Effect of Laser Optoperforation of the Zona Pellucida on Mouse Embryo Development in vitro
E. O. Zakharchenko1, A. D. Zalessky1,2, A. A. Osychenko1, A. S. Krivokharchenko1, A. K. Shakhbazyan1, A. V. Ryabova3, and V. A. Nadtochenko1,4,5*
1Semenov Institute of Chemical Physics, Russian Academy of Sciences, 119991 Moscow, Russia; E-mail: nadtochenko@gmail.com2Moscow Institute of Physics and Technology, State University, 141700 Dolgoprudny, Moscow Region, Russia
3Prokhorov General Physics Institute, Russian Academy of Sciences, 119991 Moscow, Russia
4Institute of Problems of Chemical Physics, Russian Academy of Sciences, 142432 Chernogolovka, Moscow Region, Russia
5Lomonosov Moscow State University, Faculty of Chemistry, 119991 Moscow, Russia
* To whom correspondence should be addressed.
Received January 13, 2015; Revision received February 23, 2015
The effect of laser optical perforation of the zona pellucida on the viability and development of mouse embryos has been studied. Operations of zona pellucida thinning and single or double perforation were carried out on 2-cell embryo, morula, and blastocyst stages with a laser pulse (wavelength 1.48 µm, pulse duration 2 ms). Embryo development up to the blastocyst stage and hatching efficiency were statistically analyzed. It was found that 2-cell or morula stage embryo zona pellucida thinning or single perforation did not affect development to the blastocyst stage and number of hatched embryos, but it accelerated embryo hatching compared to control groups one day earlier in vitro. Double optoperforation on 2-cell embryo or morula stage did not significantly affect development to the blastocyst stage, but it strongly decreased the number of hatched embryos. Also, zona pellucida perforation at the blastocyst stage had a negative effect: hatching did not occur after this manipulation. Blastocyst cell number calculation after single zona pellucida perforation at 2-cell and morula stages showed that cell number of hatching or hatched blastocysts did not differ from the same control groups. This fact points out that the laser single optoperforation method is a useful and safe experimental tool that allows further manipulations within the zona pellucida.
KEY WORDS: biophotonics, photobiology, laser optoperforation, embryo, laser hatchingDOI: 10.1134/S0006297915060127
Laser operations on cells and embryos are an important field of current photobiology and biophotonics. The high power density of tightly focused laser irradiation provides an efficient impact on matter of cells or embryos. Precise focusing of the laser spot allows strictly controlled perforation of the membrane. The present work was devoted to studying the influence of optoperforation of mammalian embryonic zona pellucida with a tightly focused laser beam with 1.48-µm wavelength on further development of the embryo. Such a laser operation was proposed for application in in vitro fertilization (IVF) practice and intracytoplasmic sperm injection into the oocyte (ICSI). For cultured in vitro oocytes and embryos, the process of natural exiting from the zona pellucida (“hatching”) is often impaired, which decreases probability of implantation and pregnancy [1]. Assisted hatching – an artificial disruption of the embryo zona pellucida integrity during mechanical or chemical action – was proposed for increasing embryo implantation into the endometrium of the uterus [2]. Laser perforation of the zona pellucida is the most recently described method for assisted hatching [3, 4]. The laser provides high precision of action, sterility, and minor invasiveness, which makes the laser a convenient tool for performing micromanipulations. However, there are ambiguous results of using such methods. It was reported that frequency of pregnancy was significantly increased when using assisted hatching [5, 6]. At the same time, negative effect of the method was also reported [7]. Some reports indicate that there is no difference in groups with or without artificial perforation [8, 9].
The goals of the present work were to determine the influence of different manipulations on development of embryos in vitro until blastocyst formation and on the ability of blastocysts to exit zona pellucida, and the efficiencies of different laser-assisted hatching methods. Cell counting was performed to estimate quality of blastocysts developed in vitro after laser perforation.
MATERIALS AND METHODS
Obtaining embryos. Female mice of C57BL/5 strain and male mice of CBA strain were used in the experiments. All the animals were 1.5-2.0-month-old. To obtain sufficient numbers of embryos, females were stimulated by hormones. Hormones used to reach superovulation were pregnant mare serum gonadotropin (Intervet, USA) and human chorionic gonadotropin (Intervet). The hormones (10 U.I.) were injected intraperitoneally (i.p.) with 48-h intervals, usually in the evenings (4-6 p.m.). This time was chosen because ovulation in mice begins in 10-13 h after chorionic gonadotropin injection, this time approximately corresponding to the time of copulation (late night–early morning). Embryos were obtained at the 2-cell stage (second day after fertilization) by washing the oviducts with M2 medium (Sigma, USA). Manipulations with embryos were performed in a drop of M2 medium on the cover slip. Embryos were cultured in an incubator in four-well dishes (Nunc, USA) with M16 medium at 5% CO2 and 37°C.
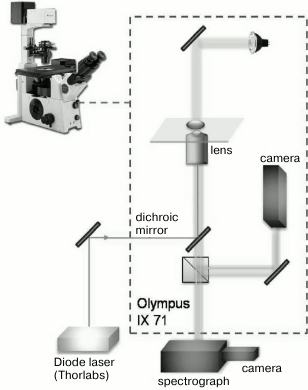
Laser perforator. Microsurgical operations were performed with a laser manipulator device designed in the Institute of Chemical Physics, Russian Academy of Sciences. The scheme of the optical block used in the experiment is shown in Fig. 1. Diode laser irradiation (Thorlabs, USA; wavelength λ = 1.48 µm, output power 500 mW, laser irradiation power at the object plane was 110 mW) was supplied via an optical fiber with microlens on the end into the optical path of an inverted microscope OLYMPUS IX71 through a dichroic mirror. Irradiation was supplied as a pulse and/or series of distinct pulses. Pulse duration was varied from 50 ns to 5 s using a controlled laser power supply that was developed based on an impulse generator (Thorlabs). In all the perforation experiments, pulse duration was 2 ms. Laser irradiation was focused with an Olympus 60× LUCPLFLN lens with numerical aperture NA = 0.7. The beam diameter was 2w0 = 1.22 λ/NA = 2.58 µm; depth of focus was z0 = (πw0)2/λ = 3.53 µm [10]; energy density at the focus area was 4.2·103 J/cm2. Absorption coefficient of water for wavelength 1.48 µm is 0.003 cm–1 [11], and the typically used pulse duration of 2 ms led to temperature jump ~75°C at the cell constriction (spot 2.58 µm in diameter). The X,Y-coordinates of the stage of the microscope and duration of laser pulses were computer-controlled. Microsurgery of embryonal zona pellucida was performed at the most distant from blastomeres area to prevent blastomere injury by the heat wave from the cell constriction spot after the laser pulse. The used laser pulse led to formation of round perforation 2 µm in size. According to the program, the laser spot moved over the zona pellucida field, impulse was applied and, thus, the layer perforation occurred sequentially. Laser perforation operations were made on the cover slip where embryos were placed into a 50-µl drop of M2 medium. After laser action, embryos were transferred to four-well dishes with M16 medium and cultured in a CO2 incubator. The operation did not last more than 2 min, and thus effects related to drop evaporation were insignificant.
Fig. 1. Schematic diagram of the laser device for perforation. Elements of the device are indicated on the figure.
Number of cells in blastocysts was estimated by two methods: making air-drying preparations using the Tarkowski method and Hoechst staining with subsequent visualization under a confocal fluorescence microscope. For making air-drying preparations by the Tarkowski method [12], we used hypotonic NaCl solution, methanol–glacial acetic acid mixture (3 : 1), and 7% solution of Giemsa stain (PanEko, Russia). The embryo was placed into a drop of hypotonic NaCl solution on the cover slip for ~5 min. When the hypotonic solution was almost completely evaporated, a drop of fixing solution (methanol–glacial acetic acid mixture) was applied over the embryo. After evaporation of the fixing mixture, the cover slip was transferred into 7% solution of Giemsa stain for 15 min. Then, nuclei of blastocysts were counted under the microscope.
Confocal microscopy. Hoechst 33342 (Sigma, USA) staining was performed in M16 medium in a CO2 incubator in 5 µg/ml dye solution for 10-20 min. Then the embryos were washed with dye-free medium, and fluorescence was registered. Fluorescence in the hatching blastocysts was localized using a Carl Zeiss LSM-710-NLO confocal laser-scanning microscope (Jena, Germany). Samples from the in vitro culturing dishes were transferred to sterile Petri dishes with thin glass bottom (0.16 mm thick) in the M2 manipulation medium. Images were obtained using a Plan_Apochromat lens with 20× magnification (aperture 0.8). Two-photon excitation of Hoechst 33342 fluorescence was executed by a Chameleon Ultra II femtosecond laser (Coherent, USA) with wavelength 770 nm and maximum power density 25 mW. Fluorescence was registered at 400-550 nm with confocal diaphragm 600 µm in diameter. To obtain images in transmitted light, a CW laser at 561 nm was used. Image dimensions were 512 × 512 pixels (0.58 nm/pixel), and scanning speed was 1.58 µs/pixel. For 3D reconstruction of blastocysts, series of images every 5.3 µm in the vertical direction were taken.
The following operations for laser optoperforation of zona pellucida were performed:
– thinning of 0.4-rad of zona pellucida arch. In this case, the zona pellucida of embryos at 2-cell stage was thinned from outside with laser with arch length of 20 µm to depth of half-layer thickness. Both experimental and control groups included 30 embryos;
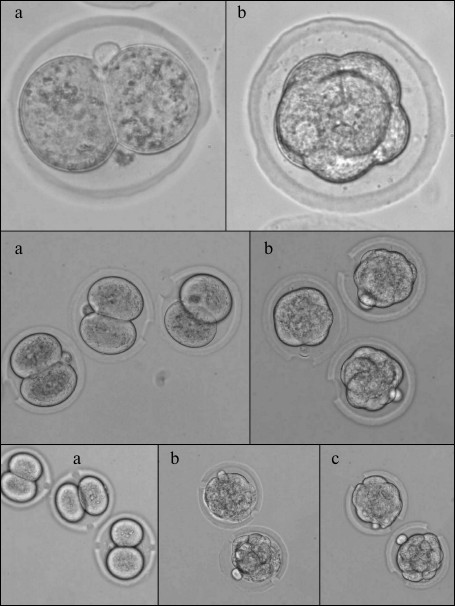
– thinning of π-rad of zona pellucida arch. In this experiment, we thinned half of the zona pellucida circle (π rad, or 180°). Operations were made on 2-cell embryos and at the morula stage. Perforation result is shown in Fig. 2. There were 30 embryos in both experimental groups and 26 embryos in the control group;
– single laser perforation of zona pellucida. We made one perforation 20 µm in diameter through the zona pellucida depth. Operations were made on 2-cell embryos and at the morula stage. The results are presented in Fig. 2. The experimental group with perforation at the stage of two blastomeres included 33 embryos, the experimental group with perforation at the morula stage – 37 embryos, and the control group – 30 embryos;
– double laser perforation of zona pellucida. In this experiment, we made two perforations at the opposing sides of the embryo. Operations were made on 2-cell embryos and at the morula stage. In the first experimental subgroup, 2-cell embryos were perforated with perforation area size of 10 µm. In the second subgroup, embryos at the morula stage were perforated likewise. In the third subgroup, two perforations 20 µm in diameter were made in the morula stage embryos. The perforation result is shown in Fig. 2. There were 30 embryos in each group;
– single laser perforation of zona pellucida of embryo at the blastocyst stage. In this experiment, the effect of zona pellucida laser perforation on the fifth day of embryonal development at the blastocyst stage was studied. Embryos were extracted from murine oviducts on day 2 of embryonal development at the 2-cell stage, and they were cultured in the M16 medium until day 5. On day 5, blastocysts were subjected to laser irradiation (perforation diameter was about 20 µm). The control group was cultured until day 6. There were 30 embryos in each group. The control group embryos were not subjected to laser action.
Fig. 2. Image of murine embryos subjected to optoperforation. Upper row: operation of thinning of π radians segment of zona pellucida arch; second row: operation of single perforation; third row: operation of double perforation. a) 2-cell embryo; b) morula stage embryo; c) hatched embryos.
Statistical analysis of the results was performed using STATISTICA 6.0 software. To validate statistical reliability, the z-test was used with significance level p = 0.05.
RESULTS AND DISCUSSION
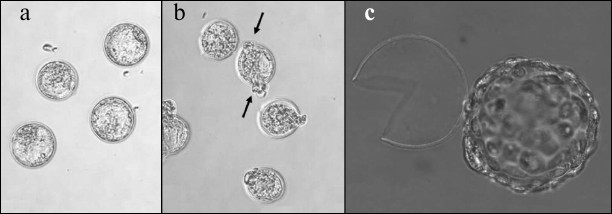
Embryos at different developmental stages – hatching or hatched blastocysts – are depicted in Fig. 3. During normal development, blastocyst formation in vivo occurs on day 4 (here, the day that follows fertilization occurring overnight or early in the morning is considered day 1). Hatching of blastocyst from the zona pellucida and its implantation into the uterus happens on day 5. In vitro developments proceeds with about 12-h delay, and on day 5 we see expanded blastocysts and on day 6 – hatching from zona pellucida [13]. The table includes the results of counting embryos that reached blastocyst stage (5th day of development), the embryos hatching on day 5, and the embryos that hatched on day 6 after several types of optoperforation: (i) thinning of 0.4 rad of zona pellucida arch; (ii) thinning of π rad of zona pellucida arch; (iii) single laser perforation of zona pellucida; (iv) double laser perforation of zona pellucida.
Results of embryo development after optoperforation
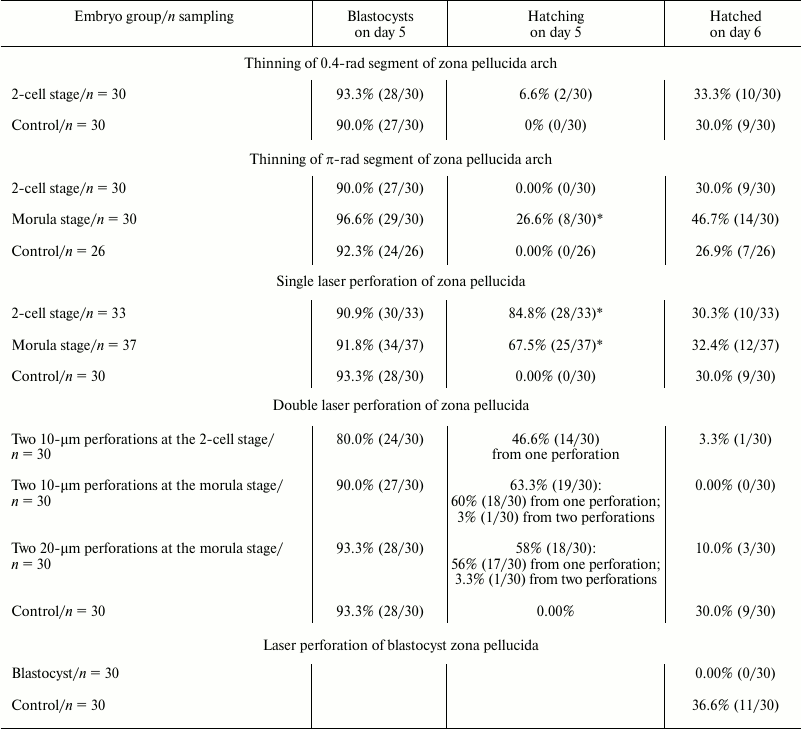
* Significant difference.
Fig. 3. Image of embryos at different developmental stages. a) Blastocyst in control group (5th day of development); b) hatching blastocyst (5th day of development). Exiting of blastocyst from two perforations is indicated with arrows; c) hatched embryo.
Analysis of these data reveals that optoperforation does not affect development of embryos until the blastocyst stage (5th day of development), significantly affecting the number of hatching blastocysts on day 5. Unlike the control groups where hatching of blastocysts from zona pellucida was not observed, hatching from zona pellucida occurred in groups with optopeforations. On day 6, number of embryos completely hatched from the zona pellucida was equal for control group and groups with single perforation and layer thinning.
Double perforation significantly reduces the number of hatching embryos. Hatching of embryo from the zona pellucida, occurring on day 5, stops, and the number of completely hatched blastocysts was small. This negative effect emerged more clearly in the case of double 10-µm perforation. In the case of double perforation at the 2-cell stage, only 3.3% of embryos hatched (1/30), and 0% of embryos hatched in the case of perforation at the morula stage (0/30). In the control group, 30% of blastocysts were completely hatched (9/30). In the group where 20-µm perforations were made at the morula stage, 10% (3/30) of blastocysts were completely hatched. For hatching, embryos predominantly used only one of the two perforations. Injurious effect of double perforation observed in our work corresponds to the data of work [14], where it was reported that two 12-15-µm perforations in the zona pellucida at the zygote stage hinder complete hatching of the blastocyst. All the embryos used both perforations for hatching and then degraded.
After the zona pellucida perforation at the blastocyst stage, hatching of the operated embryos was not observed, while embryos of the control group hatched from the zona pellucida much more successfully: 36.6% (11/30), p < 0.05.
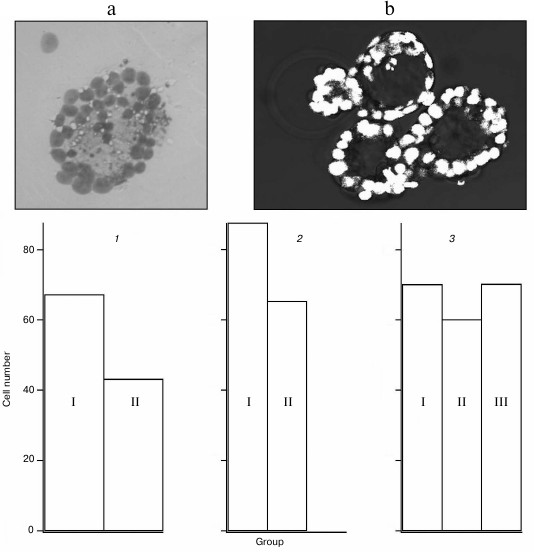
Cells in blastocysts after a single perforation were counted. Mean numbers of cells after perforation in different experimental groups are shown in Fig. 4. Statistical analysis revealed significant decrease in cell number in blastocysts that have not hatched from the zona pellucida after optoperforation at the morula stage. This observation partially corresponds to results of recently published work [15] where cell number decrease in blastocysts after zona pellucida perforation was reported. In our work, this decrease is typical for non-hatched blastocysts, while single perforation of hatching or completely hatched blastocysts had no appreciably affect.
Fig. 4. Results of counting cells in blastocysts. Insert: a) nuclei of embryo cells stained with the Tarkowski method; b) images of nuclei obtained on confocal microscope. Cell number in blastocyst: 1) not hatched from zona pellucida (I – control group, n = 7, mean value 67.3; II – group after single perforation at the morula stage, n = 6, mean value 43.2); 2) hatched from the zona pellucida (I – control group, n = 4, mean value 87.5; II – group after single perforation at the 2-cell stage, n = 13, mean value 65.3); 3) hatching from the zona pellucida (I – control group, n = 9, mean value 70.1; II – group after single perforation at the 2-cell stage, n = 7, mean value 60.1; III – group after single perforation at the morula stage, n = 9, mean value 70.2).
Utilization of assisted hatching including laser action for extracorporeal fertilization remains questionable [1, 16, 17]. In the presented work, we show experimentally that laser action on the zona pellucida at different embryo development stages did not significantly affect ability of embryos to develop until the blastocyst stage. Half circle thinning of the zona pellucida at the morula stage and single laser perforation accelerated hatching. For hatching, embryos always used the auxiliary perforation introduced by the laser and did not make new perforations. Thinning of a small area of zona pellucida as well as thinning of half-perimeter and single perforation did not affect the number of blastocysts that are completely hatched on the 6th day of development. However, multiple laser perforation decreases the fraction of hatched blastocysts. Perforation of the zona pellucida at the blastocyst stage has a negative effect on embryo development.
Analysis of cell counting revealed that after perforation, cell number is only decreased in the blastocysts that did not hatch out from the zona pellucida. After single optoperforation of the zona pellucida, cell number in hatching or completely hatched blastocysts did not differ from the control group.
This shows that single laser perforation of the zona pellucida can be widely used when it is required to penetrate this layer for additional manipulations with the embryo. From this point of view, the method of single laser optoperforation is a convenient and safe experimental approach for manipulating the embryo inside the zona pellucida.
This work was supported by the Russian Science Foundation (grant 14-14-00856).
REFERENCES
1.Mohamad, E. H., Constanze, F.-H., and Khaled, R. A.
(2011) Assisted hatching in assisted reproduction: a state of the art,
J. Assist. Reprod. Genet., 28, 119-128.
2.Cohen, J., Malter, H., Fehilly, C., Wright, G.,
Elsner, C., Kort, H., and Massey, J. (1988) Implantation of embryos
after partial opening of oocyte zona pellucida to facilitate sperm
penetration, J. Lancet, 16, 162.
3.Palanker, D., Ohad, S., Lewis, A., Simon, A.,
Shenkar, J., Penchas, S., and Laufer, N. (1991) Technique for cellular
microsurgery using the 193-nm excimer laser, J. Lasers Surg.
Med., 11, 580-586.
4.Tadir, Y. (1988) Ten years of laser-assisted
gametes and embryo manipulation, Contemp. Obstet. Gynecol.,
9, 2-10.
5.Cohen, J., Elsner, C., Kort, H., Malter, H.,
Massey, J., Mayer, M. P., and Wiemer, K. (1990) Impairment of hatching
process following IVF in the human and improvement of implantation by
assisted hatching using micromanipulation, J. Hum. Reprod.,
5, 7-13.
6.Mansour, R. T., Rhodes, C. A., Aboulghar, M. A.,
Serour, G. I., and Kamal, A. (2000) Transfer of zona-free embryos
improves outcome in poor prognosis patients: a prospective randomized
controlled study, J. Hum. Reprod., 15, 1061-1064.
7.Primi, M. P., Senn, A., Montag, M., Van der Ven,
H., Mandelbaum, J., Veiga, A., Barri, P., and Germond, M. A. (2004)
European multicentre prospective randomized study to assess the use of
assisted hatching with a diode laser and the benefit of an
immunosuppressive/antibiotic treatment in different patient
populations, J. Hum. Reprod., 19, 2325-2333.
8.Hurst, B. S., Tucker, K. E., Awoniyi, C. A., and
Schlaff, W. D. (1998) Assisted hatching does not enhance IVF success in
good-prognosis patients, J. Assist. Reprod. Genet., 15,
62-64.
9.Sifer, C., Sellami, A., Poncelet, C., Kulski, P.,
Martin-Pont, B., Bottero, J., Porcher, R., Cedrin-Durnerin, I., Hugues,
J. N., and Wolf, J. P. (2006) A prospective randomized study to assess
the benefit of partial zona pellucida digestion before frozen-thawed
embryo transfers, J. Hum. Reprod., 21, 2384-2389.
10.Silfvast, W. T. (2004) in Laser
Fundamentals (2nd Edn.) Cambridge, N. Y., pp. 102-103.
11.Hale, G. M., and Querry, M. R. (1973) Optical
constants of water in the 200 nm to 200 µm wavelength
region, Appl. Opt., 12, 555-563.
12.Tarkowski, A. K. (1966) An air-drying method for
chromosome preparations from mouse eggs, Cytogenetics, 5,
394-400.
13.Pratt, H. P. M. (1987) in Mammalian
Development: A Practical Approach (Monk, M., ed.) IRL Press,
Oxford, p. 13.
14.Montag, M., Koll, B., Holmes, P., and van der
Ven, H. (2000) Significance of the number of embryonic cells and the
state of the zona pellucida for hatching of mouse blastocysts in
vitro versus in vivo, J. Biol. Reprod., 62,
1738-1744.
15.Chailert, C., Sanmee, U., Piromlertamorn, W.,
Samchimchom, S., and Vutyavanich, T. (2013) Effects of partial or
complete laser-assisted hatching on the hatching of mouse blastocysts
and their cell numbers, J. Reprod. Biol. Endocrinol., 19,
11-21.
16.Balaban, B., Urman, B., Yakin, K., and Isiklar,
A. (2006) Laser-assisted hatching increases pregnancy and implantation
rates in cryopreserved embryos that were allowed to cleave in
vitro after thawing: a prospective randomized study, J. Hum.
Reprod., 21, 2136-2140.
17.Valojerdi, M. R., Eftekhari-Yazdi, P., Karimian,
L., Hassani, F., and Movaghar, B. (2010) Effect of laser zona thinning
on vitrified-warmed embryo transfer at the cleavage stage: a
prospective, randomized study, J. Reprod. BioMed. Online,
20, 234-242.