Interaction between Fibrinogen and Insulin-Like Growth Factor-Binding Protein-1 in Human Plasma under Physiological Conditions
N. Gligorijević* and O. Nedić
University of Belgrade, Institute for the Application of Nuclear Energy (INEP), 11000 Belgrade, Serbia; E-mail: nikolag@inep.co.rs* To whom correspondence should be addressed.
Received August 11, 2015
Fibrinogen is a plasma glycoprotein and one of the principle participants in blood coagulation. It interacts with many proteins during formation of a blood clot, including insulin-like growth factors (IGFs) and their binding proteins (IGFBP). Fibrinogen complexes were found as minor fractions in fibrinogen preparations independently of the coagulation process, and their presence influences the kinetics of polymerization. The idea of this work was to investigate whether fibrinogen in human plasma interacts with IGFBPs independently of the tissue injury or coagulation process. The results have shown that fibrinogen forms complexes with IGFBP-1 under physiological conditions. Several experimental approaches have confirmed that complexes are co-isolated with fibrinogen from plasma, they are relatively stable, and they appear as a general feature of human plasma. Several other experiments excluded the possibility that alpha-2 macroglobulin/IGFBP-1 complexes or IGFBP-1 oligomers contributed to IGFBP-1 immunoreactivity. The role of fibrinogen/IGFBP-1 complexes is still unknown. Further investigation in individuals expressing both impaired glucose control and coagulopathy could contribute to identification and understanding of their possible physiological role.
KEY WORDS: fibrinogen, IGFBP-1, human plasma, complexes, complex isolationDOI: 10.1134/S0006297916020073
Abbreviations: ConA, lectin from Canavalia ensiformis; IGF, insulin-like growth factor; IGFBP, IGF-binding protein; PBS, phosphate buffered saline.
Fibrinogen is a plasma glycoprotein and one of the principle
participants in blood coagulation. It is a 340-kDa dimer composed of
two identical monomers, each containing polypeptide chains Aα,
Bβ, and γ, linked by 29 disulfide bonds [1]. Its plasma concentration ranges from 2 to 4
g/liter, and its half-life is approximately four days [2]. Upon tissue injury, fibrinogen is cleaved by
thrombin, and the resulting peptides associate into insoluble fibrils
that are further cross-linked and stabilized by the action of the
coagulation factor XIIIa. Fibrin, together with blood cells, forms a
clot that prevents blood loss [3]. Upon tissue
healing, fibrinolysis by plasmin causes degradation of fibrin and the
clot, establishing blood flow again. Fibrinogen is also an acute-phase
reactant whose concentration increases in response to inflammation,
infection, or injury [2]. Several types of
coagulopathies are known, some of genetic origin and others acquired,
resulting in either increased bleeding or increased fibrinolysis time
[4, 5]. Molecular changes in
fibrinogen affect the structure and stability of fibrin fibrils. Gene
polymorphism, posttranslational modifications, and interactions with
other proteins influence the structure and stability of fibrinogen and
fibrin [6-9].
Fibrinogen and/or fibrin interact with many proteins, and an array of them is found in fibrin clot [7, 10-14]. Several growth factors were identified as clot constituents, including insulin-like growth factors (IGFs) and their binding proteins (IGFBP) 3 and 5 [15]. It is generally accepted that growth factors participate in wound healing by stimulating re-epithelialization [16, 17].
IGFs are involved in cellular growth, proliferation, migration, and differentiation, and they enhance tissue repair [18]. IGF bioavailability is regulated by six IGFBPs whose distribution is tissue-specific [19, 20]. IGFBP-3 is the major binding protein in the circulation, followed by IGFBP-2, and then the others. IGFBP-1 is the only one whose concentration changes several-fold during the day, as its secretion is regulated by insulin [21]. IGFBP proteolysis is a mechanism to liberate IGFs enabling their action, and plasmin was confirmed to be an IGFBP protease [22, 23]. Both IGFBP-3 and IGFBP-5 possess a heparin-binding domain that interacts with the components of the extracellular matrix, and in vitro experiments have confirmed their binding to fibrinogen [15, 24]. Other IGFBPs do not have a heparin-binding domain.
Huang and Lord [25] found that fibrinogen isolated from plasma, recombinant fibrinogen, and engineered variant Aα251 exhibit some differences upon polymerization. They demonstrated that fibrinogen complexes exist as minor fractions in all fibrinogens, but they are not exactly the same. Their presence changed the kinetics of polymerization and disturbed the formation of fibrin fibrils [25]. Taking into account these conclusions and the possibility that the presence of pre-formed fibrinogen complexes may affect clot stability, it seemed relevant to investigate whether fibrinogen in human plasma interacts with IGFBPs independently of the tissue injury or coagulation process.
MATERIALS AND METHODS
Plasma samples. Human plasma was obtained from healthy adult volunteers (n = 30), who agreed that their samples could be used for this study. Venous blood was collected from each person after overnight fasting into a vacutainer containing sodium-EDTA as anticoagulant. Plasma was separated from blood cells within 1 h and used for the isolation of fibrinogen within the next 2 h. The concentration of fibrinogen in plasma ranged from 2.5 to 3.7 g/liter. Three pools of plasma were made by using equal volumes of 10 different plasma samples.
Isolation of fibrinogen from plasma. Fibrinogen was isolated from each plasma pool using the procedure for the preparation of the first Cohn fraction [26]. Fibrinogen was precipitated from 1 ml of plasma by the addition of 176 µl of 53.3% ethanol and 1 µl of 0.8 M sodium acetate buffer, pH 4.0. The mixture was left at 4°C for 1 h and then centrifuged at 10,000g for 2 min. Fibrinogen was dissolved in 0.15 M citrate buffer, pH 6.1, and the precipitation repeated to purify the protein. Isolated fibrinogen was dissolved in the same citrate buffer (4 g/liter) and used immediately in the experiments. The concentration of fibrinogen was determined by two reagents: Fowell (specific for fibrinogen [27]) and bicinchoninic acid (BCA, general for proteins; Abcam, UK).
Electrophoresis and Western immunoblotting. Fibrinogen samples diluted to 0.4 g/liter were analyzed by polyacrylamide native (nPAGE) and SDS electrophoresis under reducing conditions (rSDS-PAGE) using 8% gels. Proteins were either stained in gels by Coomassie brilliant blue solution or transferred to nitrocellulose membrane and immunoblotted with a panel of primary antibodies: anti-fibrinogen (Abcam), anti-IGFBP-1, anti-alpha-2-macroglobulin (AbD Serotec, UK), anti-IGFBP-2 (Biogenesis, UK), anti-IGFBP-3 (DSL, USA), and anti-IGFBP-4 (Santa Cruz Biotechnology, USA). Biotinylated secondary antibody coupled with HRP-conjugated avidin (Vector, USA) or HRP-conjugated secondary antibody (AbD Serotec) and ECL reagent (Pierce Biotechnology, USA) were used for immunodetection. Immunoreactive proteins were visualized by autoradiography employing Roentgen film and the appropriate developing reagents (Kodak, France).
Immunoprecipitation. Immunoprecipitation of protein complexes fibrinogen/IGFBP-1 was performed using Pierce® Co-Immunoprecipitation Kit (Pierce Biotechnology). Following the manufacturer’s instructions, 5 µg of anti-fibrinogen or anti-IGFBP-1 antibody was separately immobilized to AminoLink®Plus Coupling Resin (50 µl) in spin columns. Isolated fibrinogen samples at 0.4 g/liter in 200 µl of PBS (provided within the kit) were incubated with each immunoaffinity resin at 4°C overnight. Unbound proteins were washed away by PBS (8 × 200 µl), specifically bound proteins were eluted with 110 µl (10 + 2 × 50 µl) of the elution buffer with pH 2.8 (provided within the kit) and immediately neutralized with 2 µl of 2 M Tris-HCl buffer, pH 8.9. Immunoprecipitated proteins were analyzed by immunoblotting.
Lectin affinity chromatography. Lectin affinity chromatography using agarose-immobilized ConA (lectin from Canavalia ensiformis; Vector, USA) was performed according to the producer’s manual. Isolated fibrinogen samples at 0.4 g/liter in 1 ml of 0.02 M HEPES buffer, pH 7.5 (containing 0.15 M NaCl and 1 mM calcium-, magnesium-, and manganese chloride each), were incubated with 2 ml of lectin resin, first by recirculation at room temperature for 1 h, and then at 4°C overnight. Unbound proteins were washed away with the same HEPES buffer (30 ml), whereas bound proteins were eluted with 0.05 M acetate buffer, pH 5.0 (containing 1 M NaCl, 15 × 1 ml), and neutralized using 2 µl of 2 M Tris buffer, pH 8.9. The fraction with the greatest amount of protein (assessed by measuring A280) was analyzed by immunoblotting.
RESULTS AND DISCUSSION
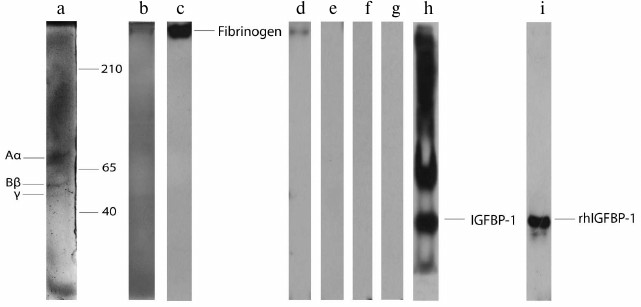
Before testing the reactivity of fibrinogen with IGFBPs, the purity of the isolated fibrinogen preparations was analyzed. Two electrophoretic approaches (nPAGE and rSDS-PAGE) showed that there were no visible protein bands in the gels except those originating from fibrinogen: three bands in rSDS-PAGE (Fig. 1a, a representative example) and one band in nPAGE (Fig. 1b). Thus, isolated fibrinogen samples were either completely pure or contained minute quantities of other plasma proteins. Immunoblotting with anti-fibrinogen antibody confirmed the identity of the isolated protein (Fig. 1c).
Fig. 1. rSDS-PAGE (a) and nPAGE (b) of isolated fibrinogen from human plasma stained with Coomassie brilliant blue. Immunoblot of the isolated fibrinogen with anti-fibrinogen (c), anti-IGFBP-1 (d), anti-IGFBP-2 (e), anti-IGFBP-3 (f), and IGFBP-4 (g) antibody. Immunoblot of plasma sample (h) and rhIGFBP-1 (i) with anti-IGFBP-1 antibody.
In the second set of experiments, the possible existence of complexes formed under physiological conditions between fibrinogen and the most abundant plasma IGFBPs was tested. Isolated fibrinogen samples were immunoblotted with anti-IGFBP-1, -2, -3, and -4 antibodies after nPAGE (representative results are shown in Fig. 1, d-g). The only immunoreactive band was detected with anti-IGFBP-1 antibody (Fig. 1d). Since fibrinogen/IGFBP-1 complexes were not previously identified, the specificity of anti-IGFBP-1 antibody was re-evaluated (apart from the producer’s declaration) using entire plasma (Fig. 1h) and rhIGFBP-1 (Fig. 1i) as samples. Immunoblotting with rhIGFBP-1 resulted in one protein band originating from IGFBP-1 monomer, whereas several immunoreactive proteins were detected in plasma corresponding to IGFBP-1 monomer, dimer, oligomers, and/or complexes [28]. According to these results, isolated fibrinogen preparations contained small quantities of fibrinogen/IGFBP-1 complexes that were formed in vivo.
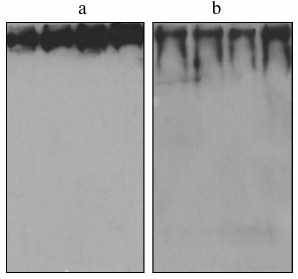
In the third set of experiments, fibrinogen was isolated from individual plasma samples (not pools) and immunoblotted to investigate whether the existence of fibrinogen/IGFBP-1 complexes is a general feature. Immunoblotting results for four representative individual fibrinogen preparations are shown in Fig. 2a (with anti-fibrinogen) and Fig. 2b (with anti-IGFBP-1 antibody). IGFBP-1 immunoreactive protein bands were detected in all individual fibrinogen samples.
Fig. 2. Immunoblot of isolated fibrinogen from four individual plasma samples with anti-fibrinogen (a) and anti-IGFBP-1 (b) antibody.
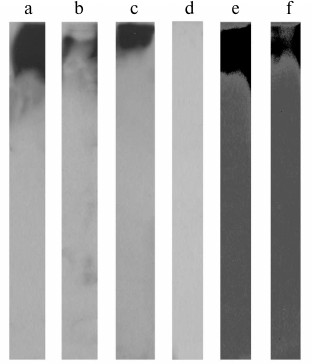
To confirm that fibrinogen/IGFBP-1 complexes exist, immunoprecipitation with anti-fibrinogen or anti-IGFBP-1 antibody of the isolated fibrinogen samples followed by immunoblotting was performed. As seen in Figs. 3a and 3b (representative results), proteins specifically eluted from fibrinogen immunoaffinity resin were IGFBP-1 immunoreactive (with anti-fibrinogen and anti-IGFBP-1 antibody, respectively), and those eluted from IGFBP-1 immunoaffinity resin were fibrinogen immunoreactive (Fig. 3c).
Fig. 3. Immunoblot of molecules isolated by immunoprecipitation with immobilized anti-fibrinogen (a, b) or anti-IGFBP-1 antibody (c) detected with anti-fibrinogen (a, c) or anti-IGFBP-1 (b) antibody. Immunoblot of isolated fibrinogen with α-2-macroglobulin antibody (d). Immunoblot of molecules isolated by ConA lectin chromatography with anti-fibrinogen (e) or anti-IGFBP-1 (f) antibody.
Since IGFBP-1 forms complexes with α-2-macroglobulin [29-31] and associates into oligomers [31-33], which all have high molecular masses, the last set of experiments was conducted to exclude the doubt that these molecules were present in fibrinogen preparations. Immunoblotting with anti-α-2-macroglobulin antibody resulted in no signal (Fig. 3d), which is in accordance with the literature data referring to α-2-macroglobulin as a protein isolated in the third Cohn fraction [34].
To eliminate IGFBP-1 oligomers as contributors to the IGFBP-1 signal in fibrinogen preparations, two experiments were performed. In the first one, a pooled serum sample (known to contain a majority of IGFBP-1 oligomers, but no fibrinogen) was subjected to ethanol precipitation in the same way as plasma. Practically no sediment was formed. After aspiration of the liquid, the rest of the procedure imitating dissolution of fibrinogen was carried out, and IGFBP-1 immunoblotting resulted in no signal (data not shown). Therefore, IGFBP-1 oligomers did not precipitate under conditions that caused precipitation of fibrinogen.
Finally, a fibrinogen sample was subjected to ConA affinity chromatography, followed by fibrinogen or IGFBP-1 immunoblotting of the specifically bound proteins. Fibrinogen is a glycoprotein whose β and γ chains are N-glycosylated, and the most dominant glycan structures are of the biantennary type containing core mannose residues [35]. Since ConA specifically recognizes such glycans [36], it was applied to re-test previous findings. The ConA-eluted fraction was found to be both fibrinogen (Fig. 3e) and IGFBP-1 immunoreactive (Fig. 3f), confirming co-isolation of IGFBP-1 with fibrinogen. IGFBP-1 oligomers could not be isolated by lectin chromatography, as IGFBP-1 is not a glycoprotein [37].
The experiments described in this work have identified IGFBP-1 as a complex-forming partner of fibrinogen under physiological conditions and independent of the coagulation process. On the other hand, the fibrinogen molecule isolated from human plasma did not exhibit IGFBP-2, IGFBP-3, or IGFBP-4 immunoreactivity. Campbell and coworkers [24] reported that rhIGFBP-3 interacted with commercial fibrinogen immobilized on a plate, and IGFBP-1 was unable to disturb that interaction. Martino and his group [15] found that rhIGFBP-3 immobilized on a plate bound recombinant HBD of fibrinogen. It is, however, questionable whether these and our results can be compared as Huang and Lord [25] have shown that the isolated, commercial, and engineered fibrinogen molecules behaved differently. Since most IGFBPs are posttranslationally modified under physiological conditions [38], they are also expected to react differently to some extent from the recombinant variants.
The possible role of fibrinogen/IGFBP-1 complexes is still unknown. IGFBP-1 is a 30-kDa protein posttranslationally modified by phosphorylation [37]. Five isoforms were identified with variable degree of phosphorylation [39]. Dephosphorylation of IGFBP-1 reduces its affinity for IGFs potentiating their activity [20, 21, 32]. IGFBP-1 synthesis occurs mainly in the liver of non-pregnant adults [21] and is inversely correlated to insulin [40]. IGFBP-1 contains the RGD sequence enabling its binding to the α5β1 integrin and stimulating cell migration [41, 42].
IGFBP-1 was identified among molecules of the fibrin clot formed in vivo [43, 44], and it is a substrate for plasmin [23]. Besides playing a general role as a regulator of IGF activity or cell migration, IGFBP-1 may perform some other functions, possibly related to insulin and glucose metabolism. The concentration of IGFBP-1 in plasma correlates with insulin sensitivity, and in patients with diabetes mellitus type 2 negative feedback control between insulin and IGFBP-1 can be lost [45]. On the other hand, vascular diseases and compromised coagulation are complications frequently occurring in patients with diabetes mellitus type 2 [46]. Further investigation on the fibrinogen/IGFBP-1 complexes in individuals expressing both impaired glucose control and coagulopathy should contribute to identification and understanding of the physiological role of these complexes.
This work was supported by the Ministry of Education, Science, and Technology of the Republic of Serbia (grant No. 173042).
REFERENCES
1.Schmidt, D., and Brennan, S. O. (2007) Modified
form of the fibrinogen Bβ chain (des-Gln Bβ), a potential
long-lived marker of pancreatitis, Clin. Chem., 53,
2105-2111.
2.Tennent, G. A., Brennan, S. O., Stangou, A. J.,
O’Grady, J., Hawkins, P. N., and Pepys, M. B. (2007) Human plasma
fibrinogen is synthesized in the liver, Blood, 109,
1971-1974.
3.Chapin, J. C., and Hajjar, K. A. (2015)
Fibrinolysis and the control of blood coagulation, Blood
Rev., 29, 17-24.
4.McMullin, N. R., Kauvar, D. S., Currier, H. M.,
Baskin, T. W., Pusateri, A. E., and Holcomb, J. B. (2006) The clinical
and laboratory response to recombinant factor VIIa in trauma and
surgical patients with acquired coagulopathy, Curr. Surg.,
63, 246-251.
5.Asselta, R., Duga, S., and Tenchini, M. L. (2006)
The molecular basis of quantitative fibrinogen disorders, J. Thromb.
Haemost., 4, 2115-2129.
6.Becatti, M., Marcucci, R., Bruschi, G., Taddei, N.,
Bani, D., Gori, A. M., Giusti, B., Gensini, G. F., Abbate, R., and
Fiorillo, C. (2014) Oxidative modification of fibrinogen is associated
with altered function and structure in the subacute phase of myocardial
infarction, Arterioscler. Thromb. Vasc. Biol., 34,
1355-1361.
7.Dunn, E. J., Philippou, H., Ariens, R. A. S., and
Grant, P. J. (2006) Molecular mechanisms involved in the resistance of
fibrin to clot lysis by plasmin in subjects with type 2 diabetes
mellitus, Diabetologia, 49, 1071-1080.
8.Brennan, S. O. (2015) Variation of fibrinogen
oligosaccharide structure in the acute phase response: possible
hemorrhagic implications, Biochim. Biophys. Acta, 3,
221-226.
9.Scott, E. M., Ariens, R. A. S., and Grant, P. J.
(2004) Genetic and environmental determinants of fibrin structure and
function, Arterioscler. Thromb. Vasc. Biol., 24,
1558-1566.
10.Schneider, D. J., Taatjes, D. J., Howard, D. B.,
and Sobel, B. E. (1999) Increased reactivity of platelets induced by
fibrinogen independent of its binding to the IIb-IIIa surface
glycoprotein, J. Am. Coll. Cardiol., 33, 261-266.
11.Makogonenko, E., Tsurupa, G., Ingham, K., and
Medved, L. (2002) Interaction of fibrin(ogen) with fibronectin: further
characterization and localization of the fibronectin-binding site,
Biochemistry, 41, 7907-7913.
12.Dowling, P., Palmerini, V., Henry, M., Meleady,
P., Lynch, V., Ballot, J., Gullo, G., Crown, J., Moriarty, M., and
Clynes, M. (2014) Transferrin-bound proteins as potential biomarkers
for advanced breast cancer patients, Biochim. Biophys. Acta,
2, 24-30.
13.Talens, S., Leebeek, F. W. G., Demmers, J. A. A.,
and Rijken, D. C. (2012) Identification of fibrin clot-bound plasma
proteins, PLoS One, 7, e41966.
14.Sahni, A., Simpson-Haidaris, P. J., Sahni, S. K.,
Vaday, G. G., and Francis, C. W. (2008) Fibrinogen synthesized by
cancer cells augments the proliferative effect of fibroblast growth
factor-2 (FGF-2), J. Thromb. Haemost., 6, 176-183.
15.Martino, M. M., Briquez, P. S., Ranga, A.,
Lutolf, M. P., and Hubbell, J. A. (2013) Heparin-binding domain of
fibrin(ogen) binds growth factors and promotes tissue repair when
incorporated within a synthetic matrix, Proc. Natl. Acad. Sci.
USA, 110, 4563-4568.
16.Haase, I., Evans, R., Pofahl, R., and Watt, F. M.
(2003) Regulation of keratinocyte shape, migration and wound
epithelialization by IGF-1- and EGF-dependent signaling pathways, J.
Cell Sci., 116, 3227-3238.
17.Werner, S., and Grose, R. (2003) Regulation of
wound healing by growth factors and cytokines, Physiol. Rev.,
83, 835-870.
18.Annunziata, M., Granata, R., and Ghigo, E. (2011)
The IGF system, Acta Diabetol., 48, 1-9.
19.LeRoith, D., and Roberts, C. T., Jr. (2003) The
insulin-like growth factor system and cancer, Cancer Lett.,
195, 127-137.
20.Firth, S. M., and Baxter, R. C. (2002) Cellular
actions of the insulin-like growth factor binding proteins,
Endocrinol. Rev., 23, 824-854.
21.Lee, P. D. K., Conover, C. A., and Powell, D. R.
(1993) Regulation and function of insulin-like growth factor-binding
protein-1, Exp. Biol. Med., 204, 4-29.
22.Marcinkiewicz, M., and Gordon, P. V. (2008) A
role for plasmin in platelet aggregation: differential regulation of
IGF release from IGF-IGFBP complexes? Growth Horm. IGF
Res., 18, 325-334.
23.Campbell, P. G., Novak, J. F., Yanosick, T. B.,
and McMaster, J. H. (1992) Involvement of the plasmin system in
dissociation of the insulin-like growth factor-binding protein complex,
Endocrinology, 130, 1401-1412.
24.Campbell, P. G., Durham, S. K., Hayes, J. D.,
Suwanichkul, A., and Powell, D. R. (1999) Insulin-like growth
factor-binding protein-3 binds fibrinogen and fibrin, J. Biol.
Chem., 274, 30215-30221.
25.Huang, L., and Lord, S. T. (2013) The isolation
of fibrinogen monomer dramatically influences fibrin polymerization,
Thromb. Res., 131, 258-263.
26.Cohn, E. J., Strong, L. E., Hughes, W. L., Jr.,
Mulford, D. J., Ashworth, J. N., Melin, M., and Taylor, H. L. (1946)
Preparation and properties of serum and plasma proteins. IV. A system
for the separation into fractions of the protein and lipoprotein
components of biological tissues and fluids, J. Am. Chem. Soc.,
63, 459-475.
27.Fowell, A. H. (1955) Turbidimetric method of
fibrinogen assay, Am. J. Clin. Pathol., 25, 340-342.
28.Lagundzin, D., Masnikosa, R., Miljus, G.,
Robajac, D., and Nedic, O. (2010) An investigation of the different
molecular forms of IGFBP-1 using immobilized metal-, immuno-, and
lectin-affinity chromatography, J. Serb. Chem. Soc., 75,
1481-1489.
29.Westwood, M., Aplin, J. D., Collinge, I. A.,
Gill, A., White, A., and Gibson, J. M. (2001) α2-Macroglobulin: a
new component in the insulin-like growth factor/insulin-like growth
factor binding protein-1 axis, J. Biol. Chem., 276,
41668-41674.
30.Sunderic, M., Malenkovic, V., and Nedic, O.
(2015) Complexes between insulin-like growth factor binding proteins
and alpha-2-macroglobulin in patients with tumor, Exp. Mol.
Pathol., 98, 173-177.
31.Shibuya, H., Sakai, K., Kabir-Salmani, M., Wachi,
Y., and Iwashita, M. (2011) Polymerization of insulin-like growth
factor-binding protein-1 (IGFBP-1) potentiates IGF-I actions in
placenta, J. Cell. Physiol., 226, 434-439.
32.Sakai, K., Busby, W. H., Jr., Clarke, J. B., and
Clemmons, D. R. (2001) Tissue transglutaminase facilitates the
polymerization of insulin-like growth factor-binding protein-1
(IGFBP-1) and leads to loss of IGFBP-1 ability to inhibit insulin-like
growth factor-I-stimulated protein synthesis, J. Biol. Chem.,
276, 8740-8745.
33.Masnikosa, R., Zivkovic, B., and Nedic, O. (2009)
IGFBP-1 forms associates with placental cell membranes, J. Serb.
Chem. Soc., 74, 707-716.
34.Dunn, S., and Spiro, R. (1967) The
α2-macroglobulin of human plasma, J. Biol. Chem.,
242, 5549-5555.
35.Adamczyk, B., Struwe, W. B., Ercan, A., Nigrovic,
P. A., and Rudd, P. M. (2013) Characterization of fibrinogen
glycosylation and its importance for serum/plasma N-glycome
analysis, J. Proteome Res., 12, 444-454.
36.Krusius, T., Finne, J., and Rauvala, H. (1976)
The structural basis of the different affinities of two types of acidic
N-glycosidic glycopeptides for concanavalin A-Sepharose, FEBS
Lett., 71, 117-120.
37.Rajaram, S., Baylink, D. J., and Mohan, S. (1997)
Insulin-like growth factor-binding proteins in serum and other
biological fluids: regulation and functions, Endocrinol. Rev.,
18, 801-831.
38.Hwa, V., Oh, Y., and Rosenfeld, R. G. (1999) The
insulin-like growth factor-binding protein (IGFBP) superfamily,
Endocrinol. Rev., 20, 761-787.
39.Weber, M. M., Spottl, G., Gossl, C., and
Engelhardt, D. (1999) Characterization of human insulin-like growth
factor-binding proteins by two-dimensional polyacrylamide gel
electrophoresis and western ligand blot analysis, J. Clin.
Endocrinol. Metab., 84, 1679-1684.
40.Hjortebjerg, R., and Frystyk, J. (2013)
Determination of IGFs and their binding proteins, Best Pract. Res.
Clin. Endocrinol. Metab., 27, 771-781.
41.Brandt, K., Grunler, J., Brismar, K., and Wang,
J. (2015) Effects of IGFBP-1 and IGFBP-2 and their fragments on
migration and IGF-induced proliferation of human dermal fibroblasts,
Growth Horm. IGF Res., 25, 34-40.
42.Jones, J., Gockerman, A., Busby, W. H., Jr.,
Wright, G., and Clemmons, D. R. (1993) Insulin-like growth factor
binding protein 1 stimulates cell migration and bind to the
α5β1 integrin by means of its Arg-Gly-Asp sequence, Proc.
Natl. Acad. Sci. USA, 90, 10553-10557.
43.Ramos-Mozo, P., Rodriguez, C., Pastor-Vargas, C.,
Blanco-Colio, L. M., Martinez-Gonzalez, J., Meilhac, O., Michel, J.-B.,
Vega de Cenga, M., Egido, J., and Martin-Ventura, J. L. (2012) Plasma
profiling by a protein array approach identifies IGFBP-1 as a novel
biomarker of abdominal aortic aneurysm, Atherosclerosis,
221, 544-550.
44.Kobayashi, M., Kawase, T., Horimizu, M., Okuda,
K., Wolff, L. F., and Yoshie, H. (2012) A proposed protocol for the
standardized preparation of PRF membranes for clinical use,
Biologicals, 40, 323-329.
45.Aneke-Nash, C. S., Parrinello, C. M., Rajpathak,
S. N., Rohan, T. E., Strotmeyer, E. S., Kritchevsky, S. B., Psaty, B.
M., Buzikova, P., Kizer, J. R., Newman, A. B., Strickler, H. D., and
Kaplan, R. C. (2015) Changes in insulin-like growth factor-I and its
binding proteins are associated with diabetes mellitus in older adults,
J. Am. Geriatr. Soc., 63, 902-909.
46.Carr, M. E. (2001) Diabetes mellitus a
hypercoagulable state, J. Diabetes Complicat., 15,
44-54.