Photoinduced Changes in Subcellular Structures of the Retinal Pigment Epithelium from the Japanese Quail Coturnix japonica
P. P. Zak1*, N. B. Serezhnikova1,2, L. S. Pogodina2, N. N. Trofimova1, T. S. Gur’eva3, and O. A. Dadasheva3
1Emanuel Institute of Biochemical Physics, Russian Academy of Sciences, 119934 Moscow, Russia; E-mail: pavelzak@mail.ru2Lomonosov Moscow State University, Faculty of Biology, 119991 Moscow, Russia
3Institute of Biomedical Problems, Russian Academy of Sciences, 123007 Moscow, Russia
* To whom correspondence should be addressed.
Received January 12, 2015; Revision received March 10, 2015
Fifteen-week-old sexually mature female Japanese quails (Coturnix japonica) grown under various lighting conditions were used in the study. It was found that the number of mitochondria and phagosomes was increased by 1.5-fold in the retinal pigment epithelium from birds reared for 95 days under blue light (440-470 nm) vs. reduced blue light component conditions. Also, it was found that egg production was increased by 15% in birds reared under blue light compared to other lightning conditions. Thus, we concluded that blue light conditions resulted in elevating metabolic activity and accelerating pace of life in Japanese quails. It is assumed that the blue light-induced effects are probably due to inhibition of melatonin synthesis.
KEY WORDS: retinal pigment epithelium, blue light, mitochondria, Japanese quail, electron microscopyDOI: 10.1134/S0006297915060140
Blue light (λmax 450 nm) is known to be an unsafe “nonvisual” factor of photobiological effects acting on the retina of humans and other animals. Short-term powerful exposure to blue light represents a real danger that has been evaluated in different animal models including primates (rhesus macaques Macaca mulatta) [1, 2]. It was demonstrated that a single short-term exposure to blue light of wavelength 450 nm (Bλ according to the lighting nomenclature for visual safety) with a dose of ~50 J/cm2 on the retinal surface results in detectable retinal degeneration, whereas the photodamage threshold doses for long wavelength visible light ranging within 500-650 nm lie within 500-1000 J/cm2 [3]. The potential role for blue light in provoking eye diseases and development of retinal aging has been extensively studied [4]. A pathogenic action of blue light is known to target phototoxic bis-retinoids of lipofuscin granules in the retinal pigment epithelium (RPE) that generate free radicals [5, 6]. In different studies, it was shown that blue light could have a damaging effect on RPE mitochondria that results in release of cytochrome c into the cytoplasm, triggering apoptotic cell death [7, 8]. The synthesis of melatonin controlled by the melanopsin blue-light-sensitive retinal cells was perhaps acting as a factor limiting negative effects of blue light [9]. Various animal models are used to examine mechanisms of the pathogenic action of blue light on the retina. Among them, use of small domestic birds such as Japanese quail Coturnix japonica is one of the most successful models [10-12]. On par with humans and primates, this species compared to routine laboratory animals (rodents, cats, dogs, etc.) has sharp central vision, being protected from blue light by lutein–zeaxanthin oxycarotenoids. On average, the biological lifetime for C. japonica is ~1.5 years, so that the retina undergoes senile changes similar to those occurring in humans by the age of 70-80 years. In particular, the concentration of lipofuscin granules in RPE of 9-month Japanese quail reaches a magnitude similar to people at the age of 80 years [13], whereas retinal visual acuity of the birds substantially declines with age [14]. Previously, Zak et al. [15] demonstrated that blue light (440-470 nm, 4 J/cm2, 40-min exposure) caused pronounced changes in the blood–retinal barrier of the RPE in C. japonica similar to the age-related changes. Also, it was shown that blue light having such parameters resulted in structural changes in some cell nuclei within the RPE that are typical of apoptosis. Moreover, it was found [15, 16] that an increased number of altered (cup-shaped) mitochondria suggesting problems in cell activity [17-19] was in fact a common feature for aging and damaging action of the blue light in the RPE cells of C. japonica. Along with using C. japonica as a model of accelerated aging, these birds are useful for examining melatonin-regulated circadian rhythms. Coturnix japonica was found to have a pronounced diurnal variation of melatonin concentration, wherein 30% of its blood pool is directly synthesized in the retinal photoreceptor cells, whereas in humans retinal melatonin accounts for only ~15% of total amount [20]. Currently, the issue of long-term effects caused by excessive blue light exposure in provoking age-related blindness [21], particularly, macular degeneration [4], has been extensively discussed. In this vein, the Scientific Committee on Emerging and Newly Identified Health Risks, the European Commission, 2012 (http://ec.europa.eu/health/scientificcommittees/policy/) made a decision to prioritize studies evaluating long-term negative effects of daily lighting regimen containing excessive blue component, particularly, from LED lighting. The study presented here was aimed at evaluating potential risks from chronic daily lighting regimen containing excessive blue component.
MATERIALS AND METHODS
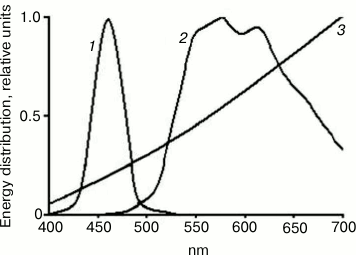
The study was conducted with 15-week-old female C. japonica housed separately from males. At the age of 1.5 month, the birds were divided into three groups depending on lighting conditions. The control group contained birds reared under the generally accepted standardized lighting conditions provided by incandescent lamp as one of the artificial white light sources according to the lighting classification. Birds in another experimental group were reared under LED blue lighting conditions (440-470 nm), whereas in the second experimental group birds were exposed to yellow light (500-650 nm) provided by a “white” LED combined with an optical filter for cutting off wavelength shorter than 500 nm. According to the aforementioned spectral characteristics (Fig. 1), the incandescent lamp and a “yellow” light source should be close to each other in terms of photobiological effects, for they possess dominant light emission band at the long wavelength spectrum (≥500 nm) without appreciable emission in blue region of the spectrum (≤500 nm).
Fig. 1. Spectrum of energy distribution emitted from three light sources: 1) blue light (dominant wavelength range 440-470 nm); 2) yellow light (dominant wavelength range 530-650 nm lacking blue light emission); 3) incandescent lamp (wavelength range with reduced blue light component and dominant yellow light emission).
In addition, all of the birds were reared using a generally accepted diurnal lighting cycle: 15-h continuous illumination, 9-h dark break. The lighting was switched on and off automatically by a timer. All three lighting regimens were equalized in power (0.002 W/cm2), and illumination was focused at the center of the birdcage (40 cm from the light source). The applied light intensity was 200-250 lx. Photo- and spectrometric calibrations were done using Avantes-2048 (Avantes, Netherlands) and MK-350 (Uptech, Taiwan) spectrometers.
At the age of 1.5-months, the birds started to be separately housed under the various lighting conditions. Overall duration of separate housing lasted for 95 days. Then birds were decapitated, and excised eyecups containing sclera, vascular retina, RPE, and neural retina were placed into fixation solution according to a method described earlier in detail [16]. Cross sections (~1 mm2) of the RPE cut at the central area of the retina (macular area) were examined using JEM-100B and JEM-1011 transmission electron microscopes (JEOL, Japan; magnification 8000× and 25,000×). The electron microscopy images were quantitatively evaluated using Adobe Photoshop CS4 software (Adobe Systems Inc., USA) and an 8 × 8 µm-square lattice test system analyzed with a standard morphometry method [22]: functionally relevant RPE subcellular structures (mitochondria, lipofuscin granules, myeloid bodies, phagosomes) were enumerated (per 100 µm2). Previously, these structures were found to concentrate mainly in the ~8-µm-thick basal region of the cytoplasm [16]. Around 50 RPE cells from different areas of the macular region were analyzed for each bird. Eyes excised from five birds from each group were used for quantitative evaluation.
The data were processed using Statistica 5.5 software by applying the non-parametric Mann–Whitney test. Significance level was set at p < 0.05. Normality for empirical distribution was checked by using the Kolmogorov–Smirnov test (α = 0.05).
Light-induced changes in overall body state of C. japonica females from different groups were assessed by evaluating average daily egg production.
RESULTS AND DISCUSSION
By evaluating an average daily egg production, it was found that in birds reared under blue illumination conditions this parameter was higher by 15% compared to birds exposed to reduced blue light component conditions, i.e. lighting provided by the yellow light source and incandescent lamp.
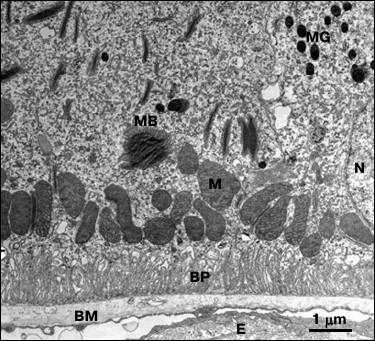
A panoramic transmission electron image demonstrating typical map of distribution and state of subcellular units at the region of the basal membrane in RPE cells from C. japonica females is presented in Fig. 2.
Fig. 2. Electron microscopy image of the cross section of a RPE cell isolated from C. japonica demonstrating extremely stratified distribution of subcellular structures. N, cell nucleus; M, mitochondria; BP, basal protrusions; BM, Bruch’s membrane; E, endothelium of blood capillaries; MB, myeloid bodies; MG, melanin granules.
According to the picture on the presented image, subcellular RPE structures examined in 15-week-old C. japonica females had a strictly stratified distribution that corresponds to their functional role and direction of metabolic processes. The most important function for RPE cells is to develop the blood–retinal barrier by the RPE cells, which is analogous to the blood–brain barrier. Bruch’s membrane consisting of connective tissue ensures passive filtration of various substances from the bloodstream. Active transport of substances between RPE and extracellular space is accomplished by numerous basal protrusions extending from the cellular membrane of the RPE. Energy for active substance transport and metabolite processing is supplied by a layer of numerous mitochondria located near the basement of the basal protrusions of the RPE. It was found that 15-week-old female quails were characterized by highly ordered position of the basal protrusions, round-shaped cellular nuclei, and mitochondria, homogenous structure of Bruch’s membrane and small number of lipofuscin granules (Fig. 2).
Birds housed under various lighting conditions significantly (p < 0.05) differed in terms of number of mitochondria and phagosomes (see table).
Subcellular structures (per cytoplasmic area
100 µm2) enumerated in the RPE cells isolated
from Japanese quail under three daily lighting regimens
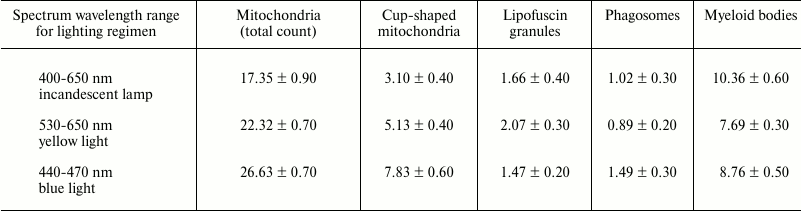
Note: The data are presented as mean values, M ± m.
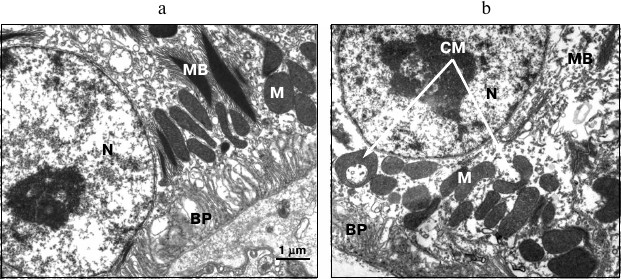
In particular, the birds reared under the blue illumination were found to have a substantially higher total number of mitochondria compared to the birds reared under reduced blue component lighting conditions provided by yellow light source and incandescent lamp (by 1.2- and 1.5-fold, respectively). An increased number of cup-shaped mitochondria resembling rings and dumbbells was found on the sections from the birds reared under blue light (Fig. 3) compared to the yellow light conditions (by 1.5-fold) and lighting conditions provided by incandescent lamp (by 2.5-fold).
Fig. 3. Typical differences in RPE ultrastructure found in birds daily reared under 95-day-exposure to illumination emitted by incandescent lamp (a) and blue light (440-470 nm) (b). N, RPE cell nucleus; BP, basal protrusions; M, mitochondria; CM, cup-shaped mitochondria; MB, myeloid bodies.
According to available publications, cup-shaped mitochondria with extended area of contact surface occur as an adaptive defense reaction to cellular stress [17-19]. It can be assumed that both increased number of total mitochondria as well as increased incidence of the cup-shaped mitochondria under the blue lighting conditions evidence elevated burden on the cellular metabolism in the RPE.
The data provided in the table show that if the birds were reared under the blue lighting conditions, it also resulted in 1.5-fold increase in the number of phagosomes compared to those exposed to yellow illumination and lighting conditions provided by incandescent lamp. These changes may be interpreted as additional evidence that daily housing under blue lighting conditions results in upregulated energy metabolic processes occurring inside the RPE cells. It is known phagocytic processes in the RPE cells capturing photoreceptor membranes are light dependent and exhibit circadian rhythm [23].
No significant differences were found in number of lipofuscin granules and myeloid bodies depending on lighting conditions.
Altogether, the data regarding number of mitochondria and phagosomes in the RPE cells as well as upregulated egg production by the birds reared under the blue lighting conditions suggest that blue light has a photostimulatory effect on energy metabolic processes and the entire body. We believe the activating effect of blue light most probably is caused by an upregulated level of melatonin because the amount of melatonin in both the blood and body organs is mainly regulated by impact triggered by blue light emitted within the 440-470 nm range [24]. It seems unlikely that the effects observed in our study somehow might be related to phototoxic free radical reactions of lipofuscin granules due to the very low daily lighting regimens.
Currently, we continue constant-age monitoring of C. japonica to assess rate of aging and corresponding RPE depending on the spectral characteristics of the applied lighting conditions.
The authors appreciate assistance provided by Dr. T. V. Lipina during the study.
This study was done with financial support from the Russian Foundation for Basic Research (grant No. 14-04-01072A).
REFERENCES
1.Ham, W. T., Mueller, H. A., and Sliney, D. H.
(1976) Retinal sensitivity to damage from short wavelength light,
Nature, 260, 153-155.
2.Ham, W. T., Mueller, H. A., Ruffolo, J. J., Guerry,
D., and Guerry, R. K. (1982) Action spectrum for retinal injury from
near-ultraviolet radiation in the aphakic monkey, Am. J.
Ophthalmol., 93, 299-306.
3.Van Norren, D., and Gorgels, T. G. (2011) The
action spectrum of photochemical to the retina: a review of
monochromatic threshold data, Photochem. Photobiol., 87,
747-753.
4.Algvere, P. V., Marshall, J., and Seregard, S.
(2006) Age-related maculopathy and the impact of blue light hazard,
Acta Ophthalmol. Scand., 84, 4-15.
5.Boulton, M., Dontsov, A., Ostrovsky, M.,
Jarvis-Evans, J., and Svistunenko, D. (1993) Lipofuscin is a
photoinducible free radical generator, J. Photochem. Photobiol.,
19, 201-204.
6.Schutt, F., Davies, S., Kopitz, J., Holz, F. G.,
and Boulton, M. E. (2000) Photodamage to human RPE cells by A2-E, a
retinoid component of lipofuscin, Invest. Ophthalmol. Vis. Sci.,
41, 2303-2308.
7.Suter, M., Reme, C., Grimm, C., Wenzel, A.,
Jaattela, M., Esser, P., Kociok, N., Leist, M., and Richter, C. (2000)
Age-related macular degeneration. The lipofusion component
N-retinyl-N-retinylidene ethanolamine detaches proapoptotic proteins
from mitochondria and induces apoptosis in mammalian retinal pigment
epithelial cells, J. Biol. Chem., 275, 39625-39630.
8.Sparrow, J. R., and Cai, B. (2001) Blue
light-induced apoptosis of A2E-containing RPE: involvement of caspase-3
and protection by Bcl-2, Invest. Ophthalmol. Vis. Sci.,
42, 1356-1362.
9.Tosini, G., Baba, K., Hwang, C. K., and Iuvone, P.
M. (2012) Melatonin: an underappreciated player in retinal physiology
and pathophysiology, Exp. Eye Res., 103, 82-89.
10.Zak, P. P., Zykova, A. V., Trofimova, N. N., Abu
Khamidakh, A. E., Fokin, A. I., Eskina, E. N., and Ostrovskiy, M. A.
(2010) Experimental model for examining mechanisms of age-related and
degenerative changes in human retina (Japanese quail C.
japonica), Doklady AN, 434, 272-274.
11.Zak, P. P., Zykova, A. V., Trofimova, N. N.,
Eskina, E. N., and Ostrovskiy, M. A. (2012) Experimental model of
accelerated retinal aging: Japanese quail Coturnix japonica,
Sensor. Sistemy, 26, 3-10.
12.Zak, P. P., Zykova, A. V., Trofimova, N. N., and
Ostrovskiy, M. A. (2013) Japanese quail Coturnix japonica as a
model for accelerated aging of human retina. Report 1. Dependence
between lipofuscin accumulation in the retinal pigment epithelium and
level of retinal oxycarotenoids, Oftal’mokhirurgiya,
1, 9-12.
13.Feeney-Burns, L., Hilderbrand, E. S., and
Eldridqe, S. (1984) Aging human RPE: morphometric analysis of macular,
equatorial, and peripheral cells, Invest. Ophthalmol. Vis. Sci.,
25, 195-200.
14.Lee, J. Y., Holden, L. A., and Djamgoz, M. B.
(1997) Effects of ageing on spatial aspects of the pattern
electroretinogram in male and female quail, Vision Res.,
37, 505-514.
15.Zak, P. P., Serezhnikova, N. B., Pogodina, L. S.,
Trofimova, N. N., and Ostrovskiy, M. A. (2014) Evaluation of
age-related sensitivity of the retinal pigment epithelium isolated from
Japanese quail Coturnix japonica to light-induced damage,
Ross. Fiziol. Zh. im. I. M. Sechenova, 100, 841-851.
16.Serezhnikova, N. B., Zak, P. P., Pogodina, L. S.,
Trofimova, N. N., Lipina, T. V., and Ostrovskiy, M. A. (2013)
Subcellular markers of aging for the retinal pigment epithelium
isolated from Japanese quail Coturnix japonica (electron
microscopy examination), Vestnik Mosk. Univer. Ser. 16.
Biologiya, 3, 9-16.
17.Liang, H., Crewther, S. G., and Crewther, D. P.
(1995) A model for the formation of ring mitochondria in retinal
pigment epithelium, Yan Ke Xue Bao, 11, 9-15.
18.Liu, X., and Hajnoczky, G. (2011) Altered fusion
dynamics underlie unique morphological changes in mitochondria during
hypoxia-reoxygenation stress, Cell Death Differ., 18,
1561-1572.
19.Lauber, J. K. (1982/83) Retinal pigment
epithelium: ring mitochondria and lesions induced by continuous light,
Curr. Eye Res., 2, 855-862.
20.Steele, C. T., Tosini, G., Siopes, T., and
Underwood, H. (2006) Time keeping by the quailʼs eye: circadian
regulation of melatonin production, Gen. Comp. Endocrinol.,
145, 232-236.
21.Behar-Cohen, F., Martinsons, C., Vienot, F.,
Zissis, G., Barlier-Salsi, A., Cesarini, J. P., Enouf, O., Garcia, M.,
Picaud, S., and Attia, D. (2011) Light-emitting diodes (LED) for
domestic lighting: any risks for the eye? Prog. Retin. Eye
Res., 30, 239-257.
22.Avtandilov, G. G. (1980) Introduction into
Quantitative Pathological Morphology [in Russian], Meditsina,
Moscow.
23.Dickson, D. H., and Morrison, C. (1993) Diurnal
variation in myeloid bodies of the chick retinal pigment epithelium,
Curr. Eye Res., 12, 37-43.
24.Rahman, S. A., Flynn-Evans, E. E., Aeschbach, D.,
Brainard, G. C., Czeisler, C. A., and Lockley, S. W. (2014) Diurnal
spectral sensitivity of the acute alerting effects of light,
Sleep, 37, 271-281.