REVIEW: Age-Related Changes in Ultrastructure of Mitochondria. Effect of SkQ1
L. E. Bakeeva
Lomonosov Moscow State University, Belozersky Institute of Physico-Chemical Biology, 119991 Moscow, Russia; fax: +7 (495) 939-3181; E-mail: bakeeva@belozersky.msu.ru
Received July 7, 2015
For many years, investigators have attempted to identify unique ultrastructural conditions of mitochondria related to aging. However, this did not result in definitive results. At present, this issue has again become of topical interest due to development of the mitochondrial theory of aging and of engineering of a novel antioxidant class known as mitochondria-targeted antioxidants. The review briefly discusses experimental results that, from our perspective, allow the most objective understanding regarding age-related changes in mitochondrial ultrastructure.
KEY WORDS: aging, mitochondrial ultrastructure, age-related changesDOI: 10.1134/S0006297915120068
Aging is a biological topic that constantly sparks great interest. As early as in the 1960s, when cell ultrastructural investigations began to develop, various subcellular factors were considered to play an important role in aging. The viewpoint arose that aging was a manifestation of events taking place directly inside the cell cytoplasm [1].
This idea marked the beginning for investigating ultrastructure of cells and their organelles during aging. Among all cell organelles, mitochondria turned out to undergo the most ultrastructural changes upon aging. It seemed extremely important to reveal ultrastructural features in mitochondria pointing to the aging process based on the crucial role mitochondria play in metabolism as well as taking into consideration highly dynamic and function-dependent parameters such as position of mitochondria inside cells and their internal organization. By now, quite a large number of publications have appeared investigating changes in cell ultrastructure, but commonly accepted ultrastructural signs unequivocally pointing to aging include accumulation of lipofuscin granules and appearance of myelin-like formations (electron-dense multilayered concentric structures).
According to numerous publications, age-related changes in mitochondrial ultrastructure within various body tissues (including myocardium and skeletal muscle) turned out to be the same: mitochondrial swelling, reduced cristae, lucent matrix, and damaged mitochondrial matrix membranes [2-7]. Using morphometric ultrastructural assays, it was also shown that upon aging the ratio of area of the inner mitochondrial membrane to mitochondrial volume in hamster myocardium was decreased [8], whereas mice of C57BL/6 strain were observed to have shortened long axis and elongated short axis of mitochondria [9]. Analysis of biopsy samples from skeletal muscle of elderly persons (69-70-year-old) revealed that the number of subsarcolemmal [10] and interfibrillar [11] mitochondria was decreased. Tomanek and Karlsson [12] conducted a thorough investigation of myocardial ultrastructure from young (3-6 months) and aged (27-28 months) rats, and they did not find these age-related changes in mitochondria such as cristae fragmentation and lowered density of mitochondrial matrix. However, they described the appearance of large clusters of mitochondria within the subsarcolemmal region that differed in ultrastructure from the major mitochondrial population. Riva et al. [13] also reveal no destructive changes in cristae using scanning electron microscopy for examining mitochondrial ultrastructure in rat myocardium upon aging.
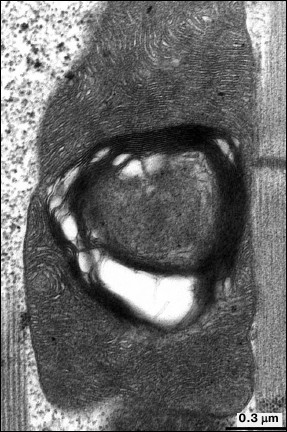
Examination of aging at the level of cellular and organellar ultrastructure in insect flight muscles is considered a promising approach. In 1943, Williams et al. for the first time experimentally demonstrated [14] that flight activity of insects significantly decreases with age. A classic study by Sacktor and Shimada [15] regarding age-related changes in mitochondrial morphology in flight muscle of the blowfly Phormia regina demonstrated that the mitochondria underwent degeneration characterized by regions of inner mitochondrial membrane locally reorganized into myelin-like structures. Later, myelin-like changes extended to the entire space of mitochondria, resulting in destruction of mitochondrial structure [16] (Fig. 1). Such changes in mitochondria were not related to lysosomal activity and were characterized by lacking oxidase activity in altered regions of the mitochondria, whereas intact areas were shown to have preserved cytochrome-oxidase activity.
Fig. 1. Mitochondrial ultrastructure in flight muscle of 52-day-old D. melanogaster.
Such features of mitochondrial ultrastructure developing in the majority of mitochondria of insect flight muscles upon aging reflect partial mitochondrial dysfunction supposedly affecting flight activity of insects. However, for long time the mechanism of age-related mitochondrial injury in flight muscles remained without explanation.
Oxidative stress is now considered as the main component in development of aging. According to this idea, oxidative stress and upregulated production of reactive oxygen species (ROS) underlie age-related tissue damage and aging. Mitochondria are considered as the major source of ROS, and their functional and structural characteristics are related to the mitochondrial theory of aging (a special case of the free-radical theory) [17].
According to current understanding regarding mechanisms of aging, Walker and Benzer [18] investigated the impact of oxidative stress on Drosophila strain white1118. It turned out that exposure of 3-4-day-old the Drosophila to hyperoxia for 4 days resulted in appearance of changes in mitochondrial ultrastructure in 35% of mitochondria from flight muscles that were typical of aging. Moreover, 7 days later after exposure, such destructive changes were found in 62% of mitochondria, whereas no/isolated changes were observed in insects from the control group.
In addition, mitochondrial areas with abnormal ultrastructure (myelin-like clusters) developed in experimental setting were shown to contain no cytochrome oxidase activity, whereas native regions of mitochondria were characterized by positive signal for oxidase activity. It was concluded that development of myelin-like clusters in mitochondria in response to oxidative stress was related to appearing defects in the mitochondrial respiratory chain, local loss of cytochrome c oxidase activity, and conformational changes in cytochrome c.
Thus, for the first time in flight muscle it was experimentally shown that oxidative stress had a determining influence on development of age-related changes in mitochondrial ultrastructure.
In connection with investigating the role of oxidative stress in development of body aging, great attention rose for creating specific mitochondrial antioxidants selectively targeting mitochondria and preventing toxic effects of ROS.
V. P. Skulachev proposed to use antioxidants conjugated to cations able to permeate through cell membranes and effectively neutralize ROS inside mitochondria – a novel type of antioxidants (SkQ). Experimentally, derivatives of SkQ having the highest permeating capacity were selected, in particular, SkQ1 [19, 20].
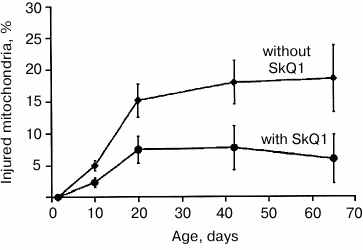
Experiments with Drosophila melanogaster [21-23] demonstrated that SkQ1 increased the lifespan of the flies without accompanying age-related lowered body activity, and the mitochondria from flight muscles had more native ultrastructure. By comparing the state of mitochondrial ultrastructure in flight muscle in insects aged 1.5 days every 10 days over the entire lifespan (65-70 days) of insects treated or not with SkQ1 (over the entire lifespan), it was found that number of destructive changes and their intensity were lower in the flies treated with SkQ1 (Fig. 2).
Fig. 2. Effect of SkQ1 on ultrastructural changes in mitochondria.
It is known that brain and muscle tissue suffer most from oxidative injury [4], and experimental studies on these tissues are of great interest. Experiments done with Wistar rats in in vivo heart ischemia/reperfusion (a model of cardiac infarction) revealed that the area of myocardial ischemia was significantly decreased after feeding animals with SkQ1-supplemented food for three weeks. Mitochondrial ultrastructure in this area matched that in animals from the control group, whereas rats not treated with SkQ1 were shown to have profound injury of all structural elements of cardiomyocytes and significantly altered mitochondrial ultrastructure [23].
In in vivo models (rat strains Wistar and OXYS, aged two years, and treated with SkQ1 over the lifespan starting from age three months), it was shown that development of age-related changes in mitochondrial ultrastructure both in cardiomyocytes and skeletal muscle was not prevented.
The OXYS rat strain has a genetically engineered metabolic deficiency, in particular, reduced resistance to oxidative stress resulting in progeria. It is assumed that disturbed structure and function of mitochondria underlie the pathogenesis of premature aging in the OXYS rat strain, and their maximum lifespan is decreased by 28% [24].
It was demonstrated that age-related changes in cardiomyocyte mitochondrial ultrastructure in the Wistar and OXYS rats differ from such changes published in the available literature as well as changes observed under various pathological myocardial states such as hypoxia, ischemia, hypertrophy, and cardiomyopathies [25]. Such changes were observed to affect both general morphology of the entire mitochondrial population as well as ultrastructure of single mitochondria. Changes in the entire mitochondrial population were only revealed in single cardiomyocytes – very tiny mitochondria, electron-transparent matrix, and small number of cristae clustered among myofibrils. No such changes were found in adjacent cardiomyocytes. In addition, these changes were detected at higher rate with more pronounced intensity in the OXYS than in the Wistar rat strain.
No destructive changes in ultrastructure of mitochondria observed in insect flight muscle were found in cardiomyocytes from aged Wistar and OXYS rats. Mainly, subsarcolemmal mitochondria succumbed to such alterations. Mitochondria from cardiomyocytes of aged rats were shown to lose proper mutually parallel arrangement of cristae in the inner mitochondrial membrane, and local myelin-like concentric formations or solitary membrane stacks occurred. The mitochondrial matrix space was substantially extended and filled with granular content [25].
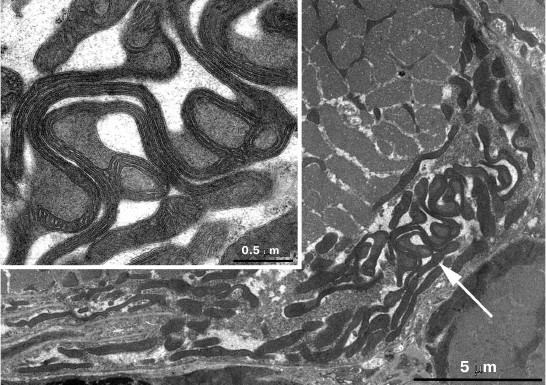
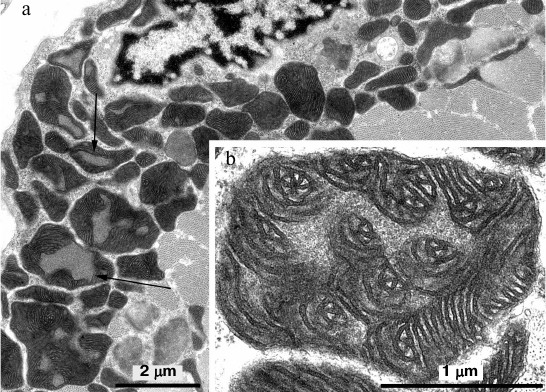
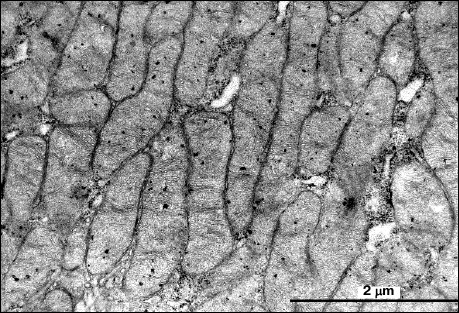
In skeletal muscle from aging Wistar and OXYS rats, changes in mitochondrial ultrastructure were found as well. The morphological organization of mitochondria did not match the well-known ultrastructure of mitochondria from skeletal muscles (Fig. 3). As a result, abnormal extended snake-like structures with local dilations occur. Such structures were formed by two-to-three orthogonally positioned elongated cristae intermitting with local dilations. The latter represent a markedly enlarged intermembrane space containing concentric layers of cristae and filled with homogenous matter. Usually, mitochondria have electron-transparent intermembrane space. At the same time, interfibrillar mitochondria contain no such changes in their ultrastructure. Such atypical mitochondria were found only in the subsarcolemmal region of solitary red muscle fibers mainly in the OXYS rats. In both Wistar and OXYS rats, the majority of red muscle fibers also contained subsarcolemmal mitochondria with marked yet not so striking changes. In all mitochondria, extended areas of intermembrane space lacking cristae and filled with content of lower than matrix electron density were developed. Cristae were documented to partially lose mutually parallel arrangement and form stellar structures (Fig. 4).
Fig. 3. Clustering of atypical mitochondria in the subsarcolemmal region of red muscle fibers from 24-month-old OXYS rats. a) Panoramic image; the arrow points to clustered mitochondria depicted in (b) with higher magnification.
Fig. 4. Mitochondrial ultrastructure in subsarcolemmal region of red muscle fibers from 24-month-old OXYS rats. a) Panoramic image, arrows point at enlarged regions of intermembrane space; b) ultrastructure of solitary mitochondrion, higher magnification.
Ultrastructural signs of developing abnormal mitochondrial changes were already observed in three-month-old OXYS rats [25], which are in good agreement with the idea that disturbed structure and function of mitochondria occur as early as at age of two-three months and then aggravate, and they play a key role in premature aging of OXYS rats [26]. It can be assumed that SkQ1 does not prevent development of age-related abnormal changes in mitochondrial ultrastructure of muscle tissue, as changes in mitochondrial ultrastructure occur much earlier than in animals that were fed with SkQ1-supplemented food. At the same time, SkQ1 influences functional and structural changes in internal ultrastructure of skeletal muscle interfibrillar mitochondria upon aging of both Wistar and OXYS rats [27]. Animals (aged 24 months) receiving SkQ1 with food were shown to have ultrastructure of interfibrillar mitochondria that matched the energized state, compared to the de-energized ultrastructure of interfibrillar mitochondria observed in the control group.
The OXYS rat strain undergoing premature aging represents a unique model for investigating the aging process in the eye [26]. In 11-month-old OXYS rats (under developing verified retinopathy), mitochondrial ultrastructure of pigment epithelium did not undergo changes. Age-matched animals treated with SkQ1 ameliorating manifestations of retinopathy were shown to develop a potent system of mitochondrial reticulum, wherein no changes in mitochondrial ultrastructure were observed [28].
In aging animals, mitochondrial ultrastructure in acinar cells from the lacrimal gland responsible for development of the condition typical for “dry eye” syndrome remains unchanged until acinar cells are fully destroyed, despite significant age-related changes in lacrimal gland tissue [29].
Thus, ultrastructural examination of various tissues in the same individual animal revealed that the mitochondrial response to aging in different tissues was not the same. In particular, upon aging, Wistar and OXYS rats underwent significant alterations of mitochondrial ultrastructure in cardiomyocytes and skeletal muscle, but not in eye tissues responsible for development of age-related pathologies (pigment epithelium, lacrimal gland). By comparing age-related changes in mitochondria [30] from various body tissues in the same individual animal, it was noted that such changes were more common in skeletal muscle than in lymphocytes.
Nevertheless, rats of the Sprague-Dawley strain (aged 25.5 months) were found to have profound changes in mitochondrial ultrastructure in solitary acini of the lacrimal gland: the space of all acinar cells was entirely filled with enlarged densely spaced mitochondria. Usually, irrespective of age, it was observed that acinar cells in Sprague-Dawley (akin to Wistar and OXYS) rats contained a small number of tiny mitochondria having single cristae (Fig. 5).
Fig. 5. Mitochondrial ultrastructure of acini of 25.5-month-old Sprague-Dawley rats.
Formation of marked mitochondrial clusters upon aging has been mainly observed in myocardium and skeletal muscle [12, 31]. Such changes were interpreted as proliferative and structural responses of these body tissues to mitochondrial dysfunction occurring upon aging. In addition, profound ultrastructural changes in mitochondrial proliferation upon aging, which differed from the well-known hypertrophy or hyperplasia of mitochondria during various cardiomyopathies and other pathological settings, were also noted [30-36].
To some degree, age-related changes in ultrastructural organization of mitochondria in various body tissues occurring upon aging and discussed in our paper are conditional. Among a great body of literature dedicated to structural and functional organization of mitochondria upon aging, it is always possible to come across papers providing results differing from our idea regarding age-related changes in mitochondrial ultrastructure. Ultrastructural versus functional changes in mitochondria developing upon aging were less investigated, underlying the fact that many reports convincingly demonstrating age-related changes in mitochondria often contained untenable ultrastructural data [37-39]. In addition, non-native biological samples are often used while analyzing mitochondrial ultrastructure in age-mediated diseases [40].
By analyzing the data of the above-mentioned studies, it can be unequivocally concluded that there is no universal unifying ultrastructural state of mitochondria typical to aging. However, at the same time special function-related ultrastructural states in mitochondria pointing to aging were found in each particular body tissue. The data on ultrastructural studies regarding age-related mitochondrial changes are of great importance for analyzing the efficacy of mitochondria-targeted antioxidants.
REFERENCES
1.Policard, A., and Bessis, M. (1968) Elements de
Pathologie Cellulaire, MASSON et Cie, Paris.
2.Martin, L., Feldman, M. L., and Navaratnam, V.
(1981) Ultrastructural changes in atrial myocardium of the ageing rat,
J. Anat., 133, 7-17.
3.Beregi, E., Regius, O., Huttl, T., and Gobl, Z.
(1988) Age-related changes in the skeletal muscle cells, Z.
Gerontol., 21, 83-86.
4.Brunk, U. T., and Terman, A. (2002) The
mitochondrial-lysosomal axis theory of aging, Eur. J. Biochem.,
269, 1996-2002.
5.Bakarev, M. A., and Nepomnyashchikh, L. M. (2004)
Structural manifestations of disturbed mitochondrial function in
skeletal muscle of prematurely aging rat strain OXYS, Bull. Exp.
Biol. Med., 138, 674-679.
6.Feher, J., Kovacs, I., Artico, M., Cavallotti, C.,
Papale, A., and Gabrieli, C. B. (2006) Mitochondrial alterations of
retinal pigment epithelium in age-related macular degeneration,
Neurobiol. Aging, 27, 983-993.
7.Corsetti, G., Pasini, E., D’Antona, G.,
Nisoli, E., Flati, V., Assanelli, D., Dioguardi, F. S., and Bianchi, R.
(2008) Morphometric changes induced by amino acid supplementation in
skeletal and cardiac muscles of old mice, Am. J. Cardiol.,
101, 26-34.
8.Sachs, H., Colgan, J., and Lazarus, M. L. (1977)
Ultrastructure of the aging myocardium: a morphometric approach, Am.
J. Anat., 150, 63-71.
9.Cheng, Z., Ito, S., Nishio, N., Thanasegaran, S.,
Fang, H., and Isobe, K. (2013) Characteristics of cardiac aging in
C57BL/6, Exp. Gerontol., 48, 341-348.
10.Crane, J. D., Devries, C., Safdar, A., Hamadeh,
M. J., and Tarnopolsky, M. A. (2010) The effect of aging on human
skeletal muscle mitochondrial and intramyocellular lipid
ultrastructure, J. Gerontol., 65, 119-128.
11.Orlander, J., Kiessling, K., Larsson, L.,
Karlsson, J., and Aniansson, A. (1978) Skeletal muscle metabolism and
ultrastructure in relation to age in sedentary men, Acta Physiol.
Scand., 104, 249-261.
12.Tomanek, R., and Karlsson, U. L. (1973)
Myocardial ultrastructure of young and senescent rats, J.
Ultrastruct. Res., 42, 201-220.
13.Riva, A., Tandler, B., Lesnefsky, E., Conti, G.,
Loffredo, F., Vazquez, E., and Hoppel, C. (2006) Structure of cristae
in cardiac mitochondria of aged rat, Mech. Ageing Dev.,
127, 917-921.
14.Williams, C. M., Barness, L. A., and Sawyer, W.
H. (1943) The utilization of glycogen by flies during flight and some
aspects of the physiological ageing of Drosophila, Biol.
Bull. (Woods Hole), 84, 263-268.
15.Sacktor, B., and Shimada, Y. (1972) Degenerative
changes in the mitochondria of flight muscle from aging blowflies,
J. Cell Biol., 52, 465-477.
16.Bakeeva, L. E., Saprunova, V. B., Pasyukova, E.
G., and Roshchina, N. V. (2007) Mitoptosis in flight muscle of
Drosophila melanogaster, Dokl. Akad. Nauk, 413,
1-3.
17.Skulachev, V. P. (2009) Functions of
mitochondria: from intracellular power stations to mediators of a
senescence program, Cell. Mol. Life Sci., 66,
1785-1793.
18.Walker, D. W., and Benzer, S. (2004)
Mitochondrial “swirls” induced by oxygen stress and in the
Drosophila mutant hyperswirl, Proc. Natl. Acad. Sci. USA,
101, 10290-10295.
19.Skulachev, V. P. (2007) A biochemical approach to
the problem of aging: “megaproject” on membrane-penetrating
ions. The first results and prospects, Biochemistry (Moscow),
72, 1385-1396.
20.Antonenko, Yu. N., Avetisyan, A. V., Bakeeva, L.
E., Chernyak, B. V., Chertkov, V. A., Domnina, L. V., Ivanova, O. Yu.,
Izyumov, D. S., Khaylova, L. S., Klishin, S. S., Korshunova, G. A.,
Lyamzaev, K. G., Muntyan, M. S., Nepryakhina, O. K., Pashkovskaya, A.
A., Pletyushkina, O. Yu., Pustovidko, A. V., Roginskiy, V. A.,
Rokitskaya, T. I., Ruuge, E. K., Saprunova, V. B., Severina, I. I.,
Simonyan, R. A., Skulachev, I. V., Skulachev, M. V., Sumbatyan, N. V.,
Sviryaeva, I. V., Tashlitskiy, V. N., Vasil’ev, Yu. M., Vysokikh,
M. Yu., Yaguzhinskiy, L. S., Zamyatnin, A. A., and Skulachev, V. P.
(2008) Mitochondria-targeted plastoquinone derivative as a means for
disrupting aging program. 1. Cationic derivatives of plastoquinone:
synthesis and in vitro examination, Biochemistry
(Moscow), 73, 1273-1287.
21.Anisimov, V. N., Bakeeva, L. E., Egormin, P. A.,
Filenko, O. F., Isakova, E. F., Manskikh, V. N., Mikhel’son, V.
M., Panteleeva, A. A., Pasyukova, E. G., Pilipenko, D. I., Piskunova,
T. S., Popovich, I. G., Roshchina, N. V., Rybina, O. Yu., Saprunova, V.
B., Samoylova, T. A., Semenchenko, A. V., Skulachev, M. V., Spivak, I.
M., Tsybul’ko, E. A., Tyndyk, M. L., Vysokikh, M. Yu., Yurova, M.
N., Zabezhinskiy, M. A., and Skulachev, V. P. (2008)
Mitochondria-targeted plastoquinone derivative as a means for
disrupting aging program. 5. SkQ1 increases lifespan and prevents
developing signs of aging, Biochemistry (Moscow), 73,
1329-1342.
22.Skulachev, V. P., Anisimov, V. N., Antonenko, Y.
N., Bakeeva, L. E., Chernyak, B. V., Erichev, V. P., Filenko, O. F.,
Kalinina, N. I., Kapelko, V. I., Kolosova, N. G., Kopnin, B. P.,
Korshunova, G. A., Lichinitser, M. R., Obukhova, L. A., Pasyukova, E.
G., Pisarenko, O. I., Roginsky, V. A., Ruuge, E. K., Senin, I. I.,
Severina, I. I., Skulachev, M. V., Spivak, I. M., Tashlitsky, V. N.,
Tkachuk, V. A., Vyssokikh, M. Y., Yaguzhinsky, L. S., and Zorov, D. B.
(2009) An attempt to prevent senescence: a mitochondrial approach,
Biochim. Biophys. Acta, 1787, 437-461.
23.Bakeeva, L. E., Barskov, I. V., Egorov, M. V.,
Isaev, N. K., Kapel’ko, V. I., Kazachenko, A. V., Kirpatovskiy,
V. I., Kozlovskiy, S. V., Lakomkin, V. L., Levina, S. V., Pisarenko, O.
I., Plotnikov, E. Yu., Saprunova, V. B., Serebryakova, L. I.,
Skulachev, M. V., Stelmashook, E. V., Studneva, I. M., Tskitishvili, O.
V., Vasil’eva, A. K., Viktorov, I. V., Zorov, D. B., and
Skulachev, V. P. (2008) Mitochondria-targeted plastoquinone derivatives
as tools to interrupt execution of the aging program. 2. Treatment of
some ROS- and age-related diseases (heart arrhythmia, heart
infarctions, kidney ischemia, and stroke), Biochemistry
(Moscow), 73, 1288-1299.
24.Shabalina, I. G., Kolosova, N. G., Grishanova, A.
Yu., Solovyov, V. N., Salganik, R. I., and Solovyova, N. A. (1995)
Oxidative phosphorylation activity, F0F1-ATPase, and cytochrome content
in liver mitochondria of rats with inherited hyperproduction of free
radicals, Biochemistry (Moscow), 66, 1563-1568.
25.Bakeeva, L. E., Vays, V. B., and Vangeli, I. M.
(2014) Age-related changes in mitochondrial ultrastructure of muscular
tissue, Biochemistry (Moscow). Suppl. Ser A: Membr. Cell
Biol., 8, 16-22.
26.Kolosova, N. G., Lebedev, P. A., Fursova, A. Zh.,
Moroskova, T. S., and Gusarevich, O. G. (2003) Prematurely aging OXYS
rat strain as a model of human senile cataract, Usp. Gerontol.,
12, 143-148.
27.Vays, V. B., Eldarov, C. M., Kolosova, N. G., and
Bakeeva, L. E. (2014) Antioxidant SkQ1 delays sarcopenia-associated
damage of mitochondrial ultrastructure, Aging, 6,
140-148.
28.Saprunova, V. B., Pilipenko, D. I., Alekseevskiy,
A. V., Fursova, A. Zh., Kolosova, N. G., and Bakeeva, L. E. (2010)
Lipofuscin granule dynamics during development of age-related macular
degeneration, Biochemistry (Moscow), 75, 130-138.
29.Vays, V. B., Vangeli, I. M., and Bakeeva, L. E.
(2014) Ultrastructural changes in lacrimal gland of Wistar rats upon
aging, Bull. Exp. Biol. Med., 157, 235-240.
30.Bourgeois, J. M., and Tarnopolsky, M. A. (2004)
Pathology of skeletal muscle in mitochondrial disorders,
Mitochondrion, 4, 441-452.
31.Feldman, M. L., and Navaratnam, V. (1981)
Ultrastructural changes in atrial myocardium of the ageing rat, J.
Anat., 133, 7-17.
32.Hubner, G., and Grantzow, R. (1983) Mitochondrial
cardiomyopathy with involvement of skeletal muscles, Virch. Arch.
Pathol. Anat., 399, 115-125.
33.James, T. N., Terasaki, F., Pavlovich, E. R., and
Vikhert, A. N. (1993) Apoptosis and pleomorphic micromitochondriosis in
the sinus nodes surgically excised from five patients with the long QT
syndrome, J. Lab. Clin. Med., 122, 309-323.
34.Klembovskiy, A. I., and Sukhorukov, V. S. (1997)
Mitochondrial insufficiency in children, Arch. Patol.,
59, 3-7.
35.Klembovskiy, A. I. (2008) A role for
mitochondrial disorders in developing diseases. Therapeutic approaches,
Vrach, 5, 21-25.
36.Beregi, E., Regius, O., Hutti, T., and Gobl, Z.
(1988) Age-related changes in the skeletal muscle cells, Z.
Gerontol., 21, 83-86.
37.Yasuda, K., Ishii, T., Suda, H., Akatsuka, A.,
Hartman, P. S., Goto, S., Miyazawa, M., and Ishii, N. (2006)
Age-related changes of mitochondrial structure and function in
Caenorhabditis elegans, Mech. Ageing Dev., 127,
763-770.
38.Kawashima, M., Kawakita, T., Okada, N., Ogawa,
Y., Murat, D., Nakamura, S., Nakashima, H., Shimmura, S., Shimmura, K.,
and Tsubota, K. (2010) Calorie restriction: a new therapeutic
intervention for age related dry eye disease in rats, Biochem.
Biophys. Res. Commun., 397, 724-728.
39.Kawashima, M., and Tsubota, K. (2011) Effect of
calorie restriction on change in lacrimal gland with age,
Cornea, 30, 29-33.
40.Feher, J., Kovaks, I., Artico, M., Cavallotti,
C., Papale, A., and Gabrieli, C. B. (2006) Mitochondrial alterations of
retinal pigment epithelium in age-related macular degeneration,
Neurobiol. Aging, 27, 983-993.