Structural and Dynamic Changes in Mitochondria of Rat Myocardium under Acute Hypoxic Hypoxia: Role of Mitochondrial ATP-Dependent Potassium Channel
E. V. Rozova1, I. N. Mankovskaya1, and G. D. Mironova2,3*
1Bogomolets Institute of Physiology, National Academy of Sciences of Ukraine, ul. Bogomoltsa 4, 01024 Kiev, Ukraine; E-mail: erozova@ukr.net2Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences, ul. Institutskaya 3, 142290 Pushchino, Moscow Region, Russia; E-mail: mironova40@mail.ru
3Pushchino State Institute of Natural Sciences, pr. Nauki 3, 142290 Pushchino, Moscow Region, Russia
* To whom correspondence should be addressed.
Received July 1, 2014; Revision received February 9, 2015
The ultrastructure and spatial localization of mitochondria (MC) in the myocardium of rats exposed to a 30-min hypoxic hypoxia were investigated. The mitochondrial structure was found to undergo changes; however, marked necrotic injuries were not observed. Changes occurring in the myocardium are aimed at the intensification of energy processes. This shows up as an increase in the number of MC in the subsarcolemmal zone of the myocardium and changes in the surface of the sublemmal membrane due to its bending around mitochondria, which improves the diffusion of oxygen into MC. In addition, the division of MC is enhanced, which partially explains the increase in their total number. In structurally altered MC with intact membrane, electron dense formations with small diameter appear, which probably represent newly formed organelles (microMC). In normoxia, changes of this kind do not occur. It was found that the ATP-dependent K+ channel is involved in the regulation of the morphological state of MC under hypoxic hypoxia. The activator of the channel diazoxide increases the number of newly formed microMC, and the channel inhibitor 5HD significantly prevents their formation. Possible mechanisms of structural and dynamic changes in rat myocardial MC under acute hypoxic hypoxia are discussed.
KEY WORDS: mitochondria, hypoxic hypoxia, ultrastructure, spatial localization, ATP-dependent K+ channel, channel modulatorsDOI: 10.1134/S0006297915080040
Abbreviations: AHH, acute hypoxic hypoxia; 5HD, 5-hydroxydecanoate; IMF MC, intramyofibrillar population of mitochondria; MC, mitochondria; microMC, micromitochondria; mitoKATP, mitochondrial ATP-dependent K+ channel; Si tot, total surface of mitochondria per unit volume of tissue; SS MC, subsarcolemmal population of mitochondria.
Changes in the functioning of cells in different physiological and
pathological states of an organism by the action of various endogenous
and exogenous factors lead to the formation of adaptive or pathological
responses. The main pathogenetic event under oxygen deficiency of
tissues in the organism is damage to mitochondria (MC), which is
accompanied by the impairment of energy supply and antioxidant
protection, as well as the disruption of membrane stability due to
enhanced formation of lipid peroxidation products [1-6]. Experimental studies
performed under hypoxic conditions have led to the conclusion that one
of the first organelles being attacked is MC, which are the most
sensitive to unfavorable impacts [7, 8]. MC are constantly reparable cell structures. Of
great importance for the normal functioning of MC under hypoxic
conditions is their intracellular distribution, which depends on their
interaction with cytoskeletal structures [9-11].
The localization of MC in the cell is controlled by external and internal factors, and it can change during their migration by means of motor proteins along microtubules and actin filaments [8-15]. In the cell, MC form a highly dynamic reticulum in which continuous remodeling occurs. The quality of MC in tissues is controlled by a balance between the removal of damaged MC through mitophagy and the generation of new full-value organelles as a result of their division and activation of biogenesis [7, 8, 11]. According to the literature, one of the factors inducing changes in the dynamic equilibrium of the mitochondrial apparatus of the cell is oxidative stress [4, 5, 13]. Changes in the dynamics of MC under prolonged stress and acute hypoxic hypoxia of different genesis lead to the development of mitochondrial dysfunction: a decrease in the maximum respiration rate and ADP-stimulated oxygen uptake, as well as a substantial reduction in the activity of creatine kinase and the level of cytochrome c. The antiapoptotic protein Bcl-2 is translocated from the inner to the outer mitochondrial membrane [4, 6, 10].
MC have a specific localization in cardiomyocytes that is essential for the functioning of such a power-consuming organ as the myocardium. As the conditions of the cell life vary, the cytoskeletal elements providing the translocation of MC to regions where O2 diffusion proceeds more easily undergo strong changes [10-12]. The mechanism of this phenomenon is still unclear, although its understanding is of importance for the elucidation of the reasons for changes in energy metabolism.
Disorders in the “correct” distribution and transport of mitochondria may lead to pathological states, in particular, the development of tissue hypoxia. One of the most frequent manifestations of cell injury induced by unfavorable factors, among them oxygen deficiency of different genesis, is an increase in the volume of the mitochondrion (swelling). In addition, under extreme conditions, MC can grow, divide, and reach giant sizes exceeding sometimes the size of the nucleus to form megamitochondria [6-8, 16, 17].
Recently, specific changes in the structure of MC of cardiomyocytes during long-term incubation (6-72 h at 20°C) of heart pieces in medium depleted of O2 were revealed [16]. In these in vitro experiments, microMC with diameter of 0.15 µm were found as structurally damaged organelles [18, 19].
It was shown that newly formed microMC totally or partially inherited the functions of “parental” MC; thus, they showed cytochrome c activity typical of normal MC [18]. Researchers consider this phenomenon as one of the pathways of apoptotic changes in MC, which enables one to interpret apoptosis as a dual “self-destruction–self-creation” process [18-21]. The mechanisms responsible for these ultrastructural changes in MC remain obscure.
It is known that the mitoKATP is involved in the protection of the myocardium in ischemia and the adaptation of an organism to hypoxia [22-24]. Many investigators have cast no doubt on the occurrence of this channel (see reviews [25, 26]). However, because of the lack of reliable evidence on the channel structure, one could not be quite sure of this [27]. Recently, some works have reported on the identification of the mitoKATP structure. It was found that it is similar to the structure of the renal outer medullary potassium channel (ROMK), which belongs to the family of potassium inward rectified (KIR) channels [28]. In addition, it was shown by immunocytochemical assay using electron microscopy that KIR channels are localized in MC [29].
The goal of the present work was to study changes in the localization and ultrastructure of MC in cardiomyocytes of animals exposed in vivo to 30-min AHH. We found that AHH induces changes in the MC ultrastructure that contribute to the enhancement of energetic processes and the adaptation of the animals. We show that mitoKATP is involved in the regulation of these changes in mitochondrial structure.
MATERIALS AND METHODS
Reagents. Glutaraldehyde, uranyl acetate, and lead citrate were from Sigma (USA), Epon-araldite was from Fluka (Switzerland), and diazoxide and 5-hydroxydecanoate (5HD) were from Schering (USA).
Thirty-four adult white laboratory male rats of the Wistar line weighing 220-300 g were used. The control group consisted of eight animals.
AHH was created in a hermetic chamber (10 liters) using a mixture of gases containing 7% oxygen in nitrogen; a rat was placed inside the chamber for 30 min. The CO2 released by the rat was absorbed by calcined soda lime. From decrease in oxygen concentration in the air expired by the animal, it is possible to determine the adaptive potential of the organism at the tissue and cell levels [30].
The activity of the mitoKATP was changed using a selective activator of mitoKATP, diazoxide (0.3 mg/100 g of body weight), or/and the selective inhibitor 5-hydroxydecanoate (5HD) (0.5 mg/100 g of body weight) [31]. The preparation was injected intravenously to animals 1 h prior to the hypoxia treatment. Tissue samples for electron microscopic examination were taken 90 min after the injection of the preparations and 30 min after the onset of hypoxia.
After the termination of the exposure to hypoxia, the rat was decapitated under weak ether narcosis, and tissue pieces of apex cordis were taken. The material was immediately fixed by 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4. Then the material was additionally fixed using the Kolfield reagent (based on a 2% solution of OsO4 in 0.1 M phosphate buffer, pH 7.4). The material was dehydrated in alcohols of increasing concentration, absolute alcohol, and acetone, followed by embedding in Epon-araldite, which was carried out by a standard method [32].
Ultrathin sections 40-60 nm thick for the electron microscopic examination were contrasted with a 1% uranyl acetate and lead citrate solution by the method of Reynolds [33]. Samples were examined on electron microscopes JEM 100CX (JEOL, Japan) and PEM-123K (Sumy, Ukraine).
Morphometric and stereometric parameters – total number of MC, number of structurally altered MC, mean diameter of MC, area of MC, and total surface of mitochondria per unit volume of tissue (Si tot) – were determined on 130-150 fields for each exposure using the Image Tool Version 3 (USA) program for morphometric calculations.
The experiments with were conducted in accordance with the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Purposes (Strasbourg, 1986) and the ethical principles formulated in the Helsinki Declaration of 2000.
The data were statistically processed with the aid of the Microsoft Excel 2003 program (USA) using Student’s t-test and Fisher’s φ-test. The differences between means were considered significant at p < 0.05.
RESULTS
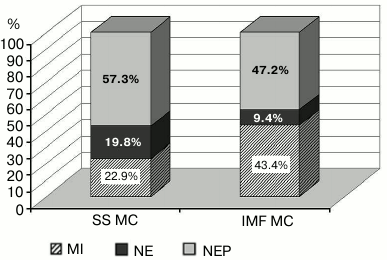
Figure 1 shows typical changes of MC in cardiomyocytes after the exposure of a rat to 30-min AHH. Necrotic changes in MC are poorly pronounced. In SS MC, they were two times more frequent and amounted to 19.8% of the total changes. Necrotic changes in IMF MC were no more than 9.4%. In the myocardium of control animals, necrosis of MC was observed in 2.8% of SS MC and in 5.0% of IMF MC.
Fig. 1. Structural changes in subpopulations of mitochondria of cardiomyocytes under acute hypoxic hypoxia. The data are given in percent; the total number of morphologically altered MC is taken as 100%. MI, mitoptotic changes; NE, necrotic changes; NEP, changes toward normalization of energetic processes; SS MC, subsarcolemmal population of mitochondria; IMF MC, intramyofibrillar population of mitochondria, p < 0.05.
The study of the mitochondrial ultrastructure in myocardium under AHH showed an increase in the number of both SS MC and IMF MC; the increase in the number of SS MC was more marked than that of the IMF subpopulation (2.7 and 1.9 times compared with control, respectively). The diameter of MC increased due to an increased permeability of mitochondrial membranes under hypoxic conditions (table). The mean area of MC increased on average by 70% in IMF MC (from 7726 ± 118 to 13,135 ± 146 arbitrary units, p < 0.05) and by 50% in SS MC (from 4864 ± 83 to 7297 ± 122 arbitrary units, p < 0.05). Together with the activation of MC morphogenesis, the changes in both characteristics led to an increase in the total surface of mitochondria per unit volume of tissue (table).
Morphometric and stereo-isometric characteristics of mitochondria in the
myocardium under acute hypoxic hypoxia (M ± m,
n = 10)
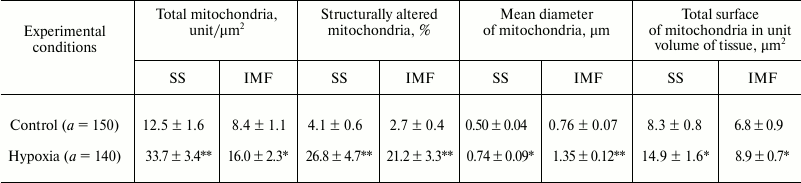
Note: a is the number of regions randomly chosen for
calculations.
* Differences are significant relative to control values (p <
0.05).
** Differences are significant relative to control values (p <
0.01).
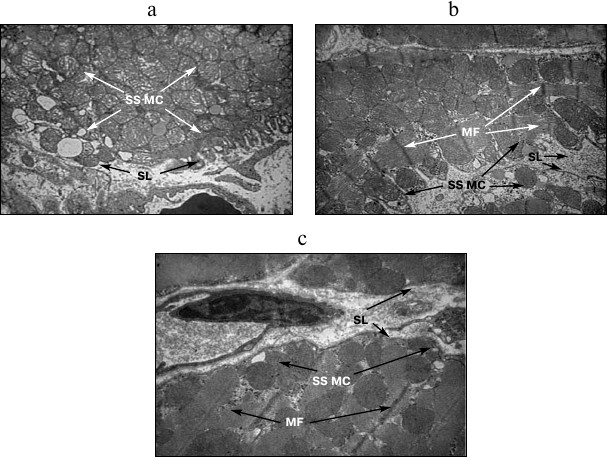
The number of structurally altered MC predominantly increased in IMF MC. In addition, the mean diameter of all MC increased 1.5-1.8-fold; as a result, the total surface of MC increased by 30.9-79.5% depending on the subpopulation of organelles (table). The SS MC often occupied the whole field of vision of the microscope and acquired rounded shape (Fig. 2a). Along with an increase in the number of MC, invaginations of the sarcolemmal edge of cardiomyocytes containing MC were often observed (Fig. 2b), which provides higher efficiency of oxygen diffusion into MC. In control experiments, neither an increase in the number of SS MC nor the invaginations of sarcolemma were observed (Fig. 2c).
Fig. 2. Structural features of subsarcolemmal population of mitochondria under acute hypoxic hypoxia. a, b) MC in invaginations of the sarcolemmal edge of cardiomyocytes under AHH; c) subsarcolemmal population of mitochondria (SS MC). SL, sarcolemma; MF, myofibrils; ×8200.
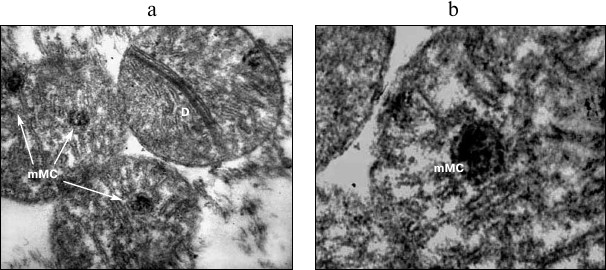
Fig. 3. Structural rearrangements in mitochondria of cardiomyocytes under acute hypoxic hypoxia. a, b) Variants of MC with incorporated microMC. MC, mitochondria; D, division of MC; mMC, microMC; ×24,500 (a); ×32,500 (b).
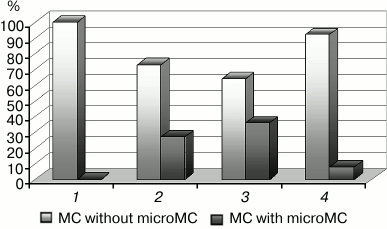
Fig. 4. Changes in the number of microMC in cardiomyocytes upon modulation of the mitoKATP: 1) control; 2) hypoxia; 3) hypoxia + diazoxide; 4) hypoxia + 5HD. The differences are significant relative to hypoxia; in both cases, p < 0.05.
DISCUSSION
The study of the ultrastructure and spatial localization of MC in the myocardium after a 30-min AHH revealed no significant necrotic injuries of the MC. On the contrary, morphological changes occurred that are commonly supposed to enhance the compensatory potential of the mitochondrial apparatus of cardiomyocytes and normalize energetic processes (Fig. 1). Thus, moderate swelling (by 25% from the initial diameter), the formation of vesicular, arched, and annular cristae, as well as their anastomosis were observed [17, 34]. Under these conditions, the cardiomyocyte mobilizes their capacity to improve oxygen diffusion into the cell and increase the energetic potency of the MC. This makes itself evident primarily as an increase in Si tot, which is more pronounced in SS MC compared with IMF MC (1.8- and 1.3-fold, respectively) (table). This parameter is of importance since it reflects the total length of mitochondrial membranes localized in a unit of the myocardial tissue [35].
Because the main function of MC, namely energy supply, depends on the work of respiratory enzymes, which are bound to membranes, the length of mitochondrial membranes may be an indirect indicator of the capability of MC to participate in the energy metabolism of cells. In this case, the total number of MC increases (which is more pronounced in SS MC) due to both enhanced division of MC and the formation of new organelles (Fig. 3). The greater extent of the above-described changes in SS MC may be related to the mode of impact since the major pathogenetic factor under hypoxia is deficient oxygen supply. Therefore, it is MC localized near the sarcolemma that should undergo changes providing more intensive capture of oxygen from blood and an enhancement of the energetic potential of the cell.
In addition, it follows from our calculations that the percentage of MC with altered ultrastructure in this model of hypoxia was in IMF MC higher than in SS MC (8.0- and 6.5-fold, respectively) (table). The data of the present work do not support the idea that SS MC are more heavily damaged under unfavorable conditions [4, 7, 8], which is quite logical since IMF MC are more remote from the capillary, and the oxygen deficit in this subpopulation is more pronounced.
The changes in the morphometric and stereometric characteristics of both mitochondrial subpopulations under hypoxic conditions show that the compensatory potential of MC of cardiomyocytes can also manifest itself in significant structural changes.
As it was found, SS MC often occupied the whole field of vision of the microscope and became rounded (Fig. 2a). Some investigators consider these changes as one of the initial stages of apoptosis when cell shrinkage occurs: the cell decreases in size, the cytoplasm becomes denser, and organelles that appear relatively normal are arranged more densely. It is assumed that the changes in the form and volume of the cell result from the activation of transglutaminase in apoptotic cells and are characteristic of the receptor-dependent (non-mitochondrial) pathway of apoptosis [13, 34, 36, 37].
We are inclined to relate the hypoxia-induced increase in the number of MC near the cytoplasm not to the activation of apoptosis but to the necessity of affording optimal energy metabolism in the myocardium. We believe that the changes in cell morphology detected under hypoxia at sites of O2 diffusion from the blood of capillaries (Fig. 2, a and b) are regulated by the active functioning of cytoskeletal systems, in particular, intermediate desmin and vimentin filaments capable of holding MC in strictly defined places [12, 15, 38].
The formation of MC with the so-called vesicular structure of cristae (Fig. 2a) is worthy of notice. As a result of the formation of arched and annular cristae and their anastomosis, one end of a crista comes in contact with the outer mitochondrial membrane. This is a necessary condition for the effective functioning of the crista [6]. Therefore, the appearance of vesicular cristae may be evidence for the switching at the cellular level of compensatory and adaptive mechanisms promoting either the retention or an increase in the energy-forming surface of cristae under hypoxic conditions.
The structural changes observed at the initial stage of hypoxia, which provide the optimization of energy metabolism, can explain, to a certain degree, the constructive effect of training with hypoxia, which is known to contribute to the normalization of energy metabolism under unfavorable conditions.
The experiments with a 30-min hypoxia showed that a great portion of MC with ultrastructural changes contained small optically dense formations structurally similar to intact MC (Figs. 3a and 3b). Sometimes, two to three formations were seen in one MC (Fig. 3a). They resembled structures found in MC in in vitro experiments with heart pieces incubated for a long time (6-72 h at 20°C) under anoxic conditions, which were called microMC [18, 20]. We proposed that this is a random process; a necessary condition for the formation of microMC is the concentration of an appropriate plastic, genetic, and energetic material in one place. It is for this reason that some MC contain a few newly formed microMC, and in other organelles, these structures are absent, which is consistent with the well-known fact of the heterogeneity of MC.
Because our experiments were carried out under in vivo conditions, the origination of microMC can be considered as one of the pathways aimed at the retention of the energetic potency of the mitochondrial apparatus under hypoxia. The examination of the effect of known drugs protecting the heart against ischemia, namely mitoKATP modulators, led to the same conclusion [22-24]. Because the channel activator diazoxide, which protects the heart against hypoxia [26, 28], enhanced the formation of microMC, and the channel inhibitor 5HD, which abolishes the cardioprotecting effect of diazoxide [23, 25, 31], prevented their formation (Fig. 4), we assume that the origination of microMC is a positive antihypoxic factor.
Until the present time, the mechanisms responsible for the formation of microMC remain poorly understood [19]. Another type of morphological changes, the fragmentation of MC, has been well studied at the molecular level, especially on yeasts. Proteins responsible for the fusion and division of MC have been isolated [39, 40]. However, the data obtained in this study indicate that, under the conditions of our experiments, not the fragmentation but the formation of new microMC inside altered “parental” MC takes place. Saprunova and coauthors [18, 20] as well as the researchers of our group showed that during the formation of microMC, the outer and inner mitochondrial membranes remain intact.
Thus, 30-min AHH induces structural and dynamic changes in rat myocardial MC, which indicates the initiation of compensatory processes in these organelles. Upon significant structural changes in MC, owing to the constructive and training effects of hypoxia, the myocardium acquires the capacity for normalization of energy metabolism. The results of this study suggest that the mitoKATP is one of the regulators of the morphological state of MC under hypoxia, which may explain the known cardioprotective effect of its activators.
This work was supported by the Government and the Ministry of Education of the Russian Federation (projects No. 14.Z50.31.0028 and DPNNiT No. 2014/281/2495).
REFERENCES
1.Vasyuk, Y. A., Kulikov, K. G., Kudryashov, O. N.,
Krikunova, A. V., and Sadulaeva, I. A. (2007) Secondary mitochondrial
dysfunction in acute coronary syndrome, Rational Pharmacol.
Cardiol., 1, 41-47.
2.Lukyanova, L. D. (1997) Bioenergy hypoxia: the
concept, mechanisms and methods of correction, Bull. Exp. Biol.
Med., 124, 244-254.
3.Cereghetti, G. M., and Scorrano, L. (2006) The many
shapes of mitochondrial death, Oncogene, 25,
4717-4724.
4.Karbowski, M., and Youle, R. J. (2003) Dynamics of
mitochondrial morphology in healthy cells and during apoptosis, Cell
Death Different., 10, 870-880.
5.Collins, T. J., Berridge, M. J., Lipp, P., and
Bootman, M. D. (2002) Mitochondria are morphologically and functionally
heterogeneous within cells, J. Eur. Mol. Biol. Organization,
21, 1616-1627.
6.Skulachev, V. P. (2001) Oxygen and the phenomenon
of programmed cell death, Russ. Biomed. J., 5,
116-126.
7.Paukov, V. S., and Khitrov, N. K. (1991)
Adaptation of Heart to Hypoxia [in Russian], Meditsina,
Moscow.
8.Rozova, E. V. (2008) Changes in the
morphofunctional state of mitochondria in cells of the lungs and heart
tissues in rats during hypoxia of different genesis, J. Acad. Med.
Sci. Ukraine, 14, 752-765.
9.Nekrasova, O. E. (2007) Studying of Mechanisms
of Intracellular Distributions of Mitochondria: Candidate’s
dissertation [in Russian], 03.00.25 – histology, cytology, cell
biology, Moscow.
10.Nekrasova, O. E., Kulik, A. V., and Minin, A. A.
(2007) Protein kinase C regulates the mitochondrial motility, Biol.
Membr. (Moscow), 24, 126-132.
11.Nekrasova, O. E., Kulik, A. V., and Minin, A. A.
(2005) Regulation by fibronectin shapes and intracellular distribution
of mitochondria, Biol. Membr. (Moscow), 22, 105-112.
12.Kulik, A. V., Nekrasova, O. E., and Minin, A. A.
(2006) Fibrillar actin regulates the mitochondrial motility, Biol.
Membr. (Moscow), 23, 42-51.
13.Pshenkina, N. N. (2008) Metabolic aspects of the
regulation of apoptosis induced by exogenous factors, Bull. Russ.
Acad. Military Med., 23, 219.
14.Milner, D. J., Mavroidis, M., Weisleder, N., and
Capetanaki, Y. (2000) Desmin cytoskeleton linked to muscle
mitochondrial distribution and respiratory function, J. Cell
Biol., 150, 1283-1298.
15.Paulin, D., and Li, Z. (2004) Desmin: a major
intermediate filament protein essential for the structural integrity
and function of muscle, Exp. Cell Res., 301, 1-7.
16.Tonshin, A. A., Saprunova, V. B., Bakeeva, L. E.,
and Yaguzhinskii, L. S. (2003) Functional activity and ultrastructure
of mitochondria isolated from apoptotic heart tissue, Biochemistry
(Moscow), 68, 875-881.
17.Sudakova, Y. V., Bakeeva, L. E., and Tsyplenkova,
V. G. (1999) Energy dependent changes in the ultrastructure of
mitochondria of human cardiomyocytes in alcoholic heart disease,
Arch. Pathol., 2, 15-20.
18.Solodovnikova, I. M., Saprunova, V. B., Bakeeva,
L. E., and Yaguzhinskii, L. S. (2006) Dynamics of changes in
mitochondrial ultrastructure of isolated myocardium cardiomyocytes in
rats during prolonged incubation under anoxia conditions,
Cytology, 48, 848-855.
19.Saprunova, V. B., Kazimirchuk, S. A., Tonshin, A.
A., Bakeeva, L. E., and Yaguzhinskii, L. C. (2002) Induction of
apoptosis in rat myocardium under conditions of anoxia, Biochemistry
(Moscow), 67, 246-253.
20.Saprunova, V. B., Bakeeva, L. E., and
Yaguzhinskii, L. S. (2003) The ultrastructure of mitochondria at rat
cardiomyocytes apoptosis induced by long action of anoxia,
Cytology, 45, 1073-1082.
21.Saprunova, V. B., Solodovnikova, I. M., and
Bakeeva, L. E. (2008) Identification of cytochrome c oxidase
activity in mitochondria of isolated myocardial tissue cardiomyocytes
during prolonged hypoxia, Cytology, 50, 268-274.
22.Garlid, K. D., Paucek, P., Yarov-Yarovoy, V.,
Murray, H. N., Darbenzio, R. B., D’Alonzo, A. J., Lodge, N. J.,
Smith, M. A., and Grover, G. J. (1997) Cardioprotective effect of
diazoxide and its interaction with mitochondrial ATP-sensitive K+
channels. Possible mechanism of cardioprotection, Circ.
Res., 81, 1072-1082.
23.Krylova, I. P., Kachaeva, E. V., Rodionova, O.
M., Negoda, A. E., Evdokimova, N. R., Balina, M. I., Sapronov, N. S.,
and Mironova, G. D. (2006) The cardioprotective effect of uridine and
uridine-5′-monophosphate: the role of the mitochondrial
ATP-dependent potassium channel, Exp. Gerontol., 41,
697-703.
24.Mironova, G. D., Shigaeva, M. I., Gritsenko, E.
N., Murzaeva, S. V., Gorbacheva, O. S., Germanova, E. L., and
Lukyanova, L. D. (2010) Functioning of the mitochondrial ATP-dependent
potassium channel in rats varying in their resistance to hypoxia.
Involvement of the channel in the process of animal’s adaptation
to hypoxia, J. Bioenerg. Biomembr., 42,
473-481.
25.Mironova, G. D., Kachaeva, E. V., Krylova, I. B.,
Rodionova, O. M., Balina, M. I., Evdokimova, N. R., and Sapronov, N. S.
(2007) Mitochondrial ATP-sensitive potassium channel. II. The role of
the channel in protecting the heart from ischemia, Bull. Russ. Acad.
Med. Sci., 2, 44-50.
26.O’Rourke, B. (2004) Evidence for
mitochondrial K+ channels and their role in
cardioprotection, Circ. Res., 94, 420-432.
27.Das, M., Parker, J., and Halestrap, A. (2003)
Matrix volume measurements challenge the existence of
diazoxide/glibenclamide-sensitive KATP channels in rat
mitochondria, J. Physiol., 547, 893-902.
28.Foster, D. B., Ho, A. S., Rucker, J., Garlid, A.
O., Chen, L., Sidor, A., Garlid, K. D., and O’Rourke, B. (2012)
Mitochondrial ROMK channel is a molecular component of
mitoKATP, Circ. Res., 111, 446-454.
29.Talanov, E. J., Pavlik, L. L., Shigaeva, M. I.,
Belosludtseva, N. V., Moshkov, D. A., and Mironova, G. D. (2013)
Detection of protein family of KIR6 in the heart and liver mitochondria
of rats by immunoelectron microcopies, Biol. Membr. (Moscow),
30, 474-478.
30.Malkin, V. B. (1977) Acute and Chronic
Hypoxia [in Russian], Nauka, Moscow.
31.Mironova, G. D., Kachaeva, E. V., and Kopylov, A.
T. (2007) Mitochondrial ATP-dependent potassium channel. I. Structure
of the channel arrangements for its functioning and regulation,
Bull. Russ. Acad. Med. Sci., 2, 34-43.
32.Karupu, V. J. (1984) Electron Microscopy
[in Russian], Vysshaya Shkola, Kiev.
33.Weekly, B. (1975) Electron Microscopy for
Beginners [Russian translation], Mir, Moscow.
34.Zaleski, V. N., and Velikaya, N. V. (2003)
Mechanisms of cytotoxic effects of active oxygen molecules and the
development of apoptosis, Modern Probl. Toxicol., 1,
11-17.
35.Tashke, K. (1980) Introduction to Quantitative
Cyto-histological Morphology, Publishing House of the Academy of
SRR, Bucharest.
36.Ushakova, T. A., Globa, A. G., and Karelin, A. A.
(2007) Cytokine profile and modulation of apoptosis under thermal
injury, Immunology, 28, 226-230.
37.Appaix, F., Kuznetsov, A. V., Usson, Y., Kay, L.,
Adrienko, T., Olivares, J., Kaambre, T., Sikk, P., Margreiter, R., and
Saks, V. (2003) Adaptation of mitochondria, Exp. Physiol.,
88, 175-190.
38.Reipert, S., Steinbock, F., and Fischer, I.
(1999) Association of mitochondria with plectin and desmin intermediate
filaments in striated muscle, Exp. Cell Res., 252,
479-491.
39.Polyakov, V. Yu., Soukhomlinova, M. Yu., and
Fais, D. (2003) Fusion, fragmentation, and fission of mitochondria,
Biochemistry (Moscow), 68, 838-849.
40.Skulachev, V. P., Bakeeva, L. E., Chernyak, B.
V., Domnina, L. V., Minin, A. A., Pletjushkina, O. Y., Saprunova, V.
B., Skulachev, I. V., Tsyplenkova, V. G., Vasiliev, J. M., Yaguzhinsky,
L. S., and Zorov, D. B. (2004) Thread-grain transition of mitochondrial
reticulum as a step of mitoptosis and apoptosis, Mol. Cell.
Biochem., 256/257, 341-358.