Cytochrome c–Cardiolipin Complex in a Nonpolar Environment
A. S. Vikulina, A. V. Alekseev, E. V. Proskurnina, and Yu. A. Vladimirov*
Lomonosov Moscow State University, 119991 Moscow, Russia; E-mail: yuvlad@mail.ru* To whom correspondence should be addressed.
Received May 26, 2015; Revision received June 9, 2015
Programmed cell death (apoptosis) plays an important role in the life of multicellular organisms and in the development of socially significant human diseases. Cytochrome c–cardiolipin complex (Cyt-CL) is formed at the very beginning of a cascade of apoptotic reactions. Nevertheless, the structure of the complex and the mechanism of its participation in lipid peroxidation in mitochondrial membranes are not yet understood. In previous work (Vladimirov, Y. A., et al. (2011) Crystallography, 56, 712-719), it was shown that the Cyt-CL complex precipitates in concentrated water solution, the sediment containing orderly nanospheres formed by cytochrome c molecules with changed conformation and surrounded by a cardiolipin monolayer, and they are essentially hydrophobic. In this work, we obtained chloroform and hexane solutions of Cyt-CL with lipid/protein ratio of 77 ± 11. The conditions are described under which the solutions were obtained. Study of the properties of Cyt-CL solutions in hydrophobic media will reveal their structure and the mechanism of their catalytic activity inside the lipid layer of biological membranes.
KEY WORDS: apoptosis, lipid peroxidation, cytochrome c, cardiolipin, hydrophobic environmentDOI: 10.1134/S0006297915100107
Abbreviations: Cyt-CL, cytochrome c–cardiolipin complex; TOCL, 1,1′,2,2′-tetraoleoylcardiolipin.
The problem of programmed cell death, apoptosis, attracts great
attention of biologists and physicians. An important role in the
development of apoptosis belongs to cytochrome c [1, 2]. In an early stage of
apoptosis, proteins of the intermembrane space of mitochondria,
including cytochrome c, are released into the surrounding
cytoplasm and trigger a cascade of reactions followed by apoptosis [3, 4]. Cytochrome c release
from mitochondria is usually preceded by mitochondrial stress, mostly
due to increased generation of free radicals. It begins with the
reception of apoptotic signal from outside or from inner causes and
ends with lipid peroxidation, matrix swelling, formation of megapores
in the outer mitochondrial membrane, cytochrome c release from
mitochondria, and triggering of apoptosis [5-7]. Lipid peroxidation is mainly catalyzed by
cytochrome c molecules bound with cardiolipin, which is part of
the inner mitochondrial membrane [1].
A number of works with our participation [8-10] were dedicated to the study of the Cyt-CL peroxidase function. The investigations resulted in partial deciphering of the schemes of Cyt-CL-catalyzed peroxidase reactions, though the structure of the complex remained unrevealed.
The first data on the structure of complexes of lipids with proteins, including cytochrome c, were obtained in model systems with the methods of optical and electron microscopy and X-ray diffraction [11-13]. In the systems formed when dry phospholipid films were dispersed in a small amount of water, layered structures formed that were thought to be made up of two phospholipid monolayers and a bilayer of cytochrome c molecules between them [11]. Under ultrasonic treatment, those structures transformed to lipid vesicles. However, cardiolipin was not used in that work; thus, it did not give much information for understanding Cyt-CL structure. An important contribution to the study of the membrane-bound Cyt-CL was made in experiments with Cyt-CL monolayers [14] and liposomes prepared from cardiolipin and phosphatidylcholine to which cytochrome c was added [15, 16]. Brown and Wuthrich suggested a scheme according to which cytochrome was bound to the surface of the membrane lipid layer mainly with electrostatic forces and collected around itself other cardiolipin molecules remaining on the membrane surface in the form of clusters. At the same time, data were obtained suggesting that when cytochrome c bound with membranes containing anionic lipids including cardiolipin, changes in conformation of the polypeptide chain took place. This was shown by measurements of absorption in the region of 695 nm [9, 17-19], circular dichroism in the region of the Soret band (400-450 nm) [20-22], cytochrome c fluorescence spectra [9, 23], and IR spectra in the absorption region of the >NH·⋅· bond (Amide I and Amide II) [24]. The data suggest that, when forming a complex with cardiolipin, the whole cytochrome c molecule partially unfolds or melts [2]. It is the change of conformation that is considered the principal cause for the fact that cytochrome c acquires enzymatic activity that causes lipid peroxidation in the inner mitochondrial membrane followed by apoptosis. The explanation of these conformational changes usually invokes the assumption that one of the cardiolipin fatty acid chains penetrates into the cytochrome c molecule and thus changes the conformation of the protein (see reviews [2, 25-29]). However, recent works with new objects (giant liposomes [30] and water-insoluble Cyt-CL complexes [31]) and application of new methods (confocal fluorescent microscopy [30], fluorescent spectroscopy [9, 23], and X-ray structural analysis [31]) caused revision of the existing view [10]. It was shown that, in the presence of cardiolipin and other anionic lipids, cytochrome c not only attaches to the surface of the membrane lipid layer, but is able “to wind” the layer around itself, thus forming spherical Cyt-CL nanostructures (nanospheres) [10, 31].
The characteristics of nanospheres were determined from the data of X-ray low-angle scattering of cytochrome c–tetraoleoylcardiolipin (TOCL) pellet that settled from a concentrated water solution when a small volume (<10%) of methanol solution of cardiolipin was mixed with excess water solution of cytochrome c. The analysis of periodic peaks on the X-ray scattering curve indicated that the pellet was composed of regularly packed nanospheres 11.2 nm in diameter with a “melted” cytochrome c molecule of 5.6 nm in the center, whereas the surface was made up of a cardiolipin monolayer (Cyt-CL complex). The cardiolipin/protein stoichiometry in the Cyt-CL complex was determined by titration of cytochrome c/cardiolipin solutions with subsequent removal of the sedimented complex after each lipid addition and determination of cytochrome c in the supernatant. Cyt-CL nanospheres poorly dissolve in water, but they must dissolve in nonpolar solvents. But, to our knowledge, no attempts were made to prepare a solution of Cyt-CL nanospheres knowingly possessing hydrophobic surfaces in hydrophobic solvents, probably because the very existence of Cyt-CL complexes became known not long ago [31]. It should be noted that Das et al. obtained cytochrome c–phosphatidylethanolamine complexes with molar lipid/protein ratio of 24 : 1 dissolved in isooctane, but they were not successful in obtaining similar Cyt-CL complexes [32]. In this work, Cyt-TOCL complex in chloroform and n-hexane was obtained, and the lipid/protein ratio in solutions was determined. The results confirm the suggested structure of the Cyt-CL complex (nanospheres Cyt-CL) and promote further clarification of the structure and mechanism of functioning of this complex in mitochondrial membranes at early stages of apoptosis.
MATERIALS AND METHODS
Reagents and equipment. Horse-heart cytochrome c was obtained from Sigma (USA). 1,1′,2,2′-Tetraoleoylcardiolipin (TOCL) was from Avanti Polar Lipids (USA). KH2PO4 and KOH used for the preparation of a 20 mM phosphate buffer solution, pH 7.4, chloroform, n-hexane, and methanol (all of analytical grade) were from Sigma. To prepare a buffer solution used as a cytochrome c solvent, bidistilled deionized water was used (obtained with a Milli-Q water purification unit; Millipore, France).
Preparation of Cyt-CL solutions in hydrophobic solvent. The sequence of operations was as follows. At the first stage, a cytochrome c solution was prepared, to which a methanol solution of cardiolipin was quickly added. The mixture was stirred – it looked like a solution of red color due to containing cytochrome. In different experiments, we added different methanol percentage to the sample (see legends to Figs. 1 and 2). At the second stage, pure chloroform or a chloroform–methanol mixture (2 : 1) was added to an equal volume of the above mixture. The mixture was thoroughly stirred and left for a time (or centrifuged) until the mixture separated into two phases. The appearance of scarlet coloration in the lower solution meant that the Cyt-CL complex transferred to the chloroform phase. Cytochrome c transfer to chloroform was never observed in the absence of cardiolipin in the mixture. The lower solution (it is generally sufficient to take 80% of the lower phase volume) was gently taken from the tube using a syringe with a long needle and put either to a cuvette for measurement of the absorption spectra and determination of the amount of cytochrome c transferred to chloroform or to a vessel of an ALSI-FT R213B rotary evaporator (Farmtekh, Russia). After all the solvent was removed with the rotary evaporator, a dark red film containing Cyt-CL complex remained at the bottom of the vessel. The film can be dissolved in a hydrophobic organic solvent, for example, hexane. The solution was pink or red and transparent.
Measurement of cytochrome c and Cyt-CL concentrations. Cytochrome c concentration in water and water–methanol solutions was measured spectrophotometrically (a Specord 200 spectrophotometer with 1-cm pathlength glass cuvettes; Analytic Jena, Germany) using calibration curves for standard solutions of the protein in the same solvents. Cyt-CL concentration was measured proceeding from the assumption that the absorption at the maximum at 407 nm is similar in cytochrome c in water solution and in cytochrome c as part of the complex dissolved in chloroform. This assumption is corroborated by the similar shape of the cytochrome c spectrum in water and water–methanol solutions and in chloroform fraction at all wavelengths except the region of the absorption maximum at 695-700 nm.
RESULTS AND DISCUSSION
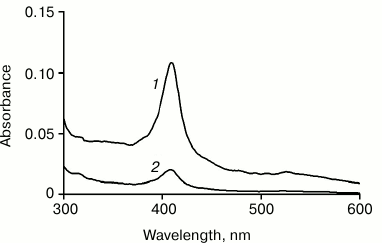
All our attempts to transfer the Cyt-CL complex prepared in water solution to low-polarity organic solvents, such as heptane or chloroform, were unsuccessful. Apparently, the hydrophobic interactions between nanospheres (or nanotubes) forming a crystal-like structure and having an expressed hydrophobic surface are so great that it is difficult to remove completely the water remaining in the sediment that the solvent is not able to detach the nanoparticles in the sediment from each other. In order that it can occur, a more polar solvent, such as methanol, should be added to the solvent. Figure 1 illustrates this situation: it shows the absorption spectra of hexane solutions of Cyt-TOCL obtained at different methanol concentrations in the mixture prior to the addition of chloroform.
Fig. 1. Absorption spectra of hexane solutions of Cyt-TOCL: 1) extraction with chloroform from cytochrome c solution in a water–methanol mixture of 10 : 11 v/v; 2) the same, but in a water–methanol mixture of 10 : 1 v/v. The initial amount of cytochrome was 20 nmol, of which 3.2 nmol (1) and 0.6 nmol (2) were transferred to hexane as determined by spectrophotometry.
These data show that the Cyt-CL complex transfers to chloroform and then to hexane significantly more actively at a higher content of methanol in a water–methanol mixture (Fig. 1, curve 1) than at a low methanol content (Fig. 1, curve 2). When the Cyt-CL mixture was dissolved in a solvent at water/methanol ratio of 10 : 1, the amount of the complex transferred to hexane was 0.6 nmol, while at the ratio of 10 : 11 it was 3.2 nmol (16% of 20 nmol cytochrome c in the initial mixture).
Our experiments showed that the transfer of the Cyt-CL complex from the water–methanol phase to chloroform did not depend on whether we added the major amount of methanol prior to the addition of chloroform or in the mixture with chloroform. In the latter case, the Cyt-CL transfer from water–buffer solution to chloroform strongly resembles the method of lipid extraction by Folch when 20-fold excess of a chloroform–methanol mixture (2 : 1) is added to tissue [33]. In our experiments, the chloroform–methanol–water ratio was 100 : 54 : 45. According to Folch, at a ratio of 100 : 50 : 37 (which is similar to ours), the upper phase consisted mainly of a water–methanol mixture (the ratio of chloroform–methanol–water was 8 : 48 : 47), and the lower phase consisted of a chloroform–methanol–water mixture (86 : 14 : 1). Most lipids in such a system pass into chloroform, but in the Cyt-CL-containing system, another species pass into chloroform, namely, hydrocarbon-covered “balls” with cytochrome c inside them.
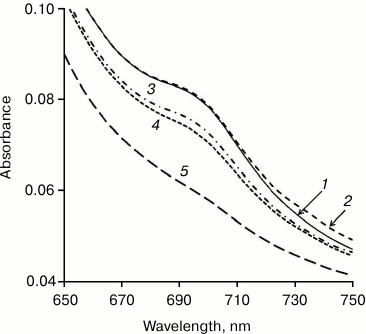
What is the role of methanol in the Cyt-CL transition in the hydrophobic phase? Cytochrome c is known to change reversibly its conformation (partial unfolding) on introduction of methanol into solution [34]. This results, in particular, in the disappearance of the absorption band at 695 nm (Fig. 2) due to the rupture of the coordination bond between heme iron and the sulfur atom of Met80. When the Cyt-CL complex is formed, the absorption band in this region also disappears [8, 9], with partial unfolding of cytochrome c confirmed by the appearance of fluorescence of tyrosine and tryptophan amino acid residues [23] and increase of the volume of the protein nuclei of the Cyt-CL nanospheres [31]. It is not yet clear whether the role of methanol in the transition of Cyt-CL to the hydrophobic phase is the consequence of its action on the protein, or that methanol, both in water and chloroform phases, modifies the properties of the lipid shell. Anyway, the addition of methanol is necessary for the transfer of the Cyt-CL complex to the hydrophobic phase.
Fig. 2. Changes in cytochrome c conformation under the action of methanol. Methanol concentration (%, v/v) in a water solution of cytochrome c: 1) 0; 2) 10; 3) 20; 4) 30; 5) 40. Disappearance of the absorption maximum at 695 nm indicates the rupture of the >Fe3+··· S(Met80) coordination bond as the result of partial unfolding of the protein molecule.
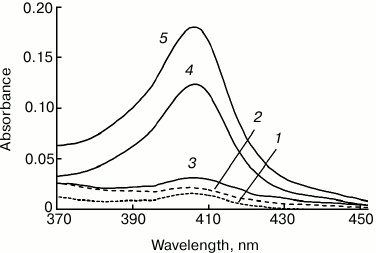
As mentioned above, in the absence of cardiolipin, cytochrome c does not pass to chloroform either in the absence or in the presence of 50% methanol. Figure 3 shows that at cardiolipin/cytochrome ratio of 10 : 1 and 15 : 1 in the initial mixture, cytochrome passes into chloroform only in small amounts (1 to 4% of the cytochrome in the system), while at a lipid/protein ratio of 20 : 1 and 30 : 1, the fraction of the hemoprotein transferred to the chloroform increases significantly – to 24 and 37%, respectively (data of spectrophotometry).
Fig. 3. Absorption spectra of Cyt-TOCL extracted to chloroform at different molar cardiolipin/cytochrome c ratios: 1) 0 : 1; 2) 10 : 1; 3) 15 : 1; 4) 20 : 1; 5) 30 : 1. The extraction was carried out by the addition of an equal chloroform volume to the solution of 20 µM cytochrome c and cardiolipin in water–methanol mixture (10 : 11, v/v).
The fact that a smaller fraction of the cytochrome dissolved in the water–methanol phase was distributed to chloroform in all our experiments is due to the presence of its excess as compared to cardiolipin, i.e. at a protein/lipid ratio greater than the final cytochrome c/TOCL ratio in chloroform. Under such conditions, Cyt-CL complex precipitated from water solution had the same composition regardless of the amount of cardiolipin added to the cytochrome solution [31].
Taking into account that on addition of Folch reagent all phospholipids pass from the initial water solution to the chloroform–methanol phase, it is evident that if we divide the amount of the added cardiolipin by the amount of cytochrome distributed to chloroform, we obtain a molar lipid/protein ratio in Cyt-CL complex in the hydrophobic phase. On the addition of a sufficient cardiolipin amount (a TOCL/Cyt ratio in the water–methanol medium of 20 : 1 and more), this ratio was found to be 77 ± 11, which was equal or slightly above the values we obtained for Cyt-CL complexes in the precipitate from water-buffer solutions [35]. Therefore, the Cyt-CL complex can be extracted by Folch solution and then transferred from chloroform to hexane.
The data corroborate the earlier suggestion of the possible existence of inverted micelles in biological membranes, which are formed by cytochrome c surrounded with cardiolipin molecules [36]. The structure of Cyt-CL nanospheres was already studied earlier [31]. Inclusion of cytochrome c in the shell of cardiolipin molecules enables the Cyt-CL complex to penetrate inside the lipid bilayer [10] and catalyze lipid peroxidation [1, 9], as well as pass though the membrane lipid bilayer [37]. Formation of Cyt-CL complex most likely plays a key role in triggering of apoptosis, though the structure of the complex and the mechanism of its participation in lipid peroxidation in mitochondrial membranes are not yet completely understood. Obtaining of Cyt-CL complex in the form of solution in nonpolar medium provides new possibilities for such investigations.
The study was financially supported by the Russian Foundation for Basic Research (grant No. 14-04-01361a).
REFERENCES
1.Kagan, V. E., Tyurin, V. A., Jiang, J., Tyurina, Y.
Y., Ritov, V. B., Amoscato, A. A., Osipov, A. N., Belikova, N. A.,
Kapralov, A. A., Kini, V. V., Vlasova, I. I., Zhao, Q., Zou, M., Di,
P., Svistunenko, D. A., Kurnikov, I. V., and Borisenko, G. G. (2005)
Cytochrome c acts as a cardiolipin oxygenase required for
release of proapoptotic factors, Nature Chem. Biol., 1,
223-232.
2.Kagan, V. E., Bayir, H. A., Belikova, N. A.,
Kapralov, A. A., Tyurina, Y. Y., Tyurin, V. A., Jiang, J., Stoyanovsky,
D. A., Wipf, P., Kochanek, P. M., Greenberger, J. S., Pitt, B.,
Shvedova, A. A., and Borisenko, G. G. (2009) Cytochrome
c/cardiolipin relations in mitochondria: a kiss of death,
Free Radic. Biol. Med., 46, 1439-1453.
3.Zhivotovsky, B., Orrenius, S., Brustugun, O. T.,
and Doskeland, S. O. (1998) Injected cytochrome c induces
apoptosis, Nature, 391, 449-450.
4.Skulachev, V. P. (1998) Cytochrome c in the
apoptotic and antioxidant cascades, FEBS Lett., 423,
275-280.
5.Skulachev, V. P. (1996) Why are mitochondria
involved in apoptosis? Permeability transition pores and apoptosis as
selective mechanisms to eliminate superoxide-producing mitochondria and
cell, FEBS Lett., 397, 7-10.
6.Skulachev, V. P. (2000) How proapoptotic proteins
can escape from mitochondria? Free Radic. Biol. Med., 29,
1056-1059.
7.Vladimirov, Y. A. (2002) Impairment of the barrier
properties of the inner and outer mitochondrial membranes, necrosis and
apoptosis, Biol. Membr. (Moscow), 19, 356-377.
8.Vladimirov, Y. A., Proskurnina, E. V., Izmailov, D.
Y., Novikov, A. A., Brusnichkin, A. V., Osipov, A. N., and Kagan, V. E.
(2006) Mechanism of activation of cytochrome c peroxidase
activity by cardiolipin, Biochemistry, 91, 989-997.
9.Belikova, N. A., Vladimirov, Y. A., Osipov, A. N.,
Kapralov, A. A., Tyurin, V. A., Potapovich, M. V., Basova, L. V.,
Peterson, J., Kurnikov, I. V., and Kagan, V. E. (2006) Peroxidase
activity and structural transitions of cytochrome c bound to
cardiolipin-containing membranes, Biochemistry, 45,
4998-5009.
10.Vladimirov, Y. A., Proskurnina, E. V., and
Alekseev, A. V. (2013) Molecular mechanisms of apoptosis. Structure of
cytochrome c–cardiolipin complex, Biochemistry
(Moscow), 78, 1086-1097.
11.Papahadjopoulos, D., and Miller, N. (1967)
Phospholipid model membranes. I. Structural characteristics of hydrated
liquid crystals, Biochim. Biophys. Acta, 135,
624-638.
12.Shipley, G. G., Leslie, R. B., and Chapman, D.
(1969) X-Ray diffraction study of the interaction of phospholipids with
cytochrome c in the aqueous phase, Nature, 222,
561-562.
13.Shipley, G. G., Leslie, R. B., and Chapman, D.
(1969) Small-angle X-ray scattering studies of cytochrome
c–phospholipid complexes, Biochim. Biophys. Acta,
173, 1-10.
14.Quinn, P. J., and Dawson, R. M. (1969) The
interaction of cytochrome c with monolayers of
phosphatidylethanolamine, Biochem. J., 113, 791-803.
15.Brown, L. R., and Wuthrich, K. (1977) A spin
label study of lipid oxidation catalyzed by heme proteins, Biochim.
Biophys. Acta, 464, 356-369.
16.Brown, L. R., and Wuthrich, K. (1977) NMR and ESR
studies of the interactions of cytochrome c with mixed
cardiolipin–phosphatidylcholine vesicles, Biochim. Biophys.
Acta, 468, 389-410.
17.De Jongh, H. H., Ritsema, T., and Killian, J. A.
(1995) Lipid specificity for membrane mediated partial unfolding of
cytochrome c, FEBS Lett., 360, 255-260.
18.Lee, I., Salomon, A. R., Yu, K., Doan, J. W.,
Grossman, L. I., and Huttemann, M. (2006) New prospects for an old
enzyme: mammalian cytochrome c is tyrosine-phosphorylated in
vivo, Biochemistry, 45, 9121-9128.
19.Nantes, I. L., Faljoni-Alario, A., Vercesi, A.
E., Santos, K. E., and Bechara, E. J. (1998) Liposome effect on the
cytochrome c-catalyzed peroxidation of carbonyl substrates to
triplet species, Free Radic. Biol. Med., 25, 546-553.
20.Sinibaldi, F., Fiorucci, L., Patriarca, A.,
Lauceri, R., Ferri, T., Coletta, M., and Santucci, R. (2008) Insights
into cytochrome c–cardiolipin interaction. Role played by
ionic strength, Biochemistry, 47, 6928-6935.
21.Nantes, I. L., Zucchi, M. R., Nascimento, O. R.,
and Faljoni-Alario, A. (2001) Effect of heme iron valence state on the
conformation of cytochrome c and its association with membrane
interfaces. A CD and EPR investigation, J. Biol. Chem.,
276, 153-158.
22.Letellier, L., and Shechter, E. (1973)
Correlations between structure and spectroscopic properties in membrane
model system. Fluorescence and circular dichroism of the cytochrome
c–cardiolipin system, Eur. J. Biochem., 40,
507-512.
23.Kapralov, A. A., Yanamala, N., Tyurina, Y. Y.,
Castro, L., Samhan-Arias, A., Vladimirov, Y. A., Maeda, A., Weitz, A.
A., Peterson, J., Mylnikov, D., Demicheli, V., Tortora, V.,
Klein-Seetharaman, J., Radi, R., and Kagan, V. E. (2011) Topography of
tyrosine residues and their involvement in peroxidation of
polyunsaturated cardiolipin in cytochrome c/cardiolipin
peroxidase complexes, Biochim. Biophys. Acta, 1808,
2147-2155.
24.Heimburg, T., and Marsh, D. (1993) Investigation
of secondary and tertiary structural changes of cytochrome c in
complexes with anionic lipids using amide hydrogen exchange
measurements: an FTIR study, Biophys. J., 65,
2408-2417.
25.Kagan, V. E., Borisenko, G. G., Tyurina, Y. Y.,
Tyurin, V. A., Jiang, J., Potapovich, A. I., Kini, V., Amoscato, A. A.,
and Fujii, Y. (2004) Oxidative lipidomics of apoptosis: redox catalytic
interactions of cytochrome c with cardiolipin and
phosphatidylserine, Free Radic. Biol. Med., 37,
1963-1985.
26.Bayir, H., Fadeel, B., Palladino, M. J., Witasp,
E., Kurnikov, I. V., Tyurina, Y. Y., Tyurin, V. A., Amoscato, A. A.,
Jiang, J., Kochanek, P. M., DeKosky, S. T., Greenberger, J. S.,
Shvedova, A. A., and Kagan, V. E. (2006) Apoptotic interactions of
cytochrome c: redox flirting with anionic phospholipids within
and outside of mitochondria, Biochim. Biophys. Acta,
1757, 648-659.
27.Kagan, V. E., Tyurina, Y. Y., Bayir, H., Chu, C.
T., Kapralov, A. A., Vlasova, I. I., Belikova, N. A., Tyurin, V. A.,
Amoscato, A., Epperly, M., Greenberger, J., Dekosky, S., Shvedova, A.
A., and Jiang, J. (2006) The “pro-apoptotic genies” get out
of mitochondria: oxidative lipidomics and red ox activity of cytochrome
c/cardiolipin complexes, Chem.-Biol. Interact.,
163, 15-28.
28.Kagan, V. E., Wipf, P., Stoyanovsky, D.,
Greenberger, J. S., Borisenko, G. G., Belikova, N. A., Yanamala, N.,
Samhan Arias, A. K., Tungekar, M. A., Jiang, J., Tyurina, Y. Y., Ji,
J., Klein-Seetharaman, J., Pitt, B. R., Shvedova, A. A., and Bayir, H.
(2009) Mitochondrial targeting of electron scavenging antioxidants:
regulation of selective oxidation vs random chain reactions, Adv.
Drug Deliv. Rev., 61, 1375-1385.
29.Huttemann, M., Pecina, P., Rainbolt, M.,
Sanderson, T. H., Kagan, V. E., Samavati, L., Doan, J. W., and Lee, I.
(2011) The multiple functions of cytochrome c and their
regulation in life and death decisions of the mammalian cell: from
respiration to apoptosis, Mitochondrion, 11, 369-381.
30.Beales, P. A., Bergstrom, C. L., Geerts, N.,
Groves, J. T., and Vanderlick, T. K. (2011) Single vesicle observations
of the cardiolipin–cytochrome c interaction: induction of
membrane morphology changes, Langmuir, 27, 6107-6115.
31.Vladimirov, Y. A., Nohl, J. Z., and Volkov, V. V.
(2011) Protein–lipid nanoparticles responsible for the life or
death of living cell, Crystallography, 56, 712-719.
32.Das, M. L., Haak, E. D., and Crane, F. L. (1965)
Proteolipids. IV. Formation of complexes between cytochrome c
and purified phospholipids, Biochemistry, 4, 859-865.
33.Folch, J., Lees, M., and Sloane Stanley, G. H.
(1957) A simple method for the isolation and purification of total
lipides from animal tissues, J. Biol. Chem., 226,
497-509.
34.Jain, R., Sharma, D., and Kumar, R. (2013)
Effects of alcohols on the stability and low-frequency local motions
that control the slow changes in structural dynamics of ferrocytochrome
c, J. Biochem., 154, 341-354.
35.Alekseev, A. V. (2014) The Structure and
Function of the Cytochrome c–Cardiolipin Complex: Ph. D.
Thesis on Biological Sciences [in Russian], Moscow State University
Publishers, Moscow.
36.De Kruijff, B., and Cullis, P. R. (1980)
Cytochrome c specifically induces non-bilayer structures in
cardiolipin-containing model membranes, Biochim. Biophys. Acta,
602, 477-490.
37.Bergstrom, C. L., Beales, P. A., Lv, Y.,
Vanderlick, T. K., and Groves, J. T. (2013) Cytochrome c causes
pore formation in cardiolipin-containing membranes, Proc. Natl.
Acad. Sci. USA, 110, 6269-6274.