Glutamic Acid – Amino Acid, Neurotransmitter, and Drug – Is Responsible for Protein Synthesis Rhythm in Hepatocyte Populations in vitro and in vivo
V. Y. Brodsky1*, L. A. Malchenko1, D. S. Konchenko2, N. D. Zvezdina2, and T. K. Dubovaya2
1Koltzov Institute of Developmental Biology, Russian Academy of Sciences, 117808 Moscow, Russia; E-mail: brodsky.idb@bk.ru2Pirogov Russian State Medical University, 117513 Moscow, Russia
* To whom correspondence should be addressed.
Received April 21, 2016
Primary cultures of rat hepatocytes were studied in serum-free media. Ultradian protein synthesis rhythm was used as a marker of cell synchronization in the population. Addition of glutamic acid (0.2 mg/ml) to the medium of nonsynchronous sparse cultures resulted in detection of a common protein synthesis rhythm, hence in synchronization of the cells. The antagonist of glutamic acid metabotropic receptors MCPG (0.01 mg/ml) added together with glutamic acid abolished the synchronization effect; in sparse cultures, no rhythm was detected. Feeding rats with glutamic acid (30 mg with food) resulted in protein synthesis rhythm in sparse cultures obtained from the rats. After feeding without glutamic acid, linear kinetics of protein synthesis was revealed. Thus, glutamic acid, a component of blood as a non-neural transmitter, can synchronize the activity of hepatocytes and can form common rhythm of protein synthesis in vitro and in vivo. This effect is realized via receptors. Mechanisms of cell–cell communication are discussed on analyzing effects of non-neural functions of neurotransmitters. Glutamic acid is used clinically in humans. Hence, a previously unknown function of this drug is revealed.
KEY WORDS: glutamic acid, kinetics of protein synthesis, direct cell–cell communication, ultradian rhythms, non-neural function of neurotransmitters, hepatocytesDOI: 10.1134/S0006297916080101
Glutamic acid is an important component of blood and of the extracellular space in mammals. Also, glutamic acid is a neurotransmitter and therefore can function together with other blood-transferred neurotransmitters, such as serotonin and noradrenalin. The detailed non-neural role of these neurotransmitters is poorly understood. Their effects on the kinetics of protein synthesis are unknown. This study began from an appropriate model of ultradian rhythm in cell cultures [1, 2]. The very fact of common rhythm in a cell population indicates direct cell–cell communication and synchronization of the cells. Linear kinetics unequivocally shows the nonsynchronous cell population. Our work was carried out to show possible involvement of glutamic acid in protein kinetics. Unlike serotonin and other neurotransmitters with non-neural functions, glutamic acid penetrates the cell membrane. Another difference between glutamic acid and serotonin and noradrenaline is that glutamic acid is used as a drug in clinics.
MATERIALS AND METHODS
Isolation and cultivation of hepatocytes. Like our previous studies (for details, see [3]), hepatocytes were isolated from the 3-4-month-old Wistar rats (weight 250-280 g) using perfusion of the liver with calcium-free Hank’s solution with 0.05% collagenase (Sigma, USA) and 0.5 mM EGTA. The suspension of hepatocytes contained about 90% of viable cells. Serum-free Medium 199 supplemented with 0.2 mg/ml albumin for cell cultures (Sigma) and 0.5 µg/ml insulin (Sigma) was used for cell cultivation. The gas phase was 10% carbon dioxide and 90% air. Suspensions containing either 106 or 105 hepatocytes/ml were placed in Petri dishes containing collagen-coated slices. Dense and sparse cultures were correspondingly obtained from cells of one for a certain experimental rat. The medium was refreshed after 2 h, and after 24 h the cultures were ready for use. Before sampling, one-day cultures were washed and transferred to fresh medium with or without (control) glutamic acid. Samples of three cultures each were taken with 10-min interval. Five cultures per sample were studied earlier, and the same peaks and troughs were detected in the 2-h curve [3].
Protein synthesis kinetics. Each sample was incubated for 10 min at 37°C in medium with 3H-labeled leucine (25-30 µCi/ml, specific activity 70-100 Ci/mmol; IMG, Russia). Then the cultures were washed with cold physiologic solution containing an excess of unlabeled leucine and treated with 5% perchloric acid for 60 min. The cultures were then washed with ethyl alcohol, and cell protein was dissolved in benzethonium hydroxide (Sigma). Incorporation of 3H-labeled leucine into proteins and free intracellular leucine radioactivity in the same individual culture were measured using a Perkin Elmer 2810TR scintillation counter.
Relative leucine incorporation collected for pool size (acid-soluble fraction) was calculated for each culture: Icorr = Ii × Pm/Pi (cpm, %), where Ii is the measured incorporation, and Pi = Ii + pi is the total radioactivity, where pi is the corresponding radioactivity of free 3H-labeled leucine in the same culture, and Pm is the mean total radioactivity over the entire experiment, i.e. at all the time-points under the curve. These relative values corrected the pool along with various numbers of cells in different cultures [3]. The values in percent were calculated when the mean Icorr values of a certain variant of the experiment were taken as 100%. The total protein radioactivity was estimated. Preliminary studies of albumin fraction alone led to the same conclusions. Sensitivity of the total analysis is higher than that of partial analysis. In addition, the main point, our criterion of cell–cell communication and cell synchrony is a rhythm per se, and not the protein analysis.
Experimental influences. A drugstore table of glutamic acid was reduced to powder, transferred to warm physiologic solution, and centrifuged. The supernatant was used in physiologic solution with glutamic acid dose of 0.2 mg/ml (1.4 mM) and 0.6 mg/ml. The dose of glutamic acid was chosen according to doses used in human clinics, taking in account the weight of the rat and its blood volume. The dose of 0.6 mg/ml was also tested in early experiments with the same effect. In some experiments, 0.01 mg/ml (50 µM) of the antagonist of metabotropic glutamine receptors MCPG (α-methyl-4-carboxyphenylglycine) (Sigma) was added to the medium of the hepatocyte culture. For study of the mechanism of protein synthesis rhythm, the protein kinase inhibitor H7 (1-(5-isoquinolinylsulfonyl)-2-methylpiperazine dihydrochloride) (Sigma) was used at the concentration of 14.56 µg/ml (40 µM).
RESULTS
To begin with, the effect of glutamic acid on dense and sparse hepatocyte cultures was compared. During 20 years of our studies, we have shown [2] that protein synthesis rhythm can be revealed in dense cultures in a few minutes after washing and refreshing the medium. Sparse cultures do not have rhythm under similar conditions. The neurotransmitter changed protein synthesis kinetics only in the sparse cultures, organizing protein synthesis rhythm. Further, we studied only these cultures. The dose of glutamic acid was selected close to that used in human clinics (see “Materials and Methods”).
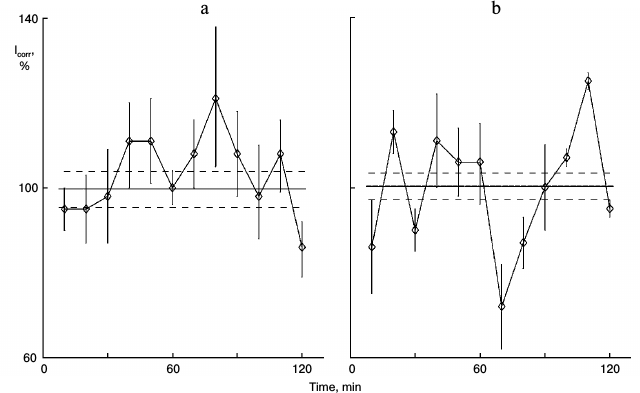
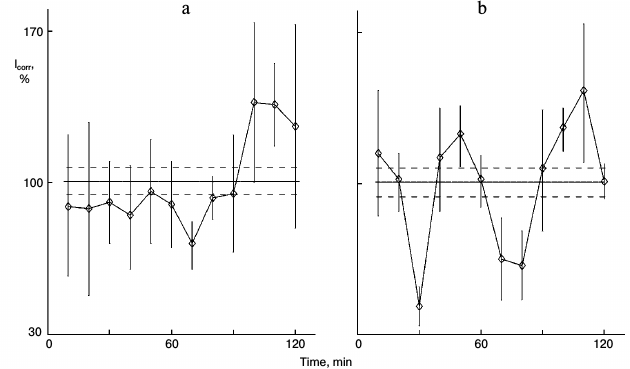
Figure 1 shows the action of glutamic acid in sparse (nonsynchronous) cultures. In control sparse cultures, the protein synthesis kinetics was linear (Fig. 1a). Eleven samples out of 12 did not differ significant from each other and comprised the range of the mean ± standard error of the mean for the whole experiment (36 cultures). Samples were taken one by one with 10-min intervals for the entire 2-h study. Protein synthesis rhythm was detected in similar cultures from the same pool of isolated hepatocytes after 1-h incubation of the cultures with 0.2 mg/ml glutamic acid (Fig. 1b). Clear peaks and troughs were detected. Thus, glutamic acid synchronized individual oscillations forming the common rhythm of the cell population.
Fig. 1. Protein synthesis kinetics in 1-day sparse cultures of rat hepatocytes; effect of glutamic acid. a) Control nonsynchronous cultures after washing and transfer to fresh medium for 60 min, and then samples of the cultures were studied. b) Similar cultures from the same cell suspension after washing were transferred to medium with 0.2 mg/ml glutamic acid for 60 min and then were studied. Here and in subsequent figures: abscissa is time (min), ordinate is relative incorporation of 3H-labeled leucine corrected for intracellular pool of free leucine and total radioactivity (see “Materials and Methods”); every sample consisted of three cultures; incorporation and pool were estimated for each culture separately; each point is for 10 min mean ± standard error for three cultures; the total mean for all points is shown with solid line, and ranges of standard error for the curve mean are shown as dotted line above and below the mean line. Each experiment (Figs. 1 and 2 or others) was carried out on hepatocyte cultures from the same rat.
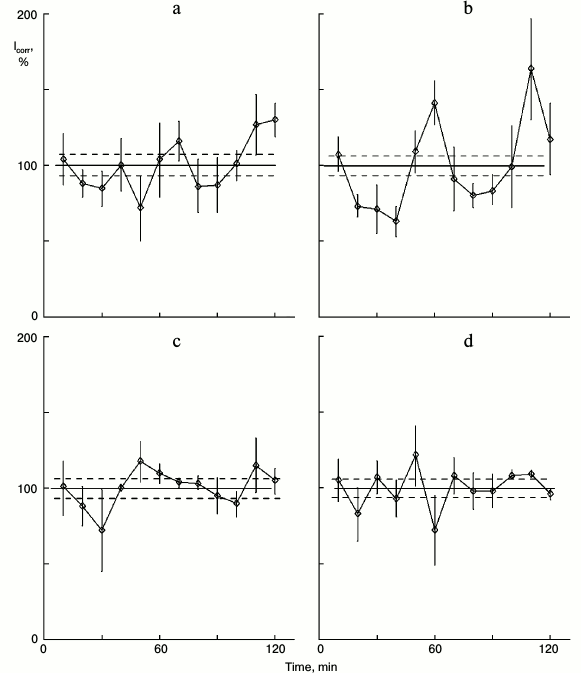
Unlike noradrenalin and serotonin, glutamic acid penetrates cell membranes. Does glutamic acid affect protein synthesis kinetics either via cytoplasm processes or via its specific receptors on the cell membrane? The nonselective metabotropic antagonist of glutamic acid MCPG [4] was used to test this. The protein synthesis kinetics was not rhythmic in control sparse cultures (Fig. 2a). Glutamic acid added to the culture medium initiated rhythm; peaks and troughs were detected (Fig. 2b). The receptor antagonist MCPG per se did not affect the cultures; protein synthesis kinetics was linear like the control (Fig. 2c). Glutamic acid together with MCPG displayed the control kinetics (Fig. 2d). Thus, glutamic acid synchronized protein synthesis oscillations via its membrane receptors, like other neurotransmitters such as noradrenalin and serotonin.
Fig. 2. Effect of glutamic acid together with the antagonist of these neurotransmitter receptors MCPG on protein synthesis kinetics in sparse cultures of hepatocytes. Before the protein synthesis was studied: a) control washed sparse cultures were incubated in fresh medium for 4 h; b) similar cultures were incubated in fresh medium for 3 h, and then 0.2 mg/ml glutamic acid was added to the medium for 1 h; c) the cultures were incubated in medium with 0.1 mg/ml of glutamine acid antagonist MCPG for 4 h; d) the cultures were incubated in medium with MCPG for 3 h, and then glutamic acid (0.2 mg/ml) was added to this medium for 1 h.
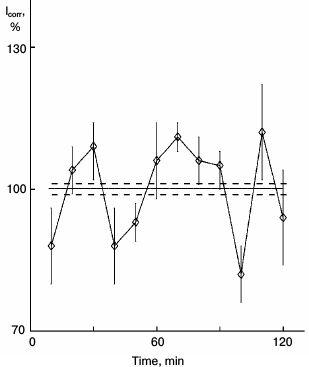
In earlier studies, we revealed the chain of reactions in cytoplasm that induces protein synthesis rhythm: from efflux of calcium ions and action on adenylate cyclase to activation of protein kinases [5], which is a key process. The activity of protein kinase inhibitors H7 and H8 abolished protein synthesis rhythm in dense cultures, and the protein kinase activator phorbol ester PMA (phorbol-12-myristate-13-acetate) induced rhythm in sparse cultures. Like other signal factors, the effect of glutamic acid is realized through protein kinases. Glutamic acid with protein kinase inhibitor H7 does not induce rhythm in sparse cultures (Fig. 3).
Fig. 3. Effect of glutamic acid with protein kinase inhibitor H7 on protein synthesis kinetics in sparse cultures of hepatocytes. Before estimation: a) control: cultures were washed and then incubated in fresh medium for 90 min; b) similar cultures were incubated in fresh medium for 30 min, then 0.2 µg/ml of glutamic acid was added to the medium for 60 min; c) cultures were incubated in medium with 40 µM H7 for 30 min, then glutamic acid was added to the medium for 60 min.
In next experiments, glutamic acid was administered to rats. The slightly hungry rats consumed 30 mg of glutamic acid inside a small lump of bread moistened with oil. The feeding was repeated in a day, then hepatocytes were isolated, and sparse cultures were obtained, while for another day protein synthesis was studied. Protein synthesis rhythm was detected in the sparse cultures taken from rat fed with glutamic acid several days earlier. Clear protein synthesis rhythm was detected (Fig. 4).
Fig. 4. Kinetics of protein synthesis in sparse hepatocyte cultures obtained from rat who consumed glutamic acid in food. One day before isolation of hepatocytes, the rat consumed 30 mg of glutamic acid in a small lump of bread moistened with oil. The feeding was repeated once more 1 h just before isolation of hepatocytes. Sparse hepatocyte cultures were studied by the schedule of Fig. 1.
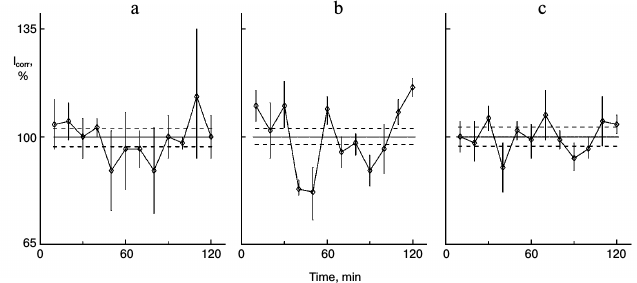
Earlier, in experiments with melatonin that was administered to rats, NaCl physiologic solution was used as control [6]. This control is not sufficient for the present study. Some active factors may be present in plant oil that saturated the bread to attract the rats. As control to the experiments like in Fig. 4, the rats were fed with bread moistened with oil, but without glutamic acid. In cultures similar to those in Fig. 4, protein synthesis rhythm was not detected (Fig. 5a). Thus, the oil does not affect the protein synthesis kinetics, and the changes demonstrated in Fig. 4 result from glutamic acid. The cultures in the experiment depicted in Fig. 5 were able to respond to glutamic acid; after addition of 0.2 mg/ml glutamic acid to the medium, rhythm was detected (Fig. 5b).
Fig. 5. Control of experiment in Fig. 4. Another rat consumed bread moistened with oil as in the previous experiment, but without glutamic acid. a) Control sparse cultures obtained from this rat; b) 0.2 mg/ml glutamic acid was added to the medium of similar cultures of this rat.
DISCUSSION
Glutamic acid is one of the common amino acids and a usual component of mammalian blood. Addition of this acid to medium with cell cultures seems only to enhance the pool of protein precursors. However, apart from this case, glutamic acid functions as a non-neural transmitter. For other non-neural transmitters, a chain of processes was revealed from receptor signal to cytoplasm calcium and protein kinases, and these have common rhythm of the cell population. A similar mechanism of cell synchronization can be assumed for the neurotransmitter glutamic acid. Along with other non-neural functions of neurotransmitters, glutamic acid functions in vitro and in vivo as a trigger. After its single administration, the chain of synchronizing processes was preserved for a long time. The effect in vivo can be detected 2-3 days after administration to a rat.
G. A. Buznikov [7, 8] was the first to note the occurrence of neurotransmitters in animals that do not have a nervous system and even in Protozoa. Changes in some neurotransmitters at early stages of embryogenesis of different animals are a long-term topic of his laboratory [9]. High effects of non-neural neurotransmitters (“pre-neural” by Buznikov’s term) on embryonic development and particularly on development of the nervous system have been subjects of many interesting studies (for instance [10-13]). In our studies, significance of the non-neural neurotransmitters spreads to postnatal cell functions (see reviews [2, 14]). Serotonin, noradrenalin, and their derivatives, such as melatonin and phenylephrine, promote cell–cell communication resulting in formation of synchronization of cell populations, whereas dopamine abrogates the rhythm. Here, glutamic acid supplements the list. Unlike many signal factors, neurotransmitters are ubiquitous, including in Bacteria, and can synchronize a microbial population [15-17]. Thus, synchronization of cell populations is a primary function of transmitters. This is proved by data on the influence of non-neural transmitters on differentiated mammalian cells. A useful hypothesis relates to earlier embryological and neurophysiological studies [7, 18]. The appearance of signal factors of cell population synchronization could be one of the factors for the formation of Metazoa and one of mechanisms of homeostasis in Metazoa [19].
The neurotransmitter glutamic acid is a constant component of blood in mammals. Glutamic acid binds and detoxifies ammonium, is involved in protein catabolism, enhances stability in hypoxia, facilitates ATP synthesis, and activates metabolism (for instance, [20, 21]). Like some signal factors, glutamic acid is used in human clinics. As a drug, glutamic acid is used in neurologic clinics: in epilepsy, in schizophrenia, in some depressive states, as well as in pediatric patients with Down’s syndrome, in cerebral paralysis, etc. In our experiments on cell cultures as well as on rats in vivo, we deliberately used the standard drug of glutamic acid purchased in usual drugstores. As noted above, doses of glutamic acid were selected to be close to those used in human clinics. The weight of man and rat, as well as the corresponding volumes of blood, were taken into account. An unknown peculiarity of this drug, such as its involvement in regulation of protein synthesis kinetics, was thus found.
Acknowledgements
We are grateful to Leonid Z. Pevzner, MD, PhD for valuable comments.
This study was supported by the Russian Foundation for Basic Research (project No. 14-04-00189).
REFERENCES
1.Brodsky, V. Y. (1975) Protein synthesis rhythm,
J. Theor. Biol., 55, 167-200.
2.Brodsky, V. Y. (2014) Circahoralian (ultradian)
metabolic rhythms, Biochemistry (Moscow), 79,
483-495.
3.Brodsky, V. Y., Nechaeva, N. V., Zvezdina, N. D.,
Prokazova, N. V., Golovanova, N. K., Novikova, T. E., Gvasava, I. G.,
and Fateeva, V. I. (2000) Ganglioside-mediated synchronization of the
protein synthesis activity in cultured hepatocytes, Cell Biol.
Int., 24, 211-222.
4.Garcia, S., Lopez, E., and Lopez-Colome, A. (2008)
Glutamate accelerates RPE cell proliferation through EPK ½
activation via distinct receptor specific mechanisms, J. Cell.
Biochem., 104, 377-390.
5.Brodsky, V. Y., Zvezdina, N. D., Fateeva, V. I.,
and Malchenko, L. A. (2007) Involvement of protein kinases in
self-organization of the protein synthesis rhythm by direct
cell–cell communication, Cell Biol. Int., 31,
65-73.
6.Brodsky, V. Y., and Zvezdina, N. D. (2010)
Melatonin as the most effective organizer of the protein synthesis
rhythm in hepatocytes in vitro and in vivo, Cell Biol.
Int., 34, 1199-1204.
7.Buznikov, G. A. (1967) Low Molecular Weight
Regulators of Development [in Russian], Nauka, Moscow.
8.Buznikov, G. A. (1990) Neurotransmitters in
Embryogenesis, Harwood Academic Publishers, New York.
9.Shmukler, Y., and Nikishin, D. (2012) Transmitters
in blastomere interactions, in Cell Interactions, Chap. 2,
InTech, New York, pp. 31-65.
10.Ugrumov, M. V. (2002) Magnocellular vasopressin
system in ontogenesis: development and regulation, Microscop. Res.
Tech., 56, 164-171.
11.Pronina, T., Ugrumov, M., Calas, A., Seif, I.,
and Tramu, G. (2003) Influence of monoamine of differentiating
gonadotropin-releasing hormone neurons in fetal mice, J.
Neuroendocrinol., 15, 925-932.
12.Voronezhskaya, E. E., Khabarova, M. Yu., and
Nezlin, L. P. (2004) Apical sensory neurons mediate developmental
retardation induced by conspecific environmental stimuli in freshwater
pulmonate snails, Development, 131, 3671-3680.
13.Mirochnik, V., Bosler, O., Tillet, Y., Calas, A.,
and Ugrumov, M. (2005) Long-lasting effects serotonin deficiency on
differentiating peptidergic neurons in the rat suprachiasmatic nucleus,
Int. J. Dev. Neurosci., 23, 85-91.
14.Brodsky, V. Y. (2006) Direct cell–cell
communication. A new approach derived from recent data on the nature
and self-organization of ultradian (circahoralian) intracellular
rhythms, Biol. Rev. Camb. Philos. Soc., 82, 143-162.
15.Lyte, M., and Ernst, S. (1992) Catecholamine
induced growth of gram-negative bacteria, Life Sci., 50,
203-212.
16.Oleskin, A. V., Kirovskaya, T. A., Botvinko, I.
V., and Lisak, L. V. (1998) Action of serotonin on bacterial growth,
Microbiology (Moscow), 67, 305-312.
17.Oleskin, A. V. (2001) Biopolitics [in
Russian], MSU, Moscow.
18.Sakharov, D. A. (1974) Genealogy of
Neurons [in Russian], Nauka, Moscow.
19.Brodsky, V. Y., and Lloyd, D. (2008)
Self-organized intracellular ultradian rhythms provide direct
cell–cell communication, in Ultradian Rhythms from Molecules
to Mind (Lloyd, D., and Rossi, E., eds.) Springer, London, pp.
85-104.
20.Storto, M., De Grazia, U., Knopfel, T., Canonico,
P., Copani, A., Richelmi, P., Nicoletti, F., and Vaireti, M. (2000)
Selective blockade of mGlu5 metabotropic glutamate receptors protects
rat hepatocytes against hypoxic damage, Hepatology, 31,
649-655.
21.Brosnan, M. E., and Brosnan, J. T. (2009) Hepatic
glutamate metabolism: a tale of hepatocytes, Am. J. Clin. Nutr.,
90, 857S-861S.