Participation of Intracellular and Extracellular pH Changes in Photosynthetic Response Development Induced by Variation Potential in Pumpkin Seedlings
O. N. Sherstneva, V. A. Vodeneev, L. A. Katicheva, L. M. Surova, and V. S. Sukhov*
Lobachevsky State University of Nizhny Novgorod, 603950 Nizhny Novgorod, Russia; E-mail: vssuh@mail.ru* To whom correspondence should be addressed.
Received January 12, 2015; Revision received March 9, 2015
Electrical signals presented in plants by action potential and by variation potential (VP) can induce a reversible inactivation of photosynthesis. Changes in the intracellular and extracellular pH during VP generation are a potential mechanism of photosynthetic response induction; however, this hypothesis requires additional experimental investigation. The purpose of the present work was to analyze the influence of pH changes on induction of the photosynthetic response in pumpkin. It was shown that a burning of the cotyledon induced VP propagation into true leaves of pumpkin seedlings inducing a decrease in the photosynthetic CO2 assimilation and an increase in non-photochemical quenching of fluorescence, whereas respiration was activated insignificantly. The photosynthetic response magnitude depended linearly on the VP amplitude. The intracellular and extracellular concentrations of protons were analyzed using pH-sensitive fluorescent probes, and the VP generation was shown to be accompanied by apoplast alkalization (0.4 pH unit) and cytoplasm acidification (0.3 pH unit). The influence of changes in the incubation medium pH on the non-photochemical quenching of fluorescence of isolated chloroplasts was also investigated. It was found that acidification of the medium stimulated the non-photochemical quenching, and the magnitude of this increase depended on the decrease in pH. Our results confirm the contribution of changes in intracellular and extracellular pH to induction of the photosynthetic response caused by VP. Possible mechanisms of the influence of pH changes on photosynthesis are discussed.
KEY WORDS: Cucurbita pepo, pH, CO2 assimilation, variation potential, non-photochemical quenching, chloroplastsDOI: 10.1134/S0006297915060139
It is well known [1-3] that local stimulations can induce generation and propagation of electric signals in plants. Non-damaging stimuli induce action potential (AP), whereas damaging influences result in propagation of variation potential (VP) [1, 2]. In seems that AP and VP are different in their ionic nature and propagation mechanisms. An actively propagating AP [4] is associated mainly with Ca2+, Cl–, and K+ flows [1, 3, 5, 6], although a reversible inactivation of H+-ATPase definitely contributes to its generation [6]. For VP, the major mechanism is a decrease in H+-ATPase activity [3, 4, 7] that seems to be induced by entrance of calcium ions [8, 9]. Chloride and potassium ions seem to participate mainly in formation of individual “AP-like” spikes [10] that in some cases develop during a slow and long-term depolarization underlying VP [2, 3, 11]. However, the VP propagation is not an active process, and it is thought to be a local electric reaction to the propagation of a hydraulic and/or chemical signal [3, 10, 12-14].
It is supposed [3, 15] that just the generation and propagation of AP and VP can be a mechanism responsible for systemic functional response of a plant to local stimuli. In particular, a local stimulation is shown to induce in non-stimulated parts of the plant an activation of expression of some genes [16, 17], synthesis of phytohormones [17-19], changes in activities of transfer processes [20], activation of respiration [21], and a photosynthetic response with complex dynamics [19, 22, 33]. It is thought [34] that these functional changes can finally result in an increase in the plant resistance to the action of unfavorable factors, and this is confirmed by some experimental works [33-35].
Photosynthetic responses are studied most actively among functional responses of plants to local stimuli. First of all, it is a rapid inactivation of photosynthesis described for plants of different species, which reaches its maximum within the first 2-15 min after a local stimulation [23-33]. This inactivation is manifested by a decrease in CO2 assimilation (A), an increase in non-photochemical quenching of fluorescence (NPQ), a decrease in quantum yields of photosystems I and II (фPSI and фPSII), and in biphasic change in the magnitude of cyclic electron flow. In addition to the rapid inactivation of photosynthesis, relatively slow changes in photosynthetic activity reaching a maximum in 20-50 min after stimulation were described in some works [23, 32].
As discriminated from slowly developing photosynthetic responses that can be associated not only with electric but also with hormonal signals [19, 23], an association between the rapid inactivation of photosynthesis with AP and VP was confirmed in some works [27, 29, 31, 32]. Nevertheless, the mechanism of the influence of the electric reaction on photosynthesis is still under discussion. Studies on the influence of AP on photosynthesis in Charophyta revealed that the entrance of calcium ions is the most likely mechanism of the response development [25]. The variation potential mainly observed in higher plants has specific mechanisms of generation and propagation [7-14], and this suggests that a specific pathway of the influence of VP on photosynthesis should exist. Considering the key role of H+-ATPase inactivation in the development of VP [3, 4, 7], its influence can be associated with proton entrance into the cell [27, 31]. Grams et al. [27] showed that the propagation of VP in maize was accompanied by cytoplasm acidification and apoplast alkalization. Photosynthesis of isolated chloroplasts depended on the incubation medium pH, whereas its dependence on calcium concentration was not observed. We found earlier [31] that proton entrance really could be a mechanism of development of photosynthetic response caused by VP in pea seedlings. However, it is still unclear whether this mechanism is universal for higher plants. Earlier, it was found [30] that the reversible inactivation of photosynthesis caused in pumpkin seedlings by local burning and possibly by VP propagation was significantly different from responses of other objects studied [29, 31, 32]. In particular, only a small increase in NPQ and a decrease in A were recorded in pumpkin, whereas there was no pronounced decrease in фPSI and фPSII.
Thus, studies on the influence of changes in the intra- and extracellular pH on the photosynthetic response induction in pumpkin seedlings is important for assessment of a universality of the role of protons in development of the VP-induced rapid inactivation of photosynthesis in higher plants. Such analysis is presented in this work.
MATERIALS AND METHODS
The study was performed on 2-3-week-old pumpkin seedlings (Cucurbita pepo L., Mozoleevskaya cultivar). The seedlings were grown in a hydroponic system (keramzit, 50% Hoagland–Arnon medium) in a climate chamber (KBW-240; Binder, Germany) with 16-h illumination under luminescent lamps and at 24°C.
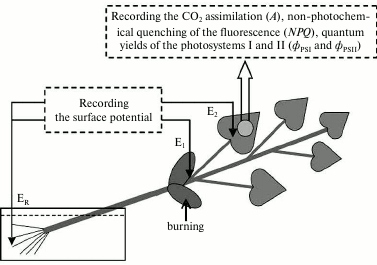
Electrophysiological measurements were performed extracellularly after adaptation of the seedlings for 1.5 h (Fig. 1) using two pairs of Ag/AgCl-macroelectrodes (EVL-1MZ; Gomel Plant of Measuring Devices, Belarus), a high-impedance amplifier (IPL-113; Semiko, Russia), and a computer. The measuring electrodes were in contact with the leafstalk basis (E1) and the leaf surface, on the border with the photosynthesis measurement zone (E2) through a drop of an electroconductive gel. The reference electrode (ER) was in contact with the solution washing the roots. Variation potential was induced by burning one of the cotyledon leaves (an open flame for 3-4 s, about 2-3 cm2 of the surface).
Fig. 1. Scheme of the burning, electrode location, and recording the photosynthesis parameters in pumpkin seedlings. E1 and E2 are measuring electrodes, ER is the reference electrode. The arrow indicates the area of the local burning of a cotyledon leaf (open flame, 3-4 s, ~2-3 cm2 of the surface).
Photosynthetic measurements were performed concurrently with recording the electric activity. This was realized with a standard system for investigation of photosynthetic processes (Heinz Walz GmbH, Germany) that included a gas analyzer (GFS-3000), a PAM-fluorimeter capable of recording the light absorption by PS I (Dual-PAM-100), and a measuring head for their concurrent use (Dual-PAM gas-exchange Cuvette 3010-Dual). In experiments on chloroplasts, only the PAM-fluorimeter was used. The dark adaptation time was 20 min (intact plants) or 2 min (isolated chloroplasts), the intensity of the subsequent actinic light (460 nm) was 240 µmol·m–2·s–1 (intact plants) or 100 µmol·m–2·s–1 (isolated chloroplasts). Saturating flashes were every 10 s. In experiments with the intact plants, the carbon dioxide concentration in the environment of the leaf was 360 µl/liter and the relative humidity was about 60%. In the work we studied the level of CO2 assimilation (A), the non-photochemical quenching of fluorescence (NPQ), and quantum yields of PS I and II (фPSI and фPSII); the parameters were calculated automatically basing on approaches from works [36-38]. To study the leaf respiration, its gas exchange was measured under conditions of full darkening.
Chloroplasts were isolated as described in previous works [30, 31]. True leaves (2 g) from one pumpkin seedling were homogenized in 15 ml of the isolation medium (0.35 M NaCl in 0.06 M phosphate buffer (pH 7.1)) at 4°C. At the same temperature, the resulting homogenate was centrifuged at 200g for 5 min, then the supernatant was centrifuged again for 15 min at 2000g. The precipitated chloroplasts were resuspended in the isolation medium (4 ml). The majority of chloroplasts retained their integrity as assessed visually by images obtained with a Carl Zeiss LSM 510 META laser scanning microscopy complex (Carl Zeiss Microscopy GmbH, Germany) that included an inverted Axiovert 200M microscope, a laser scanning module, and a Carl Zeiss 23 META spectral module.
Changes in pH of the cytoplasm and apoplast associated with VP were determined by a ratiometric method [31] using pH-sensitive fluorescent probes BCECF-AM and FITC-dextran (Life Technologies, USA).
FITC-dextran, which was used to measure pH in the apoplast, was loaded by incubation in the dark for 12-14 h of a pumpkin stalk piece with removed epidermis (2·10–5 M probe), and then the stalk was washed clean. The fluorescence was measured with a Shimadzu RF-5301PC spectrofluorimeter (Shimadzu, Japan) and a special module for investigating solid specimens (Granat, Russia). The ratio of the fluorescence intensity (520 nm) at the exciting wavelength of 490 nm to its intensity at the exciting length of 460 nm was used as the parameter of pH. To obtain the calibration curve, this ratio was measured at dilutions of FITC-dextran in buffers 20 mM Tris-MES (Sigma-Aldrich, USA) with pH values from 5.0 to 8.0.
BCECF-AM, which was used to measure pH in the cytoplasm, was loaded by incubation for 12-14 h in the dark of a seedling stalk piece with removed epidermis (2·10–5 M probe) at 4°C, then the stalk was washed clean at room temperature for at least 2 h. The ratio of the fluorescence intensity (530 nm) with exciting wavelength of 490 nm to its intensity at the exciting length of 450 nm was used to determine the cytoplasmic pH. The calibration curve was determined according to the curve for FITC-dextran (pH value changed from 5.5 to 9.0).
The selectivity of BCECF-AM localization in the cytoplasm and of FITC-dextran in the apoplast was confirmed by measurements of fluorescence of probes in different parts of the intra- and extracellular volume using a Carl Zeiss LSM 510 META laser scanning microscopy complex (Carl Zeiss Microscopy GmbH).
Biological repetition of experiments was 5-15. In Figs. 2-7, either typical experiments are presented or mean values and their standard errors. For experimental series with isolated chloroplasts, a scattering diagram is presented.
RESULTS
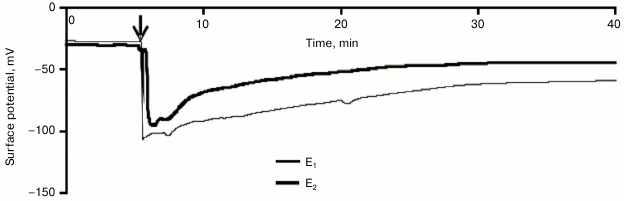
Influence of variation potential on photosynthesis of pumpkin seedlings. A local burn of the cotyledon leaf induced a propagation along the pumpkin seedling of an electric signal with a variable shape (Fig. 2). The signal amplitude was in the range from 14 to 84 mV (67 ± 4 mV in the leafstalk and 50 ± 6 mV in the leaf lamina), the repolarization phase could be 20 min and more, and the propagation rate was no more than some mm/s. The electric signal entered the leaf lamina no more than 1 min after the burning of the cotyledon leaf. Parameters of the electric signal allowed us to classify it as a variation potential [1-3, 11], which was in good agreement with data of our previous works [30] that had shown the VP generation and propagation after burning of the cotyledon leaf of pumpkin seedlings.
Fig. 2. A typical example of changes in surface potential (n = 15) caused by local burning. The arrow indicates the moment of burning.
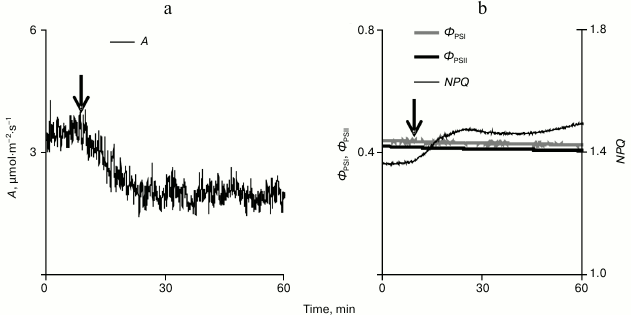
The local burning influenced photosynthetic processes inducing a long-term decrease in the CO2 assimilation under conditions of illumination (Fig. 3a). This decrease was 1.0 ± 0.2 µmol·m–2·s–1, which was ~22% of the total assimilation of CO2 on illumination (4.5 ± 0.2 µmol·m–2·s–1). The time from the beginning of the VP development in the leaf lamina to the beginning of the decrease in CO2 assimilation was 76 ± 15 s. The decrease in CO2 assimilation was maximal approximately 12 min after the stimulation.
Fig. 3. A typical example of changes in the intensity of carbon dioxide absorption (a) and in parameters of the photosynthesis light stage (b) caused by a local burning in pumpkin seedling leaf under conditions of illumination (n = 15). A is CO2 assimilation, фPSI and фPSII are quantum yields of PS I and II, and NPQ is the non-photochemical quenching of fluorescence.
Studies on the influence of a local burning on parameters of the photosynthesis light stage (Fig. 3b) revealed only a small slowly developing increase (0.16 ± 0.03) in the non-photochemical quenching of fluorescence. The time from the beginning of the VP development in the leaf lamina and the beginning of the NPQ increase was 134 ± 23 s, which was significantly longer than a similar time for the beginning of the decrease in CO2 assimilation. In the majority of experiments, no changes were observed in quantum yields of PS I and II. However, in individual experiments when a high amplitude of changes in A was observed (~2 µmol·m–2·s–1 and more), the local burning induced a small decrease in quantum yields of PS I and II.
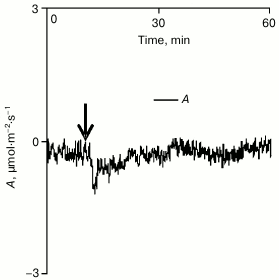
Analysis of the influence of cotyledon local burning on gas exchange in a true leaf in the dark revealed that under such conditions only a small and transient decrease in A occurred (Fig. 4). This decrease seemed to be due to the burning-induced activation of respiration that was earlier shown for some plants [29, 31]. However, the small value (0.4 ± 0.1 µmol·m–2·s–1) and the duration (several minutes) of the reaction suggested that its contribution to changes in gas exchange of the pumpkin leaf observed after the burning under conditions of illumination was insignificant. Therefore, it was supposed that changes in gas exchange of the leaf caused by a local burning should be mainly due to the photosynthetic response.
Fig. 4. A typical example of changes in intensity of leaf gas exchange caused by local burning in the dark (n = 5). A is the CO2 assimilation (negative values of A correspond to absorption of carbon dioxide). The arrow indicates the moment of burning.
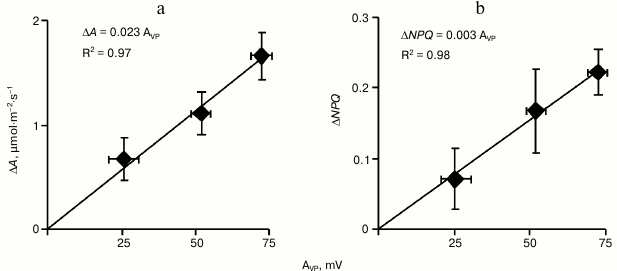
To analyze the relation between the amplitude of VP in the leaf lamina and changes in A and NPQ in it, all plants in this series were ranked by increase in amplitude and were then divided into three equal groups (with low, medium, and high amplitude of variation potential, n = 5). In Fig. 5a the dependence of the CO2 assimilation decrease on VP amplitude in these groups is shown. This dependence was linear, and the difference between the decrease in A in the group with the low VP amplitude and in the group with the high amplitude was significant. A similar approach for analyzing the increase in NPQ (Fig. 5b) revealed that the non-photochemical quenching of fluorescence also depended linearly on the amplitude of VP. The differences in NPQ between the groups with low and high VP amplitude were significant. Because the VP began to develop earlier than the photosynthetic parameters began to change, these results indicated that just the variation potential propagation could be a mechanism inducing the photosynthetic response in pumpkin seedlings.
Fig. 5. Dependence of maximal decrease in CO2 assimilation (a) and of maximal increase in non-photochemical quenching of fluorescence (b) on variation potential amplitude (AVP) after burning a cotyledon leaf in pumpkin seedlings under illumination (n = 5). R2 is the determination coefficient.
Changes in intracellular and extracellular pH during variation potential generation. According to our [31] and literature [27] data, changes in the intracellular and extracellular pH can be a mechanism of VP influence on photosynthesis of higher plants. To test this hypothesis, we studied pH of the apoplast and cytoplasm of pumpkin cells during variation potential generation.
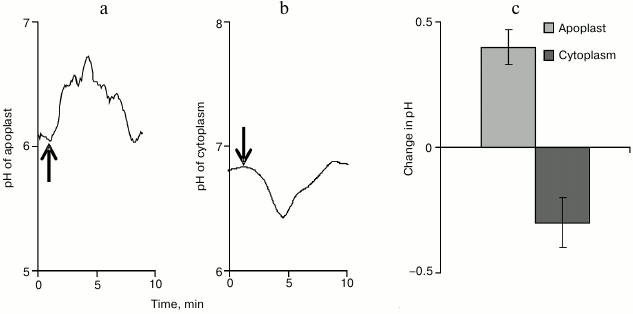
The apoplast pH was determined using a ratiometric pH-sensitive fluorescent FITC-dextran probe. Under conditions of rest, the apoplast pH value was highly variable and was, on average, 5.80 ± 0.14. The VP propagation was associated with alkalization of the apoplast (Fig. 6a), and pH maximally increased by 0.40 ± 0.07 (Fig. 6c). The total time of increase in the apoplast pH value varied significantly and, on average, was 8 min.
Fig. 6. Typical examples of changes in pH of the apoplast (a) and of the cytoplasm (b) after induction of variation potential and maximal values of such changes (c) (n = 8-12). The arrow indicates the moment of burning.
The cytoplasmic pH was determined using the ratiometric pH-sensitive fluorescent probe BCECF-AM. Under conditions of rest, the cytoplasmic pH in the pumpkin seedling cells was 6.90 ± 0.10. The VP propagation was associated with acidification of the cytoplasm (Fig. 6b), and pH maximally decreased by 0.30 ± 0.10 (Fig. 6c). The total time of changes in the cytoplasmic pH was ~8 min.
Influence of incubation medium acidification on non-photochemical quenching of isolated pumpkin chloroplasts. Changes in the apoplast and cytoplasmic pH associated with VP stimulate the important question whether these changes can influence the photosynthesis of pumpkin seedlings. Earlier, we qualitatively demonstrated [30] that incubation medium acidification could increase the non-photochemical quenching in isolated chloroplasts of pumpkin seedlings. The present work was designed to study the dependence of the NPQ increase on incubation medium acidification, which imitated acidification of the cytoplasm during the development of VP.
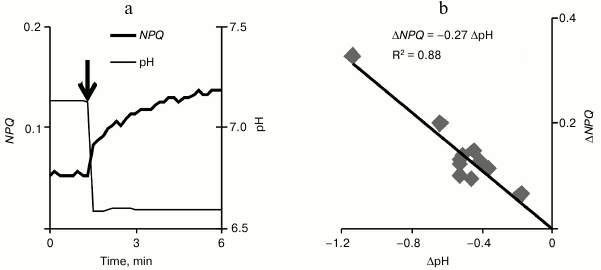
The acidification of the incubation medium of pumpkin chloroplasts (Fig. 7a) (by adding different volumes of 0.1 M HCl) caused in them an increase in NPQ that developed within several minutes after the acidification. In the range of pH changes under study (the initial level was ~7.1 and the minimal level was ~6.0), the increase in NPQ grew linearly with increase in acidification (Fig. 7b). It should be noted that a significant increase in non-photochemical quenching (~40% of the initial level) was observed on decrease in pH by 0.3 unit, which corresponded to decrease in the cytoplasmic pH during VP.
Fig. 7. An example of non-photochemical quenching of fluorescence change in isolated pumpkin chloroplasts during a decrease in the incubation medium pH (a) and the scattering diagram that shows the dependence of the NPQ increase on the decrease in pH (b) (n = 12). The incubation medium pH was changed by adding 0.1 M HCl, and the addition moment is shown by the arrow. R2 is the determination coefficient.
No significant changes were observed in фPSI and фPSII (data not presented), which was in agreement with the absence of their changes in experiments on an intact plant and our previous data [30].
DISCUSSION
Many works [19, 22-33] have shown that a local stimulation of a plant can cause a rapid and reversible inactivation of photosynthesis in non-stimulated leaves, which is usually thought to be associated with the generation and propagation of electric signals. However, the involvement of electric signals in the inactivation of photosynthesis in higher plants has been studied experimentally only in a limited number of works [27, 29, 32]. There are two groups of arguments in favor of the role of such signals in development of the photosynthetic response. First, it was shown [27] that on an increase in the distance from conducting bundles that are the major channel of VP propagation, photosynthesis was inactivated later. Moreover, the photosynthetic response began later also when the distance from the zone of damage increased. Second, it was established [29, 32] that the entrance of VP into a leaf was necessary for development of a rapid inactivation of photosynthesis. An important result of the present work is the detection of a relation between the VP amplitude and the photosynthetic response magnitude. On one hand, this is a new argument in favor of a key role of electric signals in the development of photosynthesis inactivation and, on the other hand, is a basis for analyzing mechanisms of the influence of variation potential on photosynthesis.
The reversible inactivation of the plant cell plasma membrane H+-ATPase is believed to be the major mechanism of the VP generation [3, 7, 11]. An important consequence of such inactivation is acidification of the cytoplasm and alkalization of the apoplast of plant cells [27, 31], the size of which correlates with the VP amplitude [31]. According to the earlier hypothesis [31] based on experiments with pea seedlings, changes in the intra- and extracellular pH can be a key mechanism in the development of the rapid inactivation of photosynthesis caused by VP in higher plants. Changes in the apoplast and cytoplasm pH shown by us that accompanied the variation potential development in pumpkin seedlings, as well as increase in the NPQ of chloroplast suspension on the imitation of cytoplasm acidification confirmed this hypothesis and show that it is true for various plant materials. Therefore, a significant question arises – how can cytoplasmic acidification and apoplast alkalization cause a reversible inactivation of photosynthesis in plants?
According to some works [28, 29, 31-33], the decrease in the dark stage activity is an initial stage in development of the photosynthetic response caused by electric signals. The results of the present work also are in agreement with this hypothesis. Only a relatively small decrease in CO2 assimilation was observed in pumpkin seedlings in response to VP (on average, ~22% of the assimilation under illumination) and in the majority of cases there were no changes in the quantum yields of PS I and II. However, the decrease in the assimilation in some seedlings could be significantly higher (>40%), and in this case the VP was also associated with a small decrease in фPSI and фPSII. Our earlier studies [31, 32] revealed that in pea seedlings VP caused a 55% decrease in the assimilation and also a decrease by ~0.10 in the quantum yield of the photosystems. In geranium the assimilation decreased by ~100% of the CO2 absorption under illumination [29], whereas the quantum yields of the photosystems decreased by 0.15-0.20. Therefore, we conclude that just the decrease in the CO2 absorption can be one of key mechanisms of development of the photochemical response to VP, and if this decrease is lower than a definite level (<40% of the CO2 assimilation under illumination), it does not significantly influence the quantum yields of the photosystems.
A decrease in CO2 entrance into the cell can be one of the mechanisms of decrease in the activity of the photosynthesis dark stage during VP [31]. It is known [39, 40] that in higher plants CO2 penetrates across biological membranes mainly in the neutral form, whereas charged forms, first of all, hydrocarbonate ions are transferred with difficulties. Neglecting the reverse transfer of carbon dioxide from the cytoplasm into the medium, we can assume that CO2 flow into the cell across the plasma membrane of plants (JCO2) is described by the Eq. (1):
where C is the total concentration of CO2, pK = 6.35 is the negative decimal logarithm of the equilibrium constant of the reaction CO2 + H2O ↔ HCO3– + H+ [39], pHa is pH of the apoplast, and k is the proportionality constant depending on the plasma membrane permeability for CO2. Taking C and k as constant, we evaluate the decrease in the CO2 flow into the cell on apoplast alkalization during the VP development using the Eq. (2):
where ΔpHa is the maximal change in the apoplast pH during VP. On admitting that changes in pHa under conditions of their recording and during the photosynthetic response development are approximately equal, placing into Eq. (2) of values pHa = 5.8 and ΔpHa = 0.4 (Fig. 6c) allows us to evaluate a decrease in CO2 flow associated with apoplast alkalization during VP. This decrease is about 25%, which is rather close to the decrease in CO2 assimilation (22%) observed in the experiment. Thus, the decrease in CO2 entrance across the plasma membrane can be a mechanism of the influence of changes in apoplast pH on photosynthesis.
However, the influence of pH on NPQ was also observed in the chloroplast suspension where this mechanism was absent. It was supposed that in this case incubation medium acidification could lead to entrance of protons into the stroma and lumen of the chloroplasts [31]. The decrease in the lumen pH, in turn, is the major mechanism of development of energy-dependent quenching of fluorescence [37], i.e. the increase in NPQ. This mechanism of the inactivation of photosynthesis should not already be associated with a decrease in its dark stage activity, and this is in a good correlation with our earlier data [29, 31, 33] on the existence of a direct influence of VP on the light stage of photosynthesis in higher plants.
The question should be considered separately why the VP-induced decrease in NPQ did not cause a decrease in quantum yields of the photosystems in pumpkin seedlings. It could be associated with a relatively low magnitude of such decrease observed in the pumpkin seedlings (ΔNPQ = 0.15). We demonstrated earlier [31, 32] that the decrease in the external concentration of carbon dioxide to close to zero values led to suppression of CO2 assimilation, which that allowed us to exclude dark stage inactivation from the VP-caused photosynthetic response. Under such conditions, the VP-induced changes in the light parameters were ΔNPQ ≈ 0.75, ΔфPSI ≈ –0.04, and ΔфPSII ≈ –0.04 [31]. This result indicates that even an increase in NPQ fivefold greater than the response revealed by VP in the pumpkin seedlings induces only relatively small changes in quantum yields of the photosystems. Therefore, we can conclude that the VP-caused changes in the non-photochemical quenching in pumpkin seedlings are too small for inducing a significant decrease in quantum yields of the photosystems.
Thus, our results have demonstrated that changes in the intra- and extracellular pH associated with the generation of VP are an important mechanism of development of the rapid inactivation of photosynthesis in pumpkin seedlings, which suggests the universality of this mechanism in higher plants. The further development of the response can be associated with both a decrease in the activity of the photosynthesis dark stage [28, 31-33] and pH-dependent changes in processes during the light stage [31]. It is also supposed [32, 33] that the VP-induced inactivation of photosynthesis can result in accumulation of excess ATP in the cell [28, 29, 32], activation of the cyclic flow around the PS I [33], generation of reactive oxygen species [41-43], increase in the non-photochemical quenching of fluorescence [31-33], etc. It seems that all these processes can finally result in increase in the resistance of the photosynthetic apparatus and the plant as a whole to action of unfavorable factors [32, 34, 35].
The work was supported by the Russian Federation Ministry of Education and Science (project No. 6.2050.2014/K).
REFERENCES
1.Opritov, V. A., Pyatygin, S. S., and Retivin, V. G.
(1991) Bioelectrogenesis in Higher Plants [in Russian], Nauka,
Moscow.
2.Davies, E. (2006) in Plant Electrophysiology.
Theory and Methods (Volkov, A. G., ed.) Springer-Verlag,
Berlin-Heidelberg-N. Y., pp. 407-422.
3.Fromm, J., and Lautner, S. (2007) Electrical
signals and their physiological significance in plants, Plant Cell
Environ., 30, 249-257.
4.Dziubinska, H. (2003) Ways of signal transmission
and physiological role of electrical potential in plants, Acta Soc.
Bot. Polon., 72, 309-318.
5.Felle, H. H., and Zimmermann, M. R. (2007) Systemic
signaling in barley through action potentials, Planta,
226, 203-214.
6.Vodeneev, V. A., Opritov, V. A., and Pyatygin, S.
S. (2006) Reversible changes in extracellular pH during action of
variation potential in a higher plant Cucurbita pepo, Russ.
J. Plant Physiol., 53, 538-545.
7.Stahlberg, R., and Cosgrove, D. J. (1996) Induction
and ionic basis of slow wave potentials in seedlings of Pisum
sativum L., Planta, 200, 416-425.
8.Vodeneev, V. A., Akinchits, E. K., Orlova, L. A.,
and Sukhov, V. S. (2011) The role of Ca2+, H+,
Cl– ions in generation of variation potential in
pumpkin plant Cucurbita pepo L., Russ. J. Plant Physiol.,
58, 974-981.
9.Katicheva, L., Sukhov, V., Akinchits, E., and
Vodeneev, V. (2014) Ionic nature of burn-induced variation potential in
wheat leaves, Plant Cell Physiol., 55, 1511-1519.
10.Sukhov, V., Akinchits, E., Katicheva, L., and
Vodeneev, V. (2013) Simulation of variation potential in higher plant
cells, J. Membr. Biol., 246, 287-296.
11.Stahlberg, R., Robert, E., Cleland, R. E., and
van Volkenburgh, E. (2006) in Communication in Plants. Neuronal
Aspects of Plant Life (Baluska, F., Mancuso, S., and Volkmann, D.,
eds.) Springer-Verlag, Berlin-Heidelberg, pp. 291-309.
12.Malone, M. (1994) Wound-induced hydraulic signals
and stimulus transmission in Mimosa pudica L., New
Phytol., 128, 49-56.
13.Mancuso, S. (1999) Hydraulic and electrical
transmission of wound-induced signals in Vitis vinifera,
Aust. J. Plant Physiol., 26, 55-61.
14.Vodeneev, V., Orlova, A., Morozova, E., Orlova,
L., Akinchits, E., Orlova, O., and Sukhov, V. (2012) The mechanism of
propagation of variation potentials in wheat leaves, J. Plant
Physiol., 169, 949-954.
15.Pyatygin, S. S., Opritov, V. A., and Vodeneev, V.
A. (2008) Signaling role of action potential in higher plants, Russ.
J. Plant Physiol., 55, 285-291.
16.Stankovic, B., and Davies, E. (1996) Both action
potentials and variation potentials induce proteinase inhibitor gene
expression in tomato, FEBS Lett., 390, 275-279.
17.Fisahn, J., Herde, O., Willmitzer, L., and
Pena-Cortes, H. (2004) Analysis of the transient increase in cytosolic
Ca2+ during the action potential of higher plants with high
temporal resolution: requirement of Ca2+ transients for
induction of jasmonic acid biosynthesis and PINII gene expression,
Plant Cell Physiol., 45, 456-459.
18.Dziubinska, H., Filek, M., Koscielniak, J., and
Trebacz, K. (2003) Variation and action potentials evoked by thermal
stimuli accompany enhancement of ethylene emission in distant
non-stimulated leaves of Vicia faba minor seedlings, J. Plant
Physiol., 160, 1203-1210.
19.Hlavackova, V., Krchnak, P., Naus, J., Novak, O.,
Spundova, M., and Strnad, M. (2006) Electrical and chemical signals
involved in short-term systemic photosynthetic responses of tobacco
plants to local burning, Planta, 225, 235-244.
20.Fromm, J., and Bauer, T. (1994) Action potentials
in maize sieve tubes change phloem translocation, J. Exp. Bot.,
45, 463-469.
21.Filek, M., and Koscielniak, J. (1997) The effect
of wounding the roots by high temperature on the respiration rate of
the shoot and propagation of electric signal in horse bean seedlings
(Vicia faba L. minor), Plant Sci., 123, 39-46.
22.Fromm, J., and Fei, H. (1998) Electrical
signaling and gas exchange in maize plants of drying soil, Plant
Sci., 132, 203-213.
23.Pena-Cortes, H., Fisahn, J., and Willmitzer, L.
(1995) Signals involved in wound-induced proteinase inhibitor II gene
expression in tomato and potato plants, Proc. Natl. Acad. Sci.
USA, 92, 4106-4113.
24.Bulychev, A. A., Kamzolkina, N. A., Luengviriya,
J., Rubin, A. B., and Muller, S. C. (2004) Effect of a single
excitation stimulus on photosynthetic activity and light-dependent pH
banding in Chara cells, J. Membr. Biol., 202,
11-19.
25.Krupenina, N. A., and Bulychev, A. A. (2007)
Action potential in a plant cell lowers the light requirement for
non-photochemical energy-dependent quenching of chlorophyll
fluorescence, Biochim. Biophys. Acta, 1767, 781-788.
26.Krupenina, N. A., Bulychev, A. A., Roelfsema, M.
R. G., and Schreiber, U. (2008) Action potential in Chara cells
intensifies spatial patterns of photosynthetic electron flow and
non-photochemical quenching in parallel with inhibition of pH banding,
Photochem. Photobiol. Sci., 7, 681-688.
27.Grams, T. E. E., Lautner, S., Felle, H. H.,
Matyssek, R., and Fromm, J. (2009) Heat-induced electrical signals
affect cytoplasmic and apoplastic pH as well as photosynthesis during
propagation through the maize leaf, Plant Cell Environ.,
32, 319-326.
28.Pavlovic, A., Slovakova, L., Pandolfi, C., and
Mancuso, S. (2011) On the mechanism underlying photosynthetic
limitation upon trigger hair irritation in the carnivorous plant Venus
flytrap (Dionaea muscipula Ellis), J. Exp. Bot.,
62, 1991-2000.
29.Sukhov, V., Orlova, L., Mysyagin, S., Sinitsina,
J., and Vodeneev, V. (2012) Analysis of the photosynthetic response
induced by variation potential in geranium, Planta, 235,
703-712.
30.Sukhov, V. S., Surova, L. M., Sherstneva, O. N.,
Rumyantsev, E. A., and Vodeneev, V. A. (2013) Influence of a variation
potential on photosynthesis in pumpkin seedlings (Cucurbita pepo
L.), Biophysics, 58, 361-365.
31.Sukhov, V., Sherstneva, O., Surova, L.,
Katicheva, L., and Vodeneev, V. (2014) Proton cellular influx as a
probable mechanism of variation potential influence on photosynthesis
in pea, Plant Cell Environ., 37, 2532-2541.
32.Sukhov, V., Surova, L., Sherstneva, O., and
Vodeneev, V. (2014) Influence of variation potential on resistance of
the photosynthetic machinery to heating in pea, Physiol. Plant.,
152, 773-783.
33.Sukhov, V., Surova, L., Sherstneva, O.,
Katicheva, L., and Vodeneev, V. (2015) Variation potential influence on
photosynthetic cyclic electron flow in pea, Front. Plant Sci.,
5, 766.
34.Retivin, V. G., Opritov, V. A., and Fedulina, C.
B. (1997) Generation of action potential induces preadaptation of
Cucurbita pepo L. stem tissues to freezing injury, Russ. J.
Plant Physiol., 44, 432-442.
35.Retivin, V. G., Opritov, V. A., Lobov, C. A.,
Tarakanov, S. A., and Khudyakov, V. A. (1999) Changes in the resistance
of photosynthesizing cotyledon cells to cooling and heating as induced
by the stimulation of the root system with KCl solution, Russ. J.
Plant Physiol., 46, 689-696.
36.Klughammer, C., and Schreiber, U. (2008)
Saturation pulse method for assessment of energy conversion in PS I,
PAM Application Notes, 1, 11-14.
37.Maxwell, K., and Johnson, G. N. (2000)
Chlorophyll fluorescence – a practical guide, J. Exp.
Bot., 51, 659-668.
38.Von Caemmerer, S., and Farquhar, G. D. (1981)
Some relationships between the biochemistry of photosynthesis and the
gas exchange of leaves, Planta, 153, 376-387.
39.Bulychev, A. A., Cherkashin, A. A., Vredenberg,
V., Rubin, A. B., Zykov, V. S., and Muller, S. H. (2001) Fluorescence
and photosynthetic activity of chloroplasts in acid and alkaline zones
of Chara corallina, Russ. J. Plant Physiol., 48,
326-332.
40.Tholen, D., and Zhu, X.-G. (2011) The mechanistic
basis of internal conductance: a theoretical analysis of mesophyll cell
photosynthesis and CO2 diffusion, Plant Physiol.,
156, 90-105.
41.Kim, K., and Portis, A. R., Jr. (2004)
Oxygen-dependent H2O2 production by Rubisco,
FEBS Lett., 571, 124-128.
42.Mubarakshina, M. M., and Ivanov, B. N. (2010) The
production and scavenging of reactive oxygen species in the
plastoquinone pool of chloroplast thylakoid membranes, Physiol.
Plant., 140, 103-110.
43.Mubarakshina-Borisova, M. M., Kozuleva, M. A.,
Rudenko, N. N., Naydov, I. A., Klenina, I. B., and Ivanov, B. N. (2012)
Photosynthetic electron flow to oxygen and diffusion of hydrogen
peroxide through the chloroplast envelope via aquaporins, Biochim.
Biophys. Acta, 1817, 1314-1321.