Activity of Redox Enzymes in the Thallus of Anthoceros natalensis
A. V. Chasov1*, R. P. Beckett2, and F. V. Minibayeva1
1Kazan Institute of Biochemistry and Biophysics, Kazan Scientific Center, Russian Academy of Sciences, 420111 Kazan, Russia; fax: (843) 292-7347; E-mail: chasov@kibb.knc.ru2School of Life Sciences, University of KwaZulu-Natal, Private Bag X01, Pietermaritzburg, Scottsville 3209, South Africa; fax: +27-33-260-5105
* To whom correspondence should be addressed.
Received January 16, 2015; Revision received April 15, 2015
Anthocerotophyta (hornworts) belong to a group of ancient nonvascular plants and originate from a common ancestor with contemporary vascular plants. Hornworts represent a unique model for investigating mechanisms of formation of stress resistance in higher plants due to their high tolerance to the action of adverse environmental factors. In this work, we demonstrate that the thallus of Anthoceros natalensis exhibits high redox activity changing under stress. Dehydration of the thallus is accompanied by the decrease in activities of intracellular peroxidases, DOPA-peroxidases, and tyrosinases, while catalase activity increases. Subsequent rehydration results in the increase in peroxidase and catalase activities. Kinetic features of peroxidases and tyrosinases were characterized as well as the peroxidase isoenzyme composition of different fractions of the hornwort cell wall proteins. It was shown that the hornwort peroxidases are functionally similar to peroxidases of higher vascular plants including their ability to form superoxide anion-radical. The biochemical mechanism was elucidated, supporting the possible participation of peroxidases in the formation of reactive oxygen species (ROS) via substrate–substrate interactions in the hornwort thallus. It has been suggested that the ROS formation by peroxidases is an evolutionarily ancient process that emerged as a protective mechanism for enhancing adaptive responses of higher land plants and their adaptation to changing environmental conditions and successful colonization of various ecological niches.
KEY WORDS: hornwort, peroxidase, superoxide, catalase, tyrosinases, dehydration, rehydrationDOI: 10.1134/S0006297915090060
Abbreviations: CW, cell wall; DOPA, 3,4-dihydroxyphenylalanine; ECS, extracellular solution; O2•–, superoxide anion-radical; PIB, post-infiltration buffer; ROS, reactive oxygen species; SOD, superoxide dismutase; XTT, 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide.
The hornworts belong to a group of ancient land plants that emerged
about 450 million years ago and that includes, according to different
sources, from 200 to 300 species [1]. Until
recently, the hornworts were considered as a class of mosses [2], but now they are considered as a separate division
in plant classification [3, 4].
Due to the cytological and morphological similarity of hornworts to
both algae and higher plants, they have become the subject of
particular interest for molecular biologists and geneticists [3, 5]. For example, hornworts have
chloroplasts characteristic of algae with central pyrenoid containing
ribulose biphosphate carboxylase and, hence, have a carbon assimilation
mechanism unique for land plants [1]. Unlike the
liverworts, which have symbiosis with blue-green algae only in four
orders out of more than 340, in hornworts symbiosis was found among
representatives of all 14 orders [1, 6]. From a practical point of view, the hornworts are
investigated as sources of rosmarinic acid and other biologically
active compounds [2]. Phylogenetic and structural
analyses suggest that evolutionarily hornworts might be a sister group
to modern vascular plants [3, 5]. It is likely that the ancient hornwort-like plants
were intermediate upon transition from organisms with life cycle in
which the generation with haploid gametophyte dominates to the
organisms with diploid sporophyte [5].
The majority of hornworts inhabit disrupted and grass-free locations, being the first to occupy an open moist soil. At the same time, it is known that the hornworts are extremely drought resistant. Unlike the vegetative organs of higher vascular plants that are extremely sensitive to dehydration, the hornwort thallus exhibits phenomenal ability to retain viability after the loss of 95% of water [7, 8]. Due to their high tolerance to the action of adverse environmental factors, the hornworts represent a unique model for investigation of mechanisms for the formation of resistance in higher plants. Despite the obvious importance, the biochemical mechanisms of resistance including kinetic characteristics of redox reactions in plants remain topics of discussions. In particular, elucidation of the dual role of peroxidases and switching from the antioxidant functioning mode to the prooxidant one is still a hot topic. In the cells of vascular plants such as, for example, wheat roots, the apoplastic peroxidases capable of both decomposition of H2O2 and formation of reactive oxygen species (ROS) are among the key enzymes of redox metabolism under stress [9, 10]. This multifunctionality of peroxidases as well as a multitude of isoforms and genes encoding them defines the important role of peroxidases in plant resistance. Despite intensive investigation of the reaction chemistry and evolution of the peroxidase structures [11-13], the possible mechanisms and factors facilitating ROS-forming activity of the peroxidases are still unsolved. Investigation of previously unknown features of peroxidases from hornworts that are on a lower level of evolution in comparison with vascular plants is a promising approach for solving this problem.
An increase in ROS level can occur under stress in the cells of bryophytes similarly to that in the majority of plants. Previously we showed for the first time that a number of lichens, mosses, as well as liverworts and hornworts demonstrate high redox activity such as formation of superoxide anion-radicals (O2•–) [8]. It is remarkable that the highest activity was observed in the thallus of Anthoceros natalensis Steph. Despite obvious importance, the biochemical mechanisms of resistance mediated by redox enzymes including peroxidases in hornwort cells are practically unstudied. In this context, the aim of this study was to analyze the kinetic characteristics of hornwort redox enzymes. Particular emphasis was placed on the investigation of the changes in peroxidases activities under the stress induced by dehydration and the following rehydration of the hornwort thallus, as well as on the possible participation of peroxidases in ROS formation.
MATERIALS AND METHODS
Object of study and preparation of samples for determination of enzyme activity. Experiments were conducted with thallus of A. natalensis Steph. collected in the Botanical Garden of the University of KwaZulu Natal, South Africa. The hornwort thallus was dehydrated followed by rehydration in experiments on investigation of the activity of redox enzymes under stress conditions [8, 14]. Aliquots (0.5 g) of thallus samples (water content – 30 g H2O per 1 g dry mass) were placed into weighing beakers. The thallus was dehydrated in a desiccator by keeping samples over saturated CaCl2 solution for 0 to 68 h. For calculation of the relative water content, the material was weighed at the following time intervals: 2.5, 20, 23.5, 24, 44, 48, 67, and 68 h. The samples were taken for determination of enzyme activity after 24, 48, and 68 h. The thallus after dehydration was subsequently rehydrated in distilled H2O for 1 and 3 h. The activities of redox enzymes were analyzed in the intracellular fraction and in the extracellular solution (ECS). Following the rehydration of the hornwort thallus in distilled H2O, the thallus was homogenized in 5 ml of 0.05 M Sorensen’s phosphate buffer (Na2HPO4/KH2PO4, pH 7.0), centrifuged at 4300g for 15 min at 5°C, and intracellular enzyme activity was determined in the supernatant. The solution in which the thallus was rehydrated (ECS) was used for determination of extracellular enzyme activity.
To investigate the dependence of redox activity in the cells and apoplast of the hornwort thallus on the degree of the enzyme binding to the structural elements of cell wall (CW), the activity of the redox enzymes was analyzed in the soluble intracellular fraction and in the fraction of the CW proteins. For this purpose, the proteins were extracted from the hornwort thallus using sequentially: Tris-HCl buffer, Sorensen’s phosphate buffer, digitonin, and NaCl [15]. The following fractions were obtained: intracellular soluble fraction (C) and fractions of proteins bound to CW by hydrogen bonds (B1), van der Waals bonds and hydrophobic interactions (B2), and ionic bonds (B3).
A post-infiltration buffer (PIB) of wheat roots containing apoplastic peroxidases was used for comparison of the spectral characteristics of oxidation of substrates of the hornwort and the wheat root peroxidases. For this purpose, excised roots of 5-day-old seedlings of spring wheat (Triticum aestivum L.) cultivar Kazanskaya Yubileinaya were vacuum infiltrated in 0.1 M Na-citrate buffer, pH 7.0 [16].
Determination of activity of redox enzymes. The activity of enzymes cited below was determined spectrophotometrically from accumulation of the respective product or consumption of the respective substrate using a Lambda 25 spectrophotometer (Perkin Elmer, USA) and presented in nmol/(s·g dry mass). In all cases, the reaction was initiated by the addition of substrate into the reaction mixture (total volume 0.5 ml). The activity of tyrosinase (3,4-dihydroxyphenylalanine (DOPA) oxidase) (EC 1.14.18.1) was assessed based on formation of DOPAchrome (ε475 = 3.6 mM–1·cm–1). The reaction mixture contained 0.08 M Na-citrate buffer, pH 7.0, 1 mM DOPA, and 0.05 ml of enzyme extract. The activities of ascorbate peroxidase (EC 1.11.1.11) and ascorbate oxidase (EC 1.10.3.3) were determined from the decline of ascorbic acid in the reaction mixture according to methods described previously [9, 17], and the activity of catalase (EC 1.11.1.6) from the decline of H2O2 in the reaction mixture [17]. A solution of 1 mM o-dianisidine (ε460 = 30.0 mM–1·cm–1) or 1 mM DOPA in 0.07 M Na-citrate buffer, pH 5.5, with addition of 1 mM H2O2 were used as substrates for recording peroxidase activity (EC 1.11.1.7) [9].
Solutions of 0.08 M Na-citrate buffer with pH from 2.5 to 8.0 were used for determination of the tyrosinase and peroxidase pH optima with the rest of the reaction mixture composition being the same as described above for those enzymes.
For determination of substrate specificity of the peroxidase in fraction B1, the reaction mixture contained 50 µl of the substrate at the respective concentration, 5 µl of fraction B1, 0.1 mM H2O2, and 0.07 M Na-citrate buffer, pH 5.5. The following substrates were used: o-dianisidine and ascorbic, sinapic (ε305 = 10.61 mM–1·cm–1), chlorogenic (ε323 = 16.90 mM–1·cm–1), p-coumaric (ε285 = 17.21 mM–1·cm–1), caffeic (ε311 = 9.91 mM–1·cm–1), and ferulic (ε310 = 13.74 mM–1·cm–1) acids.
Spectral analysis. Absorption spectra of different compounds were recorded in the range from 1100 to 190 nm. Various concentrations of NADH and ferulic acid were used. Changes of the absorption spectrum of mixture containing 50 µl of the enzyme extract corresponding to the respective fraction of the hornwort thallus, 0.11 mM NADH, and 0.1 mM of ferulic acid in 0.73 µM Na-citrate buffer, pH 5.5 or 7.0, were registered for 20 min in the presence or absence of 1 mM KCN with scanning rate of 1920 nm/min and period of 1 min. The total volume of the reaction mixture was 0.5 ml; a cuvette with distilled H2O was used as the reference.
Intensity of superoxide anion-radical formation. The optical density of the mixture of compounds containing 100 µl of the respective fraction of hornwort thallus, 0.1 mM NADH, and 0.1 mM 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) in 0.08 M Na-citrate buffer, pH 5.5 or 7.0, in the presence or absence of 250 units/ml of superoxide dismutase (SOD) was scanned at the rate of 1920 nm/min for 50 min. The XTT in the presence of O2•– was converted into XTT formazan, formation of which was detected by the difference in optical density (ε470 = 21.6 mM–1·cm–1) [18].
Electrophoretic protein separation. Electrophoresis was conducted in a 10% polyacrylamide gel in the modified Laemmli system [19] using a Mini-PROTEAN Tetra Cell (Bio-Rad, USA) under native conditions without addition of SDS, mercaptoethanol, and the sample heating. Peroxidase activity was visualized using 5 mM o-dianisidine prepared in the Na-citrate buffer, pH 5.5, with addition of 1 mM H2O2.
Isoelectrofocusing was conducted using an instrument (Hiiu Kalur, Estonia) in 5% polyacrylamide gel with addition of Ampholine of pH 3.5-10.0 (LKB, Sweden). A set of IEF-M1A standards (3.6-9.3) (Sigma, USA) was used for determination of isoelectric points of the proteins. Following reverse dialysis, a 25-µl aliquot of the sample was loaded into a well. The peroxidase activity in the loaded samples was 1.0, 0.3, 0.3, and 1.2 mmol of oxidized o-dianisidine/(min·liter) in the fractions C, B1, B2, and B3, respectively. The activity of peroxidase isoenzymes in the gel was visualized as described above.
The experiments were conducted in at least three biological replicates. Representative results for each experimental series ae given. All experimental data demonstrated normal distribution. Table 1 and Figs. 1 and 2 present arithmetic mean values and their standard deviations, where n is the number of data points used for calculation of the mean. The data in Fig. 2 were statistically processed using Microsoft Excel software (Student’s t-test).
The following reagents were used: XTT (Alfa Aesar, UK); NADH (Reanal, Hungary); SOD (Serva, Germany); ferulic acid (Fluka, Switzerland); DOPA, o-dianisidine, and N,N′-methylene-bis-acrylamide (Acros Organics, Belgium); caffeic and p-coumaric acids (Ferak, Germany); acrylamide (Sigma, USA). All other reagents were of the chemically pure or pure for analysis grades (Reakhim, Russia).
RESULTS
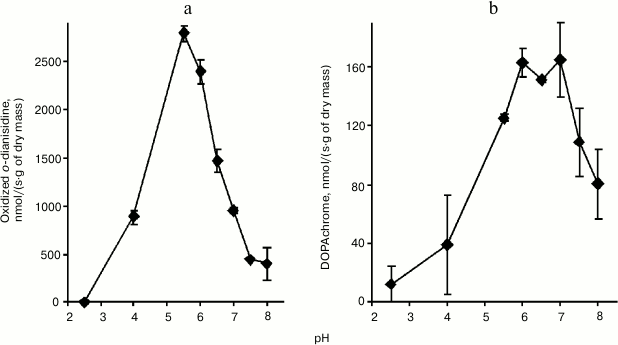
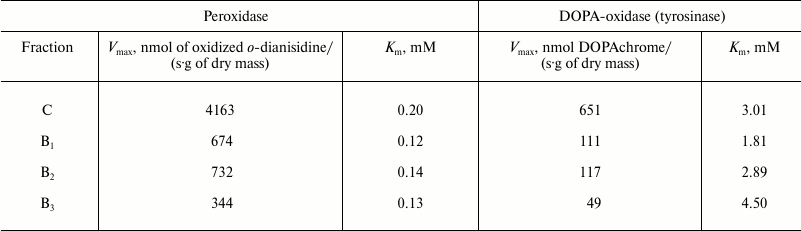
Enzyme activity. Catalase demonstrated the lowest activity among the redox enzymes of the intracellular fraction of the hornwort thallus (Table 1). The activities of the ascorbate oxidase and ascorbate peroxidase were several-fold higher, and the activities of the DOPA-oxidase and DOPA-peroxidase exceeded the catalase activity by an order of magnitude. The o-dianisidine activity of peroxidase was in turn 25-fold higher than the DOPA peroxidase activity (Table 1). The maximum activity of the intracellular peroxidase was observed at pH 5.5 (Fig. 1a), and the optimum of the intracellular tyrosinase activity – in the range from pH 6.0 to 7.0 (Fig. 1b). Kinetic analysis of the peroxidase activity in different fractions of the thallus proteins showed that the intracellular peroxidases and tyrosinases had the maximum reaction rates (Table 2). The apoplastic peroxidases from fractions B1-B3 exhibited the highest substrate affinity because they had the lowest Michaelis constants (Table 2). The Michaelis constants for tyrosinases increased in the series: B1 < B2 < C < B3 (Table 2).
Table 1. Enzyme activities in the
intracellular fraction of A. natalensis thallus expressed as a
rate of substrate (S) decomposition or product (P) formation
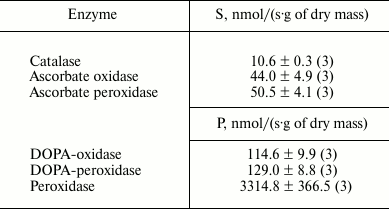
Table 2. Maximum rate
(Vmax) and Michaelis constant (Km)
for the reaction of oxidation of o-dianisidine by peroxidases
and of 3,4-dihydroxyphenylalanine by tyrosinase from different
fractions of thallus from A. natalensis
Note: C – intracellular fraction. Fractions of proteins bound
to cell wall: B1 – by hydrogen bonds,
B2 – by van der Waals forces and hydrophobic
interactions, B3 – by ionic bonds.
Fig. 1. pH dependences of the activities of peroxidase (a) and tyrosinase (b) from intracellular fraction of thallus of A. natalensis (n = 3).
Ascorbic acid demonstrated the highest affinity to the apoplastic peroxidase (B1). The Michaelis constant increased in the series: ascorbic acid < o-dianisidine < sinapic acid < caffeic acid < ferulic acid < chlorogenic acid < p-coumaric acid (Table 3). The maximum velocity of the reaction increased in the series: sinapic acid < ascorbic acid < o-dianisidine < p-coumaric acid < caffeic acid < ferulic acid < chlorogenic acid (Table 3). Among the natural phenolic substrates, the peroxidases demonstrated the highest sensitivity to sinapic acid (Table 3).
Table 3. Maximum rate
(Vmax) and Michaelis constant (Km)
for the reaction of oxidation of different substrates by apoplastic
peroxidases of fraction B1 from thallus of A.
natalensis
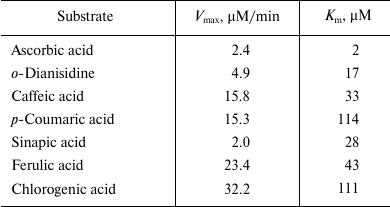
Note: Standard 0.5-ml reaction mixture contained 50 µl of
substrate at respective concentration, 5 µl of fraction
B1, 0.1 mM H2O2, and 0.07 M
Na-citrate buffer, pH 5.5.
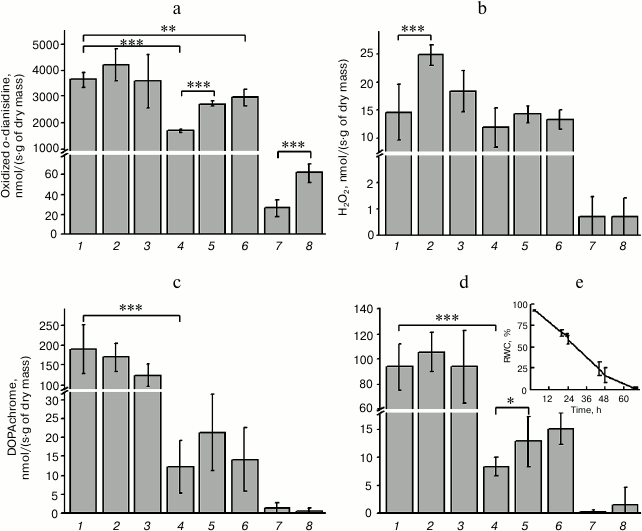
It was shown that the relative water content in the thallus of the hornwort plant decreased gradually during dehydration over saturated solution of CaCl2, reaching 58% after 24 h, 17% after 48 h, and only 2% after 68 h (Fig. 2e). The activities of the intracellular peroxidase, tyrosinase, and DOPA-peroxidase decreased significant by this time (Fig. 2, a, c, d). The activity of peroxidase increased significantly within 1 h during the following rehydration and showed a trend for increase after 3 h, but still was less than the initial level observed prior to dehydration of the thallus (Fig. 2a). It is interesting to note that peroxidase activity was also detected in the solution where the rehydration occurred (ECS). The peroxidase activity in the ECS increased significantly after 3 h of rehydration in comparison with the ECS level after 1 h of rehydration (Fig. 2a). The activity of intracellular tyrosinase increased slightly but reliably following the rehydration (Fig. 2d). Only trace amounts of the DOPA-peroxidase, tyrosinase, and catalase were found in the ECS (Fig. 2, b-d). The catalase activity increased significantly following 24 h of rehydration, decreased after 48 h, and remained on a level close to that observed prior to the dehydration even after 68 h of incubation during rehydration (Fig. 2b).
Fig. 2. Activity of redox enzymes – (a) peroxidase, (b) catalase, (c) DOPA-peroxidase, and (d) tyrosinase in intracellular fraction of thallus of A. natalensis; e) relative water content (RWC) in the thallus of the plant: 1) prior to dehydration (control); 2-4) after dehydration for 24, 48, and 68 h, respectively; 5, 6) after rehydration for 1 and 3 h, respectively; 7, 8) extracellular solution obtained 1 and 3 h after incubation and removal of the thallus from the rehydration solution, respectively. Difference is significant at P ≤ 0.05 (*), P ≤ 0.01 (**), P ≤ 0.001 (), n = 6.
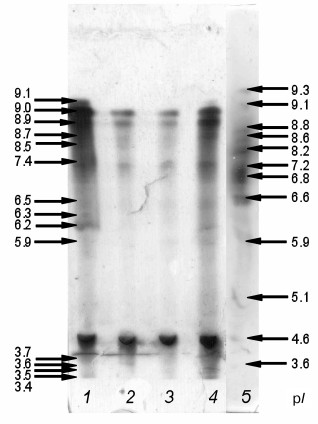
Electrophoretic separation and isoelectrofocusing of proteins. Analysis of results of electrophoretic separation of proteins under native conditions revealed the presence of five peroxidase isoenzymes in the intracellular fraction of the hornwort with Rf 0.99, 0.82, 0.54, 0.53, and 0.52 (data not shown). Isoelectrofocusing revealed 14 different isoenzymes, the majority of them being cationic (Fig. 3). Fraction C contained 12 isoenzymes with pI 3.4, 3.5, 3.6, 3.7, 5.9, 6.2, 6.5, 7.4, 8.7, 8.9, 9.0, and 9.1; fraction B1 – seven isoenzymes with pI 3.4, 5.9, 7.4, 8.5, 8.7, 8.9, and 9.0; fraction B2 – six isoenzymes with pI 3.7, 7.4, 8.5, 8.7, 8.9, and 9.0; and fraction B3 – 10 isoenzymes with pI 3.4, 3.7, 6.2, 6.3, 6.5, 7.4, 8.5, 8.9, 9.0, and 9.1 (Fig. 3).
Fig. 3. Isoelectric points of peroxidase isoenzymes from A. natalensis. Cell wall protein fractions: 1-3) B3, B2, B1, respectively; 4) intracellular fraction; 5) markers.
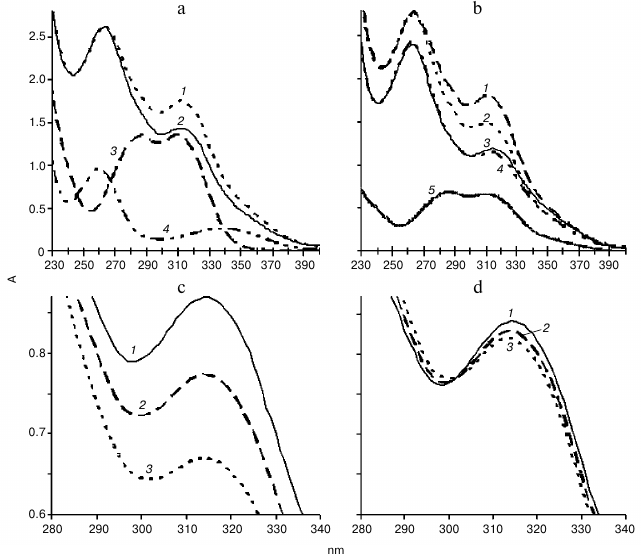
Spectral analysis. Changes in optical density in mixtures containing NADH and ferulic acid in the presence or absence of the plant extract from hornwort or wheat were investigated. Exogenous H2O2 was not added. NADH exhibits two maxima in the absorption spectrum, at 260 and 338 nm (Fig. 4a, curve 4). Decrease in optical density in the region of 338 nm and a slight increase in optical density in the region of 260 nm was observed upon addition of 50 µl of PIB of wheat root to 0.11 mM NADH, which was due to the oxidation of NADH and formation of NAD+ (data not shown). Ferulic acid exhibits two maxima in the absorption spectrum at 287 and 310 nm (Fig. 4a, curve 3), and caffeic acid – at 288 and 311 nm (Fig. 4b, curve 5). No spectral change was observed for 20 min upon introduction of 50 µl of fractions C, B1, B2, B3 of thallus or PIB of wheat to 0.1 mM ferulic acid, and similar results were obtained upon interaction of 0.1 mM caffeic acid and 50 µl of PIB (data not shown). Hence, the oxidation of phenol was not observed in the absence of NADH, which indicated the lack of polyphenol oxidase activity under these conditions.
Fig. 4. Absorption spectra. a: 1, 2) reaction mixture containing 0.11 mM NADH and 0.1 mM ferulic acid in 0.73 µM Na-citrate buffer, pH 5.5, in the presence of 50 µl of B1 fraction of hornwort thallus at the onset of the reaction and 20 min later, respectively; 3) 0.1 mM ferulic acid; 4) 55 µM NADH; b: 1, 2) same as (1, 2) in (a) but in the presence of 50 µl of PIB of wheat roots; 3, 4) oxidation reactions of 0.11 mM NADH and 0.1 mM caffeic acid in 0.73 µM Na-citrate buffer, pH 5.5, in the presence of 50 µl of the wheat root PIB at the onset of the reaction and 20 min later, respectively; 5) 62.5 µM caffeic acid; c, d: 1-3) oxidation reaction of 0.11 mM NADH and 0.1 mM ferulic acid in 0.73 µM Na-citrate buffer, pH 5.5, in the presence of 50 µl of thallus fraction B3 without KCN (c) and upon addition of 1 mM KCN (d) at the beginning of the reaction (1), and after 10 (2) and 20 min (3), respectively. The scanning rate was 1920 nm/min.
When the optical density of the mixture of compounds at pH 5.5 was periodically scanned for 20 min, it was found that the largest decline in the optical density in the mixture containing 50 µl of fraction B1 (Fig. 4a, curves 1 and 2) or B3 (Fig. 4c, curves 1-3), 0.11 mM NADH, and 0.1 mM ferulic acid was observed in the region of 310 nm, which corresponds to oxidation of ferulic acid. Similar results were obtained with the samples from fractions C and B2 of hornwort (data not shown) and with the PIB of wheat roots (Fig. 4b, curves 1 and 2). There were no changes in the absorption spectrum when 0.11 mM NADH in the reaction mixture was replaced with 0.11 mM NAD+ (data not shown). The decrease in optical density in the region of 310 nm for the mixture containing 50 µl of the B3 fraction, 0.11 mM NADH, and 0.1 mM ferulic acid was greatly suppressed by the addition of 1 mM KCN in the reaction mixture (Fig. 4, c and d, curves 1-3). Similar results were obtained for the other protein fraction of the hornwort thallus (data not shown).
It was shown that the oxidation of 0.1 mM ferulic acid by the peroxidase from PIB of wheat roots in the presence of 0.11 mM NADH at pH 5.5 occurred to a greater extent than the oxidation of the 0.1 mM caffeic acid, because the decrease in optical density in the region of 310 nm upon oxidation of ferulic acid was much larger than the decrease in optical density at 311 nm upon oxidation of caffeic acid (Fig. 4b, curves 1-4). Increase in the peak in the region of 260 nm was also observed upon oxidation of caffeic acid.
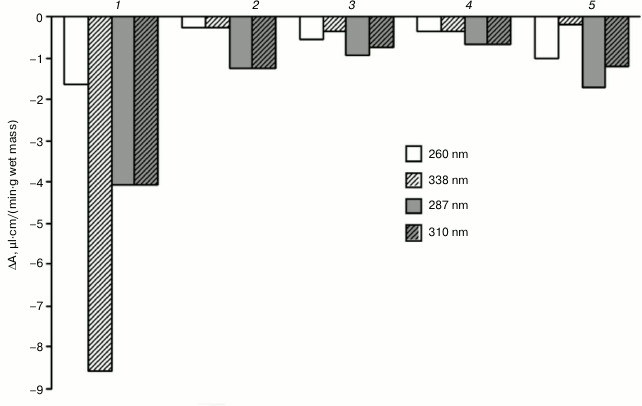
When the optical density of the mixture of compounds was periodically scanned at pH 7.0, we found that unlike for fractions B1, B2, and B3 of the CW proteins of the hornwort thallus, for fraction C the largest decrease in optical density upon oxidation of 0.1 mM NADH and 0.1 mM ferulic acid was observed in the region of 338 nm rather than 310 nm (Fig. 5). The amplitude of the decrease in optical density in the region of 310 nm increases in the series: B3 < B2 < B1 < C. The addition of a higher concentration of ferulic acid (0.2 mM) to fraction B3 resulted in almost two-fold enhancement of the ferulic acid oxidation and two-fold inhibition of the NADH oxidation, which was manifested by greater decrease in optical density in the region of 310 nm and the lesser optical density decrease in the region of 338 nm, respectively (Fig. 5).
Fig. 5. Difference calculated from the absorption spectra during the reaction of peroxidase oxidation of substrates: 0.1 mM NADH and ferulic acid in 0.73 µM Na-citrate buffer, pH 7.0, for 20 min in the presence of different hornwort fractions. Scanning rate, 1920 nm/min. 1-4) Fractions C, B1, B2, and B3, respectively, with 0.1 mM ferulic acid; 5) fraction B3 with 0.2 mM ferulic acid; 260 and 338 nm – maxima of absorption spectrum of NADH; 287 and 310 nm – maxima of absorption spectrum of ferulic acid.
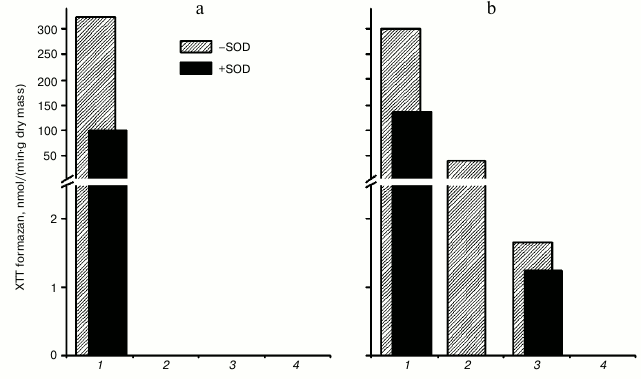
We found that the formation of XTT formazan did not occur in the B1, B2, and B3 CW protein fractions of the hornwort thallus upon oxidation of 0.1 mM NADH in the presence of 0.1 mM XTT at pH 5.5 (Fig. 6a). Formation of XTT formazan occurred in fraction C, which was inhibited by almost 70% after adding 250 units/ml of SOD. Formation of XTT formazan at pH 7.0 was observed in all fractions isolated from the hornwort thallus except for the B3 fraction, and the formation increased in the series: B2 < B1 < C (Fig. 6b). The XTT formazan formation was inhibited by 55, 100, and 25% in fractions C, B1, and B2, respectively, upon introduction of 250 units/ml of SOD.
Fig. 6. Intensity of superoxide anion-radical formation at pH 5.5 (a) and pH 7.0 (b), calculated from the absorption spectra changes during scanning of different fractions of hornwort thallus in the presence of 0.1 mM XTT and 0.1 mM NADH for 40 min in the presence or absence of 250 units/ml of SOD. Scanning rate, 1920 nm/min. 1-4) Fractions C, B1, B2, and B3, respectively.
DISCUSSION
In this work, we found that thallus of A. natalensis is characterized by high activity of redox enzymes, primarily of peroxidase (Table 1). We found that peroxidases of nonvascular plants become activated under stress similarly to peroxidases of vascular higher plants such as wheat [9]. For example, the hornwort peroxidase activity decreased significantly upon dehydration, followed by a significant increase after rehydration of the desiccated thallus (Fig. 2a). Moreover, hornwort peroxidase activity was detected in the solution in which the rehydration was conducted (Fig. 2a), which was likely due to the washing out of peroxidase isoforms weakly bound to the cell surface. It is known that the peroxidases of nonvascular plants of other divisions, such as liverworts and mosses, are also activated under stress and they are highly mobile [20, 21]. For example, the peroxidases of Marchantia polymorpha L. suspension culture were secreted into the culture medium in response to the chemical stressor, bornyl acetate [20], and the peroxidases of suspension culture of Physcomitrella patens (Hedw.) B.S.G. and Racomitrium japonicum Dozy & Molk. mosses were also activated in the culture medium in response to the fungal elicitor chitosan [21]. The mobility of apoplastic peroxidases is explained by the fact that these enzymes are present on the cell surface [11], from which they can be easily released under stress conditions even before their stress-induced synthesis begins [12, 21, 22].
The peroxidases in vascular plants are among the main components of the adaptive response involved in formation of resistance to various stresses [12, 23, 24]. It is likely that because peroxidases are so important for plants, evolution has resulted in formation of a large number of genes encoding these enzymes. For example, 138 genes located in 12 chromosomes were identified in rice (Oryza sativa L.) [11], 73 in thale cress (Arabidopsis thaliana (L.) Heynh.) [25], and 48 in P. patens moss [21]. Perhaps the availability of such a large number of genes results in the presence of several isoforms of the enzyme in the plants [10, 15]. Peroxidase activity is increased in plants that are more resistant to stressors [23]. At least two strategies are likely to exist in plants for using the protective properties of peroxidase: to keep a high level of activity of this enzyme permanently, or to increase the peroxidase activity from low to high level quickly at the location required for the plant in response to the changes in environmental conditions.
Kinetic analysis of peroxidase activity in the intracellular fraction and in the different CW protein fractions of the hornwort thallus showed that the intracellular peroxidases have the maximum reaction velocity, while the apoplastic peroxidases exhibit the highest affinity to the substrates as they had the lowest Michaelis constants (Table 2). Hence, a minimal amount of a substrate is sufficient for activation of the apoplastic peroxidase. It is known that the fast activation of apoplastic peroxidase under stress conditions could be a key factor of oxidative burst in plant cells [26, 27], and this could play an important role in initiation of signaling cascades and subsequent formation of immunity in plants [28]. Substrate-dependent peroxidase activation is also supported by the fact that peroxidase secretion under stress conditions could accompany substrate secretion as was shown, for example, for lunularin in suspension culture of M. polymorpha [20].
The availability of various redox enzymes in the apoplast of plants determines their complementary [29] or competitive interactions such as competition for substrates. In particular, considering that not only peroxidases, but also tyrosinases, interact with phenolic compounds, the negative effect of tyrosinase activation on peroxidase activity due to their competition for substrates cannot be ruled out. It is known that the tyrosinases as well as peroxidases are among the components of the immune system of living organisms [30]. Similar to the intracellular peroxidases, the intracellular tyrosinases in our experiments were the most active, and the apoplastic enzymes demonstrated the highest affinity to substrates (Table 2). The similarity of kinetic characteristics suggests that the two enzymes could be activated under the same conditions, which has been confirmed in our experiments. We showed that tyrosinase activation occurred under stress caused by dehydration, but, unlike the peroxidases, the enzymes were less mobile, because only trace amounts were found in the ECS (Fig. 2d).
Catalase can also compete with peroxidase for the substrate (H2O2). We found that one day after the beginning of dehydration, the catalase activity increased almost two-fold, while the peroxidase and tyrosinase activity only tended to increase (Fig. 2, a, b, d). It is likely that the increase in the catalase activity is related to significant increase in the H2O2 level upon dehydration, which has been mentioned in a number of publications [31, 32]. The decrease in catalase activity by the second day of dehydration to the initial level (before stress) and the lack of changes on the following day as well as after the rehydration indicated that the H2O2 content was also reduced and did not undergo significant changes.
The availability and the quantitative ratio of different substrates in vascular plants affect significantly peroxidase activity [9, 17]. It can be suggested that activation of the hornwort peroxidase upon rehydration is related not to the increase in H2O2 level, but to the release of certain substrates, inducers, or cofactors. The increase in oxidase activity of peroxidase occurs in the process, which results not in H2O2 utilization, but on the contrary in the formation of O2•– and H2O2 [33], as it occurs, for example, in the cells of vascular plants upon pathogen-induced oxidative burst [24]. Probably the emergence of some component on the cell surface that plays a role of catalyst is required for activation of the oxidation of phenolic substrates by peroxidase under stress, followed by the rapid formation of ROS (oxidative burst). It was shown that one such component should be a reductant [26] such as NADH. It is suggested that NADH can appear in the apoplast of vascular plants because of malate- or lactate-dehydrogenase activity of the plasmalemma or CW, and its interaction with the peroxidase could result in formation of ROS [10, 24, 33]. As is known, peroxidase exhibits NADH-oxidase activity, and the radical form of NAD• and H2O2 are formed in the process of interaction of this enzyme with oxygen [24, 33, 34]. A chain of interconversions can occur thereafter. It is known that during the combined oxidation by peroxidase of substrates with very different reactivity, effects of their activation or inhibition are observed [35]. It is the authors’ opinion that the activation of the slowly oxidized substrate occurs together with partial or complete inhibition of conversion of the rapidly oxidized substrate (activator). Hence, the phenol radicals and/or radical from of the reductant can interact with each other and with oxygen, forming O2•– [33-35].
To evaluate the possibility of this redox mechanism functioning in the cells of nonvascular higher plants, we analyzed features of the combined oxidation of NADH and ferulic acid, which is a natural phenolic substrate of the peroxidase, by the peroxidase of the hornwort thallus. Various derivatives of hydroxycinnamic acids have been found in hornworts [2]. It was found that at pH 5.5 when NADH and ferulic acid are present and exogenous H2O2 is absent, the largest decrease in optical density was observed in the region of 310 nm, which was likely due to the oxidation of ferulic acid rather than NADH (Fig. 4a). The significant inhibition of this reaction by the addition of cyanide, such as for example in fraction B3 (Fig. 4, c and d), indirectly confirms participation of peroxidases in this process. Despite the fact that NADH can be a slowly oxidizing substrate of peroxidase [35], it is possible that in our experiments NADH plays the role of activator of oxidation of phenolic acids. To test this possibility, the enzyme of vascular plants (extracellular wheat peroxidase from PIB) was used. It was found that similarly to the hornwort peroxidases, the wheat peroxidase at pH 5.5 oxidized ferulic acid in the presence of NADH and ferulic acid, and in the absence of exogenous H2O2 even to a greater degree than caffeic acid (Fig. 4b). It is known that for peroxidase-mediated oxidative burst to occur, the change in pH toward alkaline values is required additionally to the presence of reductant [26]. Therefore, it was important to test if the oxidation of ferulic acid in the presence of NADH by the hornwort peroxidases would depend on pH of the reaction mixture. It was found that at pH 7.0 as well as at pH 5.5 the oxidation of ferulic acid occurred to a greater degree than the oxidation of NADH in all fractions of the hornwort except for the fraction C, which was manifested by the larger decrease in optical density at 310 nm than at 338 nm (Fig. 5). All soluble compounds are present in the cytoplasmic fraction including endogenous phenols and other peroxidase substrates that may be why NADH is oxidized to a larger degree in this case than exogenous ferulic acid. The peroxidases from hornwort fraction B3 oxidized ferulic acid the least; however, its oxidation increased almost two-fold with two-fold increase in the ferulic acid concentration (0.2 mM), and the NADH oxidation decreased two-fold in the process (Fig. 5). This is a possible indication of the competition between these substrates during the oxidation catalyzed by peroxidase.
We showed that the hornwort peroxidases are capable of O2•– formation (Fig. 6), and that this process is pH-dependent. The O2•– acceptor XTT [18] was converted into XTT formazan in the presence of 0.1 mM NADH at pH 5.5 only in the intracellular hornwort fraction, and this reaction was sensitive to SOD (Fig. 6a). The SOD-sensitive formation of XTT formazan at pH 7.0 indicates that the O2•– was formed in all fractions except for fraction B3 (Fig. 6b). Such significant difference in this reaction at acidic and neutral pH is probably because more alkaline medium is required for both O2•– formation by peroxidase and for the reaction of O2•– with XTT [18, 26]. The differences in the inhibition of XTT formazan formation by fractions C, B1, and B2 following SOD addition and the lack of the XTT oxidation by the peroxidases from fraction B3 (Fig. 6b) are likely due to the different ability of the peroxidase isoforms from different fractions for O2•– formation. We found that the composition of peroxidase isoforms was different in the fractions (Fig. 3). It is possible that the peroxidases capable of O2•– formation are lacking in fraction B3, in particular, the isoform with pI 8.7 is absent in the B3 fraction, while it is present in the other fractions. Two anionic isoforms with pI 3.5 and 3.6 are present in fraction C that are absent in other fractions. The peroxidase isoform with pI 5.9 is present only the fractions C and B1 (Fig. 3), and it is exactly in these fractions the formation of XTT formazan exceeds its production in fraction B2 by one order of magnitude (Fig. 3). Hence, we suggest that the isoforms of the hornwort peroxidases have different ability for production of O2•–. In future, detailed analysis of the specificity of the O2•–-forming activity of the separate peroxidase isoforms would allow elucidating their contribution to oxidative burst under stress.
Hence, assuming that the release of NADH or any other reductant into the apoplast happens under stress conditions, the amount of it required for the induction of oxidative burst would be very small. Because of the differential oxidation of substrates by peroxidase, such a reductant can play the role of an inducer stimulating conversion of other substrates such as phenols released under stress into the apoplast. Considering that a pool of soluble peroxidases is always present in apoplast, possibly together with slowly oxidizing substrates, it can be suggested that the release of a small amount of the readily oxidized substrate or any cofactor would be sufficient for the immediate response. The following increase in the response could be through either the release of additional enzyme or the emergence of substrates. Thus, the key role of the extracellular peroxidase comprises the regulation of the ROS balance in the apoplast of plant cells, and this regulation is carried out due to the competitive and complementary interactions of different peroxidase substrates.
It was shown for the first time in this work that, along with known cytological and morphological similarity with the vascular plants, the hornworts demonstrate certain similarity in functioning features of their redox enzymes. The kinetic characteristics of peroxidases and tyrosinases of hornworts were analyzed for the first time, and the possibility of participation of the peroxidase of the hornwort thallus in anti- and prooxidant processes was demonstrated. We suggest that the revealed biochemical mechanism of the possible participation of peroxidases in ROS formation through substrate–substrate interaction is of importance for adaptation and survival of the hornwort under stress conditions such as dehydration/rehydration. It seems important to decipher in future the elements of the signaling pathways mediated by the changes in the redox state and facilitating formation of the protective responses in hornworts. We suggest that the formation of ROS by peroxidases is an evolutionarily ancient process that emerged as a protective mechanism of the land higher plants that enhances the adaptive mechanisms to ensure their adaptation to the changing environmental conditions and successful colonization of various ecological niches.
This work was financially supported by the Russian Foundation for Basic Research (grant 14-04-93962), by the Program of the President of the Russian Federation for supporting of leading scientific schools (grant NSh-825.2012.4), and by the Program of Fundamental Research of the Presidium of the Russian Academy of Sciences “Molecular and Cell Biology” (leader A. N. Grechkin).
REFERENCES
1.Villarreal, J. C., Cargill, D. C., Soderstrom, L.,
Hagborg, A., and Renzaglia, K. S. (2010) A synthesis of hornwort
diversity: patterns, causes and future work, Phytotaxa,
9, 150-166.
2.Asakawa, Y. (1995) in Progress in the Chemistry
of Organic Natural Products (Herz, W., Kirby, G. W., Moore, R. E.,
Steglich, W., and Tamm, Ch., eds.) Vol. 65, Springer, Vienna, pp.
1-562.
3.Troitsky, A. V., Ignatov, M. S., Bobrova, V. K.,
and Milyutina, I. A. (2007) Contribution of genosystematics to current
concepts of phylogeny and classification of bryophytes, Biochemistry
(Moscow), 72, 1368-1376.
4.Chang, Y., and Graham, S. W. (2011) Inferring the
higher-order phylogeny of mosses (Bryophyta) and relatives using a
large, multigene plastid data set, Am. J. Bot.,
98, 839-849.
5.Qiu, Y.-L., Li, L., Wang, B., Chen, Z., Knoop, V.,
Groth-Malonek, M., Dombrovska, O., Lee, J., Kent, L., Rest, J.,
Estabrook, G. F., Hendry, T. A., Taylor, D. W., Testa, C. M., Ambros,
M., Crandall-Stotler, B., Duff, R. J., Stech, M., Frey, W.,
Quandt, D., and Davis, C. C. (2006) The deepest divergences in land
plants inferred from phylogenomic evidence, PNAS, 103,
15511-15516.
6.Adams, D. G., and Duggan, P. S. (2008)
Cyanobacteria–bryophyte symbioses, J. Exp. Bot.,
59, 1047-1058.
7.Wood, A. J. (2007) The nature and distribution of
vegetative desiccation-tolerance in hornworts, liverworts and mosses,
Bryologist, 110, 163-177.
8.Minibayeva, F., and Beckett, R. P. (2001) High
rates of extracellular superoxide production in bryophytes and lichens,
and an oxidative burst in response to rehydration following
desiccation, New Phytologist, 152, 333-341.
9.Chasov, A. V., and Minibayeva, F. V. (2009) Effect
of exogenous phenols on superoxide production by extracellular
peroxidase from wheat seedling roots, Biochemistry (Moscow),
74, 766-774.
10.Minibayeva, F., Kolesnikov, O., Chasov, A.,
Beckett, R. P., Lüthje, S., Vylegzhanina, N., Buck, F., and
Böttger, M. (2009) Wound-induced apoplastic peroxidase activities:
their roles in the production and detoxification of reactive oxygen
species, Plant Cell Environ., 32, 497-508.
11.Passardi, F., Longet, D., Penel, C., and Dunand,
C. (2004) The class III peroxidase multigenic family in rice and its
evolution in land plants, Phytochemistry, 65,
1879-1893.
12.Almagro, L., Gómez Ros, L. V.,
Belchi-Navarro, S., Bru, R., Ros Barceló, A., and
Pedreño, M. A. (2009) Class III peroxidases in plant defense
reactions, J. Exp. Bot., 60, 377-390.
13.Mathe, C., Barre, A., Jourda, C., and Dunand, C.
(2010) Evolution and expression of class III peroxidases, Arch.
Biochem. Biophys., 500, 58-65.
14.Mayaba, N., and Beckett, R. P. (2003) Increased
activities of superoxide dismutase and catalase are not the mechanism
of desiccation tolerance induced by hardening in the moss Atrichum
androgynum, J. Bryol., 25, 281-286.
15.Li, J. L., Sulaiman, M., Beckett, R. P., and
Minibayeva, F. V. (2010) Cell wall peroxidases in the liverwort
Dumortiera hirsuta are responsible for extracellular superoxide
production, and can display tyrosinase activity, Physiol.
Plant., 138, 474-484.
16.Chasov, A. V., and Minibayeva, F. V. (2014)
Methodological approaches for studying apoplastic redox activity: 1.
Mechanisms of peroxidase release, Russ. J. Plant Physiol.,
61, 556-563.
17.Chasov, A. V., and Minibayeva, F. V. (2014)
Methodological approaches for studying apoplastic redox activity: 2.
Regulation of peroxidase activity, Russ. J. Plant Physiol.,
61, 626-633.
18.Sutherland, M. W., and Learmonth, B. A. (1997)
The tetrazolium dyes MTS and XTT provide new quantitative assays for
superoxide and superoxide dismutase, Free Rad. RPS, 27,
283-289.
19.Laemmli, U. K. (1970) Cleavage of structural
proteins during the assembly of the head of bacteriophage T4,
Nature, 227, 680-685.
20.Hirata, T., Ashida, Y., Mori, H., Yoshinaga, D.,
and Goad, L. J. (2000) A 37-kDa peroxidase secreted from liverworts in
response to chemical stress, Phytochemistry, 55,
197-202.
21.Lehtonen, M. T., Akita, M., Kalkkinen, N.,
Ahola-Iivarinen, E., Rönnholm, G., Somervuo, P., Thelander, M.,
and Valkonen, J. P. (2009) Quickly-released peroxidase of moss in
defense against fungal invaders, New Phytol., 183,
432-443.
22.Van Loon, L. C., Rep, M., and Pieterse, C. M. J.
(2006) Significance of inducible defence-related proteins in infected
plants, Ann. Rev. Phytopathol., 44, 135-162.
23.Pshenichnov, E., Khashimova, N., Akhunov, A.,
Golubenko, Z., and Stipanovic, R. D. (2011) Participation of
chitin-binding peroxidase isoforms in the wilt pathogenesis of cotton,
AJPS, 2, 43-49.
24.O’Brien, J. A., Daudi, A., Butt, V. S., and
Bolwell, G. P. (2012) Reactive oxygen species and their role in plant
defense and cell wall metabolism, Planta, 236,
765-779.
25.Tognolli, M., Penel, C., Greppin, H., and Simon,
P. (2002) Analysis and expression of the class III peroxidase large
gene family in Arabidopsis thaliana, Gene, 288,
129-138.
26.Bolwell, G. P., Bindschedler, L. V., Blee, K. A.,
Butt, V. S., Davies, D. R., Gardner, S. L., Gerrish, C., and
Minibayeva, F. (2002) The apoplastic oxidative burst in response to
biotic stress in plants: a three-component system, J. Exp. Bot.,
53, 1367-1376.
27.Lehtonen, M. T., Akita, M., Frank, W., Reski, R.,
and Valkonen, J. P. T. (2012) Involvement of a class III peroxidase and
the mitochondrial protein TSPO in oxidative burst upon treatment of
moss plants with a fungal elicitor, MPMI, 25,
363-371.
28.Tarchevskii, I. A. (2001) Metabolism of Plants
under Stress [in Russian], Fen, Kazan.
29.Roach, T., Colville, L., Beckett, R. P.,
Minibayeva, F. V., Havaux, M., and Kranner, I. (2015) A proposed
interplay between peroxidase, amine oxidase and lipoxygenase in the
wounding-induced oxidative burst in Pisum sativum seedlings,
Phytochemistry, 112, 130-138.
30.Mayer, A. M. (2006) Polyphenol oxidases in plants
and fungi: going places? A review, Phytochemistry, 67,
2318-2331.
31.Lee, B. R., Kim, K. Y., Jung, W. J., Avice, J.
C., Ourry, A., and Kim, T. H. (2007) Peroxidases and
lignification in relation to the intensity of water-deficit
stress in white clover (Trifolium repens L.), J. Exp.
Bot., 58, 1271-1279.
32.Chen, Q., Yang, L., Ahmad, P., Wan, X., and Hu,
X. (2011) Proteomic profiling and redox status alteration of
recalcitrant tea (Camellia sinensis) seed in response to
desiccation, Planta, 233, 583-592.
33.Halliwell, B. (1978) Lignin synthesis: the
generation of hydrogen peroxide and superoxide by horseradish
peroxidase and its stimulation by manganese (II) and phenols,
Planta, 140, 81-88.
34.Lebedeva, O. V., and Ugarova, N. N. (1997)
Steady-state kinetics of NADH oxidation by hydrogen peroxide in the
presence of horseradish peroxidase, Biochemistry (Moscow), 62,
212-216.
35.Lebedeva, O. V., and Ugarova, N. N. (1996)
Mechanism of peroxidase-catalyzed oxidation. Substrate–substrate
activation in horseradish peroxidase-catalyzed reactions, Russ.
Chem. Bull., 45, 18-25.