Study of Immunomodulatory Effects of Extracellular HSP70 in a Mouse Model of Allergic Airway Inflammation
M. A. Shevchenko1, N. I. Troyanova1, E. A. Servuli1, E. L. Bolkhovitina1, A. S. Fedorina1, and A. M. Sapozhnikov1,2*
1Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, 117997 Moscow, Russia; E-mail: amsap@mx.ibch.ru2Lomonosov Moscow State University, Faculty of Biology, 119991 Moscow, Russia
* To whom correspondence should be addressed.
Received May 25, 2016; Revision received July 27, 2016
Immunostimulatory properties of extracellular heat shock proteins 70 kDa (HSP70) became interesting for investigators a long time ago. However, in recent years a series of works showing a significant relation of the immunostimulating effects of recombinant HSP70 to contamination of the protein samples with bacterial endotoxins (lipopolysaccharide, LPS) has been published. The authors showed that intensive elimination of LPS from the protein samples resulted in inversion of immunostimulating effects of HSP70 to immunosuppressive activity of the protein. Nevertheless, at present the conception of immunostimulating, proinflammatory action of extracellular HSP70 is the most common. In this work, we studied immunomodulatory effects of exogenous HSP70 in a mouse model of allergic inflammation of airways. We also analyzed the dynamics of the level of the extracellular pool of HSP70 in the site of inflammation. The results demonstrated a considerable content of extracellular HSP70 in bronchoalveolar lavages with dynamics reflecting the stages of development of the induced inflammation. Oropharyngeal injection of exogenous HSP70 in the acute phase of allergic inflammation of airways resulted in significant suppression of the inflammatory process, which conforms to published data demonstrating an immunosuppressive activity of the extracellular pool of HSP70.
KEY WORDS: heat shock protein 70 kDa, HSP70, airway allergic inflammation, immunomodulatory effectsDOI: 10.1134/S0006297916110158
Abbreviations: APC, antigen presenting cells; BAL, bronchoalveolar lavage; BSA, bovine serum albumin; EDTA, ethylenediaminetetraacetic acid; ELISA, enzyme-linked immunosorbent assay; EU, endotoxin units; FDA, USA Food and Drug Administration; FSC/SSC, forward small-angle light scattering/side light scattering; HSP70, heat shock protein 70 kDa; Ig, immunoglobulin; IL, interleukin; LPS, lipopolysaccharide; OVA, chicken egg albumin; OVA/OVA mice, mice with ova-induced allergic airway inflammation (ones that got ova both i.p. and o.p.); PBS, phosphate buffered saline; Th2, T-helper type 2.
At present, a growing body of literature reports that heat shock protein
70 kDa (HSP70) has diverse immunomodulating effects. Both pro- and
antiinflammatory properties have been shown to be mediated by HSP70.
Whereas proinflammatory HSP70 activity is related to synthesis and
secretion of TNF-α, IFN-γ, IL-1β, IL-6, and IL-12 [1, 2], the evidences of
antiinflammatory role of HSP70 are associated with its potential to
activate IL-10 producing cells [3, 4]. Recent studies demonstrate that previously
reported proinflammatory activity of HSP70 is particularly caused by
bacterial endotoxin (lipopolysaccharide, LPS) contamination [5]. Nevertheless, in some cases purification of the
protein preparations from LPS does not affect HSP70 proinflammatory
potential; stimulation of proinflammatory cytokine production was also
shown for LPS-free HSP70 [6]. Therefore, the
reasons and the mechanisms of bidirectional effects of HSP70 in
inflammation remain unclear.
Most common approaches to estimating immunomodulating effects of HSP70 include the testing of exogenous HSP70 effects in different models and analyzing the correlation between circulating endogenous HSP70 level and inflammatory disease development. Allergic inflammation, and particularly bronchial asthma, is a disease characterized by a considerable increase in serum extracellular HSP70 level [7]. Other evidence of involvement of HSP70 in allergic asthma manifestation is based on the observation that increased serum level of HSP70-specific antibodies is detected in patients with asthma, and the extent of the increase correlates with elevation of IgE and IL-4 levels in peripheral blood serum of the patients [8]. However, the links between immunomodulating function of extracellular HSP70 and hyperimmune response are still not clear. The study of the role that HSP70 plays in pathogenesis of allergic airway inflammation is obviously essential for both basic and clinical immunology, because it may be useful for elaboration of new approaches to treatment of allergy and other diseases associated with inflammatory processes. Here we investigated some aspects of involvement of extracellular HSP70 in allergic airway inflammation using a well-established mouse model of bronchial asthma [9].
MATERIALS AND METHODS
Animals. 10-to-12-week-old BALB/c mice (Central Laboratory of the Animal Breeding Facility, Russian Academy of Medical Sciences, Moscow, Russia) were used in the study. The mice were fed OVA-free laboratory food and tap water ad libitum and were held in regular 12-h dark/light cycles at 22°C. Animal experiments were performed in concordance with the Guide for the Care and Use of Laboratory Animals under a protocol approved by the Institutional Animal Care and Use Committee of the Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences.
Ovalbumin-induced allergic airway inflammation. BALB/c mice were sensitized with 10 µg OVA Grade V (albumin from chicken egg, grade V; Sigma-Aldrich, USA) adsorbed to 1.5 mg of Imject Alum (Thermo Scientific, USA) diluted in PBS. OVA inoculation was carried out on days 0 and 7 via intraperitoneal (i.p.) injection. The animals were then subjected to oropharyngeal application of 0.1% OVA dissolved in PBS (50 µl) on days 19, 20, and 21. The negative control groups were exposed to PBS instead of oropharyngeal OVA applications.
Purification of HSP70. HSP70 was obtained from homogenized BALB/c mouse kidney and liver by affinity chromatography using an ATP-agarose column [10]. PAGE and Western blotting were performed using HSP70-specific antibodies BRM22 (Sigma). HSP70 was purified from ATP by two-step dialysis. LPS carrier was measured and consisted of 21.6 EU/mg (such concentration is classified as “LPS low”). The total concentration of LPS per mouse per injection was 0.4 EU/ml, which was less than current FDA limits on concentration of endotoxin for medical devices and parenteral drugs (0.5 EU/ml).
Induction of humoral immune response by multiple HSP70 injections. HSP70 that was isolated from syngeneic mice kidney and liver was injected into mice at concentration 10 µg per mouse via the intraperitoneal route five times per week for seven weeks. Mice from the control group received BSA instead of HSP70.
HSP70-induced allergic airway inflammation. Mice received two intraperitoneal injections of HSP70/Alum (Imject Alum; Thermo Scientific, USA) at days 0 and 7. The total concentration of HSP70 was 10 µg per mouse per injection. On days 19, 20, and 21 mice received oropharyngeal (o.p.) injections of 0.1% HSP70 solution in PBS, at dose 50 µl per mouse per injection. Control mice received OVA instead HSP70. In both cases, severity of the airway inflammation was evaluated by comparison to the group of mice receiving protein (OVA or HSP70) intraperitoneally and PBS by oropharyngeal application.
Oropharyngeal HSP70 administration. Mice with OVA-induced allergic airway inflammation received 50 µl of HSP70 dissolved in PBS at concentration 20 µg/ml. The dose was applied 24 h after the last allergen inhalation. Control mice received the denatured form of HSP70 (30 min, 100°C) solution at the same concentration.
Total and differential BAL cell count. Animals were sacrificed by cervical dislocation. Bronchoalveolar lavage (BAL) was performed twice with 0.8 ml of ice-cold sterile PBS. The total volume was 1.3 ml. Cells were quantified using a Goryaev chamber (Minimed, Russia) and diluted to a concentration of 1·106 cells/ml. Cells were then transferred onto glass slides using a Cytospin II centrifuge (Shandon, UK) for 5 min at 100g in concentration 100,000 cells per glass slide. Supernatants were aliquoted and stored at –20°C until use. Cells were stained using a Diff-Quik stain set (Diachem, Russia), and the differential cell counts were assessed with an Axiovert 40 microscope (Zeiss, Germany).
Extracellular HSP70 level detection. The levels of extracellular HSP70 in BALs of mice were estimated by ELISA using a HSP70 detection kit (DuoSet IC; R&D Systems, Inc., USA) in accordance with manufacturer’s recommendation. Tests were carried out in 96-well ELISA plates (Linbro, UK). Visualization was carried out using substrate solution (Immunotech, Russia), and the reaction was terminated with 10% sulfuric acid. Absorbance was detected at 450/620 nm using Multiskan FC (Thermo Scientific, Germany).
Specific immunoglobulin detection. Antigen-specific IgG, IgG1, IgG2a, IgG2b, IgG3, IgE, and IgA were detected by ELISA using HRP-conjugated antibodies (Phara-Mingen, USA) in accordance with manufacturer’s recommendation. Antibody dilutions are indicated in the figure legends. For blocking, 10% inactivated calf serum was used (Biosera, USA). Visualization was performed using substrate solution and 10% sulfuric acid as a stop solution. Absorbance was measure at 450/620 nm using a Multiskan FC device.
Cytokine secretion detection. Cytokine production was estimated by ELISA. IL-4 and IL-5 were measured using ELISA MAX Standard Sets (Biolegend, USA) and IL-13 was measured using ELISA Development Kit (PeproTech, USA) in accordance with manufacturer’s recommendations in 96-well plates (Linbro, UK). Visualization and detection were carried out as described above.
Isolation of neutrophils from mouse bone marrow cells. Bone marrow cells were obtained from mouse bones (tibia and femur) and washed twice with PBS (10 min, 1000 rpm). Neutrophils were obtained by negative selection using a Neutrophil Isolation Kit (mouse) for magnetic separation (Miltenyi Biotec, USA) in accordance with manufacturer’s recommendations. Neutrophils were then transferred into HBSS (PanEco, Russia) or (if indicated) RPMI (PanEco) supplemented with 10% inactivated calf serum.
To estimate homogeneity of the neutrophil population, an aliquot of cell suspension was transferred onto a glass slide with a Cytospin II cyto-centrifuge (Shandon) and stained with Diachem-DiffQick (Diachem, Russia). Then cells were subjected to morphological analyses.
An aliquot of cell suspension was transferred into FACS buffer (0.1% BSA, 0.05% NaN3, PBS) and stained with FITC-conjugated anti-mouse Gr-1 (clone RB6-8C5), PE-Cy5-conjugated anti-mouse CD11b, and PE-conjugated anti-mouse F4/80 (all from eBioscience, USA). Percentage of Gr-1+F4/80–CD11b+ cells was estimated by flow cytometry using a FACS Calibur device (BD Biosciences, USA). Neutrophils were distinguished according to morphology (FSC/SSC) and Gr-1 and CD11b expression. Because anti-Gr-1 antibodies bind both Ly-6G and Ly-6C, anti-mouse F4/80 were used in combination with of Gr-1 and CD11b to separate neutrophils from Ly6C+ monocytes. Every sample was measured as not less than 10,000 events per region, and the data were analyzed using FlowJo V10.0.7 software (FlowJo Enterprise, USA).
Bone marrow neutrophil activation. Mouse neutrophils isolated from bone marrow were activated by phorbol-12-myristate-13-acetate (PMA) (Sigma) at concentration 0.005-50 µM. To analyze the influence of HSP70 on neutrophil activation, HSP70 was added at concentration 2 nM-0.2 µM simultaneously with PMA. Denatured form (30 min, 100°C) of HSP70 was used for a control.
Extracellular nucleotide detection. Extracellular nucleotides were detected according to the protocol described by Barrientos et al. [11]. Neutrophils were diluted to the concentration of 1·106 cells/ml and immediately seeded in total volume of 200 µl per well of black 96-well plates (SPL, Switzerland) in the presence of 5 µM SYTOX Green Nucleic Acid Stain (Molecular Probes, USA). The cells were incubated for 4 h (37°C, 5% CO2) in the presence of different doses of PMA and HSP70. Fluorescence was detected each hour using a GloMax-Multi Detection System (Promega, USA). The level of fluorescence was detected in relative fluorescence units.
Spontaneous apoptosis detection in primary neutrophil culture. Neutrophils were selected from mouse bone marrow seeded in 5 ml polypropylene round bottom tubes (Falcon, USA) in concentration 1·105 cells per tube and incubated for 20 h in RPMI in the presence of 10% calf serum (Biosera) in 5% CO2 at 37°C. Apoptosis was detected using an AnnexinV-FITC Apoptosis Detection Kit I (BD Biosciences) according to the manufacturer’s recommendation. HSP70 was added to the neutrophils at concentration 1-10 µg/ml just prior to the incubation. Denatured HSP70 (100°C, 30 min) was used as a control. Staurosporine (Sigma) was used as a positive control as an apoptosis inducing agent.
Statistical analysis. Data are expressed as mean ± SEM and analyzed using Student’s t-test. Tests were performed using GraphPad Prism version 4.03 for Windows (GraphPad Software, USA). Differences with p < 0.05 are considered statistically significant.
RESULTS
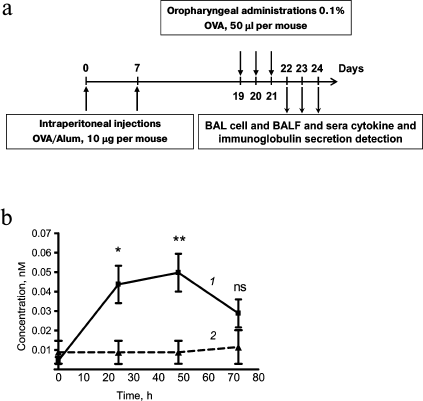
Time course of extracellular HSP70 level in BAL fluid of mice with different stages of induced allergic airway inflammation. To estimate the role of HSP70 in allergic bronchial asthma inflammatory processes, we used a model of ovalbumin-induced allergic airway inflammation. Mice were sensitized via i.p. injections with OVA/Alum at total protein concentration of 10 µg per mouse on days 0 and 7. To transfer the inflammation to the airways, the mice then received oropharyngeal applications of 0.1% OVA in PBS in total volume of 50 µl per mouse on days 19, 20, and 21 (Fig. 1a). BAL cell composition and humoral factor levels were monitored over 72 h after the last allergen challenge. One of the tasks of the present study was to detect the dynamics of extracellular HSP70 level in BAL of mice at the different phases of allergic airway inflammation.
Fig. 1. Time course of extracellular HSP70 secretion in response to inhalation of OVA to OVA-sensitized mice. a) Allergic airway inflammation was induced in mice by two intraperitoneal injections of OVA/Alum and consequent oropharyngeal allergen application. b) Time course of HSP70 in BAL of mice with induced allergic airway inflammation at different time points after the last allergen application (1); the level of HSP70 in BAL fluids of intact mice (2). The data were obtained by ELISA, BAL fluid dilution 1 : 1. Mean and SEM are presented for three independent experiments with five mice per group. Significant difference between group of mice with induced allergic inflammation and group of mice without airway inflammation (time 0): * p ≤ 0.05; ** p ≤ 0.01; ns, not significant.
Extracellular HSP70 concentration in BAL was determined by ELISA 24, 48, and 72 h after the last allergen challenge. A significant increase in HSP70 level was detected at 24 h as well as at 48 h after the last allergen challenge. At 72 h after last allergen challenge, the level of extracellular HSP70 decreased and did not differ significantly from the level of extracellular HSP70 that was detected for intact mice (Fig. 1b).
The increase in extracellular HSP70 level may serve as a stress-induced “danger signal” that occurs in inflammation [6]. In addition, extracellular HSP70 is known to be internalized by different types of cells, improving their resistance to environmental damaging factors [12]. Taking these things together brings us to the proposal that the increase in extracellular HSP70 levels in the airway during allergic inflammation may have a protective function.
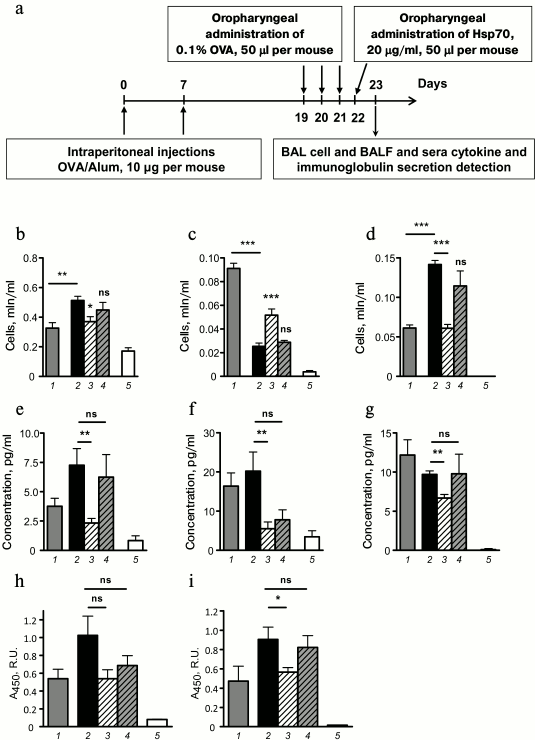
Local HSP70 administration arrests development of allergic airway inflammation. We carried out a series of experiments to estimate the potential of extracellular HSP70 in modulating allergic airway inflammation. HSP70, derived from visceral organs of mice, was administrated to syngeneic mice at the acute phase of induced allergic airway inflammation according to the scheme given in Fig. 2a. The effect of HSP70 application was assessed by monitoring BAL cells, cytokine, and specific immunoglobulin secretion within 24 h (48 h after the last allergen application).
Fig. 2. Effect of exogenously supplied HSP70 on allergic airway inflammation. a) Mice received HSP70 24 h after the last allergen challenge. Total cell number (b), neutrophil (c), and eosinophil (d) counts in BAL of mice with induced allergic airway inflammation. BAL fluid levels of IL-4 (e), IL-5 (f), and IL-13 (g) of mice with induced allergic airway inflammation and intact mice. IgE peripheral blood serum levels (h) and BAL fluid IgA levels (i) of mice with induced allergic airway inflammation and intact mice. Serum dilution for IgE detection 1 : 10, BAL fluid dilution for IgA detection 1 : 1. Columns: 1) mice with induced allergic airway inflammation 24 h after the last allergen challenge; 2) 48 h after the last allergen challenge; 3) mice that were treated with HSP70; 4) mice that were treated with denatured HSP70; 5) intact mice. Data are presented as mean ± SEM for three independent experiments with five mice per group. Significant difference from the data that were acquired for mice with induced allergic airway inflammation at acute phase (24 h after the last allergen challenge) or between other groups is indicated; * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; ns, not significant.
It was demonstrated that oropharyngeal administration of HSP70 24 h after the last allergen challenge (in acute inflammation phase) impaired airway infiltration by immune cells (Fig. 2b). In particular, mice that were treated with HSP70 showed significantly less airway eosinophilia – the main marker of allergic airway inflammation, compared to OVA/OVA mice or to mice that received denatured HSP70 (Fig. 2d). Furthermore, 48 h after the allergen application the number of neutrophils was significantly increased in BAL of HSP70-treated mice compared to the control groups (Fig. 2c). Based on the data presented by Kusmartsev et al. [13] that demonstrated the potential of neutrophils to suppress the activation and proliferation of Th2, we conclude that HSP70 had a protective effect in allergic airway inflammation.
Allergic airway inflammation is known to be driven by the effects of Th2-associated cytokines, particularly IL-4 and IL-13 on epithelial cells and IL-5 on eosinophils [14]. In the present study using the model of induced allergic airway inflammation, we monitored pro-allergic cytokine levels at different stages of allergic airway inflammation and estimated the effect of HSP70 administration on IL-4, IL-5, and IL-13 secretion. We demonstrated that HSP70-treated mice showed significant decrease in IL-4, IL-5, and IL-13 levels at 48 h after the last allergen challenge compared to mice treated with denatured HSP70 or untreated mice (Fig. 2, e-g).
Then we estimated the influence of HSP70 on allergen-specific serum IgE and BAL fluid IgA. Previously, we have shown that the levels of these antigen-specific immunoglobulins increased from acute to effector phase in the model of induced allergic airway inflammation [15]. The results demonstrated that HSP70 administration in acute phase impaired the increment of allergen-specific IgA in BAL fluid of mice with induced allergic airway inflammation (Fig. 2i). At the same time, HSP70 application did not alter serum allergen-specific IgE level (Fig. 2h) that can most likely be explained by the fact that HSP70 was supplied locally, but not via the systemic route.
Thus, we have shown that augmentation of HSP70 in the site of inflammation at acute phase of allergic airway inflammation normalizes immune cell infiltration, impaired eosinophilia, and inhibits pro-allergic cytokine secretion.
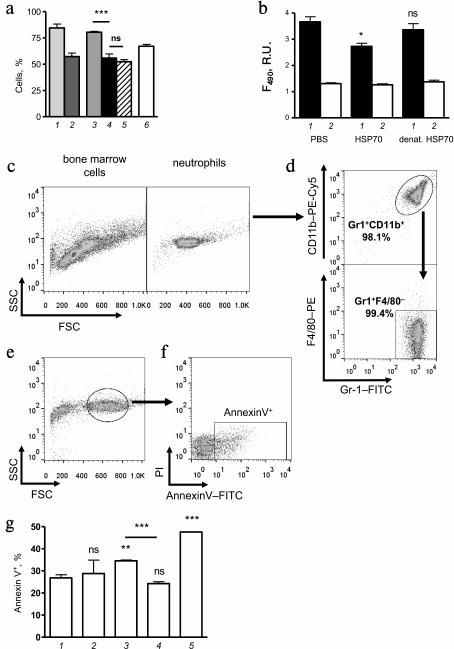
HSP70 affects death program selection in the neutrophil population. As shown above, HSP70 administration at acute phase of allergic airway inflammation increased BAL neutrophil amount, and this increment was detected up to 48 h after the last allergen challenge (24 h after HSP70 treatment) (Fig. 2c). To clarify whether these neutrophils migrated de novo in response to HSP70 administration or were retained in the airways due to increased concentration of HSP70 in the site of inflammation, which could prevent neutrophil death, we carried out a number of tests. First, we analyzed the percentage of neutrophils in bone marrow 24 and 48 h after the last allergen challenge. Because bone marrow is the main source of neutrophils that recruit to periphery in response to exogenous pathogen or trauma, we assumed that de novo recruitment of neutrophils to the airways should decrease the number of neutrophils in bone marrow. Mice with induced allergic airway inflammation showed decreased neutrophil percentage in bone marrow at both 24 and 48 h after last allergen challenge compared to sensitized but not challenged mice (Fig. 3a), whereas local administration of HSP70 did not alter neutrophil percentage in bone marrow (Fig. 3a). Therefore, HSP70 administration did not induce de novo migration of neutrophils but somehow affected the neutrophils that were recruited previously in response to allergen challenge. Thus, the results support the hypothesis that extracellular HSP70 possesses protective activity [12] and can promote survival of neutrophils at the site of inflammation.
Fig. 3. Effect of HSP70 on migration and cell death of bone marrow neutrophils. a) Percentage of neutrophils among the total bone marrow cells at different stages of allergic airway inflammation. Columns: 1) sensitized mice that received PBS instead of allergen inhalations 24 h after the last PBS application; 2) 48 h after the last PBS challenge; 3) for mice with induced allergic airway inflammation 24 h after the last OVA challenge; 4) 48 h after the last OVA challenge; 5) mice with induced allergic airway inflammation that were treated with HSP70 at acute phase of inflammation (data obtained 48 h after the last allergen challenge and at 24 h after HSP70 application, respectively); 6) intact mice. Mean ± SEM are presented for the experiment with four mice per group. Significant difference: *** p ≤ 0.001; ns, not significant. b) Effect of HSP70 and denatured HSP70 (denat. HSP70) on the extracellular nucleotide level at 4 h of incubation in primary culture of bone marrow neutrophils that were treated with PMA (at the beginning of incubation) (1) and untreated (2). Significant difference from untreated neutrophils is indicated: * p ≤ 0.05; ns, not significant. c) Total bone marrow cells (left plot) and neutrophils that were obtained by negative selection from bone marrow cells using magnetic separation (right plot). d) Flow cytometric analysis of homogeneity of neutrophil population after magnetic separation from bone marrow cells. Neutrophils were distinguished as Gr-1+ CD11b+ (upper plot) and Gr-1+ F4/80– (lower plot) cells. e) 20-h incubation of neutrophils leads to morphology changes, but there is still a percentage of cells that reveal normal morphology (indicated by ellipse). f) Spontaneous apoptosis of neutrophils that were obtained from bone marrow cells was detected according to the Annexin V-FITC binding among the cells with normal morphology (region Annexin V+). g) Percentage of Annexin V+ cells in cultures of untreated neutrophils (1) and in presence of 1 µM HSP70 (2) or 10 µM HSP70 (3), as well as in presence of 10 µM denatured HSP70 (4) and in presence of 50 µM staurosporine (5). Significant difference from untreated neutrophils marked as: ** p ≤ 0.01; *** p ≤ 0.001; ns, not significant.
To investigate the effect of HSP70 on neutrophil death mechanisms more precisely, we performed a number of in vitro experiments using neutrophils that were obtained from bone marrow of mice by negative selection (Fig. 3, c and d). Using nucleotide-binding dye SYTOX-Green in the model of PMA-induced hyperactivation of neutrophils, we have shown that HSP70 can significantly decrease the level of extracellular nucleotides (Fig. 3b) and therefore prevent neutrophil death by necrosis or NETosis. Interestingly, denatured HSP70 lacked that potential (Fig. 3b). The data suggest that increased level of BAL neutrophils in mice that received HSP70 at acute phase of allergic airway inflammation (Fig. 2c) is partly due to suppression of necrosis or NETosis. It should be noted that neutrophils that were detected in BAL cytospots of HSP70-treated mice showed instant neutrophilic morphology.
To discover the fate of neutrophils that were recruited to the airway in response to allergen inhalation, we carefully investigated the effect of HSP70 on neutrophil apoptosis in vitro. Mouse bone marrow neutrophils were cultivated in RPMI with 10% of calf serum at 5% CO2 and 37°C. Using flow cytometry, we identified neutrophils with instant morphology according to plotting on an FSC–SSC diagram (Fig. 3e). Annexin V-positive cells (Fig. 3f) were identified as “going to apoptosis”, and the percentage of such cells was compared for neutrophils treated with different doses of HSP70, denatured HSP70, staurosporine, and untreated cells. The data demonstrated the potential of HSP70 to enhance neutrophil apoptosis in a dose-dependent manner (Fig. 3g). Denaturation of HSP70 abolished the effect as it was observed for NETosis/necrosis (Fig. 3g). The results indirectly support the hypothesis that the protective effect of HSP70 in OVA-induced allergic airway inflammation can be due to suppression of airway neutrophil hyperactivation that can induce necrosis or NETosis and inflammation intensification [16-18]. Simultaneously to prevention of NETosis or necrosis in the population of neutrophils that were recruited to the airways in response to allergen inhalation [19], extracellular HSP70 can promote neutrophil apoptosis in the site of inflammation and as a consequence inflammation resolution [20].
Thus, HSP70 has suppressive effects in the model of induced allergic inflammation and can be considered a prototype for anti-allergic drug design. At the same time, in some cases HSP70 family members are mentioned as immunogenic proteins [1, 2]. To clarify this question, we estimated the evidence of HSP70-mediated side effects.
Analysis of immunogenicity and pro-allergic properties of HSP70. HSP70 family members are highly conservative proteins that are characterized by lowly immunogenic properties. However, some HSP70 family members are mentioned in the list of allergens [21]. We estimated immunogenicity and allergenic properties of exogenous HSP70 in BALB/c mice.
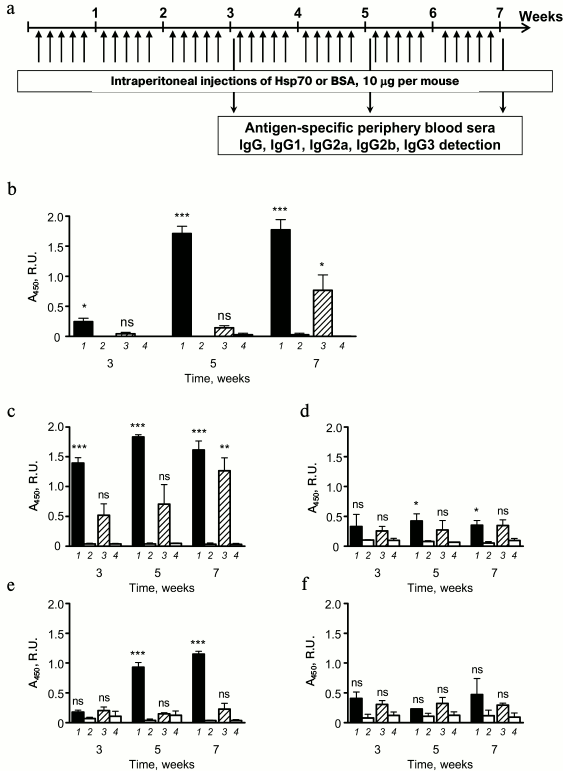
To induce HSP70-specific immune responses, mice received injections of purified HSP70 daily during 7 weeks. The level of HSP70-specific IgG was estimated at the 3rd, 5th, and 7th week after beginning the experiment (Fig. 4a). Mice from the positive control group received bovine serum albumin (BSA), which was chosen as a control due to molecular weight relevance.
Fig. 4. Systemic immunoglobulin secretion in response to multiple HSP70 injections. a) Mice received HSP70 and BSA five times a week at a dose 10 µg per mouse for seven weeks. Blood samples were collected at the 3rd, 5th, and 7th weeks. The levels of total IgG (b) and antigen-specific (BSA (1), HSP70 (3)) IgG1 (c), IgG2a (d), IgG2b (e), and IgG3 (f) in peripheral blood samples were detected by ELISA. The levels of antigen-specific antibodies in intact mouse sera were used as controls: BSA-specific (2) and HSP70-specific (4). Serum dilutions were 1 : 2000 for IgG and 1 : 200 for IgG1, IgG2a, IgG2b, and IgG3. Results are shown as mean ± SEM for two independent experiments of three and four mice per group. Significant differences from intact mice or between other groups as indicated are marked: * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; ns, not significant.
BSA-specific IgG in peripheral blood sera of mice that were treated with BSA was elevated at the third week after the beginning of immunizations. HSP70-specific antibody production against BSA was detected only after seven weeks of immunization (Fig. 4b). Thus, the HSP70 isolated from visceral organs of the mice had nonsignificant immunogenicity when it was repeatedly applied to syngeneic mice. The immunogenicity manifested by low level of specific IgG production.
To analyze antibody-mediated response to exogenously supplied autologous HSP70, we more precisely estimated the levels of peripheral blood sera HSP70-specific antibodies of different subclasses of IgG, namely IgG1 (Fig. 4c), IgG2a (Fig. 4d), IgG2b (Fig. 4e), and IgG3 (Fig. 4f). Only in case of IgG1, registered elevation of HSP70-specific antibody level was significant compare to intact mice. As mentioned for total pool of HSP70-specific IgG, upregulation was detected at seventh week after beginning the immunization; therefore, elevation of total IgG pool was most likely the result of IgG1 secretion (Fig. 4, b and c). Thus, long-term immunization of mice with HSP70 that was obtained from visceral organs of syngeneic mice were potent to induce weak elevation of antigen-specific IgG, which was represented primarily by IgG1, and that is a mark of Th2-mediated response to exogenous antigen [22]. From one side, the data support the absence of immunogenicity of HSP70 as well as disability of the protein to promote autoimmune reaction even in case of long-term routine administration. At the same time, even weak activation of Th2 that are known to play the key role in allergic inflammation [23] could be considered as a potential danger. These considerations prompted us to carry out a series of additional experiments to estimate the pro-allergic properties of HSP70.
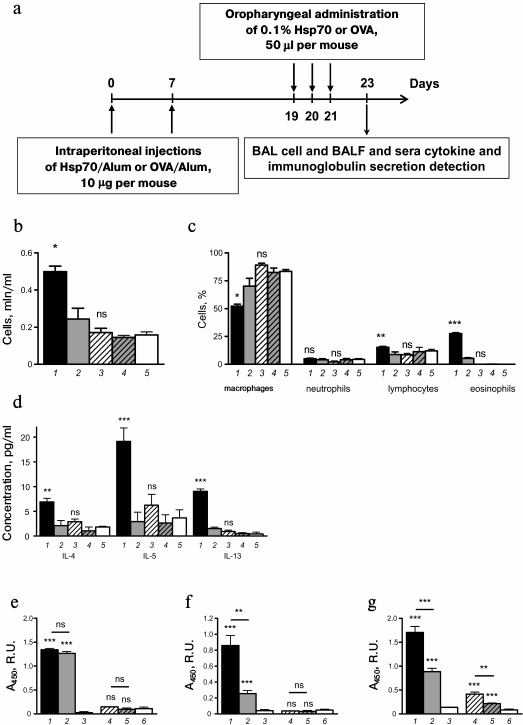
Using the standard in vivo model of induced allergic airway inflammation [24], we estimated pro-allergic potential of HSP70 by analysis of cellular and humoral immune response to HSP70 in comparison with OVA-induced asthma (Fig. 5a). We have shown that opposite to OVA-immunized animals, mice that received HSP70 did not reveal allergic airway inflammation manifestations such as total cell airway infiltration (Fig. 5b) and eosinophilia (Fig. 5c).
Fig. 5. HSP70-induced allergic airway inflammation. a) HSP70 was injected into mice by the intraperitoneal route and then oropharyngeally according to the standard scheme of allergic airway inflammation. Mice that received OVA according to the same scheme served as a positive control group. b) Total cell count and (c) percentage of macrophages, neutrophils, lymphocytes, and eosinophils as well as (d) BAL fluid cytokine levels are shown for mice with OVA-induced allergic airway inflammation (1), HSP70-induced asthma (3), sensitized but not challenged by OVA mice (2) or by HSP70 (4) and intact mice (5). Data were acquired 48 h after the last application of allergen or PBS. The levels of antigen-specific (e) IgG1 and (f) IgE in peripheral blood sera and (g) the levels of antigen-specific BAL IgA were detected by ELISA. Data are shown for mice with allergic airway inflammation that was induced by OVA (1) or by HSP70 (4), and for mice that were sensitized but not challenged with OVA (2) or HSP70 (5). The levels of specific immunoglobulins in sera of intact mice served as controls: OVA-specific (3) and HSP70-specific (6). BAL dilutions 1 : 1, serum dilutions 1 : 1000 for IgG1 and 1 : 10 for IgE. Mean ± SEM are shown for the experiment of five mice per group. Significant difference: *** p ≤ 0.001 is marked; ns, not significant.
Together with cellular response, we analyzed the alteration of humoral immune response. We did not observe elevation of IL-4, IL-5, or IL-13 in BAL of mice with HSP70-induced allergic airway inflammation (Fig. 5d).
As mentioned above, administration of exogenous antigen normally induced systemic antigen-specific IgG1 production, such as was detected in mice treated with OVA (Fig. 5e). Two intraperitoneal injections of OVA were sufficient to upregulate OVA-specific IgG1 in peripheral blood sera of mice (Fig. 5e). Injections with HSP70 did not enhance specific IgG1 production, and the level of Hsp-specific IgG1 did not differ significantly from the level in intact mice (Fig. 5e).
Elevated of serum IgE is a well-known manifestation of allergy, and OVA-specific IgE was markedly upregulated in OVA-sensitized mice and increased after OVA challenges (Fig. 5f). When allergic inflammation was induced by HSP70 application, neither intraperitoneal injection nor inhalation affected HSP70-specific IgE level, which did not differ significantly in immunized and intact mice (Fig. 5f).
Interestingly, HSP70-induced allergic airway inflammation was manifested in upregulation of BAL fluid specific IgA. Moreover, we detected increased level of HSP70-specific IgA in BAL even after intraperitoneal injections (Fig. 5g), and consequent oropharyngeal application induced further enhancement (Fig. 5g). Similar variations were found in case of OVA application (Fig. 5g).
Thus, we demonstrated that exogenously supplied HSP70 from syngeneic organisms did not have immunogenicity or pro-allergenic properties, and therefore it can be considered as a drug component for anti-allergic therapy.
DISCUSSION
There are now two main concepts on the ambivalent immunomodulatory effects of extracellular HSP70. The first, which appeared shortly after Pramod Srivastava revealed antitumor activity of HSP70 in association with tumor-associated peptides [25], is based on the results of numerous studies that have demonstrated immunostimulatory, pro-inflammatory properties of exogenous (usually recombinant) HSP70 due its activating effect on antigen-presenting cells [1]. This concept is most common; within this framework, extracellular HSP70 appearing in the body is considered as an alarm for the immune system (“alarmin”). However, in recent years a number of authors have shown the opposite, immunosuppressive activity of recombinant HSP70, carefully purified from bacterial LPS contamination. It was demonstrated that mechanism of this effect is associated with HSP70-induced activation of T regulatory cells [3]. At the same time, the immunostimulatory activity of extracellular HSP70 cannot be entirely due to LPS contamination, because proinflammatory properties of exogenous HSP70 were displayed in experiments with highly purified protein not containing the endotoxin [6]. Obviously, the expansion of existing ideas about the mechanisms of immunomodulating action of HSP70, based on analysis of the effects of these proteins in various models, is of undoubted interest. Taking into consideration the relevance of this topic, we conducted a study of immunomodulatory effects of exogenous HSP70 in a mouse model of allergic airway inflammation, and examined in this model the dynamics of the level of extracellular form of endogenous HSP70 at the site of inflammation.
Our findings demonstrate for the first time substantial content of the extracellular form of HSP70 in bronchoalveolar lavage, which reflects dynamics of the course of the induced inflammation process (Fig. 1b). We attribute the registered variations of extracellular HSP70 in the site of inflammation to stress-induced secretion of this protein that was observed in other models of inflammation [6]. Given the protective activity of extracellular HSP70 [12], we assume that one of the functions of secreted HSP70 can be connected with the need to protect against damage to cells including neutrophils localized in the site of inflammation. The possible ability of exogenous HSP70 to increase neutrophil viability indicated the results of a separate series of experiments (Fig. 3, a and b). In this series of experiments with in vitro cell culture, we demonstrated that exogenous HSP70 prevent necrosis and NETosis of neutrophils in the model of their PMA-induced hyperactivity (Fig. 3b). This confirms our assumption of the protective function of HSP70 secreted in the site of inflammation. Along with this, we found that exogenous HSP70 enhanced spontaneous apoptosis in primary culture of mouse neutrophils (Fig. 3g). We suppose that the intensification of HSP70 mediated apoptosis of neutrophils in this model is associated with switching the mechanism of cell death in a part of cultured cell population from necrotic destruction to the program of apoptosis. Under in vivo conditions, such putative functional activity of extracellular HSP70 can be directed at the completion of the inflammatory process, which is known to be associated with programmed death of neutrophils [16, 17, 20].
The most informative data related to the discussion of pro- or antiinflammatory action of extracellular HSP70 were obtained in experiments with autologous HSP70 oropharyngeal administration in the acute phase of induced allergic airway inflammation model. We found that the group of mice receiving HSP70 was characterized 48 h after inhalation of allergen by reduced level of Th2-associated cytokines IL-4, IL-5, and IL-13 compared to control groups of mice (Fig. 2, e-g). Oropharyngeal administration of HSP70 restricted also the influx of eosinophils to the lungs (Fig. 2d). In addition, inhalation of HSP70 in the acute phase of inflammation suppressed the increase in the concentration of allergen-specific IgA in the bronchoalveolar lavage (Fig. 2i). Thus, we conclude that our data demonstrate antiinflammatory properties of extracellular form of HSP70 used in our mouse model of allergic airway inflammation. This confirms the concept of the immunosuppressive activity of these proteins formulated in recent years.
Evidence of immunosuppressive, antiinflammatory activity of HSP70 not only expand existing understanding of the mechanisms of regulation of immune processes, but also point to the possibility of using these proteins to create new antiinflammatory drugs. It is well known that a number of currently incurable illnesses, such as neurodegenerative diseases, are a part of the important medical problems associated with inflammatory processes [26]. In our work with the purpose of preliminary assessment of the possibility of HSP70 application for development of antiinflammatory drugs, we tested in a mouse model the immunogenicity and allergenicity of HSP70 preparations with their prolonged administration. The results showed no significant immune response to HSP70, which suggests the potential application of the protein for development of new immunomodulatory drugs.
Acknowledgements
This work was supported by the Russian Foundation for Basic Research (project No. 16-34-01028). The study of immunogenicity and pro-allergic properties of HSP70 was supported by the Russian Science Foundation (project No. 16-15-10404).
REFERENCES
1.Asea, A., Kraeft, S. K., Kurt-Jones, E. A.,
Stevenson, M. A., Chen, L. B., Finberg, R. W., Koo, G. C., and
Calderwood, S. K. (2000) HSP70 stimulates cytokine production through a
CD14-dependant pathway, demonstrating its dual role as a chaperone and
cytokine, Nat. Med., 6, 435-442.
2.Quintana, F. J., and Cohen, I. R. (2005) Heat shock
proteins as endogenous adjuvants in sterile and septic inflammation,
J. Immunol., 175, 2777-2782.
3.Borges, T. J., Wieten, L., Van Herwijnen, M. J.,
Broere, F., Van der Zee, R., Bonorino, C., and Van Eden, W. (2012) The
anti-inflammatory mechanisms of Hsp70, Front. Immunol.,
3, 95.
4.Stocki, P., and Dickinson, A. M. (2012) The
immunosuppressive activity of heat shock protein 70, Autoimmune
Dis., 2012, doi:10.1155/2012/617213.
5.Gao, B., and Tsan, M. F. (2003) Endotoxin
contamination in recombinant human heat shock protein 70 (Hsp70)
preparation is responsible for the induction of tumor necrosis factor
alpha release by murine macrophages, J. Biol. Chem., 278,
174-179.
6.Pockley, A. G., Muthana, M., and Calderwood, S. K.
(2008) The dual immunoregulatory roles of stress proteins, Trends
Biochem. Sci., 33, 71-79.
7.Qu, B., Jia, Y., Liu, Y., Wang, H., and Ren, G.
(2015) The detection and role of heat shock protein 70 in various
nondisease conditions and disease conditions: a literature review,
Cell Stress Chaperones, 20, 885-892.
8.Yang, M., Wu, T., Cheng, L., Wang, F., Wei, Q., and
Tanguay, R. M. (2005) Plasma antibodies against heat shock protein 70
correlate with the incidence and severity of asthma in a Chinese
population, Resp. Res., 6, 18.
9.Shevchenko, M. A., Bolkhovitina, E. L., Servuli, E.
A., and Sapozhnikov, A. M. (2013) Elimination of Aspergillus
fumigatus conidia from the airways of mice with allergic airway
inflammation, Resp. Res., 14, 78.
10.Menoret, A. (2004) Purification of recombinant
and endogenous HSP70s, Methods, 32, 7-12.
11.Barrientos, L., Marin-Esteban, V., de
Chaisemartin, L., Le-Moal, V. L., Sandre, C., Bianchini, E., Nicolas,
V., Pallardy, M., and Chollet-Martin, S. (2013) An improved strategy to
recover large fragments of functional human neutrophil extracellular
traps, Front. Immunol., 4, doi:
10.3389/fimmu.2013.00166.
12.De Maio, A. (2011) Extracellular heat shock
proteins, cellular export vesicles, and the Stress Observation System:
a form of communication during injury, infection, and cell damage. It
is never known how far a controversial finding will go! Dedicated to
Ferruccio Ritossa, Cell Stress Chaperones, 16,
235-249.
13.Kusmartsev, S. A., Li, Y., and Chen, S. H. (2000)
Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T
cell activation induced through CD3/CD28 costimulation, J.
Immunol., 165, 779-785.
14.Lambrecht, B. N., and Hammad, H. (2015) The
immunology of asthma, Nat. Immunol., 16, 45-56.
15.Troyanova, N. I., Postovskaya, A. M., Servuli, E.
A., Sapozhnikov, A. M., and Shevchenko, M. A. (2015) Characteristics of
acute and effector phase of allergic inflammation of the airways in
order to select the parameters for the assessment of regulatory
properties of HSP70, Russ. J. Immunol., 9, 328-330.
16.Lu, T., Kobayashi, S. D., Quinn, M. T., and
Deleo, F. R. (2015) A NET outcome, Front. Immunol., 3,
365.
17.Kobayashi, Y. (2015) Neutrophil biology: an
update, EXCLI J., 14, 220-227.
18.Dworski, R., Simon, H. U., Hoskins, A., and
Yousefi, S. (2011) Eosinophil and neutrophil extracellular DNA traps in
human allergic asthmatic airways, J. Allergy Clin. Immunol.,
127, 1260-1266.
19.Mosca, T., Menezes, M. C., Silva, A. V.,
Stirbulov, R., and Forte, W. C. (2015) Chemotactic and phagocytic
activity of blood neutrophils in allergic asthma, Immunol.
Invest., 44, 509-520.
20.Fox, S., Leitch, A. E., Duffin, R., Haslett, C.,
and Rossi, A. G. (2010) Neutrophil apoptosis: relevance to the innate
immune response and inflammatory disease, J. Innate Immun.,
2, 216-227.
21.Radauer, C., Bublin, M., Wagner, S., Mari, A.,
and Breiteneder, H. (2008) Allergens are distributed into few protein
families and possess a restricted number of biochemical functions,
J. Allergy Clin. Immunol., 121, 847-852.
22.Stavnezer, J. (1996) Immunoglobulin class
switching, Curr. Opin. Immunol., 8, 199-205.
23.Durrant, D. M., and Metzger, D. W. (2010)
Emerging roles of T helper subsets in the pathogenesis of asthma,
Immunol. Invest., 39, 526-549.
24.Idzko, M., Hammad, H., Van Nimwegen, M., Kool,
M., Willart, M. A., Muskens, F., Hoogsteden, H. C., Luttmann, W.,
Ferrari, D., Di Virgilio, F., Virchow, J. C., Jr., and Lambrecht, B. N.
(2007) Extracellular ATP triggers and maintains asthmatic airway
inflammation by activating dendritic cells, Nat. Med.,
13, 913-919.
25.Srivastava, P. (2002) Roles of heat-shock
proteins in innate and adaptive immunity, Nat. Rev. Immunol.,
2, 185-194.
26.Collins, L. M., Thomas, A. T., Connor, J., and
Nolan, Y. M. (2012) Contributions of central and systemic inflammation
to the pathophysiology of Parkinson’s disease,
Neuropharmacology, 62, 2154-2168.