Binding of Synthetic LKEKK Peptide to Human T-Lymphocytes
E. V. Navolotskaya1*, D. V. Zinchenko1, Y. A. Zolotarev2, A. A. Kolobov3, and V. M. Lipkin1
1Branch of Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, 142290 Pushchino, Moscow Region, Russia; fax: +7 (0967) 33-0527; E-mail: navolotskaya@ibch.ru, elenanavolots@gmail.com2Institute of Molecular Genetics, Russian Academy of Sciences, 123182 Moscow, Russia; fax: +7 (495) 196-0221
3State Research Institute of Highly Pure Biopreparations, Federal Biomedical Agency, 197110 St. Petersburg, Russia; fax: +7 (812) 235-5504
* To whom correspondence should be addressed.
Received March 1, 2016; Revision received May 11, 2016
The synthetic peptide LKEKK corresponding to sequence 16-20 of human thymosin-α1 and 131-135 of human interferon-α2 was labeled with tritium to specific activity 28 Ci/mol. The [3H]LKEKK bound with high affinity (Kd = 3.7 ± 0.3 nM) to donor blood T-lymphocytes. Treatment of cells with trypsin or proteinase K did not abolish [3H]LKEKK binding, suggesting the non-protein nature of the peptide receptor. The binding was inhibited by thymosin-α1, interferon-α2, and cholera toxin B subunit (Ki = 2.0 ± 0.3, 2.2 ± 0.2, and 3.6 ± 0.3 nM, respectively). Using [3H]LKEKK, we demonstrated the existence of a non-protein receptor common for thymosin-α1, interferon-α2, and cholera toxin B-subunit on donor blood T-lymphocytes.
KEY WORDS: peptides, receptors, thymosin-α1, interferon-α, T-lymphocytesDOI: 10.1134/S0006297916080071
Abbreviations: ADP, adenosine diphosphate; BSA, bovine serum albumin; cAMP, 3′,5′-cyclic adenosine monophosphate; CT-B, cholera toxin B-subunit; GTP, guanosine-5′-triphosphate; HPLC, high performance liquid chromatography; IFN, interferon; IL, interleukin; MyD88, myeloid differentiation primary response gene 88; NAD, nicotinamide adenine dinucleotide; TIR domain, Toll/interleukin-1 receptor/resistance protein domain; TM-α1, thymosin-α1; TNF, tumor necrosis factor; TRIF, TIR-domain-containing adapter-inducing interferon-β.
In our earlier studies of structural and functional properties of
interferons-α (IFNs-α), we identified the octapeptide
LKEKKYSP (residues 131-138 of human IFN-α2) that bound
with high affinity to mouse thymocytes and human fibroblasts [1, 2]. The binding was
competitively inhibited by IFN-α2 and
thymosin-α1 (TM-α1) [1-3]. Comparison of amino acid
sequences of the octapeptide and TM-α1 revealed that
they share the same pentapeptide fragment, LKEKK, that corresponds
to residues 16-20 in TM-α1 and 131-135 in
IFN-α2 (Fig. 1). We proposed
that this fragment is responsible for TM-α1 and
IFN-α2 binding to various cells and suggested that
synthetic LKEKK peptide has similar binding capacity and exhibits
biological activity.
Fig. 1. Comparison of amino acid sequences of human thymosin-α1 and interferon-α2. Pentapeptide LKEKK is indicated; numbers correspond to the numbers of amino acid residues in the sequences.
In this work, we synthesized the LKEKK peptide and studied its binding to T-lymphocytes from donor blood.
MATERIALS AND METHODS
Human TM-α1 was from Immundiagnostik AG (Germany); phenylmethanesulfonyl fluoride (PMSF) and Tris were from Fluka (USA); cell culture medium, fetal calf serum, and HEPES were from ICN (USA); sucrose, BSA, EDTA, EGTA, and sodium azide were from Serva (Germany); Ready Gel scintillation fluid was from Beckman (USA). All other reagents and solvents were of domestic origin and were used after additional purification.
LKEKK and KKEKL peptides were synthesized with an Applied Biosystems Model 430A peptide synthesizer and a Vega Coupler Model C250 peptide synthesizer (USA) by the Boc/Bzl peptide chain elongation method and purified by preparative reversed-phase chromatography on a Waters SymmetryPrep C18 (19 × 300 mm) column (Malva, Greece) using a Gilson chromatographer (France). The synthesized peptides were characterized by reversed-phase HPLC on an XTerra RP18 column (Malva) using a Gilson chromatographer, amino acid analysis on an LKB 4151 Alpha Plus amino acid analyzer (Sweden) after hydrolysis with 6 M HCl for 24 h at 110°C, and mass spectrometry on a Finnigan mass spectrometer (USA).
Recombinant human interferon-α2 was purchased from the State Research Institute of Highly Pure Biopreparations (St. Petersburg, Russia).
[3H]LKEKK was obtained by the high-temperature solid-state catalytic isotope exchange (HSCIE) method [3]. Aluminum oxide (50 mg) was added to 2 mg of peptide dissolved in 0.5 ml of water; water was then removed by evaporation on a rotary evaporator. The resulting peptide-coated aluminum oxide was mixed with 10 mg of the catalyst (5% Rh/Al2O3) and transferred into a 10-ml ampule. The ampule was evacuated, filled with gaseous tritium to a pressure of 250 mm Hg, heated to 170°C, and incubated at this temperature for 20 min. Then, the ampule was cooled, evacuated, purged with hydrogen, and evacuated again. The labeled peptide was extracted from the reaction mixture with two portions (3 ml) of 50% ethanol in water. The extracts were combined and evaporated. To remove labile tritium, the procedure was repeated twice. The labeled peptide was purified by HPLC on a Kromasil column (4 × 150 mm; particle size, 5 µm) in a 42-70% gradient (20 min) of methanol in 0.1% trifluoroacetic acid at a flow rate of 3 ml/min. Elution was monitored at 254/280 nm with a Beckman spectrophotometer. Tritium incorporation into the peptide was determined with a liquid scintillation counter.
Mononuclear cells were isolated from the blood of healthy donors as described in [4]. T-cells were isolated by the method [5] using dense polystyrene beads coated with mouse antibodies against human CD3. The method yielded a >95% pure population of target (CD3+) T-lymphocytes.
[3H]LKEKK was bound to T-lymphocytes according to the following procedure. T-cells (106/ml) were incubated with the labeled peptide (10–10-10–7 M; three measurements for each peptide concentration) at 4°C for 40 min in 1 ml of RPMI-1640 medium containing 10 mM HEPES, 20 mM sodium azide, and 0.6 mg/ml PMSF (pH 7.4). After incubation, the reaction mixture was filtered through GF/A glass microfiber filters (Whatman, UK), and then the filters were washed three times (5 ml each wash) with ice-cold physiological buffered saline containing 10 mM HEPES (pH 7.4). The radioactivity of the filters was measured with a Beckman LS 5801 scintillation counter (USA). Specific [3H]LKEKK binding to T-lymphocytes was calculated as a difference between total and nonspecific binding; the nonspecific binding was determined in the presence of 10–4 M unlabeled peptide (1000× excess over the highest used [3H]LKEKK concentration of 10–7 M). To determine the equilibrium dissociation constant (Kd), the ratio between molar concentrations of the bound (B) and free (F) labeled peptide was plotted against molar concentration of the bound labeled peptide (B) [6].
To estimate the inhibitory effects of TM-α1, IFN-α2, and cholera toxin B-subunit (CT-B), the T-lymphocytes (106/ml) were incubated with 10 nM labeled peptide and one of the tested proteins (concentration range, 10–12-10–5 M; three measurements for each concentration) as described above. The inhibition constant (Ki) was calculated using the formula: Ki = [IC]50/(1 + [L]/Kd) [7], where [L] is the [3H]LKEKK molar concentration; Kd is the equilibrium dissociation constant of the [3H]LKEKK–receptor complex; [IC]50 is the concentration of unlabeled ligand causing 50% inhibition of the labeled peptide specific binding. [IC]50 was determined graphically from the inhibition plots. The value of Kd was determined as described above.
Treatment of T-lymphocytes with proteases. The cells (107/ml) were incubated in RPMI-1640 medium containing 5 mg/ml trypsin or 1 mg/ml proteinase K at 37°C for 30 min. Digestion was stopped by adding large volumes of the medium. The cells were washed three times with 10 volumes of the medium, and the binding reaction was carried out as described above. Each measurement was performed in triplicate.
RESULTS
The main characteristics of the synthesized LKEKK and KKEKL peptides (purity, amino acid content, and molecular mass) are shown in Table 1.
Table 1. Main characteristics of LKEKK and
KKEKL peptides

The HSCIE reaction with subsequent peptide purification yielded [3H]LKEKK with specific activity of 28 Ci/mmol. The retention time for both labeled and unlabeled peptides on a Kromasil C18 column (see “Materials and Methods”) was 11 min; the 254/280 nm absorbance ratio for the labeled and unlabeled peptides was the same, thereby confirming that hydrogen substitution with tritium did not affect chemical structure of the peptide.
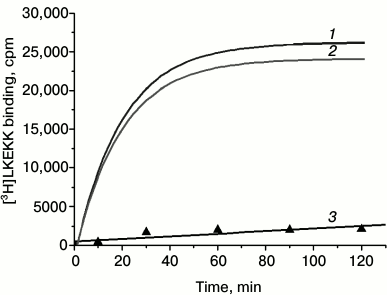
[3H]LKEKK binding to T-lymphocytes. We found that under our experimental conditions (see “Materials and Methods”), [3H]LKEKK bound specifically to T-lymphocytes. Figure 2 shows the kinetics of specific binding of [3H]LKEKK at 4°C. The receptor–peptide complex reached dynamic equilibrium after ~1 h of incubation and remained in this state for at least another hour. Therefore, to assess the equilibrium dissociation constant (Kd) for the peptide binding to T-lymphocytes, the reaction was carried out for 1 h. The nonspecific binding under these conditions was 6.9 ± 0.4% of total binding.
Fig. 2. Kinetics of total (1), specific (2), and nonspecific (3) binding of [3H]LKEKK to human blood T-lymphocytes. Specific binding was determined as the difference between total and nonspecific binding.
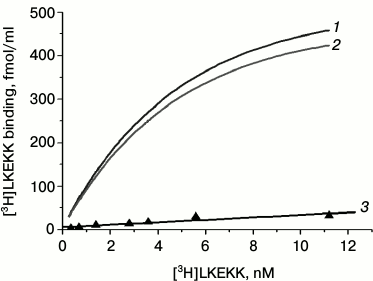
Figure 3 shows the dependence of the total (1), specific (2), and nonspecific (3) binding of [3H]LKEKK on its concentration. Curve 2 reaches a plateau, indicating the saturability of the specific binding of the peptide.
Fig. 3. Dependence of total (1), specific (2), and nonspecific (3) binding of [3H]LKEKK to human blood T-lymphocytes on the peptide concentration. Specific binding was determined as the difference between total and nonspecific binding.
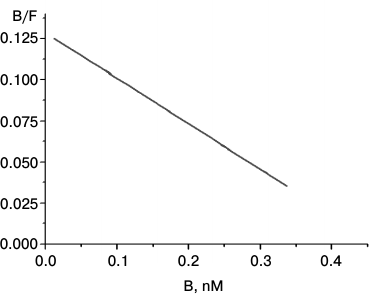
The Scatchard plot of [3H]LKEKK binding to T-lymphocytes is shown in Fig. 4. The linear character of the plot proves the presence of only one type of receptor with a high affinity (Kd = 3.7 ± 0.3 nM) for [3H]LKEKK.
Fig. 4. Scatchard plot of [3H]LKEKK specific binding to human blood T-lymphocytes. B and F, molar concentrations of bound and free [3H]LKEKK, respectively.
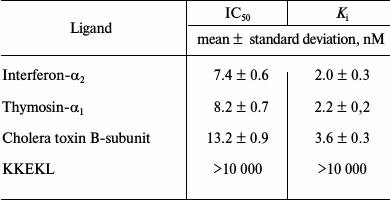
To characterize specific binding of [3H]LKEKK to T-lymphocytes, we used unlabeled LKEKK peptide, KKEKL peptide, TM-α1, IFN-α2, and cholera toxin B-subunit (CT-B) as competitive ligands (Table 2). TM-α1, IFN-α2, and CT-B strongly inhibited [3H]LKEKK binding with Ki of 2.0 ± 0.3, 2.2 ± 0.2, and 3.6 ± 0.3 nM, respectively. KKEKL did not compete with [3H]LKEKK for the binding site (Ki > 10 µM).
Table 2. Inhibition of [3H]LKEKK
specific binding to human blood T-lymphocytes by unlabeled ligands
Treatment of cells with trypsin or proteinase K did not affect [3H]LKEKK binding, thereby suggesting a non-protein nature of the receptor (or at least, of the receptor region directly involved in peptide binding).
DISCUSSION
TM-α1 is a 28-amino acid N-acetylated peptide with immunomodulating, antitumor, and direct antiviral activities [8, 9]. It is believed that such pleiotropy of action is related to the ability of TM-α1 to activate Toll-like receptors (TLRs) [10-12] that are present on the surface and in intracellular organelles of most mammalian cells. Binding of TM-α1 causes TLR dimerization and induces conformational changes required for the recruitment of various signaling molecules. TLR-mediated signal transduction occurs via two main pathways that use different sets of adaptor proteins. The first pathway utilizes MyD88 (myeloid differentiation primary response gene 88) adaptor protein and involves activation of NF-κB transcriptional factor resulting in the stimulation of production of antiinflammatory cytokines (IL-1, IL-12, and TNF-α) and induction of innate effector mechanisms [10, 11]. Most TLRs require MyD88; however, TLR3 and TLR4 utilize alternative adaptors, such as TRIF and TRAM. TRIF belongs to the family of TIR (Toll/IL-1 receptor resistant)-domain-containing proteins. TRAM (TRIF-related adaptor molecule) is involved in TLR4 signaling. The TRAM/TRIF-mediated pathway plays an important role in the induction of dendritic cell maturation and T-cell proliferation. TRIF and TRAM activate IFN-β-regulating factors IRF-3 and IRF-7 and induce late activation of NF-κB [10, 12].
The unusually broad spectrum of TM-α1 activities indicates that the mechanisms of TM-α1 action are not limited to the TLR-mediated pathways alone. Moreover, the size of the TM-α1 polypeptide chain (28 amino acids) suggests the existence of several active sites within the molecule. This suggestion is confirmed, for example, by the fact that TM-α1 competes with CT-B for binding to human fibroblasts [2], CT-B receptor being a GM1 ganglioside [13-16].
No data have been obtained so far on the binding of TM-α1 to gangliosides. However, it is known that CT-B inhibits the antiviral activity of IFN-α [17, 18] by preventing its interaction with GM1 [19, 20]. IFN-α reversibly binds to GM1 with high affinity and high specificity. The regions of the molecule directly involved in the binding are the oligosaccharide fragment of GM1 including lactose (β-D-Gal-(1→4)-Glc) and N-acetylneuraminic acid [20] and the highly conserved fragment of IFN-α (presumably, residues 131-138) [2].
The Scatchard plot of specific binding of [3H]LKEKK to T-lymphocytes (Fig. 4) shows the presence of only one type of high-affinity (Kd = 3.7 ± 0.3 nM) binding sites for [3H]LKEKK.
We also tested TM-α1, IFN-α2, CT-B, and peptide KKEKL with inverted amino acid sequence as competitive ligands. The Ki values (Table 2) demonstrated strong inhibitory capacity of TM-α1, IFN-α2, and CT-B (Ki = 2.0 ± 0.3, 2.2 ± 0.2, and 3.6 ± 0.3 nM, respectively), whereas KKEKL did not inhibit [3H]LKEKK binding (Ki > 10 µM), indicating a high specificity of the labeled peptide binding.
Treatment of cells with proteases (trypsin or proteinase K) did not affect [3H]LKEKK binding, which suggests non-protein nature of the receptor (or at least, of the receptor region directly involved in binding). These results suggest with a high degree of probability that the peptide receptor is GM1 ganglioside.
Therefore, using [3H]LKEKK, we determined that T-lymphocytes have a common binding site (receptor) for TM-α1, IFN-α2, and CT-B.
In conclusion, we should note that CT-B is now viewed as a promising immunomodulating and antiinflammatory agent. Recombinant CT-B has been recently found to stimulate humoral immunity and to induce antiinflammatory responses in vivo [21]. Regarding these data, the biological activity of the LKEKK peptide might be of considerable interest.
Acknowledgements
This work was supported by the Russian Foundation for Fundamental Research (project No. 14-04-00177) and by the Molecular and Cell Biology Program for Fundamental Research of the Presidium of the Russian Academy of Sciences (principal investigator, V. M. Lipkin).
REFERENCES
1.Zav’yalov, V. P., Navolotskaya, E. V.,
Abramov, V. M., Galaktionov, V. G., Isaev, I. S., Kaurov, O. A.,
Kozhich, A. T., Maiorov, V. A., Prusakov, A. N., Vasilenko, R. N., and
Volodina, E. Yu. (1991) The octapeptide corresponding to the region of
the highest homology between α-interferon and
thymosin-α1 effectively competes with both cytokines
for common high-affinity receptors on murine thymocytes, FEBS
Lett., 278, 187-189.
2.Zav’yalov, V. P., Navolotskaya, E. V.,
Vasilenko, R. N., Abramov, V. M., Volodina, E. Y., Roslovtseva, O. A.,
Prusakov, A. N., and Kaurov, O. A. (1995) The sequence 130-137 of human
interferon-α2 is involved in the competition of interferon,
prothymosin α and cholera toxin B subunit for common receptors on
human fibroblasts, Mol. Immunol., 32, 425-431.
3.Zolotarev, Y. A., Dadayan, A. K., Bocharov, E. V.,
Borisov, Y. A., Vaskovsky, B. V., Dorokhova, E. M., and Myasoedov, N.
F. (2003) New development in the tritium labeling of peptides and
proteins using solid catalytic isotopic exchange with
spillover-tritium, Amino Acids, 24, 325-333.
4.Boyum, A., Berg, T., and Blomhoff, R. (1983)
Fractionation of mammalian cells, in Iodinated Density Gradient
Media – a Practical Approach (Rickwood, D., ed.) Oxford,
pp. 147-170.
5.Patel, D., Rubbi, C. P., and Rickwood, D. (1995)
Separation of T- and B-lymphocytes from human peripheral blood
mononuclear cells using density perturbation methods, Clin. Chim.
Acta, 240 187-193.
6.Pennock, B. E. (1973) A calculator for finding
binding parameters from a Scatchard plot, Anal. Biochem.,
56, 306-309.
7.Cheng, Y. C., and Prusoff, W. (1973) Relationship
between the inhibition constant (Ki) and the
concentration of inhibitor which causes 50% inhibition
(IC50) of an enzymatic reaction, Biochem. Pharmacol.,
22, 3099-3108.
8.Pierluigi, B., D’Angelo, C., Fallarino, F.,
Moretti, S., Zelante, T., Bozza, S., De Luca, A., Bistoni, F., Garaci,
E., and Romani, L. (2010) Thymosin alpha1: the regulator of regulators?
Ann. N. Y. Acad. Sci., 1194, 1-5.
9.Romani, L., Moretti, S., Fallarino, F., Bozza, S.,
Ruggeri, L., Casagrande, A., Aversa, F., Bistoni, F., Velardi, A., and
Garaci, E. (2012) Jack of all trades: thymosin α1 and its
pleiotropy, Ann. N. Y. Acad. Sci., 1269, 1-6.
10.Brikos, C., and O’Neill, L. A. (2008)
Signalling of toll-like receptors, Handb. Exp. Pharmacol.,
183, 21-50.
11.Kawai T., and Akira, S. (2011) Toll-like
receptors and their crosstalk with other innate receptors in infection
and immunity, Immunity, 34, 637-650.
12.Kabelitz, D. (2007) Expression and function of
Toll-like receptors in T-lymphocytes, Curr. Opin. Immunol.,
19, 39-45.
13.Cuatrecasas, P. (1973) Interaction of Vibrio
cholerae enterotoxin with cell membranes, Biochemistry,
12, 3547-3558.
14.Cuatrecasas, P. (1973) Gangliosides and membrane
receptors for cholera toxin, Biochemistry, 12,
3558-3566.
15.Lencer, W. I., and Tsai, B. (2003) The
intracellular voyage of cholera toxin: going retro, Trends Biochem.
Sci., 28, 639-645.
16.Fujinaga, Y., Wolf, A. A., Rodighiero, C.,
Wheeler, H., Tsai, B., Allen, L., Jobling, M. G., Rapoport, T., Holmes,
R. K., and Lencer, W. I. (2003) Gangliosides that associate with lipid
rafts mediate transport of cholera and related toxins from the plasma
membrane to endoplasmic reticulum, Mol. Biol. Cell, 14,
4783-4793.
17.Friedman, R. M., and Kohn, L. D. (1976) Cholera
toxin inhibits interferon action, Biochem. Biophys. Res.
Commun., 70, 1078-1084.
18.Belardelli, F., Ausiello, C., Tomasi, M., and
Rossi, G. B. (1980) Cholera toxin and its B-subunit inhibit interferon
effects on virus production and erythroid differentiation of Friend
leukemia cells, Virology, 107, 109-120.
19.Besancon, F., and Ankel, H. (1974) Binding of
interferon to gangliosides, Nature, 252, 478-480.
20.Besancon, F., Ankel, H., and Basu, S. (1976)
Specificity and reversibility of interferon ganglioside interaction,
Nature, 259, 576-578.
21.Baldauf, K. J., Royal, J. M., Hamorsky, K. T.,
and Matoba, N. (2015) Cholera toxin B: one subunit with many
pharmaceutical applications, Toxins, 7, 974-996.