REVIEW: Role of Atypical Protein Kinases in Maintenance of Long-Term Memory and Synaptic Plasticity
A. A. Borodinova*, A. B. Zuzina, and P. M. Balaban
Institute of Higher Nervous Activity and Neurophysiology, Russian Academy of Sciences, 117485 Moscow, Russia; E-mail: borodinova.msu@mail.ru* To whom correspondence should be addressed.
Received August 4, 2016; Revision received October 26, 2016
Investigation of biochemical mechanisms underlying the long-term storage of information in nervous system is one of main problems of modern neurobiology. As a molecular basis of long-term memory, long-term changes in kinase activities, increase in the level and changes in the subunit composition of receptors in synaptic membranes, local activity of prion-like proteins, and epigenetic modifications of chromatin have been proposed. Perhaps a combination of all or of some of these factors underlies the storage of long-term memory in the brain. Many recent studies have shown an exclusively important role of atypical protein kinases (PKCζ, PKMζ, and PKCι/λ) in processes of learning, consolidation and maintenance of memory. The present review is devoted to consideration of mechanisms of transcriptional and translational control of atypical protein kinases and their roles in induction and maintenance of long-term synaptic plasticity and memory in vertebrates and invertebrates.
KEY WORDS: protein kinase Mζ, atypical protein kinases, synaptic plasticity, memory, epigenetic regulation, histone acetylation, methylationDOI: 10.1134/S0006297917030026
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolpropionic acid receptor; aPKC, atypical protein kinase C; CaMKII, Ca2+/calmodulin-dependent protein kinase II; cAMP, cyclic adenosine monophosphate; CBP, CREB-binding protein; CRE, cAMP response-element; CREB, a protein binding cAMP-sensitive element (CRE-binding protein); DAG, diacylglycerol; DNMT, DNA methyltransferase; DTEs, dendritic targeting elements; E-LTP/L-LTP, early/late long-term potentiation; ERK, extracellular signal-regulated kinase; GluA, subunit of glutamate receptor AMPA; GluA1-AMPA/GluA2-AMPA, glutamate AMPA-receptor containing GluA1/GluA2 subunit; HDAC, histone deacetylase; LTP, long-term potentiation; mTOR, mammalian target of rapamycin; PICK1, protein interacting with C kinase; PI3K, phosphoinositide-3-kinase; PIN1, peptidyl-prolyl cis-trans isomerase NIMA-interacting 1; PKA, protein kinase A; PKC, protein kinase C; PKCλ, protein kinase Cλ; PKMζ, protein kinase Mζ; PS, phosphatidylserine; shRNA, short hairpin RNA; TSA, trichostatin A; uORF, upstream open reading frame; UTRs, untranslated regions; ZIP, Zeta Inhibitory Peptide.
Investigation of mechanisms underlying long-term storage of information
in nervous system is one of main problems of modern neurobiology. There
are many data in the literature on involvement of very different
molecular systems and linked signaling cascades in formation of the
memory trace, but their participation in the memory storage seems
doubtful because blockade of these molecular systems results in
nonspecific changes in nervous system activity including disorders in
memory. Until recently, there was no common opinion about a specific
key mechanism responsible for long-term maintenance of memory, and as a
basis for long-term memory there were considered long-term changes in
kinase activities, an increase in receptor density and changes in their
subunit composition in synaptic membranes, local activity of prion-like
proteins, epigenetic modifications of chromatin, or a combination of
these factors [1, 2].
During the last 15-20 years, it has been found that some atypical protein kinases play in the nervous system of mammals [3-6] and invertebrates [7-10] an important role in long-term potentiation and other forms of synaptic plasticity that present a physiological substrate for long-term memory. It was shown convincingly that selective inhibition in a definite brain structure of atypical protein kinase C (aPKC) with a peptide specific for these molecules affected memory that was normally formed with participation of just this region of the brain [6, 11-14]. The erasure of memory observed in experiments was not accompanied by disorders in other vital functions and, moreover, did not influence consolidation of a new memory trace or the repeated development of the same memories [15, 16]. It should be noted that these newly developed memories could be erased again on repeated inhibition of aPKC [16]. These data induced great interest of researchers in atypical protein kinases C, and especially to one of their isoforms, the protein kinase Mζ.
STRUCTURAL FEATURES AND CELLULAR LOCALIZATION OF ATYPICAL PROTEIN
KINASES
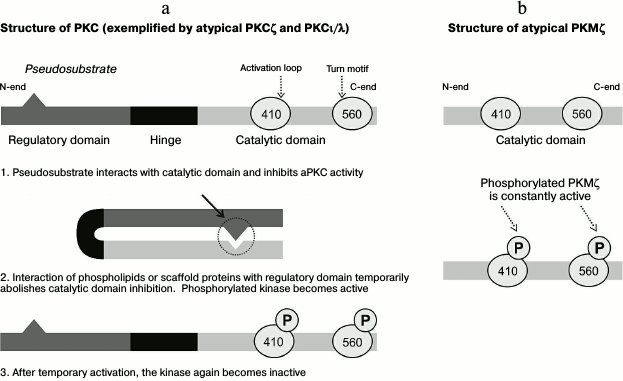
Representatives of the PKC family are similar in structure. The typical protein molecule consists of regulatory (N-end) and catalytic (C-end) domains connected with a hinge (Fig. 1a) [17-19]. The N-terminal regulatory domain of the protein molecule contains a region that functions as a pseudosubstrate, and its binding with an active site of the C-terminal catalytic domain inhibits the protein kinase activity (Fig. 1a) [18]. Interaction of secondary mediators (in the case of classical and novel PKC) or of phospholipids and scaffold proteins (in the case of atypical PKC; Fig. 1a) with a sequence of the PKC regulatory domain triggers transient conformational changes leading to withdrawal of autoinhibition of the catalytic domain and transfer of the active kinase onto the membrane [17, 19]. Upon destruction of the secondary messenger, PKC is again changed into inactive conformation (Fig. 1a). Detailed consideration of the PKC family has revealed that the catalytic domain of different PKC isoforms is rather conservative, whereas regulatory domains of individual kinases are different. Based on the interaction character with secondary messengers, PKCs can be conventionally divided into three groups: classical protein kinases (α, β, γ), whose activities are regulated by calcium ions, diacylglycerol (DAG), and phosphatidylserine (PS); novel protein kinases (δ, ε, η, θ), whose regulation is mediated through interaction of the kinase regulatory domain only with DAG and PS; and atypical protein kinases (ζ, ι, λ), whose regulation is not quite clear but is only shown to be independent of the presence of calcium ions and DAG, but it can be increased in the presence of some phospholipids [17-21].
Fig. 1. Structural features underlie differences in characteristics of transient activities of different atypical protein kinases C (aPKC). a) PKCζ and PKCι/λ consist of regulatory and catalytic domains. The pseudosubstrate sequence in the regulatory domain binds with a site of the kinase domain and thus inhibits its activity. The interaction of phospholipids or scaffold proteins with the regulatory domain leads to transient conformational rearrangements that determine activated states of PKCζ and PKCι/λ. Thus, the kinase activities are limited by the time of interaction of these molecules with the regulatory domain. b) In the PKMζ protein molecule, there is no regulatory domain; therefore, the kinase activity is persistent. In the scheme, the aPKC catalytic domain contains two sites whose phosphorylation leads to increase in kinase activity and to prolongation of the molecule lifetime.
According to many recent studies, representatives of atypical protein kinases PKMζ and PKCι/λ are important for generating and maintaining long-term changes in synaptic plasticity and memory in vertebrate and invertebrate animals [1, 3-5, 7, 8, 11, 13]. For neurobiologists, the PKCζ isoform – protein kinase Mζ – is especially interesting (Fig. 1b). In the nervous system of invertebrates, PKCζ is subjected to proteolysis with separation of the regulatory domain and formation of shortened constitutively active PKMζ [9, 22, 23]. For a long time, PKMζ was considered only as a product of PKCζ proteolysis [24], until in animals with PKCζ gene knockout, data were obtained that demonstrated the possibility of PKMζ synthesis in mammalian brain even in the complete absence of PKCζ protein molecules [23]. Considering the problem of the neuronal PKMζ origin, one should consider data on the PKCζ gene organization in vertebrates [25]. It is known that on the 5′- and 3′-ends of the gene, there are exon clusters encoding, respectively, the regulatory and catalytic domains [25]. During promoter activation on the 5′-end of the gene, a long transcript is produced that encodes PKCζ. During evolutionary development of vertebrates, an addition promoter that separates the 5′- and 3′-clusters of exons inside the PKCζ gene intron has been generated. Activation of this promoter results in production of an alternative (short) transcript, which contains the catalytic domain sequence and corresponds to PKMζ [1, 23, 25]. Based on results of a classical work on PKMζ and on the presence of PKCζ traces in the nervous system (see below), it is now believed that the major source of protein molecules of PKMζ in the mammalian brain is de novo protein synthesis from short PKMζ-transcripts [23].
Analysis of untranslated 5′- and 3′-regions of PKMζ mRNA 5′- and 3′-UTRs revealed another interesting feature. These 5′- and 3′-UTRs were shown to contain specific dendritic targeting elements (DTEs), which determine the further transplacing of the transcripts into strictly defined cellular compartments where protein molecules are synthesized “in place” “by demand” [26, 27]. It was shown that 5′-DTE was responsible for somato-dendritic distribution of PKMζ mRNA, whereas 3′-DTE provided the intracellular transfer of transcripts into distal dendrites and synapses [26]. Data on the distribution of PKMζ transcripts are in a good agreement with results of analysis of the intracellular localization of aPKC protein molecules in invertebrates and mammals. Some data have indicated that PKCζ and PKMζ proteins are concentrated in synaptic contacts of nerve cell processes where they are involved in mechanisms of long-term potentiation and synaptic plasticity [28, 29]. Some works have described nuclear localization of PKMζ and PKCζ proteins in activated [30, 31] and inactivated cells [28], where their presence seems to be necessary for realizing and regulating various epigenetic programs that will be discussed below.
The distribution of aPKC in the organism was studied in the early 2000s. Screening of different tissues revealed a wide distribution of two isoforms (PKCζ, PKCι/λ) in internal organs (liver, kidneys, lungs, etc.) and significantly less in the brain [23]. On the other hand, the short constitutively active PKMζ was found only in brain, especially in such structures as hippocampus, neocortex, striatum, and hypothalamus [23, 32]. Careful study of localization of different aPKC in nervous tissue showed that PKMζ is the major isoform of aPKC specific for the brain structures. Note that alongside PKMζ, PKCι/λ is expressed in the hippocampus, and trace amounts of PKCζ are found in the cerebellum [23, 32].
Thus, PKMζ is the most widespread neuron-specific isoform of atypical protein kinases C, and the constitutive activity of this isoform in strictly defined synaptic compartments seems to be necessary for realizing programs of memory storage in vertebrates and invertebrates.
PROPOSED ACTION MECHANISM OF ATYPICAL PROTEIN KINASES. ATYPICAL
PROTEIN KINASES IN THE CONTEXT OF SYNAPTIC PLASTICITY
As mentioned, in the brain atypical protein kinases are mainly represented by isoform PKMζ. Protein molecules of PKMζ synthesized de novo are rapidly phosphorylated at two sites in the catalytic domain (Figs. 1 and 2), and after that the protein kinase becomes activated (see below). Since the PKMζ sequence lacks the regulatory domain containing the pseudosubstrate region, the activation of the protein kinase is persistent [23, 25]. In this aspect, PKMζ is significantly different from PKCζ and PKCι/λ whose phosphorylation is limited by the presence of the regulatory domain and can be realized only under certain conditions (e.g. at LTP, see below [18, 19]).
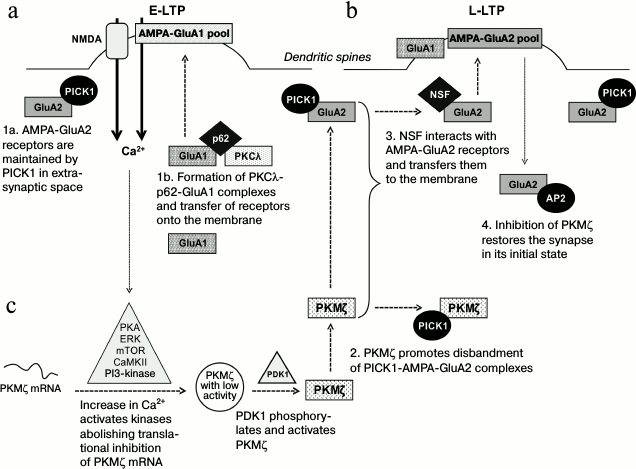
Fig. 2. Role of atypical protein kinases in the induction and maintenance of long-term changes in synaptic plasticity. a) During the early potentiation phase, PKCι/λ regulates the intracellular transfer of calcium-permeable GluA1-AMPA receptors and increases their incorporation into the postsynaptic membrane. b) During the late potentiation phase, PKMζ promotes the formation of calcium-impermeable GluA2-AMPA receptor complexes with the protein carrier NSF and their incorporation into the postsynaptic membrane. c) Increase in intracellular calcium concentration in potentiated synapses promotes activation of some kinases, which remove the translational block from mRNA of PKMζ. The newly produced PKMζ is phosphorylated by the PDK1 kinase and becomes constitutively active. The active PKMζ is responsible for maintenance of the changed (increased) efficiency of potentiated synapses for a long time.
According to some data, the conservative catalytic domain of PKC is characterized by low substrate specificity; therefore, for the PKC family including aPKC a wide spectrum of targets involved in the interaction of the cytoskeleton with the cell membrane is known [33-35]. The wide choice of targets and absence of substrate specificity make us doubt the existence of specific substrates whose phosphorylation would be crucial for the unique functions ascribed to individual isoforms of the aPKC family in the nervous system. The literature data do not answer the question about mechanisms of substrate selective phosphorylation by PKMζ. However, directed phosphorylation of substrates by other aPKC isoforms can be explained from the standpoint of the addressed delivery of protein kinases to various cellular targets [33]. Substrate isolation of protein kinases, or, on the contrary, the transfer of aPKC to targets into strictly determined cellular compartments was shown to be due to the interaction of aPKC with scaffold proteins such as p62 and Par (the protein determining cell polarity). These proteins not only correctly orient aPKC in the space, but can also control their activities through conformational rearrangements leading to release of the catalytic domain from the complex with the pseudosubstrate [35, 36]. With these data in mind, it is necessary to consider mechanisms of synaptic plasticity and memory using a complex approach with analyzing enzymatic activities of atypical PKC, their interaction with scaffold proteins, and the intracellular targeted transport of aPKC to its substrates.
Mechanisms of formation and storage of memory are often studied using in vitro models. The best-studied and generally accepted model of long-term synaptic potentiation (LTP) is represented by development of long-term changes in synaptic efficiency using high-frequency stimulation of afferent fibers resulting in activation of the postsynaptic cell. For the first time, LTP was convincingly shown in the work by Bliss and Lomo [37]. Long-term potentiation is represented by at least two temporary phases: the early phase (E-LTP) and the late phase (L-LTP), which are results of different molecular mechanisms of potentiation. E-LTP is considered as a short-term (less than 2 h) increase in synapse efficiency. The further transition from short-term changes in the synapse efficiency (E-LTP) to long-term changes (L-LTP) is necessary for formation and storage of the memory trace. Many works indicate an important role of aPKC in long-term memory formation; therefore, some molecular mechanisms underlying the short-term and the long-term synaptic modifications will be considered below.
It was recently shown that during LTP induction many glutamate calcium-permeable receptors of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA receptors with GluA1-subunit composition, GluA1-AMPA) were incorporated into the postsynaptic membranes of the neurons of the hippocampus [38]. It is believed that the observed increase in receptor density in the postsynaptic ends is mediated through PKC, because activation and transfer onto the membrane of its individual isoforms (α, βI, βII, δ, η, and ζ) are recorded during LTP induction [22, 39]. Note that although 10 min after LTP induction an increase is observed in the number of atypical PKMζ molecules in the cytosol, its presence is nevertheless not essential for development of short-term changes in plasticity [4, 39]. According to some data, another atypical protein kinase, PKCλ, is a crucial factor for the development of E-LTP, which determines phosphorylation and incorporation of GluA1-AMPA receptors into the membrane in active synapses (Fig. 2a) [36]. The presence in cells of scaffold protein p62 interacting with aPKC facilitates the spatial convergence of the kinase and receptors and also determines formation of the triple complex PKCλ-GluA1-p62 in the potentiated synapses during the early phase of LTP (Fig. 2a), which seems to determine the further trafficking and incorporation of calcium-permeable GluA1-AMPA receptors into the synaptic membrane [36]. On the contrary, calcium-impermeable receptors AMPA (with the GluA2-subunit composition, GluA2-AMPA) during LTP induction are kept in the intracellular space by the protein PICK1 (a protein interacting with C-kinase; Fig. 2a), which binds with the GluA2-subunit of receptors and prevents their transfer and incorporation into the postsynaptic membrane. According to some data, the initial activation of calcium-permeable GluA1-AMPA receptors promotes disbanding of PICK1-GluA2 complexes (during the first 20 min of LTP induction) and triggers the transfer of GluA2-AMPA receptors onto the membrane [38]. Thus, PKCλ can play a role in LTP induction, regulating the incorporation of GluA1-AMPA receptors into the postsynaptic membrane. The triggered processes change the binding of protein PICK1 with another subtype of receptors, GluA2-AMPA, and thus control transient changes in the subunit composition of glutamate AMPA-receptors in active synapses during the early stages of LTP (Fig. 2).
It is well known that during LTP induction, NMDA receptors are activated and calcium entrance through them seems to stimulate various intracellular channels triggering withdrawal of the translational block from PKMζ transcripts and, correspondingly, the subsequent synthesis of the constitutively active protein kinase [22, 39].
Thus, the atypical protein kinase PKCλ mediates E-LTP development, whereas the presence of PKMζ is believed to be necessary and sufficient for further transforming short-term changes in the synapse efficiency to long-term ones [3, 4].
The development of L-LTP depends on generation of new proteins, and the use of nonspecific inhibitors of protein synthesis prevents development of the late phase of potentiation [3, 4]. Data of in vitro and in vivo experiments demonstrate an increase in the number of the PKMζ protein molecules during L-LTP and during learning [22, 23, 27, 39, 40]. Some works have shown that increased level of PKMζ positively correlates with the enhancement of postsynaptic currents across AMPA receptors [3, 5, 22, 23, 39, 41, 42], which is usually thought to characterize synapse efficiency and occur during memory formation. Suppression of the PKMζ activity with Zeta Inhibitory Peptide (ZIP) abolishes the already formed long-term potentiation and affects memory storage [3-5, 11, 13].
It is thought that during potentiation the newly formed functionally active PKMζ mediates the redistribution of AMPA receptors between the synaptic and extrasynaptic cellular compartments and regulates their incorporation into the postsynaptic membrane, i.e. changes the synapse efficiencies (Fig. 2b) [5, 41]. Analysis of the fraction of synaptosomes isolated from activated sections of rat hippocampus revealed a significant decrease in the membrane fraction of calcium-permeable GluA1-AMPA receptors and an increase in the fraction of calcium-impermeable GluA2-AMPA receptors incorporated into the membrane [5]. By analogy with in vitro experiments, in vivo works revealed that on learning animals in fear conditioning test, the increase in PKMζ amount positively correlated with the density of GluA2-AMPA membrane receptors in the prelimbic cortex [40] and basolateral amygdala [13]. The molecular mechanism of PKMζ-dependent trafficking of GluA2-AMPA receptors in potentiated synapses was studied in detail in one work, and it was shown that PKMζ production in potentiated synapses promoted the release of GluA2-AMPA receptors from complexes with the PICK1 protein, which held them in the intracellular space (Fig. 2b) [5]. According to the authors’ opinion, this could occur due to direct interaction of the PKMζ with the PICK1 protein, because PKMζ–PICK1 complexes were precipitated from homogenates of the hippocampus. Therefore, a tyrosine-enriched region on the C-end of free GluA2-subunits within AMPA-receptors remained open for binding with the protein carrier NSF responsible for transfer of receptors onto the postsynaptic membrane (Fig. 2b) [5]. Another AP2 protein (clathrin adaptor complex), which is a modulator of endocytosis in the plasma membrane, could bind with the C-terminal region of the GluA2-subunit and overlap the binding site with NSF [43]. In this case, AMPA-receptors were actively removed from the postsynaptic membrane, restoring the synapse to the initial non-potentiated state (Fig. 2b). It should be noted that prevention of incorporation of AMPA-receptors into the membrane on injection of the peptide pep2m imitating the NSF-binding site of the GluA2-subunit not only inhibited the potentiation, but completely eliminated the L-LTP [5]. These experiments fully repeated results of suppressing PKMζ activity with ZIP. It seems that maintenance of an increased number of postsynaptic AMPA-receptors (and, consequently, increase in postsynaptic currents through these receptors) needs a persistent presence of the active PKMζ, and these changes can underlie the long-term enhancement of the synapse efficiency and memory storage.
Thus, PKCλ is a necessary component for induction of LTP due to promoting the incorporation of the GluA1-AMPA receptors into the postsynaptic membrane. On the other hand, PKMζ is responsible for maintenance of the long-term potentiation and memory due to stabilization on a certain level of postsynaptic AMPA receptors (either due to suppressing their internalization or through increasing the NSF-mediated cellular transfer of receptors to the membrane) [1, 44].
ROLE OF ATYPICAL PROTEIN KINASES IN MEMORY FORMATION AND
RETENTION IN MAMMALS AND INVERTEBRATES in vivo
Numerous works have shown an important role of atypical protein kinases, in particular of PKMζ, in learning and memory trace formation in invertebrates and vertebrates [1]. According to experimental data, the number of protein molecules of atypical PKMζ noticeably increases after some types of learning [6, 40, 45] and seems to be retained at a certain level for a long time, because an inhibition of the PKMζ activity disturbs the maintenance and storage of long-term memory formed long before the introduction of the inhibitor [11, 16, 46]. At present, PKMζ is thought to be a key link in the chain of events leading to formation and storage of memory and to mediate the selective retention of certain memory forms, such as context, cued, food aversion, and spatial memories. Moreover, it is reasonable to think that this molecule is also necessary for reconsolidation initiated by a reminder [47].
The presence of active PKMζ is a crucial factor for maintenance of contextual (environmental) memory in rats. In a recently published work, contextual memory was studied in the fear conditioning test on the background of selective PKMζ overexpression in the rat hippocampus [42]. The animals with the PKMζ overexpression were found to learn better and to demonstrate an enhanced response to repeated context stimulus a week after the training [42]. On the contrary, local injection of the PKMζ inhibitor ZIP into the basolateral amygdala [12, 13, 46, 48] and/or the CA3 field (cornu ammonis) of the hippocampus [11, 12, 46] of the trained animals prevented storage of the memory trace of the context stimulus in the fear conditioning test. Note that injection of the inhibitor into the dorsolateral hippocampus did not influence the memory. The authors think that if the observed memory deficiency were caused by disorders in information extraction, the inhibitor effects would be transient, whereas problems of information maintenance (storage) upon ZIP injection would be persistent [11]. In experiments with repeated contextual reminder, the ability to learn and memory of rats were decreased both 2 h and a week after the injection of the PKMζ inhibitor [11]. Thus, the findings evidenced more in favor of a long-term ZIP-mediated disturbance in the long-term storage of spatial memory than in favor of transient problems of its retrieval. Because suppression of the PKMζ activity was accompanied by a decrease in response of AMPA-receptors [11] and, respectively, by a decrease in GluA2-AMPA number [13] only in the potentiated synapses of the trained animals (but not in the control), it was supposed that the PKMζ-mediated memory storage should be realized through maintenance of a certain number of GluA2-AMPA receptors on the postsynaptic membrane.
It should be noted that data obtained in experiments on mammalian brain are reproduced well in the simple nervous systems of invertebrates. Thus, recently contextual memory was studied in the terrestrial snail Helix in a contextual aversion behavior model [10]. The selective inhibitor of PKMζ, ZIP, but not its inactive analog (scrambled ZIP (scr-ZIP)), led to disappearance of the context memory in the trained animals. It is interesting that the inhibitor action was observed in relation to the already formed memory when novel PKMζ molecules had been formed. On the other hand, under condition of triggering generation of novel PKMζ molecules on memory activation by reminder (a reconsolidation for 4-6 h), the inhibitor effects were not manifested [10].
Along with context memory, in the fear conditioning model another parameter is also frequently assessed – the so-called signaling or cued memory. The reaction of a rat to a signaling (cued) stimulus after placing it in a new context was shown to decrease after suppression of the PKMζ activity in the basolateral amygdala [12, 46], but it did not change on the background of decrease in the amount of active PKMζ in the hippocampus [12, 42]. Thus, formation and storage of context and cued memory seemed to be associated with PKMζ activity in individual zones of the brain [11, 13, 42, 46, 48].
PKMζ-mediated aversive memory was studied in a series of works using the conditioned taste aversion (CTA) test. This type of learning is based on using a negative reinforcement and development in animals of negative reaction to presentation of initially neutral (irrelevant) food stimulus. Injection of ZIP during a training series or immediately after it did not cause disorders in the learning, which suggested PKMζ-independent formation of short-term memory [16]. However, an injection of the PKMζ inhibitor three days, one month, or three months after the learning into the insular region of the rat cortex resulted in a decrease in the food aversion at all time points. This suggested an effective erasure of the aversive memory in the experimental model used and indicated that functionally active PKMζ was necessary for maintenance of the long-term changes [15, 16]. On the other hand, the local injection of ZIP into the dorsal hippocampus did not influence the aversive memory; therefore, it was concluded that storage of the aversive memory in mammals was region-specific [15]. In in vivo experiments, direct proof was obtained that the long-term PKMζ-mediated phosphorylation of targets was a necessary condition for aversive memory storage in cortical regions of the brain [45]. The local overexpression of lentiviral constructions encoding the PKMζ native form positively correlated with the long-term changes and even led to enhancement of the already existent memory. On the other hand, overexpression of a PKMζ mutant form with inactive catalytic site disturbed the maintenance of long-term changes [45]. Thus, the persistent presence of active PKMζ in the insular region of the cortex is necessary for maintenance of aversive memory for a long time.
The role of PKMζ in aversive memory storage was also studied on invertebrates in a series of works. Long-term aversive memory in the cockroach Leucophaea maderae developed by combination of an unpleasant odor with food reinforcement could be erased by systemic injections of chelerythrine or ZIP [49]. Similar data were obtained in a work on Drosophila melanogaster: feeding the flies with the aPKC inhibitor chelerythrine or an increase in expression of the genes suppressing the PKMζ activity disturbed formation of the defensive reflex to odor and, consequently, induced erasure of aversive memory in the insects [7]. On the contrary, activation of PKMζ gene expression strengthened the aversive memory. Analysis of behavior of the snail Helix lucorum in a model of conditioned taste aversion revealed that the defensive reaction to the taste conditioned stimulus disappeared after the trained animals were injected with the PKMζ inhibitor ZIP, but not with its inactive analog scr-ZIP [10]. It is important to emphasize that the disturbance of the ZIP-mediated memory was not irreversible and that the once-erased aversive memory could be redeveloped to the same relevant stimulus [15, 16]. It is interesting that the newly formed memory could be erased by injecting a new portion of ZIP [16].
A kind of spatial memory was studied in a work by Serrano et al. [12] in rats using the active place avoidance test. Injection of chelerythrine into the dorsal hippocampus region was shown to cause disorders in memory, which the authors thought to be caused by PKMζ-mediated mechanisms of spatial information storage. However, recent data on the possible activation by chelerythrine of other intracellular targets make it necessary to carefully revise the conclusions. Moreover, studies on spatial memory of rats in the Morris water maze revealed an interesting feature. It was found that in the dorsal hippocampus active PKMζ maintained only very specific information about the spatial position that was necessary for an accurate orientation in the maze [12]. However, the protein kinase was not essential for retention of the general spatial or contextual information needed for realization of the search strategy in the space. A similar absence of positive correlations of spatial memory with PKMζ level was found in studies on mice in a water maze [50]. The amount of protein was found to increase only for PKCγ in the class C protein kinases (6 h later) in the hippocampus of the trained animals, which was in correlation with the time spent by the mouse in the goal quadrant of the water maze. Thus, it cannot be excluded that formation and retrieval of spatial memory in the hippocampus could be determined by the PKCγ amount, or that the current methods for determination of changes in the protein product amount are insufficiently accurate for detecting local changes due to the memory formation.
On the other hand, in a series of behavior tests (object location and object-in-place) aimed to study associative spatial memory, it was shown that the association memory storage was determined by the presence of active PKMζ [13, 14, 42]. In these works, a classical approach was used based on suppression of the enzyme activity by ZIP in structures of the hippocampus and medial region of the prefrontal cortex. The authors found that the inhibition of PKMζ led to serious disorders in reproduction of the already existent memory in the brain structures under study [14]. According to one of the works, the PKMζ-dependent maintenance of spatial memory in the object location test was realized via an increase in the number of GluA2-AMPA receptors incorporated into the postsynaptic membrane, because difficulties in their removal from the membrane prevented the ZIP-dependent loss of the memory [13]. On the other hand, the local overexpression of PKMζ in rat hippocampus with the aid of adenoviral constructs did not influence the storage of memory about the spatial location of subjects [42]. According to the findings, storage of the spatial component of the association memory was sensitive to decrease in the active PKMζ level, but it did not depend on production of excess amount of the enzyme.
Thus, numerous experimental data indicated exceptional importance of PKMζ for storage of different kinds of memory: while PKMζ mediates maintenance of contextual and cued memory in the amygdala and also of aversive memory in the insular zone of the cortex, the reaction to context and signaling stimuli does not need the presence of the enzyme in the hippocampus. As to spatial memory, mechanisms of its formation and storage can be more complicated, because they depend on activities of different PKC isoforms. On this basis, it can be supposed that the storage of different kinds of memory in mammals should be region-specific, and PKMζ should be selective in their maintenance in mammals. Moreover, results of experiments on mollusks and insects are similar to data obtained on rats and mice, and this directly indicates the common character of molecular mechanisms of storage and maintenance of some kinds of memory in invertebrates and mammals, notwithstanding a fundamental difference in the systemic mechanisms of memory.
CONDUCTOR OF LONG-TERM PLASTIC REARRANGEMENTS: PKMζ OR
PKCλ?
Most data indicating the involvement of PKMζ in maintenance of LTP and long-term memory (see above) are based on inhibition of the protein kinase activity with ZIP. From this standpoint, some works where alternative approaches were used for investigation of long-term changes in plasticity induced a question whether other molecules of this family could exist in addition to PKMζ actively participating in the long-term memory maintenance.
Revision of the concept of the role of PKMζ was mainly caused by results obtained recently on mice with the inactivated PKCζ/PKMζ gene [6, 51, 52]. Paradoxically, the conventional knockout of the PKCζ/PKMζ gene did not influence the storage and retrieval of the association, spatial, and motor memories in mice in some behavioral tests [6, 51, 52]. Data obtained in experiments in vivo were supplemented by a series of in vitro experiments in which stimulation of the hippocampus sections from these animals led to development of LTP notwithstanding the absence of PKMζ in the cells [6, 52]. The authors demonstrated that the long-term potentiation in the hippocampus sections from the control and PKCζ/PKMζ–/– animals was removed similarly in the presence of ZIP, which could suggest the presence of other targets. The atypical protein kinase PKCλ was a candidate for this role.
To study the role of PKCλ in the maintenance of long-term memory, Ren et al. [36] developed genetic constructs with shRNA (short hairpin RNA) for suppressing the PKCλ gene expression. The constructed shRNA complementarily bound with mRNA of the target gene and caused its degradation. It was found that the shRNA-caused decrease in the PKCλ level in rat hippocampus caused by shRNA was accompanied by a decrease in the potentiation amplitude. Further experiments with selective inhibitors revealed that PKCλ activation promoted the incorporation of GluA1-AMPA receptors into the postsynaptic membrane and, consequently, mediated the maintenance of changes in synaptic plasticity [36]. Based on experimental data, the authors suggested that PKCλ should be a necessary component for long-term memory formation.
In addition, in the laboratory of R. Y. Tsien dynamical changes were studied in the amount of atypical protein kinases in chemically stimulated neuron cultures [53]. For these studies, the authors developed genetically modified constructs containing the PKMζ or PKCλ sequences with fluorescent labels, which allowed them to follow fates of the corresponding newly generated protein molecules. Analysis of the fluorescence signal intensity in rat cortical neurons showed that the PKMζ basal level in non-stimulated cultures was maintained at a constant level, but the PKMζ amount significantly increased in dendritic spines after chemical stimulation of neurons. It should be noted that the rapid production of labeled molecules of protein kinase was replaced by a similarly rapid degradation of PKMζ [53]. On the contrary, the PKCλ level only slightly increased in the dendritic spines after the chemical induction of LTP, but absence of transient changes in the fluorescence signal in the analyzed cultures indicated stability of the newly produced PKCλ. According to these data, mechanisms of maintenance of long-term changes in the brain could be realized mainly with participation of PKCλ, but not of PKMζ.
The disagreement of the views concerning the participation of atypical protein kinases in long-term memory maintenance was solved by a group of researchers from the laboratory of T. C. Sacktor. They found that both atypical protein kinases could participate in the maintenance of long-term changes, because in pyramidal cells of the mouse hippocampus CA1 field, ZIP equally suppressed the potentiation caused by both PKMζ and PKCλ [6]. This was consistent with earlier works in which PKCλ was shown to be important during the early phase of synaptic potentiation [27, 36], whereas the maintenance of LTP could be controlled by both PKMζ and PKCλ [6, 14]. According to the latter data, an increase in the basic amount of PKCλ protein molecules was observed in the hippocampus of PKCζ/PKMζ–/– mice, and their lifetime was also increased in the potentiated cells [6]. Normally, the maintenance of LTP in vitro and of long-term memory in vivo is mediated through PKMζ (see above); however, the authors showed that in the absence of the active protein kinase, the leading role in maintaining long-term changes in the plasticity in the hippocampus and in spatial memory storage was passed to PKCλ [6]. Thus, it was confirmed experimentally that for replacing the basic mechanism of LTP maintenance in the brain of knockout PKCζ/PKMζ–/– animals, an additional PKCλ-dependent mechanism was triggered for long-term storage of plastic rearrangements.
Note in conclusion that formation and storage of the memory trace is a complex process that normally includes participation of both atypical protein kinases. According to some data, PKCλ is important for formation of memory because it regulates the number of postsynaptic GluA1-AMPA receptors during the LTP early phase (see above). On the other hand, PKMζ is necessary and sufficient for maintenance of long-term potentiation and memory. Moreover, on insufficiency of PKMζ expression, a compensatory PKCλ-mediated storage of the memory trace is switched on in the brain.
TRANSLATIONAL REGULATION OF PKMζ
So far, only protein molecules of the atypical protein kinases PKMζ and PKCλ has been proved to be necessary and sufficient for memory maintenance [1, 6, 44]. Moreover, inhibition of PKMζ can lead to erasure of old memory [13, 16, 46, 48]. However, studies on memory mechanisms inevitably raise a key question: if the protein molecule life is limited to a rather a short time, how can information be stored for a long time? The answer can be represented by the existence of a mechanism that is triggered on learning and leads to changes in the translation level of particular proteins. Upon activation, this mechanism maintains for a long time the number of molecules of these proteins on a new, changed level. Now only for the PKMζ molecule the long-term maintenance of the changed amount of the protein kinase has been shown experimentally in neurons due to training and to existence of unique mechanisms of regulation of PKMζ mRNA translation. Therefore, this section will be dedicated to consideration of some aspects of PKMζ translation.
Regulation of PKMζ translational activity. Mechanisms of translational regulation of the protein kinases are partially determined by their structural features. In the 5′-UTR of PKMζ mRNA, there are seven sites with upstream open reading frames (uORF) that, based on data of in vitro experiments, make difficult its translation under conditions of rest [23, 54]. It is supposed that inhibition of PKMζ translation could be caused by competition between uORF of the sites with the main coding frame for binding of translation initiation factors and formation of translation initiation complexes [54]. Artificial shortening of this 5′-UTR by removal of the first four uORF sites abolishes the translational block and promotes an increase in the target fragment synthesis in vitro [23]. A similar increase in the translational activity is also achieved on point shutdown of uORF sites by inserting into them mutations with retention of the total length of 5′-UTR of PKMζ mRNA [54]. However, in active neurons, the translation inhibition is overcome, and such type of template with uORF sites in the 5′-UTR region gets preference during the translation, which is manifested by an increase in the number of the PKMζ protein molecules in dendrites [54].
It seems that the above-described general mechanism of the uORF-mediated regulation of PKMζ translation can be supplemented in dendrites by local control mechanisms of the PKMζ translational activity. In a recent work, translational regulation of PKMζ by a protein interacting with PIN1 protein (peptidyl-prolyl-cis-trans-isomerase 1) was shown [55]. Under conditions of rest, a large amount of the active protein PIN1 is present in dendrites and suppresses the local translation of many targets including PKMζ. On the contrary, the shutdown of the PIN1 gene leads to a twofold increase in the amount of PKMζ and PKCζ proteins in the cortex and hippocampus of PIN1–/– mice compared to the control group [55].
It has been shown that in activated neurons the PIN1-mediated translational block disappears and the protein expression increases [55], but the mechanism of removal of this block is not established in detail (see below).
Activation of translation and maintenance of stable PKMζ level. As mentioned earlier, mRNA of PKMζ under conditions of rest is subjected to translational suppression [1]. On the contrary, the activation of neurons is accompanied by removal of the translational block and rapid development of new PKMζ protein molecules. It is known that the early phase of LTP (induction) is associated with activation of the translation and de novo synthesis of proteins, including PKMζ. The amount of PKMζ in hippocampal sections can be increased 1.5-2.0 times 30-60 min after the LTP induction [22, 23, 27, 39, 40]. Increase in intracellular calcium level stimulates activities of several kinases (Ca2+/calmodulin-dependent protein kinase II, CaMKII; phosphoinositide-3-kinase, PI3K; extracellular signal-regulated kinase, ERK; protein kinase A, PKA; mammalian target of rapamycin kinase, mTORK) that are necessary for induction of protein synthesis (Fig. 2c). It has been shown in a work by Kelly et al. [27] that inhibition of any of these kinases inhibits PKMζ translation and prevents the development of LTP. These data make us think that signaling cascades mediated by CaMKII, PI3K, ERK, PKA, and mTORK kinases meet in the same point, and that the maintenance of phosphorylated status of their targets is crucial for activation of PKMζ translation. It seems that PIN1 can be such a target, because in its sequence there are phosphorylation sites for some kinases [55, 56].
The newly produced atypical protein kinases PKCζ and PKMζ are characterized by relatively low activity, and their acquisition of the most active conformation is determined by interaction of the so-called activation loop in the catalytic domain (T410 in aPKC) with phosphoinositide-dependent protein kinase 1 (PDK1) (Figs. 1a, 1b, and 2c) [18, 19, 27]. It has been shown that various kinases involved in induction of the PKMζ translation do not influence the level of its further phosphorylation and, consequently, of the enzymatic activity. Moreover, PKMζ can enhance its own kinase activity by autophosphorylation of the T560 terminal domain. While the PKMζ molecules are constitutively phosphorylated and active (Fig. 1b), the phosphorylation status and, consequently, the activity of the other atypical protein kinase, PKCι/λ, increases only upon the induction of LTP [27]. There are some data indicating that the number of phosphorylated sites of the protein kinase (Fig. 1) has a positive correlation with the lifetime of the molecule, and this can partially explain rather long-term effects mediated by PKMζ [57, 58].
For maintaining an elevated level of PKMζ protein molecules in dendrites of trained neurons, there is a positive feedback loop: the newly formed and constitutively active protein kinase can activate its own translation due to removal of the translational block [59]. On the contrary, the inhibition of PKMζ by chelerythrine and ZIP prevents production of the PKMζ protein in the stimulated sections of rat hippocampus [27]. The mechanism of the PKMζ translational block removal is realized via phosphorylation of S16 in the PIN1 molecule, which induces conformational changes leading to the loss of its functional (peptidyl-prolyl isomerase) activity [55]. Thus, the local strengthening of PKMζ translation in potentiated synapses leads to long-term maintenance in them of high PKMζ concentration and increased efficiency of the synapses with increased PKMζ concentration, which may underlie long-term memory.
Comparison of the existing data results in the following picture: the activation of neurons and elevation of intracellular calcium activate many kinases (CaMKII, PI3K, ERK, PKA and mTORK) and the associated intracellular cascades that results in phosphorylation of targets, including PIN1. Its phosphorylation removes the translational block and increases the amount of PKMζ protein in active neurons. The de novo synthetized PKMζ is phosphorylated by PDK1 and becomes constitutively active. Then this PKMζ, via the positive feedback loop, increases proportionally its translation in the activated cells and thus promotes the long-term maintenance of the new concentration of the PKMζ protein and the storage of a memory trace.
ATYPICAL PROTEIN KINASES AND EPIGENETIC CHANGES
Studies of the recent decade have shown that long-term changes in synaptic plasticity can be associated with chromatin modifications (methylation of DNA, acetylation and methylation of histones) that determine the level of DNA condensation and thus regulate the availability of transcriptional factors and accompanying translational activity of the genes [2, 60]. The acetylation of histones is usually thought to be associated with an increase in gene activities, whereas their methylation is often considered as a marker of suppression of gene transcriptional activities.
Many experimental works designed to elucidate the role of PKMζ in learning and maintenance of memory considered its regulation at the level of translation and posttranslational modifications. Nevertheless, the concept of PKMζ as of a target of epigenetic modifications, having in mind its role in the maintenance of the long-term potentiation and, finally, of memory, seems to be a promising line for investigations. On the other hand, the presence of atypical protein kinases can be crucial for realization and regulation of various epigenetic programs, especially on considering that in some recent works PKMζ and PKCζ molecules have been found in the nuclear compartment of activated [30, 31] and non-activated cells [28]. This section will consider the putative role of PKMζ as both a target and regulator of epigenetic modifications.
Acetylation and PKMζ. The level of histone acetylation in the cell is determined by the ratio of two groups of enzymes with opposite activities: histone deacetylases (HDAC) and histone acetyltransferases. According to some data, an increase in the deacetylated status of histones can be a cause of memory extinction [61, 62]. In particular, the removal by HDAC1 of acetyl groups from H3K9 (the 9th lysine of the 3rd histone) results in selective suppression of genes in the hippocampus and mediates the extinction of contextual memory in the fear conditioning model, without affecting other kinds of memory [62].
On the other hand, numerous data of in vitro experiments in which histone acetylation in the hippocampus was increased due to selective suppression of the HDAC3 activity [63] or by incubation with a nonselective HDAC inhibitor trichostatin A (TSA) have shown a significant increase in long-term potentiation (LTP), which is a characteristic of the synaptic plasticity [63-65]. These data are in a good agreement with results of in vivo experiments and demonstrate a crucial role of increased acetylation of histones during learning and memory consolidation in mammals [64-68] and invertebrates [9, 69]. Using a decrease in histone acetylation due to the controlled genetic shutdown of one of the key histone acetyltransferases, CBP (CREB-binding protein), the authors revealed a disturbance in the consolidation of spatial memory in transgenic CBP– animals in a series of behavioral tests (the Morris water maze, the novel object recognition test) [66]. However, the deficiency of memory consolidation could be prevented in the CBP– mice by an artificial increase in the fraction of acetylated histones using the HDAC inhibitor TSA. In another work, it was shown that the increase in acetylation of histones H3 and H4 in the hippocampus by addition of the HDAC inhibitor selectively enhanced contextual memory in the fear conditioning test, whereas the cued memory was not changed [65]. The authors controlled the injection time of the HDAC inhibitor and found that the observed changes were mediated by an increase in the consolidation but not in the memory retrieval. The increase in contextual memory was shown to be due to a selective increase in the transcriptional activity of individual genes as a result of recruiting complexes of the transcriptional factor CREB (cyclic AMP response element-binding protein) with histone acetyltransferase CBP into the promoter regions of these genes. Very interesting is the work by Kim et al. [70], which showed that after training animals in the fear conditioning test, in some individual neurons of the amygdala there was observed an increased expression of CREB that would be sufficient for the reactivation of memory and its further reconsolidation leading to strengthening of the memory trace. What targets can be activated on triggering the CREB-signaling pathway is still unclear. There are some data indicating that in the promoter region of PKMζ, the CREB binding sites have been detected [23, 25]. Considering the key role of the protein kinase in the learning and maintenance of memory (see above), we consider PKMζ as a potential target for epigenetic regulation of synaptic plasticity and memory.
It has been repeatedly mentioned that PKMζ is a key link of memory and that production of protein molecules of the protein kinase during the L-LTP phase mediates the selective maintenance of individual forms of memory (see above). For a long time, it has been thought that the long-term effects of PKMζ are mediated by local changes in synaptic characteristics. However, recent studies suggest PKMζ as a potential epigenetic regulator of plasticity and memory. It has been shown that the PKMζ sequence includes signals of protein nuclear localization, and on activation of the cell PKMζ is transferred into the nucleus, where it phosphorylates and activates histone acetyltransferase CBP and enhances acetylation of histones H2B and H3 [31]. Some studies in invertebrates and mammals indicated that suppression of the enzymatic activity of PKMζ by ZIP or chelerythrine caused disorders in the long-term memory, which could be prevented by a preliminary artificial decrease of histone deacetylation (using sodium butyrate or TSA) and, correspondingly, by increasing the amount of acetylated histones [9, 31]. It is interesting that artificial increase in the level of acetylated histones upon injection of inhibitors of histone deacetylases (TSA or sodium butyrate) not only improved the learning of mammals and invertebrates, but also promoted long-term storage of information, which under usual conditions was not transmitted into long-term memory [9, 67, 69].
The data presented suggest that a definite epigenetic balance in neurons plays an important role in the maintenance of long-term memory in both invertebrates and vertebrates, and that changes in the ratio of histone acetylation/deacetylation can be used as a tool for regulating plasticity and memory [60]. PKMζ is believed to play a special role in this system. Some data suggest that PKMζ can be considered as both a target and a regulator of the CBP–CREB-dependent epigenetic mechanism of enhancement of transcriptional activity of individual genes during learning and memory formation.
Methylation and PKMζ. It is emphasized in recent studies that dynamical changes in the methylation of DNA can be a critical factor for the formation and maintenance of long-term memory [2]. It was shown that the expression level of DNA methyltransferases 3A and 3B (DNMT) responsible for de novo methylation increased in rat hippocampus 30 min after training in the fear conditioning test [71]. Local injection of DNMT inhibitors into the CA1 region of the hippocampus immediately after the training led to a subsequent decrease in the animals’ ability for learning, and this confirmed that the presence of active DNA methyltransferases was necessary for the consolidation of memory [71]. Further experiments revealed that contextual learning in rats was accompanied by selective and long-term hypermethylation of DNA of genes whose activation usually was associated with deficiency of learning and memory [2]. On the other hand, methylation of genes mediating synaptic plasticity and memory (reelin, PKMζ) was decreased during learning [2, 40, 71]. Moreover, fluctuations in methylation level of individual genes can be temporarily different and thus provide fine regulation of plastic changes in the brain [72].
In 2016, a group of Chinese researchers showed that not only normal learning, but also age-related disorders in cognitive functions can be caused by changes in methylation of DNA, including DNA of PKMζ [40]. Assessment of context spatial memory (the Morris maze) and recognition of new objects in investigation of three age groups of rats (3, 9, and 24 months) revealed significant disorders in the long-term memory in the old (24 months) animals. Further experiments showed that the memory deficiency observed in the old rats was caused by local epigenetic changes in the promoter region of the PKMζ gene in the prelimbic cortex, which were accompanied by deficiency in expression of the PKMζ protein and of the associated targets (GluA2-subunit of AMPA receptors and a postsynaptic density protein 95 (PSD-95)) [40]. It was shown that on contextual learning the amount of nonmethylated DNA of PKMζ increased in the young and adult animals, whereas in the old animals it remained on the pre-training low level. In turn, it led to an increase in the amount of PKMζ mRNA and the protein in the first two groups after the training, whereas the decreased demethylated status of the PKMζ DNA in the old animals could be accompanied by a decrease in PKMζ gene transcription and by disorders in cognitive functions [40]. These data indicate that the maintenance of long-term changes in synaptic plasticity and, thus, long-term memory formation can be determined by the character of epigenetic modifications of genes, including the PKMζ gene.
In the present review, we attempted to analyze at different levels (from the molecular to the behavioral levels) the role of aPKC in synaptic plasticity and long-term memory. Since PKCζ has been found in the brain in trace amounts, the major accent in studies of memory formation mechanisms has been made mainly for PKMζ and PKCλ. According to the literature data, the formation and storage of a memory trace is a complex process that normally involves both atypical protein kinases. According to some data, PKCλ is important for the formation of early memory, because its activation regulates the incorporation into the postsynaptic membrane of glutamate calcium-permeable AMPA receptors and leads to an increase in the synapse efficiency during LTP induction. On the other hand, PKMζ is necessary and sufficient for the maintenance of long-term potentiation and memory. Changes in various intracellular characteristics, including the intracellular calcium level in the potentiated neurons during LTP induction, can stimulate the activities of kinases (CaMKII, PI3K, ERK, PKA, and mTORK) that are necessary for removal of the translational block and the de novo production of protein molecules of the constitutively active PKMζ. The newly produced PKMζ increases the amount of glutamate calcium-impermeable AMPA receptors incorporated into the postsynaptic membrane and maintains these changes in the efficiency of synaptic transmission over a long time. Existence of a positive feedback loop between the amount of PKMζ and the level of its translation guarantees long-term local maintenance of an increased level of PKMζ in potentiated synapses of the activated neurons and, thus, provides an increase in LTP and the long-term storage of memory. Some studies demonstrated the region-specific storage of different memory varieties and their selective maintenance by PKMζ in mammals.
In addition to classical studies that explain the effects of PKMζ from the standpoint of regulation of synaptic characteristics, some data have appeared on directed transport of atypical protein kinases into the nuclear compartment, where their presence can be crucial for realization and regulation of different transcriptional and epigenetic programs. Individual studies indicate a potential role of PKMζ as both a target and a regulator of epigenetic modifications.
Thus, the constitutively active PKMζ is a key component of the maintenance of long-term changes in synaptic plasticity and memory in invertebrates and vertebrates. Under the insufficient expression of PKMζ in mammalian brain, a compensatory PKCλ-mediated storage of memory trace is engaged. Numerous data obtained in invertebrates (mollusks, insects) and in vertebrates have demonstrated mostly similar results and seem to indicate the conservativeness of molecular mechanisms of the maintenance and storage of memory in invertebrates and vertebrates.
Acknowledgements
The work was supported by the Russian Foundation for Basic Research (project No. 16-34-00918) and by the Russian Science Foundation (project No. 14-25-00072; analysis of protein kinases participation in the synapse plasticity).
REFERENCES
1.Sacktor, T. C. (2012) Memory maintenance by
PKMζ – an evolutionary perspective, Mol. Brain,
5, 31.
2.Morris, M. J., and Monteggia, L. M. (2014) Role of
DNA methylation and the DNA methyltransferases in learning and memory,
Dialogues Clin. Neurosci., 16, 359-371.
3.Ling, D. S., Benardo, L. S., Serrano, P. A., Blace,
N., Kelly, M. T., Crary, J. F., and Sacktor, T. C. (2002) Protein
kinase Mζ is necessary and sufficient for LTP maintenance, Nat.
Neurosci., 5, 295-296.
4.Serrano, P., Yudong, Y., and Sacktor, T. C. (2005)
Persistent phosphorylation by protein kinase Mzeta maintains late-phase
long-term potentiation, J. Neurosci., 25, 1979-1984.
5.Yao, Y., Kelly, M. T., Sajikumar, S., Serrano, P.,
Tian, D., Bergold, P. J., Frey, J. U., and Sacktor, T. C. (2008) PKM
zeta maintains late long-term potentiation by
N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of
postsynaptic AMPA receptors, J. Neurosci., 28,
7820-7827.
6.Tsokas, P., Hsieh, C., Yao, Y., Lesburgueres, E.,
Wallace, E. J., Tcherepanov, A., Jothianandan, D., Hartley, B. R., Pan,
L., Rivard, B., Farese, R. V., Sajan, M. P., Bergold, P. J., Hernandez,
A. I., Cottrell, J. E., Shouval, H. Z., Fenton, A. A., and Sacktor, T.
C. (2016) Compensation for PKMζ in long-term potentiation and
spatial long-term memory in mutant mice, eLife, 5,
e14846.
7.Drier, E. A., Tello, M. K., Cowan, M., Wu, P.,
Blace, N., Sacktor, T. C., and Jin, J. C. (2002) Memory enhancement and
formation by atypical PKM activity in Drosophila
melanogaster, Nat. Neurosci., 5, 316-324.
8.Cai, D., Pearce, K., Chen, S., and Glanzman, D. L.
(2011) Protein kinase M maintains long-term sensitization and
long-term facilitation in aplysia, J. Neurosci., 31,
6421-6431.
9.Chen, S., Cai, D., Pearce, K., Sun, P. Y., Roberts,
A. C., and Glanzman, D. L. (2014) Reinstatement of long-term memory
following erasure of its behavioral and synaptic expression in
Aplysia, Elife, 3, e03896.
10.Balaban, P. M., Roshchin, M., Timoshenko, A. Kh.,
Zuzina, A. B., Lemak, M., Ierusalimsky, V. N., Aseyev, N. A., and
Malyshev, A. Y. (2015) Homolog of protein kinase Mzeta maintains
context aversive memory and underlying long-term facilitation in
terrestrial snail Helix, Front. Cell Neurosci., 9,
222.
11.Pastalkova, E., Serrano, P., Pinkhasova, D.,
Wallace, E., Fenton, A. A., and Sacktor, T. C. (2006) Storage of
spatial information by the maintenance mechanism of LTP,
Science, 313, 1141-1144.
12.Serrano, P., Friedman, E. L., Kenney, J.,
Taubenfeld, S. M., Zimmerman, J. M., Hanna, J., Alberini, C., Kelley,
A. E., Maren, S., Rudy, J. W., Yin, J. C., Sacktor, T. C., and Fenton,
A. A. (2008) PKMzeta maintains spatial, instrumental, and classically
conditioned long-term memories, PLoS Biol., 6,
2698-2706.
13.Migues, P. V., Hardt, O., Wu, D. C., Gamache, K.,
Sacktor, T. C., Wang, Y. T., and Nader, K. (2010) PKMzeta maintains
memories by regulating GluR2-dependent AMPA receptor trafficking,
Nat. Neurosci., 13, 630-634.
14.Evuarherhe, O., Barker, G. R., Savalli, G.,
Warburton, E. C., and Brown, M. W. (2014) Early memory formation
disrupted by atypical PKC inhibitor ZIP in the medial prefrontal cortex
but not hippocampus, Hippocampus, 24, 934-942.
15.Shema, R., Sacktor, T. C., and Dudai, Y. (2007)
Rapid erasure of long-term memory associations in the cortex by an
inhibitor of PKM, Science, 317, 951-953.
16.Shema, R., Hazvi, S., Sacktor, T. C., and Dudai,
Y. (2009) Boundary conditions for the maintenance of memory by PKMzeta
in neocortex, Learn. Mem., 16, 122-128.
17.Nishizuka, Y. (1988) The molecular heterogeneity
of protein kinase C and its implications for cellular regulation,
Nature, 334, 661-665.
18.Newton, A. C. (1997) Regulation of protein kinase
C, Curr. Opin. Cell Biol., 9, 161-167.
19.Newton, A. C. (2003) Regulation of the ABC
kinases by phosphorylation: protein kinase C as a paradigm, Biochem.
J., 370, 361-371.
20.Ono, Y., Fujii, T., Ogita, K., Kikkawa, U.,
Igarashi, K., and Nishizuka, Y. (1989) Protein kinase C zeta subspecies
from rat brain: its structure, expression, and properties, PNAS,
86, 3099-3103.
21.Nakanishi, H., Brewer, K. A., and Exton, J. H.
(1993) Activation of the zeta isozyme of protein kinase C by
phosphatidylinositol 3,4,5-trisphosphate, J. Biol. Chem.,
268, 13-16.
22.Sacktor, T. C., Osten, P., Valsamis, H., Jiang,
X., Naik, M. U., and Sublette, E. (1993) Persistent activation of the
zeta isoform of protein kinase C in the maintenance of long-term
potentiation, PNAS, 90, 8342-8346.
23.Hernandez, A. I., Blace, N., Crary, J. F.,
Serrano, P. A., Leitges, M., Libien, J. M., Weinstein, G., Tcherapanov,
A., and Sacktor, T. C. (2003) Protein kinase M zeta synthesis from
a brain mRNA encoding an independent protein kinase C zeta catalytic
domain. Implications for the molecular mechanism of memory, J. Biol.
Chem., 278, 40305-40316.
24.Bougie, J. K., Lim, T., Farah, C. A., Manjunath,
V., Nagakura, I., Ferraro, G. B., and Sossin, W. S. (2009) The atypical
protein kinase C in Aplysia can form a protein kinase M by
cleavage, J. Neurochem., 109, 1129-1143.
25.Marshall, B. S., Price, G., and Powell, C. T.
(2000) Rat protein kinase C zeta gene contains alternative
promoters for generation of dual transcripts with 5′-end
heterogeneity, DNA Cell Biol., 19, 707-719.
26.Muslimov, I. A., Nimmrich, V., Hernandez, A. I.,
Tcherepanov, A., Sacktor, T. C., and Tiedge, H. (2004) Dendritic
transport and localization of protein kinase Mzeta mRNA: implications
for molecular memory consolidation, J. Biol. Chem., 279,
52613-52622.
27.Kelly, M. T., Crary, J. F., and Sacktor, T. C.
(2007) Regulation of protein kinase Mzeta synthesis by multiple kinases
in long-term potentiation, J. Neurosci., 27,
3439-3444.
28.Hernandez, A. I., Oxberry, W. C., Crary, J. F.,
Mirra, S. S., and Sacktor, T. C. (2013) Cellular and subcellular
localization of PKMζ, Philos. Trans. R Soc. Lond. B Biol.
Sci., 369, 20130140.
29.Yerusalimsky, V. N., Borodinova, A. A., and
Balaban, P. M. (2015) Localization of atypical protein kinase Cζ
in the nervous system of the terrestrial mollusk Helix,
Neirokhimiya, 32, 295-301.
30.Neri, L. M., Martelli, A. M., Borgatti, P.,
Colamussi, M. L., Marchisio, M., and Capitani, S. (1999) Increase in
nuclear phosphatidylinositol 3-kinase activity and phosphatidylinositol
(3,4,5) trisphosphate synthesis precedes PKC-zeta translocation to the
nucleus of NGF-treated PC12 cells, FASEB J., 13,
2299-2310.
31.Ko, H. G., Kim, J. I., Sim, S. E., Kim, T., Yoo,
J., Choi, S. L., Baek, S. H., Yu, W. J., Yoon, J. B., Sacktor, T. C.,
and Kaang, B. K. (2016) The role of nuclear PKMζ in memory
maintenance, Neurobiol. Learn. Mem., 135, 50-56.
32.Naik, M. U., Benedikz, E., Hernandez, I., Libien,
J., Hrabe, J., Valsamis, M., Dow-Edwards, D., Osman, M., and Sacktor,
T. C. (2000) Distribution of protein kinase Mzeta and the complete
protein kinase C isoform family in rat brain, J. Comp. Neurol.,
426, 243-258.
33.Jaken, S. (1996) Protein kinase C isozymes and
substrates, Curr. Opin. Cell Biol., 8, 168-173.
34.Naz, F., Islam, A., Ahmad, F., and Hassan, M. I.
(2015) Atypical PKC phosphorylates microtubule affinity-regulating
kinase 4 in vitro, Mol. Cell Biochem., 410,
223-228.
35.Tobias, I. S., and Newton, A. C. (2016) Protein
scaffolds control localized protein kinase Cζ activity, J.
Biol. Chem., 291, 13809-13822.
36.Ren, S. Q., Yan, J. Z., Zhang, X. Y., Bu, Y. F.,
Pan, W. W., Yao, W., Tian, T., and Lu, W. (2013) PKCλ is
critical in AMPA receptor phosphorylation and synaptic incorporation
during LTP, EMBO J., 32, 1365-1380.
37.Bliss, T. V., and Lomo, T. (1973) Long-lasting
potentiation of synaptic transmission in the dentate area of the
anaesthetized rabbit following stimulation of the perforant path, J.
Physiol., 232, 331-356.
38.Jaafari, N., Henley, J. M., and Hanley, J. G.
(2012) PICK1 mediates transient synaptic expression of GluA2-lacking
AMPA receptors during glycine-induced AMPA receptor trafficking, J.
Neurosci., 32, 11618-11630.
39.Osten, P., Valsamis, L., Harris, A., and Sacktor,
T. C. (1996) Protein synthesis-dependent formation of protein kinase
Mzeta in long-term potentiation, J. Neurosci., 16,
2444-2451.
40.Chen, C., Meng, S. Q., Xue, Y. X., Han, Y., Sun,
C. Y., Deng, J. H., Chen, N., Bao, Y. P., Zhang, F. L., Cao, L. L.,
Zhu, W. G., Shi, J., Song, W. H., and Lu, L. (2016) Epigenetic
modification of PKMζ rescues aging-related cognitive impairment,
Sci. Rep., 6, 22096.
41.Ling, D. S., Benardo, L. S., and Sacktor, T. C.
(2006) Protein kinase Mzeta enhances excitatory synaptic transmission
by increasing the number of active postsynaptic AMPA receptors,
Hippocampus, 16, 443-452.
42.Schuette, S. R., Fernandez-Fernandez, D., Lamla,
T., Rosenbrock, H., and Hobson, S. (2016) Overexpression of protein
kinase Mζ in the hippocampus enhances long-term potentiation and
long-term contextual but not cued fear memory in rats, J.
Neurosci., 36, 4313-4324.
43.Lee, S. H., Liu, L., Wang, Y. T., and Sheng, M.
(2002) Clathrin adaptor AP2 and NSF interact with overlapping sites of
GluR2 and play distinct roles in AMPA receptor trafficking and
hippocampal LTD, Neuron, 36, 661-674.
44.Sacktor, T. C. (2011) How does PKMzeta maintain
long-term memory? Nat. Rev. Neurosci., 12, 9-15.
45.Shema, R., Haramati, S., Ron, S., Hazvi, S.,
Chen, A., Cacktor, T. C., and Dudai, Y. (2011) Enhancement of
consolidated long-term memory by overexpression of protein kinase Mzeta
in the neocortex, Science, 331, 1207-1210.
46.Kwapis, J. L., Jarome, T. J., Lonergan, M. E.,
and Helmstetter, F. J. (2009) Protein kinase Mzeta maintains fear
memory in the amygdala but not in the hippocampus, Behav.
Neurosci., 123, 844-850.
47.Zuzina, A. B., and Balaban, P. M. (2015)
Extinction and reconsolidation of memory, Zh. Vyssh. Nerv. Deyat.
im. Pavlova, 65, 564-576.
48.Kwapis, J. L., Jarome, T. J., Gilmartin, M. R.,
and Helmstetter, F. J. (2012) Intra-amygdala infusion of the protein
kinase Mzeta inhibitor ZIP disrupts foreground context fear memory,
Neurobiol. Learn. Mem., 98, 148-153.
49.Deng, Z., Lubinski, A. J., and Page, T. L. (2015)
Zeta inhibitory peptide (ZIP) erases long-term memories in a cockroach,
Neurobiol. Learn. Mem., 118, 89-95.
50.Li, L., You, L., Sunyer, B., Patil, S., Hoger,
H., Pollak, A., Stork, O., and Lubec, G. (2014) Hippocampal protein
kinase C family members in spatial memory retrieval in the mouse,
Behav. Brain Res., 258, 202-207.
51.Lee, A. M., Kanter, B. R., Wang, D., Lim, J. P.,
Zou, M. E., Qiu, C., McMahon, T., Dadgar, J., Fischbach-Weiss, S. C.,
and Messing, R. O. (2013) Prkcz null mice show normal learning and
memory, Nature, 493, 416-419.
52.Volk, L. J., Bachman, J. L., Johnson, R., Yu, Y.,
and Huganir, R. L. (2013) PKM-ζ is not required for hippocampal
synaptic plasticity, learning and memory, Nature, 493,
420-423.
53.Palida, S. F., Butko, M. T., Ngo, J. T., Mackey,
M. R., Gross, L. A., Ellisman, M. H., and Tsien, R. Y. (2015)
PKMζ, but not PKCλ, is rapidly synthesized and degraded at
the neuronal synapse, J. Neurosci., 35, 7736-7749.
54.Bal, N., Susorov, D., Chesnokova, E., Kasyanov,
A., Mikhailova, T., Alkalaeva, E., Balaban, P., and Kolosov, P. (2016)
uORFs located in the 5′-UTR of PKMζ mRNA regulate its
translation, Front. Mol. Neurosci., 9, 103.
55.Westmark, P. R., Westmark, C. J., Wang, S.,
Levenson, J., O'Riordan, K. J., Burger, C., and Malter, J. S. (2010)
Pin1 and PKMzeta sequentially control dendritic protein synthesis,
Sci. Signal., 3, ra18.
56.Smet-Nocca, C., Launay, H., Wieruszeski, J. M.,
Lippens, G., and Landrieu, I. (2013) Unraveling a phosphorylation event
in a folded protein by NMR spectroscopy: phosphorylation of the Pin1 WW
domain by PKA, J. Biomol. NMR, 55, 323-337.
57.Smith, L., Wang, Z., and Smith, J. B. (2003)
Caspase processing activates atypical protein kinase C zeta by
relieving autoinhibition and destabilizes the protein, Biochem.
J., 375, 663-671.
58.Jalil, S. J., Sacktor, T. C., and Shouval, H. Z.
(2015) Atypical PKCs in memory maintenance: the roles of feedback and
redundancy, Learn. Mem., 22, 344-353.
59.Sacktor, T. C. (2010) PINing for things past,
Sci. Signal., 3, pe9.
60.Penney, J., and Tsai, L. H. (2014) Histone
deacetylases in memory and cognition, Sci. Signal., 7,
re12.
61.Lattal, K. M., Barrett, R. M., and Wood, M. A.
(2007) Systemic or intrahippocampal delivery of histone deacetylase
inhibitors facilitates fear extinction, Behav. Neurosci.,
121, 1125-1131.
62.Bahari-Javan, S., Maddalena, A., Kerimoglu, C.,
Wittnam, J., Held, T., Bahr, M., Burkhardt, S., Delalle, I., Kugler,
S., Fischer, A., and Sananbenesi, F. (2012) HDAC1 regulates fear
extinction in mice, J. Neurosci., 32, 5062-5073.
63.Franklin, A. V., Rusche, J. R., and McMahon, L.
L. (2014) Increased long-term potentiation at medial-perforant
path-dentate granule cell synapses induced by selective inhibition of
histone deacetylase 3 requires Fragile X mental retardation protein,
Neurobiol. Learn. Mem., 114, 193-197.
64.Levenson, J. M., O'Riordan, K. J., Brown, K. D.,
Trinh, M. A., Molfese, D. L., and Sweatt, J. D. (2004) Regulation of
histone acetylation during memory formation in the hippocampus, J.
Biol. Chem., 279, 40545-40559.
65.Vecsey, C. G., Hawk, J. D., Lattal, K. M., Stein,
J. M., Fabian, S. A., Attner, M. A., Cabrera, S. M., McDonough, C. B.,
Brindle, P. K., Abel, T., and Wood, M. A. (2007) Histone deacetylase
inhibitors enhance memory and synaptic plasticity via CREB:
CBP-dependent transcriptional activation, J. Neurosci.,
27, 6128-6140.
66.Korzus, E., Rosenfeld, M. G., and Mayford, M.
(2004) CBP histone acetyltransferase activity is a critical component
of memory consolidation, Neuron, 42, 961-972.
67.Stefanko, D. P., Barrett, R. M., Ly, A. R.,
Reolon, G. K., and Wood, M. A. (2009) Modulation of long-term memory
for object recognition via HDAC inhibition, Proc. Natl. Acad. Sci.
USA, 106, 9447-9452.
68.Hawk, J. D., Florian, C., and Abel, T. (2011)
Post-training intrahippocampal inhibition of class I histone
deacetylases enhances long-term object-location memory, Learn.
Mem., 18, 367-370.
69.Federman, N., Fustinana, M. S., and Romano, A.
(2009) Histone acetylation is recruited in consolidation as a molecular
feature of stronger memories, Learn. Mem., 16,
600-606.
70.Kim, J., Kwon, J. T., Kim, H. S., Josselyn, S.
A., and Han, J. H. (2014) Memory recall and modifications by activating
neurons with elevated CREB, Nat. Neurosci., 17,
65-72.
71.Miller, C. A., and Sweatt, J. D. (2007) Covalent
modification of DNA regulates memory formation, Neuron,
53, 857-869.
72.Miller, C. A., Gavin, C. F., White, J. A.,
Parrish, R. R., Honasoge, A., Yancey, C. R., Rivera, I. M., Rubio, M.
D., Rumbaugh, G., and Sweatt, J. D. (2010) Cortical DNA methylation
maintains remote memory, Nat. Neurosci., 13, 664-666.