culturing. Gray and white sectors, cell body size of neurons incubated in the presence or absence of NGF, respectively. II, two-dendrite neurons; III, three-dendrite neurons; IV, four-dendrite neurons.
Fig. 6. Effect of NGF on percentage content of AChE-positive neurons in septal cell cultures from 18-day-old rat embryos after 14 days of culturing: II) two-dendrite neurons; III) three-dendrite neurons; IV) four-dendrite neurons. a) Control; b) NGF.
Although cholinergic neurons of nucleus basalis of Meynert are larger and more branched than septal neurons, the number of studies on the effects of NGF on these cells is insignificant, because nucleus basalis of Meynert does not have distinct borders. Experiments with cell cultures from nucleus basalis of Meynert showed that its cholinergic neurons respond much more weakly to the presence of NGF in the culture medium than the septal neurons do; in nucleus basalis neurons, NGF increases AChE activity but does not cause elongation of the neurites [52, 54].
LOW MOLECULAR WEIGHT NGF MIMETICS AND GENE THERAPY AS APPROACHES
TO TREATMENT OF NEURODEGENERATIVE PATHOLOGIES RELATED TO NGF DEFICIT IN
THE BRAIN
NGF is involved in the growth, differentiation-associated plastic rearrangements, and survival of neurons in the central and peripheral nervous systems, both in healthy and diseased organisms. Experimental and clinical studies have demonstrated that the NGF content in the brain changes in various pathologies [56-58]. However, therapeutic application of NGF is hindered by the low permeability of the blood–brain barrier for this protein, the possibility of immune response, and adverse side effects that are due to the pleiotropic action of NGF. So far, all clinical and experimental efforts to use NGF for preventing pathological processes caused by brain trauma or AD have failed [59-61]. However, direct injection of NGF into brain structures in animal models of ischemic stroke reduced neurological deficit, infarct volume, neural cell apoptosis, and expressions of caspase-3 [62]. Therefore, design and synthesis of low molecular weight mimetics capable of interacting with neurotropic factor receptors would be a promising approach to the regulation of the activity of these factors in the central nervous system [63, 64].
Non-peptide NGF mimetics that act as TrkA receptor agonists have been developed in several studies [65-67]. These mimetics could support survival and stimulate differentiation of serum-depleted PC12 cells and cells of dorsal root ganglia and promote survival of cultured hippocampal cells in medium depleted of NGF with anti-NGF antibodies [65]. The NGF mimetic D3 reversed aged-related cognitive impairments in rats and prevented the loss of cholinergic synapses, decrease in cell body size of cholinergic neurons, and decline in ChAT activity in cortex and basal forebrain [68]. In transgenic mice expressing precursor of the mutant β-amyloid peptide, D3 significantly improved learning and short-term memory and ameliorated the mutation-associated elevation of soluble β-amyloid content in the cortex [69]. The biological activity of NGF peptide mimetics NL1L4 and L1L4 was similar to that of NGF. They induced differentiation of chicken dorsal root ganglia and stimulated tyrosine phosphorylation of TrkA receptor. In addition, L1L4 induced neuronal differentiation of PC12 cells. L1L4 reduced neuropathic behavior and restored neuronal function in a rat model of peripheral neuropathic pain in vivo, thereby suggesting a potential therapeutic role for this NGF peptidomimetic [70].
Another low molecular weight mimetic of NGF is the GK-2 peptide synthesized at the Zakusov Research Institute of Pharmacology. The structure of GK-2 (bis-(N-succinyl-glutamyl-lysine)hexamethylenediamide) is based on the structure of the NGF fourth loop β-turn. GK-2 activates TrkA and PI3K/Akt, but not MAPK/Erk [71]. Unlike full-size NGF, GK-2 easily penetrates the blood–brain barrier and causes no typical NGF side effects, such as hyperalgesia and weight loss. Neuroprotective properties of GK-2 have been demonstrated in PC12 and HT22 cell cultures and in primary cultures of rat cerebellar and hippocampal neurons subjected to oxidative stress and glutamate neurotoxicity [72-74]. Note that GK-2 did not induce differentiation of cultured rat embryo spinal ganglia. Unlike NGF, GK-2 did not stimulate neurite growth in these ganglia; however, it significantly increased the number of ganglia capable of neurite formation during culturing [74]. Similar properties were demonstrated earlier for peptides corresponding to the NGF loop β-turn [75]. GK-2 also exhibited anti-ischemic properties in various models of brain ischemia [76-78]. It restored impaired limb motor functions in rats with focal traumatic brain injury of the cortical motor area [74] and produced antiparkinsonian effects in animal models [79]. Unfortunately, there are still no data on the influence of GK-2 on plastic rearrangements of cholinergic neurons in basal ganglia; however, it was found that GK-2 restored impaired brain cognitive functions in animal AD models [80]. These data suggest low molecular weight NGF mimetics as promising agents in AD therapy [81].
In 2005, Tuszynski et al. performed a phase I trial of ex vivo NGF gene delivery by implanting autologous fibroblasts genetically modified with a viral vector to express human NGF into the basal forebrains of individuals with mild AD [82]. The treated patients demonstrated enhancement of cognitive functions, increased brain metabolism, and improved morphology of cholinergic neurons [82]. Later, brains of the same patients were studied post mortem. In all the cases, brain neurons responded to the treatment by increased growth of axons toward the NGF source despite severe ongoing neurodegenerative processes [83]. Similarly, surgical implantation of NGF-producing cells into the basal forebrain of AD patients improved the cholinergic markers in cerebrospinal fluid [84].
Another approach to the treatment of NGF deficit-related disorders that has received wide application during the last few years due to the development of viral vectors is direct injection of the vector into the brain [85]. Thus, lentivirus-mediated overexpression of NGF in the rat hippocampus prevented β-amyloid-induced long-term potentiation decline [86, 87]. Lentiviral NGF gene delivery into the basal forebrain in aged non-human primates reversed age-related neuronal atrophy in these animals [88].
NGF is synthesized in the brain structures innervated by the basal forebrain cholinergic neurons and then transported retrogradely via axons to the bodies of cholinergic neurons. This neurotrophic factor is required for normal plastic rearrangements during development and functioning of basal forebrain cholinergic neurons. The trophic support of developing and mature basal forebrain cholinergic neurons by NGF maintains the required number of these neurons, stabilizes the levels of activity of key enzymes of acetylcholine synthesis, and affects the extent of connection between cholinergic neurons and innervated targets. The levels of NGF in brain structures change in various pathologies, such as AD, ischemia, and brain trauma. However, therapeutic application of NGF is restricted due to the low permeability of the blood–brain barrier for this protein, the possibility of immune response against NGF, and adverse side effects resulting from the pleiotropic action of this factor. For this reason, development of low molecular weight NGF mimetics that would be able to interact with the corresponding receptors and have high biological activity but lack negative properties of the native NGF is of particular interest. Another promising approach is the use of gene therapy for delivery of neuroprotective compounds to damaged brain regions.
Acknowledgements
This work was supported by the Russian Science Foundation (project No. 16-15-10108).
REFERENCES
1.Levi-Montalcini, R., and Hamburger, V. (1951)
Selective growth stimulating effects of mouse sarcoma on the sensory
and sympathetic nervous system of the chick embryo, J. Exp.
Zool., 116, 321-361.
2.Levi-Montalcini, R., Meyer, H., and Hamburger, V.
(1954) In vitro experiments on the effects of mouse sarcomas 180
and 37 on the spinal and sympathetic ganglia of the chick embryo,
Cancer Res., 14, 49-57.
3.Levi-Montalcini, R. (1987) The nerve growth factor:
thirty-five years later, EMBO J., 6, 1145-1154.
4.Levi-Montalcini, R., and Cohen, S. (1960) Effects
of the extract of the mouse submaxillary salivary glands on the
sympathetic system of mammals, Ann. N. Y. Acad. Sci., 85,
324-341.
5.Ullrich, A., Gray, A., Berman, C., and Dull, T. J.
(1983) Human beta-nerve growth factor gene sequence highly homologous
to that of mouse, Nature, 303, 821-825.
6.Chao, M. V., and Hempstead, B. L. (1995) p75 and
Trk: a two-receptor system, Trends Neurosci., 18,
321-326.
7.Esposito, D., Patel, P., Stephens, R. M., Perez,
P., Chao, M. V., Kaplan, D. R., and Hempstead, B. L. (2001) The
cytoplasmic and transmembrane domains of the p75 and Trk A receptors
regulate high affinity binding to nerve growth factor, J. Biol.
Chem., 276, 32687-32695.
8.Scott, J., Selby, M., Urdea, M., Quiroga, M., Bell,
G. I., and Rutter, W. J. (1983) Isolation and nucleotide sequence of a
cDNA encoding the precursor of mouse nerve growth factor,
Nature, 302, 538-540.
9.Wiesmann, C., and De Vos, A. M. (2001) Nerve growth
factor: structure and function, Cell Mol. Life Sci., 58,
748-759.
10.Iulita, M. F., and Cuello, A. C. (2014) Nerve
growth factor metabolic dysfunction in Alzheimer’s disease and
Down syndrome, Trends Pharmacol. Sci., 35,
338-348.
11.Korsching, S., Auburger, G., Heumann, R., Scott,
J., and Thoenen, H. (1985) Levels of nerve growth factor and its mRNA
in the central nervous system of the rat correlate with cholinergic
innervation, EMBO J., 4, 1389-1393.
12.Crutcher, K. A., and Collins, F. (1982) In
vitro evidence for two distinct hippocampal growth factors: basis
of neuronal plasticity? Science, 217, 67-68.
13.Larkfors, L., Ebendal, T., Whittemore, S. R.,
Persson, H., Hoffer, B., and Olson, L. (1987) Decreased level of nerve
growth factor (NGF) and its messenger RNA in the aged rat brain,
Brain Res., 427, 55-60.
14.Seiler, M., and Schwab, M. E. (1984) Specific
retrograde transport of nerve growth factor (NGF) from neocortex to
nucleus basalis in the rat, Brain Res., 300,
33-39.
15.Korsching, S. (1986) The role of nerve growth
factor in the CNS, TINS, 9, 570-573.
16.Korsching, S., Heumann, R., Thoenen, H., and
Hefti, F. (1986) Cholinergic denervation of the rat hippocampus by
fimbrial transection leads to a transient accumulation of nerve growth
factor (NGF) without change in mRNA NGF content, Neurosci.
Lett., 66, 175-180.
17.Dunnett, S. B., and Fibiger, H. C. (1993) Role of
forebrain cholinergic systems in learning and memory: relevance to the
cognitive deficits of aging and Alzheimer’s dementia, Prog.
Brain Res., 98, 413-420.
18.Kasa, P., Rakonczay, Z., and Gulya, K. (1997) The
cholinergic system in Alzheimer’s disease, Prog.
Neurobiol., 52, 511-535.
19.Isaev, N. K., and Orlova, E. I. (1990) Choline
acetyltransferase and acetylcholinesterase dynamics in the septum
ontogenesis in rats, macro- and microlevels of brain organization, in
Proceedings of the Brain Research Institute of the Academy of
Medical Sciences of the USSR (Adrianov, O. S., ed.) [in Russian],
pp. 92-94.
20.Auburger, G., Heumann, R., Hellweg, R.,
Korsching, S., and Thoenen, H. (1987) Developmental changes of nerve
growth factor and its mRNA in the rat hippocampus: comparison with
choline acetyltransferase, Dev. Biol., 120, 322-328.
21.Richardson, P. M., Issa, V. M., and Riopelle, R.
J. (1986) Distribution of neuronal receptors for nerve growth factor in
the rat, J. Neurosci., 6, 2312-2321.
22.Weskamp, G., Lorez, H. P., Keller, H. H., and
Otten, U. (1986) Cholinergic but not monoaminergic denervation
increases nerve growth factor content in the adult rat hippocampus and
cerebral cortex, Naunyn Schmiedebergs Arch. Pharmacol.,
334, 346-351.
23.Woolf, N. J., and Butcher, L. L. (2011)
Cholinergic systems mediate action from movement to higher
consciousness, Behav. Brain Res., 221, 488-498.
24.Gould, E., Farris, T. W., and Butcher, L. L.
(1989) Basal forebrain neurons undergo somatal and dendritic remodeling
during postnatal development: a single-section Golgi and choline
acetyltransferase analysis, Brain Res. Dev. Brain Res.,
46, 297-302.
25.Gnahn, H., Hefti, F., Heumann, R., Schwab, M. E.,
and Thoenen, H. (1983) NGF-mediated increase of choline
acetyltransferase (ChAT) in the neonatal rat forebrain: evidence for a
physiological role of NGF in the brain? Brain Res., 285,
45-52.
26.Chen, K. S., Nishimura, M. C., Armanini, M. P.,
Crowley, C., Spencer, S. D., and Phillips, H. S. (1997) Disruption of a
single allele of the nerve growth factor gene results in atrophy of
basal forebrain cholinergic neurons and memory deficits, J.
Neurosci., 17, 7288-7296.
27.Hefti, F., Dravid, A., and Hartikka, J. (1984)
Chronic intraventricular injections of nerve growth factor elevate
hippocampal choline acetyltransferase activity in adult rats with
partial septo-hippocampal lesions, Brain Res., 293,
305-311.
28.Gage, F. H., Armstrong, D. M., Williams, L. R.,
and Varon, S. (1988) Morphological response of axotomized septal
neurons to nerve growth factor, J. Comp. Neurol.,
269, 147-155.
29.Montero, C. N., and Hefti, F. (1988) Rescue of
lesioned septal cholinergic neurons by nerve growth factor: specificity
and requirement for chronic treatment, J. Neurosci., 8,
2986-2999.
30.Hagg, T., Manthorpe, M., Vahlsing, H. L., and
Varon, S. (1988) Delayed treatment with nerve growth factor reverses
the apparent loss of cholinergic neurons after acute brain damage,
Exp. Neurol., 101, 303-312.
31.Conner, J. M., Franks, K. M., Titterness, A. K.,
Russell, K., Merrill, D. A., Christie, B. R., Sejnowski, T. J., and
Tuszynski, M. H. (2009) NGF is essential for hippocampal plasticity and
learning, J. Neurosci., 29, 10883-10889.
32.Altavista, M. C., Rossi, P., Bentivoglio, A. R.,
Crociani, P., and Albanese, A. (1990) Aging is associated with a
diffuse impairment of forebrain cholinergic neurons, Brain Res.,
508, 51-59.
33.Sofroniew, M. V., Pearson, R. C., and Powell, T.
P. (1987) The cholinergic nuclei of the basal forebrain of the rat:
normal structure, development and experimentally induced degeneration,
Brain Res., 411, 310-331.
34.Springer, J. E., Tayrien, M. W., and Loy, R.
(1987) Regional analysis of age-related changes in the cholinergic
system of the hippocampal formation and basal forebrain of the rat,
Brain Res., 407, 180-184.
35.Lowes-Hummel, P., Gertz, H. J., Ferszt, R., and
Cervos-Navarro, J. (1989) The basal nucleus of Meynert revised: the
nerve cell number decreases with age, Arch. Gerontol. Geriatr.,
8, 21-27.
36.Bertoni-Freddari, C., Fattoretti, P., Casoli, T.,
Caselli, U., and Meier-Ruge, W. (1996) Deterioration threshold of
synaptic morphology in aging and senile dementia of Alzheimer’s
type, Anal. Quant. Cytol. Histol., 18, 209-213.
37.Kerbler, G. M., Fripp, J., Rowe, C. C.,
Villemagne, V. L., Salvado, O., Rose, S., and Coulson, E. J. (2014)
Alzheimer’s disease neuroimaging initiative. Basal forebrain
atrophy correlates with amyloid β burden in Alzheimer’s
disease, Neuroimage Clin., 7, 105-113.
38.Grothe, M., Heinsen, H., and Teipel, S. (2012)
Longitudinal measures of cholinergic forebrain atrophy in the
transition from healthy aging to Alzheimer’s disease,
Neurobiol. Aging, 34, 1210-1220.
39.Bishop, N. A., Lu, T., and Yankner, B. A. (2010)
Neural mechanisms of ageing and cognitive decline, Nature,
464, 529-535.
40.Isaev, N. K., Stelmashook, E. V., Stelmashook, N.
N., Sharonova, I. N., and Skrebitsky, V. G. (2013) Brain aging and
mitochondria-targeted plastoquinone antioxidants of SkQ-type,
Biochemistry (Moscow), 78, 295-300.
41.Aloe, L., and Rocco, M. L. (2015) NGF and
therapeutic prospective: what have we learned from the NGF transgenic
models? Ann. Ist. Super Sanita, 51, 5-10.
42.Capsoni, S., Giannotta, S., and Cattaneo, A.
(2002) Beta-amyloid plaques in a model for sporadic Alzheimer’s
disease based on transgenic anti-nerve growth factor antibodies,
Mol. Cell Neurosci., 21, 15-28.
43.Cattaneo, A., Capsoni, S., and Paoletti, F.
(2008) Towards non-invasive nerve growth factor therapies for
Alzheimer’s disease, J. Alzheimer’s Dis., 15,
255-283.
44.Origlia, N., Capsoni, S., Domenici, L., and
Cattaneo, A. (2006) Time window in cholinomimetic ability to rescue
long-term potentiation in neurodegenerating anti-nerve growth factor
mice, J. Alzheimer’s Dis., 9, 59-68.
45.Covaceuszach, S., Capsoni, S., Ugolini, G.,
Spirito, F., Vignone, D., and Cattaneo, A. (2009) Development of a
non-invasive NGF-based therapy for Alzheimer’s disease, Curr.
Alzheimer Res., 6, 158-170.
46.Cattaneo, A., and Calissano, P. (2012) Nerve
growth factor and Alzheimer’s disease: new facts for an old
hypothesis, Mol. Neurobiol., 46, 588-604.
47.Mesulam, M. M., Mufson, E. J., Levey, A. I., and
Wainer, B. H. (1983) Cholinergic innervation of cortex by the basal
forebrain: cytochemistry and cortical connections of the septal area,
diagonal band nuclei, nucleus basalis (substantia innominata), and
hypothalamus in the rhesus monkey, J. Comp. Neurol., 214,
170-197.
48.Gahwiler, B. H., Enz, A., and Hefti, F. (1987)
Nerve growth factor promotes development of the rat septo-hippocampal
cholinergic projection in vitro, Neurosci. Lett.,
75, 6-10.
49.Takei, N., Tsukui, H., and Hatanaka, H. (1988)
Nerve growth factor increases the intracellular content of
acetylcholine in cultured septal neurons from developing rats, J.
Neurochem., 51, 1118-1125.
50.Isaev, N. K., and Viktorov, I. V. (1991) Effects
of nerve growth factor on cholinergic neurons in the dissociated
cultures of septum pellucidum, Byull. Eksp. Biol. Med.,
111, 305-306.
51.Isaev, N. K., Markova, E. G., Stelmashchuk, E.
V., and Viktorov, I. V. (1994) Effects of nerve growth factor on the
selective increase in body size of cultured cholinergic neurons,
Byull. Eksp. Biol. Med., 117, 102-104.
52.Hartikka, J., and Hefti, F. (1988) Comparison of
nerve growth factor’s effects on development of septum, striatum,
and nucleus basalis cholinergic neurons in vitro, J.
Neurosci. Res., 21, 352-364.
53.Hartikka, J., and Hefti, F. (1988) Development of
septal cholinergic neurons in culture: plating density and glial cells
modulate effects of NGF on survival, fiber growth, and expression of
transmitter-specific enzymes, J. Neurosci., 8,
2967-2985.
54.Isaev, N. K. (1991) Effects of Nerve Growth
Factor on Differentiation of Cholinergic Neurons in Tissue Culture:
PhD in Biology dissertation [in Russian], Moscow State University,
Moscow.
55.Markova, E. G., and Isaev, N. K. (1992) Effects
of nerve growth factor on the dendrite development in the dissociated
culture of rat cholinergic neurons, Byull. Eksp. Biol. Med.,
113, 318-320.
56.Hellweg, R., Gericke, C. A., Jendroska, K.,
Hartung, H. D., and Cervos-Navarro, J. (1998) NGF content in the
cerebral cortex of non-demented patients with amyloid-plaques and in
symptomatic Alzheimer’s disease, Int. J. Dev. Neurosci.,
16, 787-794.
57.Lindvall, O., Ernfors, P., Bengzon, J., Kokaia,
Z., Smith, M. L., Siesjo, B. K., and Persson, H. (1992) Differential
regulation of mRNAs for nerve growth factor, brain derived neurotrophic
factor, and neurotrophin 3 in the adult rat brain following cerebral
ischemia and hypoglycemic coma, Proc. Natl. Acad. Sci. USA,
89, 648-652.
58.Chiaretti, A., Barone, G., Riccardi, R.,
Antonelli, A., Pezzotti, P., Genovese, O., Tortorolo, L., and Conti, G.
(2009) NGF, DCX, and NSE upregulation correlates with severity and
outcome of head trauma in children, Neurology, 72,
609-616.
59.Young, J., Pionk, T., Hiatt, I., Geeck, K., and
Smith, J. S. (2015) Environmental enrichment aides in functional
recovery following unilateral controlled cortical impact of the
forelimb sensorimotor area however intranasal administration of nerve
growth factor does not, Brain Res. Bull., 115, 17-22.
60.Lv, Q., Lan, W., Sun, W., Ye, R., Fan, X., Ma,
M., Yin, Q., Jiang, Y., Xu, G., Dai, J., Guo, R., and Liu, X. (2014)
Intranasal nerve growth factor attenuates tau phosphorylation in brain
after traumatic brain injury in rats, J. Neurol. Sci.,
345, 48-55.
61.Ferreira, D., Westman, E., Eyjolfsdottir, H.,
Almqvist, P., Lind, G., Linderoth, B., Seiger, A., Blennow, K., Karami,
A., Darreh-Shori, T., Wiberg, M., Simmons, A., Wahlund, L. O.,
Wahlberg, L., and Eriksdotter, M. (2015) Brain changes in
Alzheimer’s disease patients with implanted encapsulated cells
releasing nerve growth factor, J. Alzheimer’s Dis.,
43, 1059-1072.
62.Yang, J. P., Liu, H. J., Yang, H., and Feng, P.
Y. (2011) Therapeutic time window for the neuroprotective effects of
NGF when administered after focal cerebral ischemia, Neurol.
Sci., 32, 433-441.
63.Obianyo, O., and Ye, K. (2013) Novel small
molecule activators of the Trk family of receptor tyrosine kinases,
Biochim. Biophys. Acta, 1834, 2213-2218.
64.Skaper, S. D. (2008) The biology of
neurotrophins, signaling pathways, and functional peptide mimetics of
neurotrophins and their receptors, CNS Neurol. Disord. Drug
Targets, 7, 46-62.
65.Scarpi, D., Cirelli, D., Matrone, C., Castronovo,
G., Rosini, P., Occhiato, E. G., Romano, F., Bartali, L., Clemente, A.
M., Bottegoni, G., Cavalli, A., De Chiara, G., Bonini, P., Calissano,
P., Palamara, A. T., Garaci, E., Torcia, M. G., Guarna, A., and
Cozzolino, F. (2012) Low molecular weight, non-peptidic agonists of
TrkA receptor with NGF-mimetic activity, Cell Death Dis.,
3, e389.
66.Maliartchouk, S., Feng, Y., Ivanisevic, L.,
Debeir, T., Cuello, A. C., Burgess, K., and Saragovi, H. U. (2000) A
designed peptidomimetic agonistic ligand of TrkA nerve growth factor
receptors, Mol. Pharmacol., 57, 385-391.
67.Massa, S. M., Xie, Y., and Longo, F. M. (2002)
Alzheimer’s therapeutics: neurotrophin small molecule mimetics,
J. Mol. Neurosci., 19, 107-111.
68.Bruno, M. A., Clarke, P. B., Seltzer, A.,
Quirion, R., Burgess, K., Cuello, A. C., and Saragovi, H. U. (2004)
Long-lasting rescue of age-associated deficits in cognition and the CNS
cholinergic phenotype by a partial agonist peptidomimetic ligand of
TrkA, J. Neurosci., 24, 8009-8018.
69.Aboulkassim, T., Tong, X. K., Tse, Y. C., Wong,
T. P., Woo, S. B., Neet, K. E., Brahimi, F., Hamel, E., and Saragovi,
H. U. (2011) Ligand-dependent TrkA activity in brain differentially
affects spatial learning and long-term memory, Mol. Pharmacol.,
80, 498-508.
70.Colangelo, A. M., Bianco, M. R., Vitagliano, L.,
Cavaliere, C., Cirillo, G., De Gioia, L., Diana, D., Colombo, D.,
Redaelli, C., Zaccaro, L., Morelli, G., Papa, M., Sarmientos, P.,
Alberghina, L., and Martegani, E. (2008) A new nerve growth
factor-mimetic peptide active on neuropathic pain in rats, J.
Neurosci., 28, 2698-2709.
71.Seredenin, S. B., and Gudasheva, T. A. (2015)
Development of pharmacologically active small molecule with the
properties of nerve growth factor, Zh. Nevrol. Psikhiatr. im.
Korsakova, 115, 63-70.
72.Antipova, T. A., Gudasheva, T. A., and Seredenin,
S. B. (2011) In vitro neuroprotective properties of GK-2, a new
original mimetic of nerve growth factor, Byull. Eksp. Biol.
Med., 150, 607-609.
73.Antipova, T. A., Nikolaev, S. V., Gudasheva, T.
A., and Seredenin, S. B. (2014) In vitro neuroprotective
properties of GK-2(H), a new original mimetic of human nerve growth
factor, Eksp. Klin. Farm., 77, 8-11.
74.Stelmashook, E. V., Genrikhs, E. E., Novikova, S.
V., Barskov, I. V., Gudasheva, T. A., Seredenin, S. B., Khaspekov, L.
G., and Isaev, N. K. (2015) Behavioral effect of dipeptide NGF mimetic
GK-2 in an in vivo model of rat traumatic brain injury and its
neuroprotective and regenerative properties in vitro, Int. J.
Neurosci., 125, 375-379.
75.Longo, F. M., Manthorpe, M., Xie, Y. M., and
Varon, S. (1997) Synthetic NGF peptide derivatives prevent neuronal
death via a p75 receptor-dependent mechanism, J. Neurosci. Res.,
48, 1-17.
76.Povarnina, P. Yu., Gudasheva, T. A., Vorontsova,
O. N., Nikolaev, S. V., Antipova, T. A., Ostrovskaya, R. U., and
Seredenin, S. B. (2012) Neuroprotective effects of the dimeric
dipeptide nerve growth factor mimetic GK-2 in the irreversible carotid
ligature model in rats, Eksp. Klin. Farm., 75, 15-20.
77.Seredenin, S. B., Silachev, D. N., Gudasheva, T.
A., Pirogov, Yu. A., and Isaev, N. K. (2011) Neuroprotective properties
of the nerve growth factor dipeptide mimetic GK-2 in the induced focal
ischemia in the middle cerebral artery basin, Byull. Eksp. Biol.
Med., 151, 518-521.
78.Seredenin, S. B., Romanova, G. A., Gudasheva, T.
A., Shakova, F. M., Barskov, I. V., Srelmashchuk, E. V., and Antipova,
T. A. (2010) Neuroprotective and anti-amnesic properties of the nerve
growth factor dipeptide mimetic GK-2 in the experimental ischemia of
the cortex, Byull. Eksp. Biol. Med., 150, 406-410.
79.Povarnina, P. Yu., Gudasheva, T. A., Vorontsova,
O. N., Bondarenko, N. A., and Seredenin, S. B. (2011) Anti-Parkinsonian
properties of the nerve growth factor dipeptide mimetic GK-2 in
vivo, Byull. Eksp. Biol. Med., 151, 634-637.
80.Povarnina, P. Yu., Vorontsova, O. N., Gudasheva,
T. A., Ostrovskaya, R. U., and Seredenin, S. B. (2013) Original nerve
growth factor mimetic dipeptide GK-2, Acta Naturae, 5,
88-95.
81.Kazim, S. F., and Iqbal, K. (2016) Neurotrophic
factor small-molecule mimetics mediated neuroregeneration and synaptic
repair: emerging therapeutic modality for Alzheimer’s disease,
Mol. Neurodegener., 11, 50.
82.Tuszynski, M. H., Thal, L., Pay, M., Salmon, D.
P., U, H. S., Bakay, R., Patel, P., Blesch, A., Vahlsing, H. L., Ho,
G., Tong, G., Potkin, S. G., Fallon, J., Hansen, L., Mufson, E. J.,
Kordower, J. H., Gall, C., and Conner, J. (2005) A phase 1 clinical
trial of nerve growth factor gene therapy for Alzheimer’s
disease, Nat. Med., 11, 551-555.
83.Tuszynski, M. H., Yang, J. H., Barba, D., U, H.
S., Bakay, R. A., Pay, M. M., Masliah, E., Conner, J. M., Kobalka, P.,
Roy, S., and Nagahara, A. H. (2015) Nerve growth factor gene therapy:
activation of neuronal responses in Alzheimer’s disease, JAMA
Neurol., 72, 1139-1147.
84.Karami, A., Eyjolfsdottir, H., Vijayaraghavan,
S., Lind, G., Almqvist, P., Kadir, A., Linderoth, B., Andreasen, N.,
Blennow, K., Wall, A., Westman, E., Ferreira, D., Kristoffersen Wiberg,
M., Wahlund, L. O., Seiger, A., Nordberg, A., Wahlberg, L.,
Darreh-Shori, T., and Eriksdotter, M. (2015) Changes in CSF cholinergic
biomarkers in response to cell therapy with NGF in patients with
Alzheimer’s disease, Alzheimer’s Dement.,
11, 1316-1328.
85.Stepanichev, M. Yu. (2011) Modern approaches and
prospects in the gene therapy of Alzheimer’s disease,
Neirokhimiya, 28, 181-191.
86.Uzakov, S. S., Ivanov, A. D., Salozhin, S. V.,
Markevich, V. A., and Gulyaeva, N. V. (2015) Lentiviral-mediated
overexpression of nerve growth factor (NGF) prevents
beta-amyloid-induced long-term potentiation (LTP) decline in the rat
hippocampus, Brain Res., 1624, 398-404.
87.Ivanov, A. D., Tukhbatova, G. R., Salozhin, S.
V., and Markevich, V. A. (2015) NGF but not BDNF overexpression
protects hippocampal LTP from beta-amyloid-induced impairment,
Neuroscience, 289, 114-122.
88.Nagahara, A. H., Bernot, T., Moseanko, R.,
Brignolo, L., Blesch, A., Conner, J. M., Ramirez, A., Gasmi, M., and
Tuszynski, M. H. (2009) Long-term reversal of cholinergic neuronal
decline in aged non-human primates by lentiviral NGF gene delivery,
Exp. Neurol., 215, 153-159.