Selection of Progesterone Derivatives Specific to Membrane Progesterone Receptors
A. V. Polikarpova1*, A. A. Maslakova1, I. S. Levina2, L. E. Kulikova2, Y. V. Kuznetsov2, A. A. Guseva1, T. A. Shchelkunova1, I. V. Zavarzin2, and O. V. Smirnova1
1Lomonosov Moscow State University, Faculty of Biology, 119991 Moscow, Russia; E-mail: kairo911@gmail.com2Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 117913 Moscow, Russia; E-mail: li@ioc.ac.ru
* To whom correspondence should be addressed.
Received July 13, 2016; Revision received October 10, 2016
The search of selective agonists and antagonists of membrane progesterone receptors (mPRs) is a starting point for the study of progesterone signal transduction mechanisms mediated by mPRs, distinct from nuclear receptors. According to preliminary data, the ligand affinity for mPRs differs significantly from that for classical nuclear progesterone receptors (nPRs), which might indicate structural differences in the ligand-binding pocket of these proteins. In the present work, we analyzed the affinity of several progesterone derivatives for mPRs of human pancreatic adenocarcinoma BxPC3 cell line that is characterized by a high level of mPR mRNA expression and by the absence of expression of nPR mRNA. The values were compared with the affinity of these compounds for nPRs. All tested compounds showed almost no affinity for nPRs, whereas their selectivity towards mPRs was different. Derivatives with an additional 19-hydroxyl group and removed 3-keto group had the highest selectivity for mPRs. These results suggest these compounds as the most selective progesterone analogs for studying the mechanisms of progestin action via mPRs.
KEY WORDS: membrane progesterone receptors, progesterone analogs, human pancreatic adenocarcinoma BxPC3 cell line, synthesisDOI: 10.1134/S0006297917020055
Progesterone (pregn-4-ene-3,20-dione), a natural progestin, is one of the main sex hormones. In females, progesterone is synthesized in ovaries by the corpus luteum and by the placenta during pregnancy; in males, the main source of progesterone are testes and adrenal cortex. In healthy females, progesterone concentration depends on the phase of the menstrual cycle. It should be mentioned that progesterone level during the follicular phase in females is equal to progesterone level in males and is about 1-4 nmol/liter. During the luteal phase, the concentration of progesterone in blood of healthy females increases and reaches 30-80 nmol/liter due to the functioning of the corpus luteum [1]. The best-studied mechanism of action of progesterone is the regulation of expression of a target gene through nuclear receptors (nPRs). It is well known that progesterone can act by inducing a quick stimulation of signal pathways initiated on cellular membrane [2]. It is now almost accepted that several groups of receptor proteins are involved in hormone signal transduction (in addition to nPRs). These include five types of membrane progesterone receptors (mPRs type α, β, γ, δ, ε), members of the progestin and adiponectin receptor (PAQR) family, and progesterone receptor membrane components PGRMC 1 and 2 [3, 4]. The mPRs are localized on the cell surface [4]. The mPRs type α, β, and γ have similar ligand-binding specificity [3]. The effects of progestins mediated by mPRs are poorly studied because most cells contain both membrane and nuclear receptors. However, it was shown that in tumor cells of some tissues, progesterone inhibits proliferation, stimulates apoptosis, and negatively influences carcinogenesis specifically through mPRs [5, 6]. It was also shown that progesterone, acting through different types of receptors, can have opposite effects on myometrial contraction activity [7]. Therefore, creation of selective mPR ligands having agonist and antagonist activity is clearly important. Complete selective agonists and antagonists of mPRs have not been identified. The most selective progesterone analogs tested are mPRα agonists Org OD 02-0 (10-ethenyl-19-norprogesterone) and Org OD 13-0 (19α-methylprogesterone) with discrimination indexes mPRα/nPRs of 20 and 40, respectively [8]. We analyzed the affinity of several pregna-D′-pentarans to recombinant mPRα. We found that modifications such as substitution of a methyl group of C10 with ethyl or methoxy group, substitution of a 3-keto group with 3-O-methoxyimino group, or introduction of a 17α-hydroxyl group and an additional C6–C7 double bond increase the affinity of modified ligands to mPRα and reduce considerably their affinity to nPRs [9].
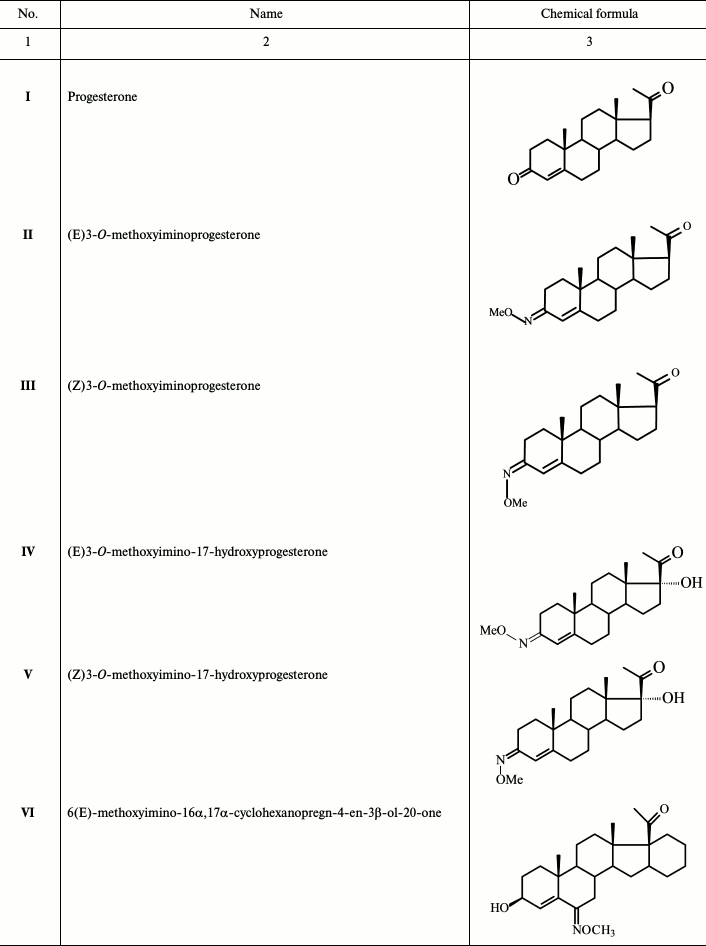
To continue these studies, derivatives of progesterone (II-X; Table 1) that probably bind mainly mPRs were synthesized. The 3-O-methoxyimino derivatives of progesterone (II, III) and 17α-hydroxyprogesterone (IV, V) were synthesized, as well as progesterone analogs without the 3-ketone group (IX, X), as we assume that the mode of interaction of ligands with mPRs and nPR around the C3 position of the steroid molecule is quite different. According to published data, modification at C19 has an opposite effect on the steroid affinity to different receptor types [8, 9]; therefore, three molecules among the compounds studied in the present work (VIII-X) have a 19-hydroxyl group. Substitution at position 6 of a steroid scaffold also plays an important role in steroid binding to mPRα. In this work, we also studied two compounds (VI and VII) bearing a 6(E)-methoxyimino group in position 6 of the steroid scaffold.
Table 1. Derivatives of progesterone used in
this work
It was shown earlier that mRNA of nPRs are not expressed in cells of human pancreatic adenocarcinoma BxPC3 cell line. However, these cells were characterized by high levels of expression of mPRα, mPRβ, and mPRγ mRNAs [10]. We used the BxPC3 cell line to study mPR binding characteristics and to measure the affinity of synthesized progesterone derivatives to the membrane receptor. For comparison, we used nuclear nPRs from rat uterine cytosol fraction having binding characteristics and structure of the ligand-binding pocket similar to that of human nPRs [11, 12]. In the absence of a ligand, nPR is localized in cytoplasm within an oligomeric complex with a heat shock protein, cochaperone, and a protein from the immunophilin family [1]. Thus, cytosolic fraction is enriched with nPRs.
MATERIALS AND METHODS
Chemicals. Growth media RPMI-1640 (with or without phenol red), supplemented with 100× L-glutamine (L-Glu), 100× antibiotics and antifungal mix, fetal bovine serum (FBS), and charcoal/dextran fetal calf serum (DFBS) were obtained from Gibco (USA). Dulbecco’s phosphate buffered saline (DPBS Ca2+/Mg2+) and Versene solution were obtained from PanEco (Russia). Radiolabeled [1,2-3H]progesterone with specific activity of 40 Ci/mmol was synthesizes by V. P. Shevchenko at the Institute of Molecular Genetics, Russian Academy of Sciences. Progesterone, cortisol, testosterone, estradiol, mifepristone (17β-hydroxy-11β-(4-dimethylaminophenyl)-17α-(1-propinyl)estra-4,9-dien-3-one), phenylmethylsulfonyl fluoride (PMSF), dithiothreitol (DTT), EDTA, glycerol, and Tris were purchased from Sigma (USA), charcoal was purchased from Serva (Germany), and Dextran-70 from Fluka (Switzerland).
Synthesis of progesterone derivatives. The 3-O-methoxyiminoprogesterones II, III and 3-O-methoxyimino-17α-hydroxyprogesterones IV, V were synthesized from progesterone or 17α-hydroxyprogesterone, respectively. Synthesis was carried out in three steps via selective protection of the 20-oxo group with ethylene ketal, then treatment of the resulting ketal with O-methylhydroxylamine hydrochloride with further removal of the protection by treatment with p-TsOH. Individual E- and Z-isomers II, III and IV, V were purified by chromatography [13]. The compounds 6(E)-methoxyimino-16α,17α-cyclohexanopregn-4-en-3β-ol-20-one VI and 6(E)-methoxyimino-16α,17α-cyclohexanopregn-4-en-3,20-dione VII were synthesized as described earlier [14]. 19-Hydroxyprogesterone VIII was synthesized as described elsewhere [15].
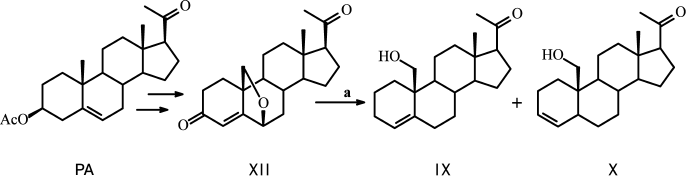
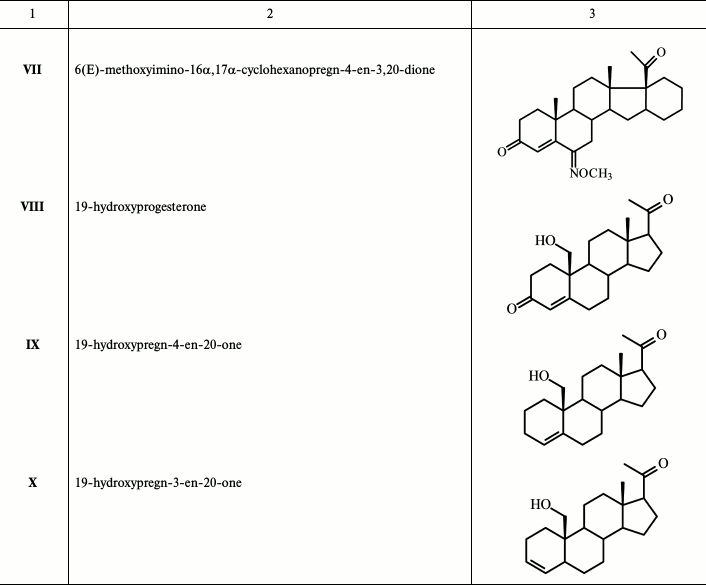
New compounds 19-hydroxypregn-4-en-20-one IX and 19-hydroxypregn-3-en-20-one X were obtained after chromatographic separation of the mixture of these isomeric alkenes generated during reductive epoxy ring opening in 6β,19-epoxypregn-4-en-3,20-dione XII (obtained in three steps from dehydropregnenolone acetate PA [16] with an excess of the activated Zn-dust in glacial AcOH with simultaneous elimination of the 3-keto group) (Fig. 1). Structures of these steroids were confirmed by physicochemical analysis. The proton H-5 configuration in compound X was determined by ge-1D NOESY experiments. Excitation of proton H-5 (δ 2.51 ppm) gave response for protons at H-19 (δ 3.51 and 3.80), while excitation of proton H-19 (δ 3.80) gave response for H-5, which defines the conformation of H-5 as β.
Fig. 1. Synthesis of 19-hydroxypregn-4-en-20-one (IX) and 19-hydroxypregn-3-en-20-one (X). Reagents and conditions: glacial AcOH, Zn-dust, 90°C, 20 min.
Chemical characterization. Melting points were recorded on a Boethius hot plate (Germany). The 1H and 13C NMR spectra (δ ppm, CHCl3, 30°C) were recorded on Bruker AM-300 (300.13 MHz for 1H and 75.5 MHz for 13C) and Bruker AV-6 (600.13 MHz for 1H and 150.9 MHz for 13C) spectrometers. Residual signal of CHCl3 (δH 7.27 ppm and δC 77.0 ppm) was used as a reference. High-resolution mass spectra were measured using a Bruker micrOTOF II instrument with electrospray ionization (ESI). The measurements were done in positive ion mode (capillary voltage 4500 V). The mass scan range was m/z 50-3000 Da, and calibration was external (Electrospray Calibrant Solution, Fluka). The compounds dissolved in MeOH were injected with a syringe (injection speed 3 liters/min). The spray gas was nitrogen (8 liters/min), and interface temperature was 200°C. Analytical TLC was performed on Silica gel 60 F254 (Merck) plates in hexane–acetone systems. Compounds were detected with 1% Ce(SO4)2 solution in 10% H2SO4 aqueous solution with subsequent heating. Preparative separation was done by column chromatography on silica gel Kieselgel 60 (0.063-0.200 µm) (Merck) with compound to sorbent ratio 1 : 40. The 16-pregnenolone acetate (PA) was a commercial reagent from Sigma. Solvents were purified according to standard techniques.
Standard treatment of organic extracts included their washing to neutral reaction of the washing water, drying with Na2SO4, and evaporation under vacuum. Yield of individual products is calculated for recrystallized samples.
A sample of 2.48 g (38.0 mmol) of activated Zn-dust was added to a solution of 0.52 g (1.58 mmol) of 6β,19-epoxypregn-4-en-3,20-dione XII in 15 ml of glacial CH3COOH with stirring; the solution was heated to 90°C for 20-25 min and then cooled; Zn-dust was collected by filtering, and the pellet was washed with methanol. The pellet obtained after the removal of the solvents was dissolved in chloroform, and the precipitated Zn(OAc)2 was filtered and washed with chloroform. The crystalline residue obtained after standard treatment of the combined filtrates was loaded on a chromatography column. Elution with hexane–acetone (3→10% acetone) gave successively: (i) 0.08 g (20%) of 19-hydroxypregn-4-en-20-one IX with m.p. 127-132°C (from ether–hexane). Mass-spectrum: experimental – m/z 317.2470 [M+H]+, m/z 339.2289 [M+Na]+. C21H32O2. Calculated: M = 316.2402. 1H NMR spectrum: 0.69 s with (3 H, 18-CH3), 2.15 s with (3 H, 21-CH3), 3.57 d and 3.85 d (on 1 H, 19-CH2, J = 9 Hz), 5.67 m (1 H, H-4). 13C NMR spectrum: 13.61 (C-18), 62.82 (C-19), 124.62 (C-4), 138.36 (C-5); and (ii) 0.05 g (10%) 19-hydroxypregn-3-en-20-one X with m.p. 140-143°C (from ether–hexane). Mass-spectrum: experimental – m/z 317.2471 [M+H]+, m/z 339.2288 [M+Na]+. C21H32O2. Calculated: M = 316.2402. 1H NMR spectrum: 0.60 s (3 H, 18-CH3), 2.09 s with (3 H, 21-CH3), 3.57 d and 3.90 d (on 1 H, 19-CH2, J = 9 Hz), 5.39 dd (1 H, H-4, J = 10 and 2 Hz), 5.66 m (1 H, H-3). 13C NMR spectrum: 13.42 (C-18), 35.70 (C-5), 65.92 (C-19), 126.51 (C-4), 132.46 (C-3).
Cell culture. The human pancreatic adenocarcinoma BxPC3 cell line was obtained from the American Type Culture Collection (ATCC). After thawing, cells were cultivated in standard conditions (37°C, 5% CO2) in RPMI-1640 medium with phenol red supplemented with 10% FBS and 1× L-Glu and 1× mix of antibiotics and antimycotic. After the third passage, the cells were transferred to RPMI-1640 medium without phenol red, supplemented with 10% DFBS, 1× L-Glu, and 1× mix of antibiotics and antimycotic. After three passages, the cells were collected with Versene solution and pelleted by centrifugation (Jouan CR 3i) at 500g for 7 min at room temperature. The cells were resuspended in DPBS Ca2+/Mg2+ and used to study binding with [3H]progesterone.
Analysis of [3H]progesterone binding in whole BxPC3 cells. BxPC3 cell suspension in DPBS Ca2+/Mg2+ (100,000-120,000 cells per 100 µl) was incubated with 100 µl steroid mix in DPBS Ca2+/Mg2+ buffer at room temperature (20-22°C) for 2 h with constant stirring. The steroid mixture consisted of 10 µl of [3H]progesterone (final concentration 3-5 nM) and 90 µl of unlabeled progesterone (concentration from 0 to 6320 nM). After incubation, the cells were pelleted at 500g for 7 min at room temperature. The cell pellet was washed with 700 µl of DPBS Ca2+/Mg2+ and centrifuged again under the same conditions. Then 250 µl of distilled water was added to the cell pellet, and the suspension was transferred to a scintillation vial. Radioactivity was measured in 6 ml of Bray dioxane scintillation liquid in a RackBeta 1217 liquid scintillation counter (LKB WALLAC, Finland). For each experimental point, the value of nonspecific binding of [3H]progesterone measured in the presence of excess cold progesterone (6320 nM) was subtracted from the value of total [3H]progesterone binding. Statistical analysis, calculation of Kd and Bmax values, and plots of competitive inhibition were done using the GraphPad Prism 6 software (GraphPad Software Inc., USA). Relative binding affinity (RBA) values for progesterone derivatives were calculated as the ratio Kd1/Kd2, where Kd1 – progesterone dissociation constant, Kd2 – dissociation constant for the studied competitor: RBA = Kd(progesterone)/Kd(studied ligand) × 100%. RBA of progesterone was considered as 100%. The results are presented as mean ± standard deviation from three or more experiments. To compare the affinity of an analog for nPRs and mPRs, we used the “discrimination index” calculated as the ratio RBAmPRs/RBAnPRs.
Analysis of [3H]progesterone binding with uterine cytosol fraction. Mongrel albino adult female rats (150-200 g) were sacrificed by decapitation. Uteri from 3-4 animals with an average weight of 2 g were placed on ice, minced, and homogenized using a glass homogenizer in 10 mM Tris-HCl buffer (pH 7.5) containing 10 mM KCl, 1 mM EDTA, 30% glycerol, 1 mM DTT, and 0.5 mM PMSF at tissue/buffer ratio 1 : 6. After centrifugation at 14,000g for 30 min at 4°C, the supernatant (cytosol) with protein concentration of 2-4 mg/ml was collected and used immediately. To measure affinity, uterine cytosol fraction (100 µl) was incubated at 4°C for 20 h with 100 µl of the steroids mixture containing 10 µl of [3H]progesterone (final concentration 3-5 nM) and 90 µl of unlabeled competitor (final concentration from 0 to 6320 nM). Protein-bound and free [3H]progesterone was separated by treatment of the incubation mix with 2% suspension of charcoal covered with 0.4% dextran (100 µl) for 5 min at 4°C. The charcoal was pelleted by centrifugation at 400g for 5 min. Radioactivity was measured in aliquots of supernatants (200 µl) and analyzed in the same way as described earlier for the whole BxPC3 cells.
RESULTS
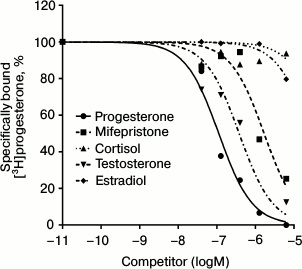
The radioligand assay using [3H]progesterone revealed the presence of a protein specifically binding progesterone in BxPC3 cells. Since this cell line demonstrated rather high level of expression of glucocorticoid receptor mRNAs (384 ± 23% of the level of GAPDH mRNA [10]), we should confirm that progesterone binding protein was an mPR and not the receptor of glucocorticoids or androgens, so we used steroids of the main classes as competing ligands. Competition curves between [3H]progesterone and other steroids are presented in Fig. 2. The relative binding affinity (RBA) values of unlabeled testosterone, estradiol, cortisol, and the nPR antagonist mifepristone are presented in Table 2. Almost total absence of estradiol binding (RBA = 1.6 ± 0.7%) and cortisol binding (RBA = 0.5 ± 0.4%) to the studied protein shows that the protein that binds specifically [3H]progesterone is a mPR. Low affinity of mifepristone (RBA = 5.1 ± 1.3%) and moderate affinity of testosterone (RBA = 10.2 ± 1.7%) also characterize mPRs and correlate with published data for MDA-MB-231 cell line, which demonstrated a high level of mPRα expression after transfection with the corresponding gene [4]. According to the results of 14 experiments, the equilibrium dissociation constant Kd of progesterone with mPRs was 141.6 ± 43.5 nM, and the concentration of binding sites Bmax was 6.1 ± 3.9 nM. Similar results were obtained for mPRα receptor expressed in CHO cells, where Kd of progesterone was 122 ± 50 nM [17]. In corpus luteum from rat containing an mPRα homolog, Kd of progesterone was 162 ± 20 nM [18]. Moreover, the Kd of progesterone with human mPRα expressed in baker’s yeast was 143 ± 43 nM [9]. Thus, the binding properties of the identified protein correlated with published data concerning mPRs.
Fig. 2. Competition curves for mPR binding for steroids from major classes in BxPC3 cells presented in percentage of maximal specific binding of [3H]progesterone.
Table 2. Relative binding affinity (RBA) of
steroids from major classes to mPRs in BxPC3 cells
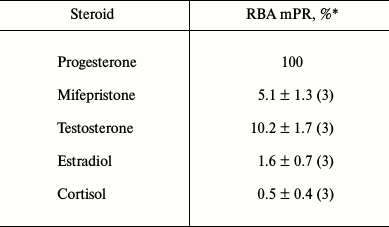
* Mean value ± standard deviation (number of measurements).
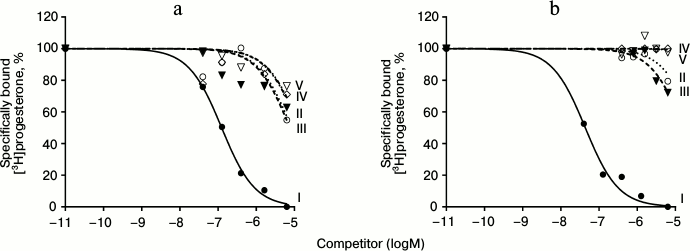
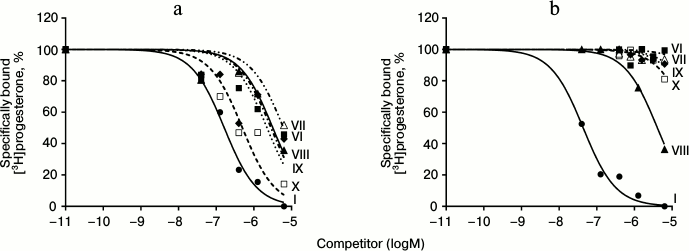
Results of typical experiments of [3H]progesterone displacement from its complexes with mPRs in BxPC3 line cells (a) and from its complexes with nPRs in rat uterine cytosol fraction (b) by cold progesterone and its analogs are presented in Figs. 3 and 4. The integrated quantitative characteristics for each compound are presented in Table 3. We see that all nine studied compounds (II-X; Table 1) bind preferentially mPRs and have discrimination indexes higher than that of progesterone. Replacement of the 3-keto group with a 3-O-methoxyimine group (compounds II and III) leads to a decrease in affinity of E- and Z-isomers both to mPRs and to nPRs, with discrimination indexes mPRs/nPRs of 31 and 16, respectively. Addition of a 17α-hydroxyl group (compounds IV and V) reduced even further the RBA of these compounds for both receptor types, but the discrimination indexes mPRs/nPRs remained the same (37 and 16, respectively).
Fig. 3. Competition curves of ligand binding. a) mPR in BxPC3 cells; b) nPR in rat uterine cytosol fraction, expressed as a percentage of maximal specific binding of [3H]progesterone. Numbers next to curves correspond to the compound number in Table 1.
Fig. 4. Competition curves of ligand binding. a) mPR in BxPC3 cells; b) nPR in rat uterine cytosol fraction, expressed as a percentage of maximal specific binding of [3H]progesterone. Numbers next to curves correspond to the compound number in Table 1.
Table 3. Relative binding affinity (RBA) of
studied compounds to nuclear and membrane progesterone receptors and
ligand binding preferences RBAmPR/RBAnPR
(discrimination index)
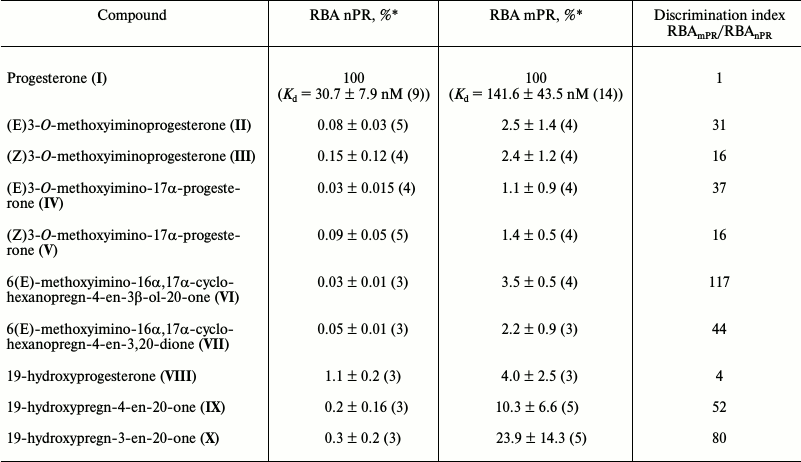
* Mean value ± standard deviation (number of measurements).
The combination of the 6-methoxyimine group with additional 16α,17α-carbocycle (compounds VI and VII) reduces the affinity of the corresponding derivatives to both receptor types according to the RBA values for these compounds to mPRs and nPRs. However, taking into the account almost complete absence of binding of compound VI with nPRs, this analog possesses the highest discrimination index mPRs/nPRs of 117.
19-Hydroxyprogesterone (compound VIII) shows relatively low affinity to both receptor types and has the smallest, compared to the other compounds, discrimination index mPR/nPR of 4. Simultaneous removal of the 3-keto group and introduction of the 19-hydroxyl group (compound IX) leads to increase in affinity to mPRs and decrease in affinity to nPRs, providing discrimination index mPRs/nPRs of 52. The combination of these modifications with changing of the double bond position in ring A (compound X) increases even more the affinity to mPRs, resulting in discrimination index mPRs/nPRs of 80. From all the tested compounds, compound X (19-hydroxypregn-3-en-20-one) showed the best combination of modifications for further increase in ligand selectivity to mPRs.
DISCUSSION
To identify progesterone analogs that are highly selective for membrane receptors, we compared the affinity of nine synthesized progestins (II-X; Table 1) to mPRs and nPRs expressed in human pancreatic adenocarcinoma cell line and in rat uterine cells, respectively. The comparison is reasonable because nPRs of rat uterus have binding characteristics and structure of ligand-binding pocket similar to those of human nPRs [11, 12]. In our work, the Kd for progesterone binding with nPRs and mPRs was 30.7 and 141.6 nM, respectively, i.e. the affinity of progesterone to nPRs is approximately five times higher than its affinity to mPRs, which correlates with published data [4]. The binding specificity of the studied receptors in BxPC3 cells for cortisol, testosterone, estradiol, and mifepristone was almost the same as the specificity of mPRs reported earlier [4, 8]. Thus, the binding we have shown is due to the mPRs, which belong to the human progestin and adiponectin receptor (PAQR) family.
Synthesis of progesterone analogs selective to mPRs is important for studying of mechanisms of progestin action. The Δ4-3-keto group is an important determinant of ligand interaction with nPRs. It forms hydrogen bonds with the amino acid residues of the ligand-binding pocket of the receptor [19]. It was shown that an increase in the size of a substituting group at C3 proportionally reduces the affinity of the ligand to nPRs [20]. The results of the analysis of the influence of various substituting groups on the affinity of corresponding compounds to nPRs and mPRα [9] suggests the existence of a cavity around C3 in the ligand-binding pocket of mPRα. The analogs (E)3-O-methoxyiminoprogesterone (II) and (Z)3-O-methoxyiminoprogesterone (III) bearing a 3-O-methoxyimine group instead of the 3-keto group were expected to have higher discrimination indexes mPRs/nPRs. However, in our work these compounds showed low affinity to mPRs with almost total absence of binding to nPRs.
The highest affinity to mPRs was shown by the compounds that lacked the 3-keto group: 19-hydroxypregn-4-en-20-one (IX) and 19-hydroxypregn-3-en-20-one (X). This result confirms a hypothesis about the different ligand-binding properties of the two receptor types. It is obvious that the network of hydrogen bonds around C3 is not essential for ligand interaction with mPRs, unlike nPRs. Introduction of a 17α-hydroxy group, as expected, decreased the affinity of (E)3-O-methoxyimino-17α-progesterone (IV) and (Z)3-O-methoxyimino-17α-progesterone (V) both to mPRs and nPRs compared to (E)3-O-methoxyiminoprogesterone (II) and (Z)3-O-methoxyiminoprogesterone (III). However, the hypothesis that the effect will be much stronger for nPRs [9] was not confirmed. Thus, the 17α-hydroxy group did not increase the discrimination index.
Modifications of a steroid molecule at C19, as shown earlier, significantly influence its affinity for different types of receptors. It was shown that introduction of a hydrophilic substituent at C19 decreased the affinity of the corresponding ligand both to nPRs [20] and mPRs [8]. However, it seems that this effect is much less significant in the case of interaction of 19-hydroxyprogesterone (VIII) with mPRs, since the introduction of the 19-hydroxy group increased the discrimination index mPRs/nPRs four-fold (Table 3). To further increase the selectivity towards mPRs, a hydrophobic group can be introduced to the C19 area, since 19-methylprogesterone showed no agonist activity to nPRs [8], but the presence of 19-methyl in 16α,17α-cycloalkanoprogesterones increased the discrimination index mPRα/nPRs from 0.05 to 83.7 [9].
It was shown that introduction of a substituent into position 6 of the B ring affords some preference in binding to mPRα: introduction of a 6α-methyl group decreased the affinity of 16α,17α-cyclohexanoprogesterone to nPRs and increased the discrimination index mPRα/nPRs [9, 20]. However, the 16α,17α-cyclohexane group itself reduced the affinity of steroids to mPRs and had no significant effect on affinity to nPRs. Introduction of an oxyimino group into position C6 of a steroid changes the general geometry of the steroid ring, leading to almost full suppression of binding with nPRs but not preventing the interaction with mPRs (Table 3). In our experiments, compounds (VI) and (VII) with 6(E)-methoxyimino group and additional 16α,17α-cyclohexane D′ cycle showed practically no binding to nPRs and gave a high discrimination index (117 and 44, respectively). Such modifications can serve as a reserve for further increase in the selectivity of progesterone derivatives to mPRs.
Thus, despite previous results with compounds bearing additional 16α,17α-carbocycle D′ [9], we found that the replacement of the 3-keto group with the 3(E or Z)-O-methoxyimino group in progesterone derivatives leads to considerable decrease in the affinity of such compounds to mPRs and, therefore, is not suitable for generation of selective ligands for these receptors. These discrepancies are probably related to differences in the carbon backbone of the steroids used.
Introduction of an additional 17α-hydroxyl group resulted in equal decrease in affinity of corresponding ligands to mPRs and nPRs, therefore also being inefficient for improvement of the discrimination index (Table 3). However, in the present work we found new modifications of the progesterone molecule increasing its affinity to the studied receptor type. Removal of the 3-keto group (compounds IX and X) as well as the introduction of an oxyimino group at C6 (compounds VI and VII) stimulates the binding of the corresponding derivatives to mPRs compared to nPRs. The 3-desoxy compounds IX and X with discrimination indexes of 52 and 80, respectively, bind preferentially mPRs compared to the earlier described 10-ethenyl-19-norprogesterone and 19α-methylprogesterone with discrimination indexes of 20 and 40, respectively [8]. It should also be mentioned that unlike reported compounds [8], 19-hydroxypregn-4-en-20-one (IX) and 19-hydroxypregn-3-en-20-one (X) have practically no binding to nPRs, and therefore can act only through mPRs. This is especially important when opposite effects of progestins via different types of receptors are expected, for example on proliferation and apoptosis of some tumor tissue cells [5, 6]. Further increase in ligand selectivity can probably be achieved through combination of the most perspective modifications, such as removal of the 3-keto group, introduction of a hydrophobic substitute at C19 instead of the 19-hydroxy group, and introduction of the oxyimino group at C6. Introduction of a C6–C7 double bond or modifications of C18 could also have a positive effect on preferential binding of progesterone derivatives with mPRs compared to nPRs.
Acknowledgements
This work was done with financial support from the Russian Foundation for Basic Research (projects Nos. 14-04-01021 and 14-04-31589).
REFERENCES
1.Smirnov, A. N. (2006) Elements of Endocrine
Regulation [in Russian], GEOTAR-Media, Moscow.
2.Tokmakov, A. A., and Fukami, Y. (2009) Nongenomic
mechanisms of progesterone, Tsitologiya, 51, 403-416.
3.Zhu, Y., Bond, J., and Thomas, P. (2003)
Identification, classification, and partial characterization of genes
in humans and other vertebrates homologous to a fish membrane progestin
receptor, Proc. Natl. Acad. Sci. USA, 100, 2237-2242.
4.Thomas, P., Pang, Y., Dong, J., Groenen, P.,
Kelder, J., De Vlieg, J., Zhu, Y., and Tubbs, C. (2007) Steroid and G
protein binding characteristics of the seatrout and human progestin
membrane receptor alpha subtypes and their evolutionary origins,
Endocrinology, 148, 705-718.
5.Atif, F., Yousuf, S., and Stein, D. G. (2015)
Anti-tumor effects of progesterone in human glioblastoma multiforme:
role of PI3K/Akt/mTOR signaling, J. Steroid Biochem. Mol. Biol.,
146, 62-73.
6.Atif, F., Sayeed, I., Yousuf, S., Ishrat, T., Hua,
F., Wang, J., Brat, D. J., and Stein, D. G. (2011) Progesterone
inhibits the growth of human neuroblastoma: in vitro and in
vivo evidence, Mol. Med., 17, 1084-1094.
7.Karteris, E., Zervou, S., Pang, Y., Dong, J.,
Hillhouse, E. W., Randeva, H. S., and Thomas, P. (2006) Progesterone
signaling in human myometrium through two novel membrane G
protein-coupled receptors: potential role in functional progesterone
withdrawal at term, Mol. Endocrinol., 20, 1519-1534.
8.Kelder, J., Azevedo, R., Pang, Y., De Vlieg, J.,
Dong, J., and Thomas, P. (2010) Comparison between steroid binding to
membrane progesterone receptor alpha (mPRalpha) and to nuclear
progesterone receptor: correlation with physicochemical properties
assessed by comparative molecular field analysis and identification of
mPRalpha-specific agonists, Steroids, 75, 314-322.
9.Lisanova, O. V., Shchelkunova, T. A., Morozov, I.
A., Rubtsov, P. M., Levina, I. S., Kulikova, L. E., and Smirnov, A. N.
(2013) Approaches to the design of selective ligands for membrane
progesterone receptor alpha, Biochemistry (Moscow), 78,
236-243.
10.Goncharov, A. I., Maslakova, A. A., Polikarpova,
A. V., Bulanova, E. A., Guseva, A. A., Morozov, I. A., Rubtsov, P. M.,
Smirnova, O. V., and Shchelkunova, T. A. (2017) Progesterone inhibits
proliferation and modulates expression of proliferation-related genes
in classical progesterone receptor-negative human BxPC3 pancreatic
adenocarcinoma cells, J. Steroid Biochem. Mol. Biol.,
165, 293-304.
11.Smirnov, A. N., Pokrovskaya, E. V., Shevchenko,
V. P., Nagaev, I. Yu., Myasoedov, N. F., Levina, I. S., Kulikova, L.
E., and Kamernitsky, A. V. (2002) Species and tissue distribution of
proteins binding 16α,17α-cycloalkanoprogesterone
derivatives, Bioorg. Khim., 28, 251-257.
12.Fedyushkina, I. V., Skvortsov, V. S., Romero
Reyes, I. V., and Levina, I. S. (2013) Molecular docking and 3D-QSAR of
16α,17α-cycloalkanoprogesterone derivatives as ligands of
the progesterone receptor, Biomed. Chem., 8, 168-176.
13.Zolottsev, V. A., Zavarzin, I. V., Shirinyan, V.
Z., and Levina, I. S. (2013) Synthesis of E- and
Z-isomeric progesterone 3-O-methyloximes, Russ. Chem.
Bull., 62, 2086-2087.
14.Semeikin, A. V., Fedotcheva, T. A., Levina, I.
S., Kulikova, L. E., Zavarzin, I. V., Tikhonov, D. A., Kareeva, E. N.,
and Shimanovskii, N. L. (2014) Synthesis and cytostatic activity of
some pregna-D′-pentarans studied on HeLa cells, Khim. Farm.
Zh., 48, 9-13.
15.Kirk, D. N., Rajagopalan, M. S., and Varley, M.
J. (1983) An improved route to 19-hydroxypregn-4-ene-3,20-dione and
synthesis of its [19-2H2] analogue, J. Chem.
Soc. Perkin Trans., 1, 2225-2227.
16.Bagli, J. F., Morand, P. F., and Gaudry, R.
(1963) Synthetic studies on C-19 oxygenated pregnanes, J. Org.
Chem., 28, 1207-1217.
17.Ashley, R. L., Arreguin-Arevalo, J. A., and Nett,
T. M. (2009) Binding characteristics of the ovine membrane progesterone
receptor alpha and expression of the receptor during the estrous cycle,
Reprod. Biol. Endocrinol., 7, 42.
18.Cai, Z., and Stocco, C. O. (2005) Expression and
regulation of progestin membrane receptors in the rat corpus luteum,
Endocrinology, 146, 5522-5532.
19.Whilliams, S. P., and Sigler, P. B. (1998) Atomic
structure of progesterone complexed with its receptor, Nature,
393, 392-396.
20.Levina, I. S., Pokrovskaya, E. V., Kulikova, L.
E., Kamernitzky, A. V., Kachala, V. V., and Smirnov, A. N. (2008) 3-
and 19-oximes of 16α,17α-cyclohexanoprogesterone
derivatives: synthesis and interactions with progesterone receptor and
other proteins, Steroids, 73, 815-827.