REVIEW: What Controls the Expression of the Core-1 (Thomsen–Friedenreich) Glycotope on Tumor Cells?
U. Karsten* and S. Goletz
Glycotope GmbH, Robert-Rössle-Str. 10, D-13125 Berlin-Buch, Germany; fax: +49-30-9489-2609; E-mail: uwe_karsten@gmx.de; steffen.goletz@glycotope.com* To whom correspondence should be addressed.
Received February 1, 2015; Revision received March 23, 2015
Malignant transformation is tightly connected with changes in the glycosylation of proteins and lipids, which in turn are contributing to the invasive and metastatic behavior of tumor cells. One example of such changes is demasking of the otherwise hidden core-1 structure, also known as Thomsen–Friedenreich antigen, which is a highly tumor-specific glycotope and potentially a cancer stem cell marker. This review summarizes what is known about the mechanism(s) of its expression on tumor cells. New data reveal a close connection between tumor metabolism and Golgi function. Based on these data, we suggest that the expression of this antigen is also a marker of aerobic glycolysis.
KEY WORDS: glycosylation, Golgi, core-1, Thomsen–Friedenreich, glycolysis, tumor metabolism, pHDOI: 10.1134/S0006297915070019
Abbreviations: TF, Thomsen–Friedenreich antigen; V-ATPase, vacuolar H+-ATPase.
Core-1 (Galβ1-3GalNAcα1-Thr/Ser) is an intermediate structure
in the biosynthesis of O-glycans of the mucin type. It was
detected by serendipity as a blood group-related antigen (occurring on
contaminated red blood cells) in the mid-twenties of the last century,
and named after the authors Thomsen–Friedenreich (TF or T)
antigen [1]. Its chemical nature was described by
Gerhard Uhlenbruck in the sixties [2]. However, it
was only in 1975 that Georg F. Springer determined that TF is in fact a
tumor antigen, or more correctly an oncofetal antigen [3, 4]. Since then many
publications have confirmed the outstanding tumor specificity [5, 6] and broad tumor distribution
(e.g. [7-11]) of this glycan
structure in adult human tissues. In spite of this fact, the TF antigen
has attracted much less attention than most other tumor antigens. One
reason may be that it is a glycan and not a protein. As such, it
requires a different experimental approach, and its expression follows
divergent and complex rules. Another fact is that specific antibodies
to this structure were difficult to generate and are relatively rare
[12].
Whereas the tumor specificity of TF in adult human tissues is very high, its sensitivity is not. Immunohistochemical studies have shown that TF is expressed on many different tumor types, especially on epithelial tumors. However, the percentage of positive cases varies among carcinoma types from almost 100% in ovarian cancer, 85% in breast cancer, 60% in colon cancer, and 50% in acute lymphatic leukemia (T cell ALL) to around 15% in renal cell cancer. In addition, the frequency of TF-positive cells within individual tumors varies. Studies with human cell lines revealed a similar expression pattern. Whereas normal (non-malignant) cell lines are throughout negative, tumor cell lines are in most but not all cases TF-positive, and often not all cells are actually stained even after cloning. In addition, the percentage of positive cells of a given cell line may vary over time for unknown reasons.
The variability of expression of the Thomsen–Friedenreich glycotope on cancer cells has an obvious impact on its potential use as a therapeutic target. Some evidence suggests that TF may be a (not necessarily exclusive) marker of cancer stem cells [13]. In this case, TF would be a promising therapeutic target irrespective of its absence on parts of the tumor. Therefore, the mechanisms leading to the expression of this exceptional tumor antigen should be of considerable interest.
CARRIER MOLECULES OF CORE-1/TF
TF does not exist as such in the body. It is exclusively expressed on carrier proteins. Whether it is also found on glycolipids is not certain. Glycolipids often carry a structurally related glycan, Galβ1-3GalNAcβ1- (the so-called TFβ antigen), which is immunologically distinct, and is not per se a tumor antigen. Two questions arise: first, which proteins are carrier proteins of TF, and second, how are these carrier molecules distributed?
TF is a ubiquitous primary (core-1) sequence of O-glycans. As such, it is present on almost all membrane glycoproteins of the mucin type, although immunologically masked through the elongation of the sugar chain. Since the expression (or more correctly demasking) of TF on tumor cells is generally considered as the result of distorted (truncated) glycosylation [14], many glycoproteins should theoretically be candidate carriers of TF after malignant transformation. However, this is apparently not the case. In Western blots of tumor cell lysates, only one or very few TF-positive bands are detected. So far, only a handful of carrier proteins have been identified [13, 15]. Each of them is apparently characteristic for a certain tumor type, e.g. CD44 for colon cancer, MUC1 for breast cancer, or CD34 for leukemia. Interestingly, most of these proteins are known stem cell markers [13]. The reason for this selectivity is not known. The simplest answer would be that the identified carrier molecule is the most prominent glycoprotein of the cell in question. However, the fact that in most cases not all cells of the tumor are TF-positive also speaks in favor of a selective process. This is clearly different from embryonic epithelial cells, and also different from normal adult cells after treatment with sialidase, in which case virtually all epithelial cells are TF+. Therefore, it is even more important to ask what may control the expression of TF on tumor cells.
EVIDENCE FOR ENZYME ACTIVITY CHANGES
The first question to be asked is whether common changes in the expression and/or activity of enzymes involved in the glycan biosynthesis, for instance glycosyltransferases, are observed in tumor cells. This has been examined in numerous studies and assessed in several reviews [16-19]. A drawback of most of these studies is that they were performed on tumor cell lines, which in most cases are derived from ascites cells and do not represent primary tumors.
The best examined tumor types in respect of glycosylation defects are colon and breast cancer. Normal colonic epithelial cells express predominantly glycans of the core-3 type (table), whereas colorectal cancers express core-1 and other short glycan chains. The enzyme responsible for the elongation of the primary GalNAc residue to core-3, core3GlcNAcT, is diminished in colon cancer [16]. This might redirect the glycosylation towards core-1. In a similar way C2GnT2, which leads to further extension of core-3, is often diminished [20]. The interpretation of data from certain other enzymes is not as easy. For example, in a study encompassing 40 primary colorectal cancer cases, four relevant glycosyltransferases were examined by RT-PCR (and partly also for their enzyme activity) and correlated with the expression of TF [21]. Three sialyltransferases, which are able to modify (mask) core-1, were found to be enhanced in colorectal cancer, but not in inverse correlation with the expression of core-1 (TF), as could have been expected. Normal breast epithelial cells express predominantly core-2-related glycans (table). Breast cancer cells carry sialyl-Tn, TF, and sialyl-TF. ST3Gal-I is consistently overexpressed in breast cancer [22]. At the same time, C2GnT1 expression is lower than in normal breast tissue [22], consistent with the observed decline in core-2 glycans and the accumulation of TF. In other studies, no mutants or major changes of the relevant transferases were found. Among hundreds of breast carcinoma samples, no sign of loss of activity of the core-1 transferase (C1GalT) was seen [23]. In a recent study on 46 cases of pancreatic cancer, no evidence for mutations in any of over 200 different transferases examined was observed [24]. Instead, the chaperone COSMC was found hypermethylated in 40% of these cases. COSMC is necessary for the activity of C1GalT1 [25]. Another aspect to be considered is the availability of sufficient amounts of substrates for the transferases, which depends on the activities of the respective transporter enzymes. For instance, transfection with UDP-Gal transporter cDNA led to the expression of TF in colon cells [26]. In accord with this, the amount of mRNA for the UDP-Gal transporter has been found to be enhanced in colon cancer tissues [26].
Structures of glycans mentioned in this review
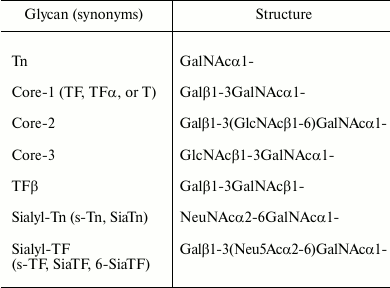
Taken together, the observed changes in the expression or activity of relevant enzymes alone are apparently not sufficient to explain the phenomenon of core-1 expression on tumor cells. We should therefore turn the attention on changes occurring in the structure and function of the Golgi apparatus during the process of malignant transformation.
EVIDENCE FOR DISORDER IN THE GOLGI, AND THE ROLE OF pH
There is clear evidence that each glycosyltransferase has a defined place in the cisternae of the Golgi stack, and that this is of utmost importance for a regular glycan biosynthesis [27, 28]. Any disturbance of its specific localization leads to the synthesis of “incorrect” glycans. This can also be seen on normal cells under experimental conditions. We have shown that the overexpression of a non-enzyme Golgi transmembrane protein, RCAS1 (EBAG9), in human embryonic kidney cells (HEK293) leads to the expression of TF and Tn at the cell membrane [29]. This observation can be explained as a consequence of mechanical dislocation of glycosyltransferases from their normal sites.
In the Golgi of malignant cells, abnormal localization of glycosyltransferases and other molecules has repeatedly been reported. For instance, certain Golgi proteins were observed at the wrong place in colon cancer cells [30]. In T-47D breast cancer cells, ST6GalNAc-1 localizes throughout the Golgi instead of its normal position in the cis-Golgi, which is considered to be responsible for the increased expression of truncated glycans [31]. In another example, GalNAc transferases T1-T3 in HeLa cells are located throughout the whole organelle [32]. As a possible reason for the redistribution of glycosyltransferases and/or the expression of TF, an enhanced pH in the lumen of the Golgi vesicles has been described [33, 34].
Interestingly, incomplete glycosylation as the result of an experimental increase of the Golgi pH in plasma cells has been demonstrated as early as in 1986 [35]. This treatment also led to the loss of polarized transport of secreted proteins, underscoring the importance of an acidic pH in the secretory pathway [36]. Recently, more sophisticated models have been used by Sakari Kellokumpu and coworkers [37]. They examined normal and malignant cell lines as well as tissue samples from normal colon and from colonic cancer, and they applied bafilomycin A1 (BafA1) and other pH-dissipating drugs to the cell lines. BafA1 is a specific inhibitor of the vacuolar H-ATPase (V-ATPase), the major proton pump of the Golgi. After treatment with BafA1, the normal pH gradient within the Golgi stack (from 6.7 in the cis-Golgi network to 6.0 in the trans-Golgi network) is replaced with a uniform luminal pH of around 6.8 [38]. In the experiments of Kellokumpu and coworkers [37], this treatment led in normal rat kidney (NRK) and other cell lines to a structural disorganization of the Golgi apparatus and to the expression of TF. Structural disorganization of the Golgi and the expression of TF were also seen in colorectal cancer cells but not in normal colonic mucosa. Another example was shown with MCF7 breast cancer cells (acidification-defective) and an acidification-competent subline, MCF7/AdrR, whereby the latter was found to have rescued normal glycosylation potential in parallel to the regained intracellular pH gradient [37]. The observed correlation between the expression of TF and an elevated Golgi pH was substantially confirmed in further studies [39]. Direct measurements of the Golgi pH revealed significant differences between normal and malignant cells (around 6.2 in normal human fibroblasts versus about 6.8 in MCF7, HT29, and SW48 tumor cells). Treatment of (non-malignant) COS-7 cells with pH gradient dissipating drugs (BafA1, chloroquine, or NH4Cl) resulted in an increase of Golgi pH and in the (intracellular) expression of TF. It was found that an increase of 0.2 pH unit was sufficient to inhibit terminal glycosylation. Finally, MCF7 cells showing a mosaic of TF+ and TF– cells were individually analyzed for their luminal Golgi pH. It was found that the median Golgi pH of TF+ cells was about 0.3 pH unit higher than that of TF– cells. Further studies have shown that an elevated Golgi pH, similar to its effect on O-glycosylation, also leads to incomplete glycosylation of N-glycans [40, 41].
Recent studies give some clue to the mechanism(s) of the observed pH-sensitivity of glycosylation. It is known for some time that Golgi vesicles contain defined heteromeric complexes of glycosyltransferases, which guarantee an orderly biosynthetic processing of the glycan chains [28]. These enzyme complexes are stable at low pH, but disintegrate at higher pH [42]. In fact, the formation of heteromers of glycosyltransferases at low pH and its disintegration and the formation of homomers at higher pH, are part of a normal trafficking cycle of these enzymes [43], which is obviously distorted in cancer cells.
In conclusion, besides occasional changes in the activity or expression of individual transferases or substrate transporters, a more general reason for the observed changes in tumor cell glycosylation is apparently the partial or complete loss of the acidic pH of the Golgi lumen.
The question arises, what can explain the loss of the pH gradient in the Golgi of malignant cells? First, a brief look on the normal pH regulation of the Golgi cisternae. Three main ion transport systems are known to be responsible: the vacuolar H+-ATPase (V-ATPase), the Golgi pH regulator (GPHR), and the AE2a exchanger [38, 44-46]. The first is continuously pumping H+ ions into the Golgi lumen. The second channel protein mediates counter-ion conductance in order to maintain the membrane potential. The third is a Golgi-specific HCO3–/Cl– exchanger responsible for buffering the Golgi pH during protein import and export processes. All three must counteract a constant passive leakage of protons from the Golgi. At present, however, there is no general explanation available, why the Golgi pH is misregulated in cancer cells [44].
We should therefore turn our attention to one of the main hallmarks of cancer, the metabolic changes occurring during malignant transformation, because they lead to massive pH changes in and around tumor cells.
METABOLIC CHANGES
In the mid-twenties of the past century, Otto Warburg demonstrated that tumor cells change their glucose metabolism from oxidative phosphorylation to glycolysis even under normoxic conditions (aerobic glycolysis) [47]. After decades of widespread negligence, this fact has again attracted much attention during recent years to become one of the major hallmarks of cancer [48]. The metabolic switch leads to a reverse pH gradient at the cell membrane with an acidic pH in the interstitial space, and an alkaline intracellular (cytoplasmic) pH [49-51]. The acidic microenvironment leads, among other effects, to the generation of acid-resistant and invasive subpopulations [52].
Could the alkaline intracellular pH (ranging between 7.12 and 7.65 [50]) be the reason for the alkalinization of the Golgi lumen? We will first have a brief look on the mechanism(s) causing the metabolic switch. Considering the current model of carcinogenesis, which proposes that a sequence of accumulating mutants of oncogenes and tumor suppressor genes is the primary cause of cancer [53, 54], it is necessary to look for genetic changes causing glycolysis. It is astonishing that very many oncogenes (e.g. PI3K, Akt, HER2, EGFR, c-Myc, HRAS) or silenced tumor suppressor genes (e.g. PTEN, p53, SIRT3, VHL) are potentially involved in this process [49, 53, 59]. Most are also growth-regulating genes, and they may act either alternatively or synergistically. One pathway involves the Na+/H+ exchanger (NHE1) [55], which is activated by several oncogenes such as HRAS. Overexpression of NHE1 is sufficient to result in an inverse pH gradient at the plasma membrane. In addition, this leads to the elevation of aerobic glycolysis. In turn, the generation of lactate during glycolysis activates the H+/lactate cotransporter (monocarboxylate transporter, MCT), which results in a further increase of intracellular pH [55]. Another case [56] is initiated by the overexpression of c-Myc, which results in a change in the expression pattern of pyruvate kinase (PKM), a key enzyme in the metabolic regulation. PKM exists in two isoenzymes. The prevailing isoenzyme PKM1 forms stable tetramers, which direct pyruvate towards oxidative phosphorylation. c-Myc, however, leads to the preferential expression of PKM2, which mainly exists as a dimer and guides the metabolism towards glycolysis. In addition, PKM2 gives a positive feedback signal to c-Myc [56]. c-Myc also induces the upregulation of lactate dehydrogenase (LDH-A) [49]. In most cases, the metabolic switch towards glycolysis is coordinated by the transcription factor HIF-1 (hypoxia-inducible factor 1) [57]. It is upregulated either under hypoxia (which arises already in very small tumors such as in carcinoma in situ) or by oncogenes or silenced tumor suppressor genes, in this case also under normoxic conditions. In the latter case, it remains constitutively activated. HIF-1 is composed of two subunits, HIF-1α and HIF-1β, of which the former is subject to O2-dependent degradation. Both, the constitutive overexpression of HIF-1α or the inactivation of the tumor suppressor VHL (von Hippel–Landau protein), which is involved in its degradation, results in a constantly increased HIF-1 level. HIF-1 is a versatile transformation factor targeting hundreds of genes, primarily those active in glycolysis.
It can be added that an artificially generated acidic extracellular environment without metabolic background has quite different effects. It results in lowering of the cytoplasmic pH, and it does not alter the Golgi pH [58].
CONSEQUENCES FOR THE GOLGI pH
In any case, the result of malignant transformation is an enhanced pH of the cytoplasm of tumor cells. It can be anticipated that there is a link between the rise of pH in the cytosol and the rise of pH in the Golgi lumen of tumor cells. The question is whether the high pH in the cytosol per se leads to an alkalinization of the Golgi lumen, or whether there are further mediators involved. At present, there is no definitive answer available to this question. The simplest explanation would be to assume that the (already existing) leakage of the Golgi membrane for protons is enhanced under these conditions and cannot be compensated by the V-ATPase. This effect might be potentially aggravated by the observed volume increase of the lumen of the cisternae [35, 37]. On the other hand, changes in the activities of the Golgi proton pumps are also possible. Experiments with the bovine papillomavirus E5 demonstrate that impairment of the V-ATPase activity caused by its binding to the transforming protein of the virus is sufficient to generate an alkaline Golgi lumen [60]. The activity of the V-ATPase is regulated in many ways. One of the regulatory elements is its glucose dependency [61]. Since the turnover rate of glycolysis is very high, the level of free glucose can be low. Not much is known about the regulation of the newly described Golgi pH regulator (GPHR) [45]. In conclusion, we have to admit that these possibilities are so far only speculative. In other words, there is still a “missing link” in our knowledge about the mechanism(s) connecting the alkaline cytosolic pH of tumor cells with the dissipation of the pH gradient in the Golgi.
An example for a direct connection between hypoxia and the expression of TF is provided in a study in which human placenta explants or immortalized cytotrophoblasts were exposed to normoxic or hypoxic conditions, respectively. In case of low oxygen supply, an enhanced expression of both HIF-1α and TF was observed [15]. It should be kept in mind, however, that trophoblast cells are very unusual cells [62].
HYPOTHESIS AND CONCLUSION
We propose that the expression of the truncated O-glycan, core-1/TF, is a (indirect) marker of aerobic glycolysis. This is based on the following facts. The expression of TF is linked to the dissipation of the pH gradient in the Golgi. Alkalinization of the Golgi lumen is regularly observed in tumor cells, and it leads to changes in the localization of certain glycosyltransferases, in particular to the dissociation of heteromeric enzyme complexes, which are essential for an orderly, differentiation-equivalent glycosylation. This leads to incorrect and/or incomplete glycosylation of O- and N-glycans. At the same time, the cytosolic pH is enhanced because of the basic metabolic switch of the tumor cell towards aerobic glycolysis, which is the result of the genetic changes leading to malignancy. Thus, an almost complete causal chain obviously exist from altered genes to cancer-specific glycan structures.
New data reveal that subpopulations of tumor cells with different types of glucose metabolism exist side by side, showing either pure glycolysis, oxidative phosphorylation, or glycolysis mixed with oxidative phosphorylation in different proportions [48]. Such heterogeneity conforms to the fact that the expression of TF on tumors is also often heterogeneous, perhaps restricted to cancer stem cell (CSC) areas. However, this has to be substantiated in further studies.
Some questions remain unanswered. One question is, as mentioned above, how the alkaline pH of the cytosol (about 7.4) is implementing the neutralization of the Golgi lumen. A second open question is, whether or under which circumstances expression of TF may occur on sites of anaerobic glycolysis, such as at normal stem cell niches [63]. This seems not to be the case, since in many immunohistochemical surveys for TF expression in human tissues, these areas were always TF-negative. Third, new data show that HIF-1 from cancer cells can reprogram cancer-associated fibroblasts (CAFs) towards glycolysis [64]. In return, these cells serve the tumor cells by feeding them with lactate and other metabolites (“reverse Warburg effect”). It is not known, whether TF expression can occur under these circumstances, and if so, which cells of this type of metabolic symbiosis are TF-positive.
The Thomsen–Friedenreich antigen as a blood group determination artifact and the aerobic glycosylation of cancer cells were detected almost simultaneously in the mid-twenties of the past century, about 90 years ago. Nobody could imagine at that time that there could be a connection between the two phenomena, but now it appears that just this seems to be the case.
REFERENCES
1.Friedenreich, V. (1930) The Thomsen
Hemagglutination Phenomenon, Levin & Munksgaard,
Copenhagen.
2.Kim, Z., and Uhlenbruck, G. (1966) Untersuchungen
uber T-antigen und T-agglutinin, Z. Immun. Forsch., 130,
88-99.
3.Springer, G. F., Desai, P. R., and Banatwala, I.
(1975) Blood group MN antigens and precursors in normal and malignant
human breast glandular tissue, J. Natl. Cancer Inst., 54,
335-339.
4.Springer, G. F. (1997) Immunoreactive T and Tn
epitopes in cancer diagnosis, prognosis, and immunotherapy, J. Mol.
Med., 75, 594-602.
5.Springer, G. F. (1984) T and Tn, general carcinoma
autoantigens, Science, 228, 1198-1206.
6.Cao, Y., Stosiek, P., Springer, G. F., and Karsten,
U. (1996) Thomsen–Friedenreich-related carbohydrate antigens in
normal adult human tissues: a systematic and comparative study,
Histochem. Cell Biol., 106, 197-207.
7.Cao, Y., Karsten, U., Liebrich, W., Haensch, W.,
Springer, G. F., and Schlag, P. M. (1995) Expression of
Thomsen–Friedenreich-related antigens in primary and metastatic
colorectal carcinomas: a reevaluation, Cancer, 76,
1700-1708.
8.Takanami, I. (1999) Expression of
Thomsen–Friedenreich antigen as a marker of poor prognosis in
pulmonary adenocarcinoma, Oncol. Rep., 6, 341-344.
9.Baldus, S. E., Hanisch, F. G., Monaca, E., Karsten,
U., Zirbes, T. K., Thiele, J., and Dienes, H. P. (1999)
Immunoreactivity of Thomsen–Friedenreich (TF) antigen in human
neoplasms: the importance of carrier-specific glycotope expression on
MUC1, Histol. Histopathol., 14, 1153-1158.
10.Cao, Y., Karsten, U., Otto, G., and Bannasch, P.
(1999) Expression of MUC1, Thomsen–Friedenreich antigen, Tn,
sialosyl-Tn, and α2,6-linked sialic acid in hepatocellular
carcinomas and preneoplastic hepatocellular lesions, Virchows
Arch., 434, 503-509.
11.Veerman, A. J. P., Hogeman, P. H. G., Huismans,
D. R., Van Zantwijk, C. H., and Bezemer, P. D. (1985) Peanut
agglutinin, a marker for T-cell acute lymphoblastic leukemia with a
good prognosis, Cancer Res., 45, 1890-1893.
12.Karsten, U. (2002) CD176 workshop panel report,
in Leucocyte Typing VII (Mason, D., ed.) Oxford University
Press, Oxford, pp. 202-203.
13.Karsten, U., and Goletz, S. (2013) What makes
cancer stem cell markers different? Springer Plus,
2, 301.
14.Brockhausen, I., Yang, J., Burchell, J.,
Whitehouse, C., and Taylor-Papadimitriou, J. (1995) Mechanism
underlying aberrant glycosylation of the MUC1 mucin in breast cancer,
Eur. J. Biochem., 233, 607-617.
15.Ermini, L., Bhattacharjee, J., Spagnoletti, A.,
Bechi, N., Aldi, S., Ferretti, C., Bianchi, L., Bini, L., Rosati, F.,
Paulesu, L., and Ietta, F. (2013) Oxygen governs Galβ1-3GalNAc
epitope in human placenta, Am. J. Physiol. Cell Physiol.,
305, C931-C940.
16.Brockhausen, I. (1999) Pathways of
O-glycan biosynthesis in cancer cells, Biochim. Biophys.
Acta, 1473, 67-95.
17.Brockhausen, I. (2006) Mucin-type
O-glycosylation in human colon and breast cancer: glycodynamics
and functions, EMBO Rep., 7, 599-604.
18.Goletz, S., Cao, Y., Danielczyk, A., Ravn, P.,
Schoeber, U., and Karsten, U. (2003) Thomsen–Friedenreich
antigen: the “hidden” tumor antigen, Adv. Exp. Med.
Biol., 535, 147-162.
19.Burchell, J. M., Mungul, A., and
Taylor-Papadimitriou, J. (2001) O-linked glycosylation in the mammary
gland: changes that occur during malignancy, J. Mamm. Gland Biol.
Neoplasm, 6, 355-364.
20.Vavasseur, F., Yang, J., Dole, K., Paulsen, H.,
and Brockhausen, I. (1995) Synthesis of core 3: characterization of
UDP-GlcNAc: GalNAc β3-N-acetylglucosaminyl-transferase
activity from colonic tissues. Loss of the activity in human cancer
cell lines, Glycobiology, 5, 351-357.
21.Schneider, F., Kemmner, W., Haensch, W., Franke,
G., Gretschel, S., Karsten, U., and Schlag, P. M. (2001) Overexpression
of sialyltransferase CMP-sialic acid:Galβ1,3GalNAc-R
α6-sialyltransferase is related to poor patient survival in human
colorectal carcinomas, Cancer Res., 61, 4605-4611.
22.Dalziel, M., Whitehouse, C., McFarlane, I.,
Brockhausen, I., Gschmeissner, S., Schwientek, T., Clausen, H.,
Burchell, J. M., and Taylor-Papadimitriou, J. (2001) The relative
activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine
O-glycan structure and expression of a tumor-associated epitope
on MUC1, J. Biol. Chem., 276, 11007-11015.
23.Gill, D. J., Tham, K. M., Chia, J., Wang, S. C.,
Steentoft, C., Clausen, H., Bard-Chapeau, E. A., and Bard, F. A. (2013)
Initiation of GalNAc-type O-glycosylation in the endoplasmic reticulum
promotes cancer cell invasiveness, Proc. Natl. Acad. Sci.
USA, 110, E3152-E3162.
24.Radhakrishnan, P., Dabelsteen, S., Madsen, F. B.,
Francavilla, C., Kopp, K. L., Steentoft, C., Vakhrushev, S. Y., Olsen,
J. V., Hansen, L., Bennett, E. P., Woetmann, A., Yin, G., Chen, L.,
Song, H., Bak, M., Hlady, R. A., Peters, S. L., Opavsky, R., Thode, C.,
Qvortrup, K., Schjoldager, K. T.-B. G., Clausen, H., Hollingsworth, M.
A., and Wandall, H. H. (2014) Immature truncated O-glycophenotype of
cancer directly induces oncogenic features, Proc. Natl. Acad. Sci.
USA, 111, E4066-E4075.
25.Ju, T., Otto, V. I., and Cummings, R. D. (2011)
The Tn antigen – structural simplicity and biological
complexity, Angew. Chem. Int. Ed., 50, 1770-1791.
26.Kumamoto, K., Goto, Y., Sekikawa, K.,
Takenoshita, S., Ishida, N., Kawakita, M., and Kannagi, R. (2001)
Increased expression of UDP-galactose transporter RNA in human colon
cancer tissues and its implication in synthesis of
Thomsen–Friedenreich antigen and sialyl Lewis A/X determinants,
Cancer Res., 61, 4620-4627.
27.Nilsson, T., Slusarewicz, P., Hoe, M. H., and
Warren, G. (1993) Kin recognition. A model for the retention of Golgi
enzymes, FEBS Lett., 330, 1-4.
28.De Graffenried, C. L., and Bertozzi, C. R. (2004)
The roles of enzyme localization and complex formation of Golgi
enzymes, Curr. Opin. Cell Biol., 16, 356-363.
29.Engelsberg, A., Hermosilla, R., Karsten, U.,
Schulein, R., Dorken, B., and Rehm, A. (2003) The Golgi protein RCAS1
controls cell surface expression of tumor-associated O-linked
glycan antigens, J. Biol. Chem., 278, 22998-23007.
30.Egea, G., Franci, C., Gambus, G., Lesuffleur, T.,
Zweibaum, A., and Real, F. X. (1993) Cis-Golgi resident proteins
and O-glycans are abnormally compartmentalized in the RER of
colon cancer cells, J. Cell Sci., 105, 819-830.
31.Sewell, R., Backstrom, M., Dalziel, M.,
Gschmeissner, S., Karlsson, H., Noll, T., Gatgens, J., Clausen, H.,
Hansson, G. C., Burchell, J., and Taylor-Papadimitriou, J. (2006) The
ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is
responsible for the synthesis of the tumor-associated sialyl-Tn-glycan
in human breast cancer, J. Biol. Chem., 281,
3586-3594.
32.Rottger, S., White, J., Wandall, H. H., Olivo,
J.-C., Stark, A., Bennett, E. P., Whitehouse, C., Berger, E. G.,
Clausen, H., and Nilsson, T. (1998) Localization of three human
polypeptide GalNAc-transferases in HeLa cells suggests initiation of
O-linked glycosylation throughout the Golgi apparatus, J. Cell
Sci., 111, 45-60.
33.Axelsson, M. A. B., Karlsson, N. G., Steel, D.
M., Ouwendijk, J., Nilsson, T., and Hansson, G. C. (2001)
Neutralization of pH in the Golgi apparatus cause redistribution of
glycosyltransferases and changes in the O-glycosylation of
mucins, Glycobiology, 11, 633-644.
34.Campbell, B. J., Rowe, G. E., Leiper, K., and
Rhodes, J. M. (2001) Increasing the intra-Golgi pH of cultured LS174T
goblet-differentiated cells mimics the decreased mucin sulfation and
increased Thomsen–Friedenreich antigen
(Galβ1-3GalNacα-) expression seen in colon cancer,
Glycobiology, 11, 385-393.
35.Thorens, B., and Vassalli, P. (1986) Chloroquine
and ammonium chloride prevent terminal glycosylation of immunoglobulins
in plasma cells without affecting secretion, Nature, 321,
618-620.
36.Caplan, M. J., Stow, J. L., Newman, A. P., Madri,
J., Anderson, H. C., Farquhar, M. G., Palade, G. E., and Jamieson, J.
D. (1987) Dependence on pH of polarized sorting of secreted proteins,
Nature, 329, 632-635.
37.Kellokumpu, S., Sormunen, R., and Kellokumpu, I.
(2002) Abnormal glycosylation and altered Golgi structure in colorectal
cancer: dependence on intra-Golgi pH, FEBS Lett., 516,
217-224.
38.Paroutis, P., Touret, N., and Grinstein, S.
(2004) The pH of the secretory pathway: measurement, determinants, and
regulation, Physiology, 19, 207-215.
39.Rivinoja, A., Kokkonen, N., Kellokumpu, I., and
Kellokumpu, S. (2006) Elevated Golgi pH in breast and colorectal cancer
cells correlates with the expression of oncofetal carbohydrate
T-antigen, J. Cell. Physiol., 208, 167-174.
40.Gawlitzek, M., Ryll, T., Lofgren, J., and
Sliwkowski, M. B. (2000) Ammonium alters N-glycan structures of
recombinant TNFR-IgG: degradative versus biosynthetic mechanisms,
Biotechnol. Bioeng., 68, 637-646.
41.Rivinoja, A., Hassinen, A., Kokkonen, N.,
Kauppila, A., and Kellokumpu, S. (2009) Elevated Golgi pH impairs
terminal N-glycosylation by inducing mislocalization of Golgi
glycosyltransferases, J. Cell. Physiol., 220,
144-154.
42.Hassinen, A., Pujol, F. M., Kokkonen, N.,
Pieters, C., Kihlstrom, M., Korhonen, K., and Kellokumpu, S. (2011)
Functional organization of the Golgi N- and O-glycosylation pathways
involves pH-dependent complex formation that is impaired in cancer
cells, J. Biol. Chem., 286, 38329-38340.
43.Hassinen, A., and Kellokumpu, S. (2014)
Organizational interplay of Golgi N-glycosyltransferases
involves organelle microenvironment-dependent transitions between
enzyme homo- and heteromers, J. Biol. Chem., 289,
26937-26948.
44.Rivinoja, A., Pujol, F. M., Hassinen, A., and
Kellokumpu, S. (2012) Golgi pH, its regulation and roles in human
disease, Ann. Med., 44, 542-554.
45.Maeda, Y., Ide, T., Koike, M., Uchiyama, Y., and
Kinoshita, T. (2008) GPHR is a novel anion channel critical for
acidification and functions of the Golgi apparatus, Nature Cell
Biol., 10, 1135-1145.
46.Marshansky, V., Rubinstein, J. L., and Gruber, G.
(2014) Eukaryotic V-ATPase: novel structural findings and
functional insights, Biochim. Biophys. Acta, 1837,
857-879.
47.Warburg, O., Wind, F., and Negelein, E. (1927)
The metabolism of tumors in the body, J. Gen. Physiol.,
8, 519-530.
48.Hanahan, D., and Weinberg, R. A. (2011) Hallmarks
of cancer: the next generation, Cell, 144, 646-674.
49.DeBerardinis, R. J., Lum, J. J., Hatzivassiliou,
G., and Thompson, C. B. (2008) The biology of cancer: metabolic
reprogramming fuels cell growth and proliferation, Cell Metab.,
7, 11-20.
50.Gillies, R. J., Raghunand, N., Karczmar, G. S.,
and Bhujwalla, Z. M. (2002) MRI of the tumor microenvironment, J.
Magn. Reson. Imag., 16, 430-450.
51.Damaghi, M., Wojtkowiak, J. W., and Gillies, R.
J. (2013) pH sensing and regulation in cancer, Front. Physiol.,
4; http://dx.doi.org/10.3389/fphys.2013.00370.
52.Gatenby, R. A., Smallbone, K., Maini, P. K.,
Rose, F., Averill, J., Nagle, R. B., Worrall, L., and Gillies, R. J.
(2007) Cellular adaptions to hypoxia and acidosis during somatic
evolution of breast cancer, Brit. J. Cancer, 97,
646-653.
53.Vogelstein, B., and Kinzler, K. W. (2004) Cancer
genes and the pathways they control, Nature Med., 10,
789-799.
54.Kroemer, G., and Pouyssegur, J. (2008) Tumor cell
metabolism: cancer’s Achilles heel, Cancer Cell,
13, 472-482.
55.Cardone, R. A., Casavola, V., and Reshkin, S. J.
(2005) The role of disturbed pH dynamics and the
Na+/H+ exchanger in metastasis, Nature Rev.
Cancer, 5, 786-795.
56.Wong, N., De Melo, J., and Tang, D. (2013) PKM2,
a central point of regulation in cancer metabolism, Int. J. Cell
Biol.; http://dx.doi.org/10.1155/2013/242513.
57.Semenza, G. L. (2013) HIF-1 mediates metabolic
responses to intratumoral hypoxia and oncogenic mutations, J. Clin.
Invest., 123, 3665-3671.
58.Soonthornsit, J., Yamaguchi, Y., Tamura, D.,
Ishida, R., Nakakoji, Y., Osako, S., Yamamoto, A., and Nakamura, N.
(2014) Low cytoplasmic pH reduces ER–Golgi trafficking and
induces disassembly of the Golgi apparatus, Exp. Cell Res.,
328, 325-339.
59.Finley, L. W. S., Carracedo, A., Lee, J., Souza,
A., Egia, A., Zhang, J., Teruya-Feldstein, J., Moreira, P. I., Cardoso,
S. M., Clish, C. B., Pandolfi, P. P., and Haigis, M. C. (2011) SIRT3
opposes reprogramming of cancer cell metabolism through HIFα
destabilization, Cancer Cell, 19, 416-428.
60.Schapiro, F., Sparkowski, J., Adduci, A.,
Suprynowicz, F., Schlegel, R., and Grinstein, S. (2000) Golgi
alkalinization by the papillomavirus E5 oncoprotein, J. Cell
Biol., 148, 305-315.
61.Sautin, Y. Y., Lu, M., Gaugler, A., Zhang, L.,
and Gluck, S. L. (2005) Phosphatidylinositol 3-kianse-mediated effects
of glucose on vacuolar H+-ATPase assembly, translocation,
and acidification of intracellular compartments in renal epithelial
cells, Mol. Cell. Biol., 25, 575-589.
62.Jeschke, U., Richter, D. U., Hammer, A., Briese,
V., Friese, K., and Karsten, U. (2002) Expression of the
Thomsen–Friedenreich antigen and of its putative carrier protein
mucin 1 in the human placenta and in trophoblast cells in vitro,
Histochem. Cell Biol., 117, 219-226.
63.Ito, K., and Suda, T. (2014) Metabolic
requirements for the maintenance of self-renewing stem cells, Nature
Rev. Mol. Cell Biol., 15, 243-256.
64.Martinez-Outschoorn, U. E., Lisanti, M. P., and
Sotgia, F. (2014) Catabolic cancer-associated fibroblasts transfer
energy and biomass to anabolic cancer cells, fueling tumor growth,
Seminars Cancer Biol., 25, 47-60.