Construction of a Fusion Enzyme Exhibiting Superoxide Dismutase and Peroxidase Activity
M. G. Sharapov*, V. I. Novoselov, and V. K. Ravin
Institute of Cell Biophysics, Russian Academy of Sciences, 142290 Pushchino, Moscow Region, Russia; E-mail: sharapov.mars@gmail.com* To whom correspondence should be addressed.
Received December 8, 2015; Revision received January 19, 2016
A chimeric gene construct encoding human peroxiredoxin 6 and Mn-superoxide dismutase from Escherichia coli was developed. Conditions for expression of the fusion protein in E. coli cell were optimized. Fusing of the enzymes into a single polypeptide chain with peroxiredoxin 6 at the N-terminus (PSH) did not affect their activities. On the contrary, the chimeric protein with reverse order of enzymes (SPH) was not obtained in a water-soluble active form. The active chimeric protein (PSH) exhibiting both peroxidase and superoxide dismutase activities was prepared and its physicochemical properties were characterized.
KEY WORDS: superoxide dismutase, peroxiredoxin 6, chimeric protein, oxidative stressDOI: 10.1134/S0006297916040131
Reactive oxygen species (ROS) formed in living organisms under normal as well as adverse conditions are capable of inactivating most important biological macromolecules. Excess of ROS initiates oxidative stress and development of a broad spectrum of pathological conditions (diseases of cardiovascular, respiratory, and nervous systems, etc.) [1-3]. Antioxidant preparations can be used to reduce ROS level in cells and correct or neutralize the development of oxidative stress. The use of antioxidant enzymes is considered a promising approach for the development of preparations with antioxidant activity because they are several orders of magnitude more effective than the low molecular weight compounds used in current medical practice [2].
The following enzymes are the most important catalytic antioxidants detoxifying ROS: superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione transferase (GST), thioredoxin (Trx), and peroxiredoxin (Prx).
Superoxide dismutases (EC 1.15.1.1) catalyze the dismutation of superoxide into molecular oxygen and hydrogen peroxide:
2O2˙ˉ + 2H+ → H2O2 + O2. (1)
Secondary organic hydroperoxides are formed in free radical interaction with superoxide:
O2˙ˉ + RH → ROOH, (2)
where R is an organic molecule.
The formed hydrogen peroxide and organic hydroperoxides are, in turn, reduced by catalase and other peroxidases:
2H2O2 → 2H2O + O2 (catalase); (3)
2R′SH + H2O2 → R′S-SR′ + 2H2O (GPx, Trx, Prx); (4)
2R′SH + ROOH → R′S-SR′ + H2O + ROH (GPx, Trx, Prx), (5)
where R′ is a cysteine of the enzyme active center or low molecular weight thiol-based reductant.
Hydrogen peroxide as well as organic peroxides formed in reactions (1) and (2) migrate until they meet peroxidase. Before reaching a peroxidase molecule, these peroxides can oxidize important macromolecules, thus possibly resulting in the loss of their functions. This can be avoided if the peroxidase enzyme would be located at near where the peroxide is formed. It was exactly this approach that was used by Maksimenko and coauthors, who chemically conjugated superoxide dismutase with catalase from bovine liver producing one molecule [4, 5]. Despite the fact that the activity of enzymes in the composition of the conjugate reduced significantly (by more than one order of magnitude) the final therapeutic effect (prevention of vascular thrombosis and infarction) was much higher than in the case when the two enzymes functioned independently [4]. Chemical conjugation of proteins is a nonspecific reaction that can lead to shielding of active centers of enzymes, and, hence, reflect negatively on their activity. Preparation of fused enzymes with the methods of genetic engineering, i.e. construction of genes encoding two enzymes in a single polypeptide chain, is a promising solution to this problem. It must be noted that this type of study has been conducted previously [6-8], but authors most often used one type of enzyme, such as two isoforms of Cu/Zn-superoxide dismutase (extracellular and intracellular) [6]. It is obvious that these chimeric enzymes catalyze a very narrow spectrum of neutralization of ROS, and, hence, cannot be sufficiently effective from the therapeutic point of view.
In this work, we decided to build upon the successful work of Maksimenko et al. Superoxide dismutase was used as the first component of the chimeric protein. It is an irreplaceable enzyme in the dismutation reaction of superoxide, which is a key form of ROS. Three types of superoxide dismutases are present in humans (and other mammals): SOD1 (Cu,Zn) – in cytoplasm, SOD2 (Mn) – in mitochondria, and SOD3 (Cu,Zn) – an extracellular form. The important physiological role of superoxide dismutases was demonstrated experimentally using SOD-knockout mouse models. The Cu/Zn-SOD1-knockout mice exhibit a broad spectrum of pathologies including developing carcinoma, cataract, muscle dystrophy, and reduced lifespan [9]. Mitochondrial Mn-SOD2-knockout mice die within a few days after birth from the development of strong oxidative stress [10]. Mice lacking the gene for the extracellular form of Cu/Zn-SOD3 do not exhibit significant deviations from the norm of physiological development and lifespan, but are more susceptible to hyperoxia in comparison with the wild type animals [11]. Hence, the most severe consequences have been observed for the manganese-dependent superoxide dismutase (Mn-SOD2) knockouts, and it is likely that this enzyme plays the most important role in protection from superoxide and can be used in practice. However, in practice the activity of superoxide dismutase in vivo depends strongly on the source of the enzyme. Surprisingly, it was shown in experiments with rats (edema model) that the enzyme from rats themselves was ineffective. The highest antiinflammatory effects were observed for the enzymes from phylogenetically distant species such as manganese-containing superoxide dismutase from E. coli [12]. The fact that the Mn-SOD from E. coli (unlike other superoxide dismutases) is not inactivated by H2O2 formed in the course of superoxide dismutation also counts in favor of this enzyme [13-15].
We decided to use a peroxidase demonstrating the broadest possible spectrum of neutralization of hydroperoxides as the second component of the chimeric protein – a peroxiredoxin. Six types of peroxiredoxins (Prx1-Prx6) are present in mammals. These peroxiredoxins contain one or two cysteine residues in the active center (1-Cys-peroxiredoxin Prx6 and 2-Cys-peroxiredoxins Prx1-5, respectively), and they neutralize a broad spectrum of hydroperoxides (both organic and inorganic) [16, 17]. We selected peroxiredoxin 6 (Prx6) because it demonstrates the broadest spectrum of hydroperoxide neutralization and, unlike the remaining representatives of the Prx family, it is capable of reducing phospholipid peroxides and peroxynitrite [18, 19]. The mice lacking a functional Prx6 gene are highly susceptible to hyperoxia despite high level of other peroxidases [20]. Moreover, high therapeutic activity of Prx6 was demonstrated on skin damage, lung inflammation, and ischemia–reperfusion-induced injury of kidney, colon, and isolated rat heart [21-26]. It seems likely that Prx6 can have important medical application for treatment of pathologies caused by free radicals. Hence, the chimeric protein could be able to neutralize all major types of ROS. Based on this reasoning, the following combination was selected for construction of the chimeric protein: human Prx6 and Mn-SOD from E. coli.
MATERIALS AND METHODS
Oligonucleotides used in this work are presented in Table 1.
Table 1. Oligonucleotides
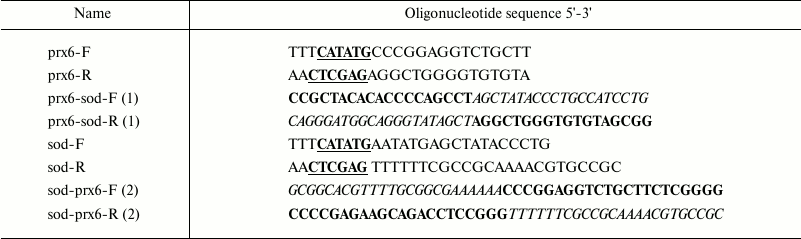
Notes: Restriction sites (NdeI, XhoI) are shown in bold and underlined.
Complementary sites of prx6-sod-F,R (1) and sod-prx6-F,R (2)
oligonucleotides corresponding to the Mn-SOD gene are shown in
italic, and the sites corresponding to the Prx6 gene are shown
in bold.
Mn-SOD gene from E. coli was constructed using a PCR method with the Tersus high-fidelity DNA polymerase (Evrogen, Russia) and gene-specific primers sod-F and sod-R (Table 1). Genomic DNA of E. coli was used as a template. PCR conditions: denaturation at 94°C – 2 min; then 30 cycles: denaturation at 94°C – 30 s, primer annealing at 54°C – 30 s, DNA synthesis at 72°C – 1 min; DNA elongation at 72°C – 5 min.
The human Prx6 gene was constructed using the PCR method with a Tersus high-fidelity DNA polymerase and gene-specific primers prx6-F and prx6-R (Table 1), a plasmid containing the human Prx6 gene being used as the template. PCR conditions: denaturation at 94°C – 2 min; then 30 cycles: denaturation at 94°C – 30 s, primer annealing at 56°C – 30 s, DNA synthesis at 72°C – 1 min; DNA elongation at 72°C – 5 min.
Genes encoding fusion proteins were constructed using the PCR method with Tersus high-fidelity DNA polymerase (Evrogen) and overlapping primers sod-prx6 and prx6-sod (Table 1) complementary to both the human Prx6 gene and the Mn-SOD gene from E. coli. Two variants of spatial arrangement of the genes are possible: 5′-Prx6-Mn-SOD-3′ and 5′-Mn-SOD-Prx6-3′. Two PCR fragments were prepared for each construct. Procedure for preparation of 5′-Prx6-Mn-SOD-3′: the first fragment was produced with primers prx6-F and sod-prx6 that contained an NdeI restriction site at the 5′-terminus. The second fragment was produced with primers prx6-sod + sod-R with XhoI restriction site at the 3′-terminus. PCR conditions for both fragments were as follows: heating at 94°C – 3 min, then 30 cycles: heating at 94°C – 20 s, annealing of primers at 55°C – 20 s, synthesis at 68°C – 60 s; and elongation at the end of RCR – 5 min at 68°C. Both fragments contained overlapping complementary sequences at the ends. The fragments were purified from primers by electrophoresis in 1% agarose gel, cut from the gel, and the DNA was isolated using silica gel columns (Evrogen). The isolated fragments were mixed at equimolar ratio (50 ng of each in a 50-µl volume), heated at 95°C for 5 min, and slowly (2°C/min) cooled to 52°C. The fragments were “glued” together via their complementary ends as a result of annealing. Next the PCR was conducted with the hybrid fragment using forward and reverse primers of both genes: prx6-F and sod-R (for the 5′-Prx6-Mn-SOD-3′ construct) and sod-F + prx6-R (for the 5′-Mn-SOD-Prx6-3′ construct). PCR conditions were the following: chain elongation after “gluing” of fragments at 68°C – 10 min, then 30 cycles: heating at 94°C – 20 s, primer annealing at 58°C – 20 s, synthesis at 68°C – 60 s; and elongation at the end of RCP for 5 min at 68°C. As a result, the target DNA molecules were produced consisting of two adjacent genes.
The Prx6, Mn-SOD, and fused hybrid genes were cloned into expression vector pET23b at the NdeI and XhoI restriction sites according to standard procedures [27]. The resulting constructs were verified by sequencing.
Preparation and purification of proteins. A separated biomass (5 g) of an E. coli strain producer (Mn-SOD, Prx6, or hybrid proteins) was resuspended in 25 ml of buffer (12 mM Tris-HCl, pH 7.8, 10 mM imidazole) prior to loading onto a column, and the cells were disrupted with ultrasound at 4°C using a UDZN-2T sonicator (Russia). Cell debris was removed by centrifugation at 14,000g for 30 min, the supernatant was filtered by passing through a 0.45-µm pore size filter (Corning, USA) and then loaded onto a column with a Ni-NTA-agarose (Invitrogen, USA) that was pre-equilibrated with buffer, in which the cells were resuspended and disrupted. The bacterial lysate was incubated with Ni-NTA-agarose for 40 min with shaking at 4°C. Next the column was washed with 300 ml of 12 mM Tris-HCl buffer, pH 7.8, with 20 mM imidazole. The protein was eluted with 10 ml of the same buffer supplemented with 250 mM imidazole. The protein was concentrated using a Vivaspin concentrator with a 30-kDa cut-off membrane (Sartorius, Germany) and then dialyzed in the same unit against phosphate buffer (1.7 mM KH2PO4, 5.2 mM Na2HPO4, 150 mM NaCl, pH 7.4). Protein concentration was determined with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, USA).
Determination of activity. Peroxidase activity and constants of the Michaelis–Menten equation were determined according to Kang et al. [28] with minor modification as described earlier [29]. Superoxide dismutase activity was determined with a commercial Superoxide Dismutase Assay Kit (Calbiochem, USA) in accordance with the supplier’s instructions.
Determination of thermal stability. Protein solutions (1 mg/ml) were heated in an MJMini BioRad thermocycler from 37 to 90°C (deviation from the target temperature 0.2°C). The sample volume was 100 µl, and the duration of heating was 30 min. The residual peroxidase activity was determined at 37°C, and superoxide dismutase activity at 25°C.
RESULTS AND DISCUSSION
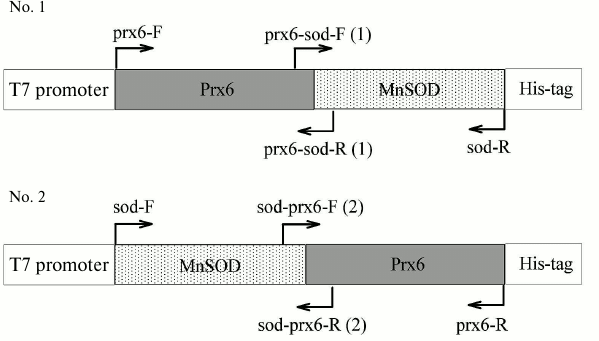
Preparation of genetic constructs. Schematic representations of the genetic constructs are given in Fig. 1. In the construct encoding the PSH protein (Prx6-MnSOD-6His) the Prx6 gene (without stop codon) is located at the 5′-terminus followed by the Mn-SOD gene (without stop codon), and after that the sequence encoding His-tag is placed followed by the stop codon. In the construct encoding the SPH protein (Mn-SOD-Prx6-6His) the Mn-SOD gene (without stop codon) is located at the 5′-terminus followed by the Prx6 gene (without stop codon), and the sequence encoding six histidine residues (His-tag) is located at the 3′-terminus followed by the stop codon.
Fig. 1. Schematic representation of genetic constructs encoding chimeric proteins PSH (Prx6-MnSOD-6His) and SPH (MnSOD-Prx6-6His).
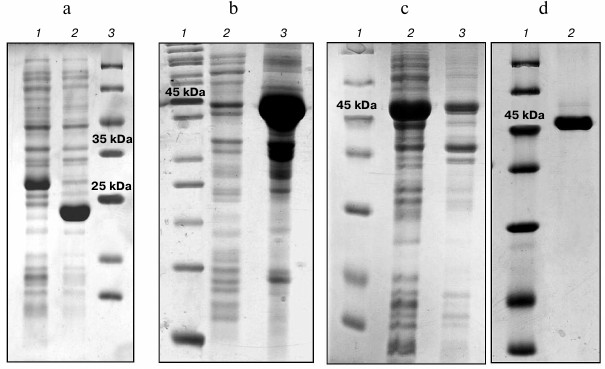
Isolation and purification of PSH. Electrophoregrams of the lysates of the producers of the investigated proteins are presented in Fig. 2. The SPH protein synthesized by construct No. 2 in E. coli cells aggregates almost completely (>90%), forming water-insoluble inclusion bodies (Fig. 2b).
Fig. 2. Polyacrylamide gel (12.5 %) electrophoresis in the presence of 0.1% SDS. a: 1) Prx6 producer; 2) Mn-SOD producer; 3) molecular weight markers; b: SPH producer: 1) molecular weight markers; 2) water-soluble protein fraction; 3) water-insoluble protein fraction; c: PSH producer: 1) molecular weight markers; 2) water-soluble protein fraction; 3) water-insoluble protein fraction; d: 1) molecular weight markers; 2) purified PSH protein.
The low content of the water soluble fraction of SPH provides insufficient quantities of the protein for determination of enzymatic activities, and isolation of the protein from inclusion bodies under denaturing conditions using 8 M urea followed by protein folding via dialysis against phosphate buffer is accompanied by the loss of activities. Hence, this variant of the hybrid protein was not used in further work.
Construct No. 1 encodes PSH, which is synthesized in E. coli cells in a water-soluble form (>90%). The amount of the hybrid PSH synthesized in the cells of the producing bacterium reaches 40-50% of the total protein. The yield of purified PSH was ~40 mg per liter of culture. The purity degree of the protein estimated form the polyacrylamide electrophoresis was ≥95% (Fig. 2d). The enzyme preparation was stored in phosphate buffer (1.7 mM KH2PO4, 5.2 mM Na2HPO4, 150 mM NaCl, pH 7.4) at concentration of 10-20 mg/ml at –20°C. No activity loss was observed during the two months of storage.
To evaluate the effect of covalent conjugation of two enzymes – Mn-SOD and Prx6 – on their activity in the composition of the chimeric PSH protein, these enzymes (Prx6 and Mn-SOD) were produced using the procedure described above for PSH.
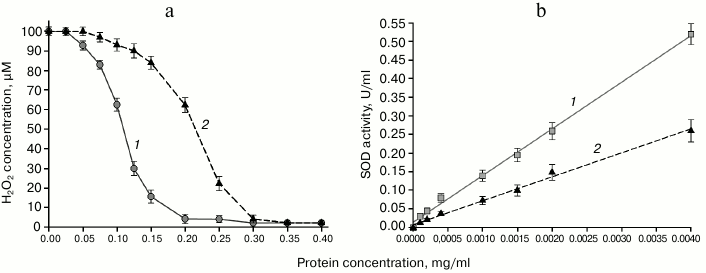
Determination of enzymatic activity of the chimeric PSH protein. The data on enzymatic activities of the chimeric PSH protein are presented in Fig. 3.
Fig. 3. a) Peroxidase activity of PSH (curve 2) and Prx6 (curve 1) with respect to hydrogen peroxide; b) superoxide dismutase activity of PSH (curve 2) and Mn-SOD (curve 1).
The peroxidase activity of the PSH with respect to hydrogen peroxide was ~100 nmol/min per mg protein, which was approximately 2-fold lower than the activity of pure human Prx6 (Fig. 3a), and similar results were obtained with tert-butyl hydroperoxide (an organic hydroperoxide) as the substrate. The substrate specificity (apparent Michaelis–Menten constants) of the chimeric PSH protein with respect to H2O2 and tert-butyl hydroperoxide (tBOOH) is in agreement with that for human Prx6 (Table 2).
Table 2. Peroxidase activity and
Michaelis–Menten constants

Superoxide dismutase activity of the PSH was 0.073 U/µg of protein, which was ~2-fold lower than the activity of the pure Mn-SOD protein from E. coli (0.139 U/µg of protein) (Fig. 3b).
Considering that molecular weight of the PSH is ~47 kDa (Prx6 ~25 kDa, Mn-SOD ~22 kDa), it is obvious that 1 mg of the PSH contains approximately twice less of the Prx6 and Mn-SOD molecules than 1 mg of the Prx6 protein or the Mn-SOD protein, and, therefore approximately two-fold reduction of activities per mg of PSH is expected. Hence, it can be concluded that both the peroxidase and superoxide dismutase activities in the composition of the PSH protein have been fully retained.
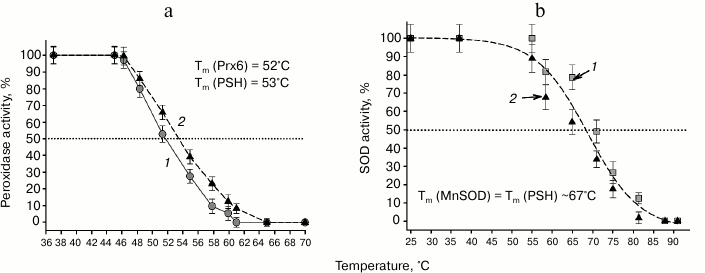
Thermal stability of the enzymes. To evaluate the effect of combining the two enzymes (Prx6 and Mn-SOD) on the stability of their structures, the thermal stability of the chimeric and initial proteins was investigated. The peroxidase activity of PSH is preserved under the same temperatures as for the human Prx6: ~50% of activity is retained following 30 min incubation at 53°C (Fig. 4a).
Fig. 4. Thermal stability of proteins. a) Determination of thermal stability of the PSH (curve 2) and Prx6 (curve 1) from the residual peroxidase activity; b) determination of thermal stability of the PSH (curve 2) and Mn-SOD (curve 1) from the residual superoxide dismutase activity.
It should be mentioned that protein aggregation was observed for human Prx6 protein solution at temperatures above 50°C, which was manifested by the increase in light scattering, while aggregate formation for the chimeric PSH protein was observed only above 60°C (data not shown). It seems likely that the Mn-SOD prevents aggregation of the chimeric protein; nevertheless, inactivation of peroxidase activity of PSH occurs at the same temperatures as in the case of Prx6 (Fig. 4).
The thermal stability of the PSH and Mn-SOD determined from the superoxide dismutase activity was also found to be the same: ~50% of the chimeric PSH and of Mn-SOD retained their superoxide dismutase activity after 30 min incubation at 67°C (Fig. 4b). Hence, it could be concluded that combining of Prx6 and Mn-SOD into the chimeric PSH protein did not affect either their enzymatic activities or their physicochemical properties.
PSH exhibiting peroxidase and superoxide dismutase activity can be considered as a bifunctional enzyme. Such enzymes have been found in many prokaryotic organisms. It has been suggested that they emerge during evolution as a result of in frame fusion of two adjacent genes [30-32]. Such enzymes can be also created artificially, but some modification of at least one gene is required [33, 34]. The gene encoding the PSH was obtained by fusion of two genes (peroxiredoxin 6 and manganese-containing superoxide dismutase), and the ATG start codon of the superoxide dismutase gene was removed in the process. Hence, the Prx6 is located at the N-terminus of the chimeric protein followed by the Mn-SOD and the sequence encoding six histidine residues. It seems likely that the C-terminus of the Prx6 is not essential for its activity [35-38]. In any case, the removal of ~40 a.a. from the C-terminus of the Prx6 protein results only in a slight loss of activity (90% of peroxidase activity is retained) [38, 39]. On the other hand, the first 30 a.a. at the N-terminus of superoxide dismutase are highly variable and, probably, are not so important for its activity [40]. Hence, it could be expected that fusion of these two sites would not result in the disruption of the structure and the loss of activity of the respective enzymes, which indeed was observed in the experiments. Testing of the PSH enzyme in animal models of various pathologies accompanied by the development of oxidative stress could be an important confirmation of the functional activity of the protein. As of now, successful testing of this protein in experimental models of thermal and chemical burn of the upper respiratory tract [23], ischemia/reperfusion-induced injury of kidney [41], and rat heart [42] has been conducted. The results indicate that high protective and therapeutic activity of the PSH protein is observed in all the mentioned pathologies. It must be noted that the use of Mn-SOD and Prx6 proteins separately in the abovementioned models did not provide the same outstanding therapeutic results as in the case with the PSH.
We believe that PSH offers considerable promise for practical application as a component of perfusion solution for conserving isolated organs as well as for treatment of various diseases of respiratory organs.
This work was financially supported by the Russian Foundation for Basic Research (project No. 15-04-04021a).
REFERENCES
1.Halliwell, B., and Gutteridge, J. M. (2007) Free
Radicals in Biology and Medicine, 4th Edn., Oxford University
Press.
2.Menshchikova, E. B., Lankin, V. Z., Zenkov, N. K.,
Bondar, I. A., Krugovykh, N. F., and Trufakin, V. A. (2006)
Oxidative Stress. Prooxidants and Antioxidants [in Russian],
Slovo, Moscow.
3.Bruskov, V. I., Karp, O. E., Garmash, S. A.,
Shtarkman, I. N., Chernikov, A. V., and Gudkov, S. V. (2012)
Prolongation of oxidative stress by long-lived reactive protein species
induced by X-ray radiation and their genotoxic action, Free Radic.
Res., 46, 1280-1290.
4.Maksimenko, A. V. (2005) Experimental antioxidant
biotherapy for protection of the vascular wall by modified forms of
superoxide dismutase and catalase, Curr. Pharm. Des., 11,
2007-2016.
5.Maksimenko, A. V., Golubykh, V. L., and Tischenko,
E. G. (2004) The combination of modified antioxidant enzymes for
anti-thrombotic protection of the vascular wall: the significance of
covalent connection of superoxide dismutase and catalase activities,
J. Pharm. Pharmacol., 56, 1463-1468.
6.Stenlund, P., and Tibell, L. A. (1999) Chimeras of
human extracellular and intracellular superoxide dismutases. Analysis
of structure and function of the individual domains, Protein
Eng., 12, 319-325.
7.Isarankura-Na-Ayudhya, C., Yainoy, S.,
Tantimongcolwat, T., Bulow, L., and Prachayasittikul, V. (2010)
Engineering of a novel chimera of superoxide dismutase and
Vitreoscilla hemoglobin for rapid detoxification of reactive
oxygen species, J. Biosci. Bioeng., 110, 633-637.
8.Seetharaman, S. V., Taylor, A. B., Holloway, S.,
and Hart, P. J. (2010) Structures of mouse SOD1 and human/mouse SOD1
chimeras, Arch. Biochem. Biophys., 503, 183-190.
9.Elchuri, S., Oberley, T. D., Qi, W., Eisenstein, R.
S., Jackson Roberts, L., Van Remmen, H., Epstein, C. J., and Huang, T.
T. (2005) Cu,Zn-SOD deficiency leads to persistent and widespread
oxidative damage and hepatocarcinogenesis later in life,
Oncogene, 24, 367-380.
10.Li, Y., Huang, T. T., Carlson, E. J., Melov, S.,
Ursell, P. C., Olson, J. L., Noble, L. J., Yoshimura, M. P., Berger,
C., Chan, P. H., Wallace, D. C., and Epstein, C. J. (1995) Dilated
cardiomyopathy and neonatal lethality in mutant mice lacking manganese
superoxide dismutase, Nat. Genet., 11, 376-381.
11.Sentman, M. L., Granstrom, M., Jakobson, H.,
Reaume, A., Basu, S., and Marklund, S. L. (2006) Phenotypes of mice
lacking extracellular superoxide dismutase and copper- and
zinc-containing superoxide dismutase, J. Biol. Chem.,
281, 6904-6909.
12.Michelson, A. M., Puget, K., and Jadot, G. (1986)
Anti-inflammatory activity of superoxide dismutases: comparison of
enzymes from different sources in different models in rats: mechanism
of action, Free Radic. Res. Commun., 2, 43-56.
13.Keele, B. B., Jr., McCord, J. M., and Fridovich,
I. (1970) Superoxide dismutase from Escherichia coli B. A new
manganese-containing enzyme, J. Biol. Chem., 245,
6176-6181.
14.Beyer, W. F., Jr., and Fridovich, I. (1987)
Effect of hydrogen peroxide on the iron-containing superoxide dismutase
of Escherichia coli, Biochemistry, 26,
1251-1257.
15.Sheng, Y., Abreu, I. A., Cabelli, D. E., Maroney,
M. J., Miller, A. F., Teixeira, M., and Valentine, J. S. (2014)
Superoxide dismutases and superoxide reductases, Chem. Rev.,
114, 3854-3918.
16.Wood, Z. A., Poole, L. B., and Karplus, P. A.
(2003) Peroxiredoxin evolution and the regulation of hydrogen peroxide
signaling, Science, 300, 650-653.
17.Sharapov, M. G., Ravin, V. K., and Novoselov, V.
I. (2014) Peroxiredoxins as multifunctional enzymes, Mol. Biol.
(Moscow), 48, 600-628.
18.Chen, J. W., Dodia, C., Feinstein, S. I., Jain,
M. K., and Fisher, A. B. (2000) 1-Cys peroxiredoxin, a bifunctional
enzyme with glutathione peroxidase and phospholipase A2 activities,
J. Biol. Chem., 275, 28421-28427.
19.Manevich, Y., Shuvaeva, T., Dodia, C., Kazi, A.,
Feinstein, S. I., and Fisher, A. B. (2009) Binding of peroxiredoxin 6
to substrate determines differential phospholipid hydroperoxide
peroxidase and phospholipase A2 activities, Arch.
Biochem. Biophys., 485, 139-149.
20.Nagy, N., Malik, G., Fisher, A. B., and Dipak, K.
D. (2006) Targeted disruption of peroxiredoxin 6 gene renders the heart
vulnerable to ischemia-reperfusion injury, Am. J. Physiol. Heart
Circ. Physiol., 291, 2636-2640.
21.Kumin, A., Huber, C., Rulice, T., Wolf, E., and
Werner, S. (2006) Peroxiredoxin 6 is a potent cytoprotective enzyme in
the epidermis, Am. J. Pathol., 169, 1194-1205.
22.Novoselov, V. I., Ravin, V. K., Sharapov, M. G.,
Sofin, A. D., Kukushkin, N. I., and Fesenko, E. E. (2011) Modified
peroxiredoxins as prototypes of drugs with powerful antioxidant action,
Biophysics (Moscow), 56, 873-880.
23.Volkova, A. G., Sharapov, M. G., Ravin, V. K.,
Gordeeva, A. E., Karaduleva, E. A., Mubarakshina, E. K., Temnov, A. A.,
Fesenko, E. E., and Novoselov, V. I. (2014) Effect of various enzymes
antioxidants on tracheal epithelial regeneration following chemical
burn, Pulmonologiya, 12, 84-90.
24.Novoselov, V. I., Amelina, S. E., Kravchenko, I.
N., Novoselov, S. V., Yanin, V. A., Sadovnikov, V. B., and Fesenko, E.
E. (2000) The role of peroxiredoxin in the antioxidant system of
respiratory organs, Dokl. Biophys., 373-375, 64-66.
25.Chuchalin, A. G., Novoselov, V. I., Shifrina, O.
N., Soodaeva, S. K., Yanin, V. A., and Barishnikova, L. M. (2003)
Peroxiredoxin VI in human respiratory system, Respir. Med.,
97, 147-151.
26.Manevich, Y., and Fisher, A. B. (2005)
Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant
defense and lung phospholipid metabolism, Free Radic. Biol.
Med., 38, 1422-1432.
27.Sambrook, J., and Russell, D. W. (2001)
Molecular Cloning: a Laboratory Manual, Cold Spring
Harbor, Cold Spring Harbor Laboratory Press, N. Y.
28.Kang, S. W., Baines, I. C., and Rhee, S. G.
(1998) Characterization of a mammalian peroxiredoxin that contains one
conserved cysteine, J. Biol. Chem., 273, 6303-6311.
29.Sharapov, M. G., Novoselov, V. I., and Ravin, V.
K. (2009) The cloning, expression, and comparative analysis of
peroxiredoxin 6 from various sources, Mol. Biol. (Moscow),
43, 505-511.
30.Yourno, J., Kohno, T., and Roth, J. R. (1970)
Enzyme evolution: generation of a bifunctional enzyme by fusion of
adjacent genes, Nature, 228, 820-824.
31.Bulow, L. (1990) Preparation of artificial
bifunctional enzymes by gene fusion, Biochem. Soc. Symp.,
57, 123-133.
32.Zhang, W., Fisher, J. F., and Mobashery, S.
(2009) The bifunctional enzymes of antibiotic resistance, Curr.
Opin. Microbiol., 12, 505-511.
33.Luo, G., Liu, W., Sun, Q., Ding, L., Zhu, Z.,
Yan, G., and Yang, T. (1996) Preparation of bifunctional enzyme with
both superoxide dismutase and glutathione peroxidase activities by
using chemical mutation, Ann. N. Y. Acad. Sci., 50,
799.
34.Yan, F., Yang, W. K., Li, X. Y., Lin, T. T., Lun,
Y. N., Lin, F., Lu, S. W., Yan, G. L., Liu, J. Q., Shen, J. C., Mu, Y.,
and Luo, G. M. (2008) A trifunctional enzyme with glutathione
S-transferase, glutathione peroxidase and superoxide dismutase
activity, Biochim. Biophys. Acta, 1780, 869-872.
35.Fisher, A. B. (2011) Peroxiredoxin 6: a
bifunctional enzyme with glutathione peroxidase and phospholipase A2
activities, Antioxid. Redox Signal., 15, 831-844.
36.Choi, H. J., Kang, S. W., Yang, C. H., Rhee, S.
G., and Ryu, S. E. (1998) Crystal structure of a novel human peroxidase
enzyme at 2.0 A resolution, Nat. Struct. Biol., 5,
400-406.
37.Smeets, A., Loumaye, E., Clippe, A., Rees, J. F.,
Knoops, B., and Declercq, J. P. (2008) The crystal structure of the
C45S mutant of annelid Arenicola marina peroxiredoxin 6 supports
its assignment to the mechanistically typical 2-Cys subfamily without
any formation of toroid-shaped decamers, Protein Sci.,
17, 700-710.
38.Nekrasov, A. N., Radchenko, V. V., Shuvaeva, T.
M., Novoselov, V. I., Fesenko, E. E., and Lipkin, V. M. (2007) The
novel approach to the protein design: active truncated forms of human
1-CYS peroxiredoxin, J. Biomol. Struct. Dyn., 24,
455-462.
39.Radchenko, V. V., Nekrasov, A. N., Shuvaeva, T.
M., and Lipkin, V. M. (2005) Information Structure Analysis of
Protein Sequences. Design and Purification of Recombinant Truncated
Forms of Human 1-CYS Peroxiredoxin (PrxVI), Moscow Conf. on
Computational Molecular Biology, Moscow, pp. 311-313.
40.Sheng, Y., Abreu, I. A., Cabelli, D. E., Maroney,
M. J., Miller, A. F., Teixeira, M., and Valentine, J. S. (2014)
Superoxide dismutases and superoxide reductases, Chem. Rev.,
114, 3854-3918.
41.Palutina, O. A., Sharapov, M. G., Temnov, A. A.,
and Novoselov, V. I (2016) Nephroprotective effect of exogenous
antioxidant enzymes during ischemia/reperfusion-induced damage of renal
tissue, Bull. Exp. Biol. Med., 160, 322-326.
42.Karaduleva, E. V., Mubarakshina, E. K., Sharapov,
M. G., Volkova, A. E., Pimenov, O. Yu., Ravin, V. K., Kokoz, Yu. M.,
and Novoselov, V. I. (2016) Cardioprotective effect of modified
peroxiredoxins during retrograde perfusion of isolated rat heart under
oxidative stress conditions, Bull. Exp. Biol. Med., 160,
584-588.