REVIEW: Photobiosensors Containing Luminescent Bacteria
A. D. Ismailov* and L. E. Aleskerova
Lomonosov Moscow State University, Faculty of Biology, 119991 Moscow, Russia; E-mail: anvaris@list.ru* To whom correspondence should be addressed.
Received January 20, 2015; Revision received March 2, 2015
The scientific basis for producing luminescent biosensors containing free and immobilized luminescent bacteria is discussed. Modern technologies for engineering target objects, procedures used to immobilize bacteria in different carriers, as well as procedures for integral and specific biodetection of toxins are presented. Data regarding generation and application of biomonitoring for ecotoxicants derived from natural and genetically engineered photobacterial strains are analyzed. Special attention is given to immobilization of photobacteria in polyvinyl alcohol-containing cryogel. The main physicochemical, biochemical, and technological parameters for stabilizing luminescence in immobilized bacteria are described. Results of the application of immobilized photobacterial preparations both during discrete and continuous biomonitoring for different classes of ecotoxicants are presented.
KEY WORDS: bioluminescence, biomonitoring, luminescent bacteria, biosensors, immobilization, gels, polyvinyl alcoholDOI: 10.1134/S0006297915060085
PHOTOBACTERIA IN BIOMONITORING OF ECOTOXICANTS
Analytical systems containing different functional enzymatic systems, cells, and microbes are widely used for scientific and applied biomonitoring tasks. Recently, special attention has begun to be paid to photobacteria-containing bioluminescent biosensors. The technology used in this process is based on substrate, inhibitory, or inductive action of substances on specific targets of biosensors that change both energy and/or structural metabolism inside cells. The intensity of luminescence emitted from an object serves as a quantitative indicator of a biological object responding to an external impact. The results of such studies are presented in a number of reviews, books, and scientific papers [1-4].
The breadth of practical application of photobacteria in environmental biomonitoring is related to the sensitivity level of cellular bioluminescent reactions to a wide range of substances having cytotoxic and/or genotoxic activity [5].
Photobacteria transform chemical energy into luminous signal at a level available to rapid and quantitative measurements that can be estimated using standard photodetectors. Emission triggered by a wide range of toxic substances correlates well with responses in standard bioassays using fish, crustacean, and protozoa, i.e. 50% emission quenching (EC50) correlates with LD50, with correlation coefficient reaching 0.80-0.95.
During biomonitoring, natural and genetically engineered microorganisms are used as bioluminescent test materials. Application of cells for analytical purposes has a number of advantages compared to cell-free systems, primarily due to the high-level of spontaneous light emission as well as multiple targets available for action of various chemical agents such as toxins, mutagens, carcinogens (xenobiotics, heavy metals, carbohydrates), and physical factors (UV radiation and other types of radiation).
Both free and immobilized photobacteria are used as luminescent objects for detection of toxic substances [6, 7]. Several test systems are now commercially available, i.e. Microtox (Azur Enviromental, USA) [8], Toxalert (Merck, USA) containing Photobacterium phosphoreum [9], as well as Mutatox (USA) [10], Vitotox (GENTAUR Molecular Products, Belgium) [11] containing Vibrio fischeri, etc. Protocols for producing lyophilized and immobilized photobacteria of different strains as well as technological procedures for using such products in discrete and continuous biomonitoring of ecotoxicants have been developed [12, 13]. A large number of studies of recent years have used luxCDABE plasmids derived from E. coli strains and other bacterial strains for specific detection of stressful conditions as well as DNA-damaging and membrane-targeting agents of organic and inorganic origin [8, 14, 15].
The available data suggest photobacterium-based assays as a universal method for large-scale application of measures for ecological control of environmental pollutants. Due to its high sensitivity, high speed, and economic efficiency compared to other assays, technologies of bioluminescent assays are considered promising for rapid detection of herbicides, insecticides, and heavy metals present in soil, water, and preparative forms as well as for monitoring of toxin biotransformation and degradation [10, 15, 16].
A substantial number of publications have considered technologies for engineering of microbial biosensors containing photobacteria [4, 17, 18]. According to these tasks, special attention has been given to engineering test objects, biodetection systems, and technologies for their application. The emission response from a biosensor is measured using inducible and constitutive modes. In the inducible approach, reporter lux-genes are “ligated” to specific sensor genes, wherein both types of genes are driven by a common promoter. A promoter reaction to certain chemical substances results in induced luminescence. With the constitutive approach, reporter lux-genes are linked to a continuously expressed promoter. Both types of biosensor are constantly being improved as evidenced by an increasing number of relevant publications. In a substantial number of publications, the main attention is paid to engineering novel lux-marked recombinant bacterial strains as well as technological procedures for cell immobilization, storage, and application of biosensors both in discrete and continuous biomonitoring of toxicants.
TECHNOLOGY OF PHOTOBACTERIAL DETECTION OF TOXINS
Technically, a bioassay system is a combined complex composed of a bacterial light emitter and a light signal converter inside a photodetector. The following light emitters are used: 1) natural luminescent bacteria P. phosphoreum, Vibrio harveyi, V. fischeri, etc.; 2) targeted mutant bacteria V. harveyi and V. fischeri; 3) recombinant strains of numerous bacteria containing cloned luciferase genes. A cellular luminescent biosensor contains: 1) a sensor element (S); 2) a reporter element (R).
The sensor element includes the structural elements of the cell responsible for interaction with the toxicant. Sensor elements in natural microbial strains used for evaluation of integral (nonspecific) toxicity are represented by plasma membrane and energy turnover chain exposed into the periplasm as well as intracellular structural components that are related directly or indirectly to the luminescence system. A biosensor reaction is exhibited as emission quenching. The sensor element in mutant and recombinant microbial strains is a promoter of specifically expressed genes responsible for defense against organic xenobiotics, heavy metals, mutagens including DNA-damaging agents, membrane-targeting agents, and inhibitors of enzymes of lipid biosynthesis, protein transport, heat shock, and oxidative stress proteins. A biosensor reaction is exhibited as activation of emission.
The reporter element in natural photobacteria is composed of five genes within the bioluminescence operon luxCDABE together with luxI- and luxR-regulating genes. The reporter element in genetically engineered strains is a luxCDABE promoterless transposon. The latter is usually engineered as a composite structure containing two linked genetic elements driven by a single promoter – a specific sensor element provides responses to chemical agents, whereas an indicator element is responsible for emission of bioluminescent signal.
The optical detecting element (D) is composed of photoelectric multipliers or photodiodes having spectral sensitivity in the range 400-700 nm.
Thus, a biomonitoring system (SRD) is based on two sequential energy converters: a biological converter performing a chemical-to-light energy transformation, and a photoelectric converter transforming a light input into an electric signal.
BIOSENSORS BASED ON IMMOBILIZED PHOTOBACTERIA
Biosensors containing cell suspensions have some drawbacks: rapid speed of assay is affected by procedures of supplementary and main steps used to obtain reference preparations from lyophilized cells. In contrast, immobilized cells are more cost-effective and standardized. The main costs are generally related to technology used to develop recombinant microbial strains. Moreover, it should be mentioned that during a specific assay with genetically engineered microbial strains, light emission is observed after a lag-phase lasting for many hours.
Photobacteria and bioluminescent enzymatic system have been successfully immobilized on different types of organic and inorganic carriers. Use of preparations with immobilized cells leads to heterogeneous catalysis that in many cases provides economic and practical efficiency. The advantages of this approach are primarily related to simplification of the manufacturing process as well as the opportunity to switch from occasional output of desired products or expected substrate reactions towards a continuous process. In addition, immobilized cells allow using preparations multiple times and for longer period compared to single application of cells in a homogeneous suspension. Moreover, the technology of immobilization in many cases enhances enzymatic activity. Also, immobilized preparations have increased resistance to various physicochemical factors (temperature, acidity, pH) and lesser sensitivity to the action of pathogenic organisms. Immobilizing photobacteria increases stability of a biosensor upon its storage as well as duration of its use [19, 20]. Also, continuous biomonitoring of toxins places increased demands on stability of light emission generated by the cells in test system.
Similarly to the free cells in suspension, in the case of immobilized preparations, an integral toxicity assay is performed by natural photobacteria, whereas specific toxin detection is mediated by mutant and genetically engineered microbial strains containing cloned genes of a bioluminescent system [13].
In most cases, agar, agarose, and alginate gels are used as the carriers for immobilized luminescent bacteria. Ca2+- and Sr2+-alginate gels are the carriers most commonly used in manufacturing bioluminescent toxicological biosensors [21].
Combined systems are considered especially promising for practical application of immobilized cells of type “biosensor–photoconducting cell–photodetector” that contain a gel with sensor cells covering an fiber optic [15, 22, 23].
Various reporter bacteria (e.g. different strains of Pseudomonas and Salmonella) are used for specific analysis to detect certain substances. A study conducted to develop biosensors containing genetically engineered bioluminescent bacteria with a reporter element for detection of catabolic repression for continuous real-time biomonitoring of salicylate and naphthalene serve as an example [13]. Moreover, biosensors were also used to analyze catabolic repression caused by industrial waste. Pseudomonas fluorescens strain HL44 was selected for use as reporter bacteria that contain a transcriptional nahG-luxCDABE fusion construct. As a result, exposure to salicylate and naphthalene induce luminescence of these bacteria. A reporter microbial culture was immobilized and fixed on the surface of a photodetector element using Sr-alginate gel. Immobilized biosensors for detecting such substances were used both during discrete and continuous monitoring. Conditions and concentrations range for substances triggering light emission were optimized. It was found that both the maximum emission and rate of emission induction depended on incubation time and concentration of substances (time/dose-dependence). However, exposing a biosensor to other substances (glucose, toluene, and integrated culture medium) induced luminescence that was minor compared to luminescence induced by salicylate and naphthalene. It was concluded that this system was suitable for real-time analysis of environmental pollutants and industrial waste as well as evaluation of metabolic status of various biological materials. Continuous cell culturing allowed real-time detection of toxicants. Cells immobilized in Ca-alginate gel effectively functions during the bioassay. A linear dependence between induced bioluminescent response and concentration of the substances was noted using both free and immobilized bacteria. The authors attracted attention to high stability of the alginate–glycerol cell suspension at –70°C. The data presented in [12] were obtained using recombinant Salmonella typhimurium strain TA1535 designed from a plasmid with an inducible SOS-promoter linked to the luxCDABE operon derived from Photobacterium leiognathi. The latter was immobilized in agar carrier, a simple procedure that includes a 96-well plate matrix (10 µl of mixture added into the wells) incubated for 30 min for polymerization and further storage at 4°C. The principle protocol used for analysis is similar to others that use recombinant biosensors and includes emission induction upon incubation with toxic substance. Model experiments on emission induction with this bacterial strain were made with DNA-damaging genotoxic agent mitomycin C. The immobilized cells retained activity at 4°C for 6 weeks. A response of 4-week-old bacterial culture exposed to mitomycin C was indistinguishable from that triggered by a fresh immobilized culture. Both the magnitude of the maximum response and duration of the lag-phase depend on the concentration of mitomycin C. The maximum activation response is obtained at approximately 1 µg/ml concentration of mitomycin C. It is crucial that further increase in concentrations results in abrupt quenching of the emission. Apparently, this is due to a cytotoxic action of the antibiotic on the bacteria similar to toxins affecting recombinant bacteria of other strains treated with high concentrations. Bacterial biosensors immobilized in thin cellulose-agar films were used to detect various chemical agents such as phenol, hydrogen peroxide, copper, and cadmium. It was found that immobilized cells revealed high sensitivity to these agents, and inhibitory effect was observed at concentrations close to those detected by free bacteria (Microtox; Azur Environmental, USA). The final pattern of thin films did not hinder diffusion of the examined toxic agents. The stability of immobilized photobacteria was examined and showed that AWS culture media was optimal for both storage and application of the preparations. The preparation was stable when dissolved in AWS culture medium for 4 weeks at 4°C. However, incubation in 3% NaCl solution significantly decreased stability. Along with toxicology experiments, data regarding temperature- and pH-dependent relations with luminescence of both free and immobilized bacterial preparations as well as spectral and kinetic characteristics are presented as well. Unfortunately, it should be noted that some results cause doubts, e.g. a spectral pattern that corresponds to V. harveyi rather than P. phosphoreum. The same is true for temperature dependence, whereas pH-dependence is close to that of P. phosphoreum. At the same time, the results of model experiments can be considered promising to apply a multilayer system for bioassay of environmental pollutions [24].
An optical fiber sensor containing light-generating enzymes is considered as a multifunctional biosensor that can be used to analyze amounts of ATP (firefly luciferase) and NADH+ (bacterial oxidoreductase/luciferase). Enzymatic systems were immobilized in polyamide membranes. Limits of detection for ATP and NADH+ ranged within 0.1 pmol to 0.5 µmol. Polyvinyl alcohol was also used as a matrix along with the mentioned carrier. This biosensor was found to be effective (>40-fold) in analysis of luciferase substrates [25].
Apart from bioassays containing intact photobacteria, cell-free systems based on photobacteria can also be used in toxicological analysis. Data on using a combined test system including immobilized bioluminescent enzymatic complex associated with a detection system are presented in [26]. Therein, two types of luciferase (from firefly and bacteria) and a peroxidase (for chemiluminescent analysis) were used. Manufacturing issues on immobilization for obtaining membrane biosensors were discussed in several reports [14, 27, 28]. Practical application of biosensors in biotechnology was also elucidated.
A number of articles published at the beginning of the new millennium opened up a novel direction in manufacturing biosensors to be used in environmental toxicological analysis [18, 29]. Many of these were aimed at development of genetically engineered constructs containing sensor genes (SOS-system, anti-heat shock defense system, action of DNA- and membrane-damaging agents) specific to various toxins, and reporter genes derived from Photorhabdus luminescens. Moreover, great was given to technology of cell immobilization. Therein, Ca-alginate gel was used as a carrier. Different types of genetically engineered bioluminescent photobacteria were immobilized at the sidewalls of optic fiber strands. In such systems, each biosensor specifically reacted to a certain type of toxins. The developed test system was effective in detecting pure inorganic and organic toxins as well as admixtures of toxic substances in aqueous medium, soil, and other samples. Natural photobacteria were used together with genetically engineered counterparts, where toxic agents had an inhibitory impact on their luminescence. Application of such bacteria stems from a need to have a reference control delineating the cytotoxic action of the examined substances. Relevant computer software allows both discrete and continuous monitoring of toxicants by assessing specific and total toxicity in the samples [29]. It was found that thin films consisting of gel and cells were stable for 6 h at 26°C and able to detect mitomycin C at concentrations up to 25 µg/liter. Concentrations of cells up to (1-3)·107 contained in a carrier were effective for analytical procedures. Biosensor stability within a combined system sensitive to temperature impact as well as other chemical and physical parameters of the biodetector system were analyzed in detail [18] in response to substances causing a stressful reaction in the cells (heat shock, action of SOS-agents, impaired protein biosynthesis, peroxide and oxidative stress). The results of a 10-year-long examination evaluating technologies creating genetically engineered microbial strains as well as immobilization procedures, storage, and application of genetically engineered microbial strains in both laboratory and practical application of biosensors in toxicological biomonitoring were thoroughly described [15]. A fundamental difference for such systems was that they contained a combined complex made of optical fiber detector together with a biodetector. Results on improving technology of generating combined biosensors containing natural and recombinant microbial strains immobilized on optical fiber strands and used for cytotoxicity and genotoxicity assays are presented in [18, 23, 30].
However, it should be noted that cells immobilized in agar, agarose, and alginate are characterized by insufficiently high stability of luminescence due to the sensitivity of the cells to relatively high (up to 30-50°C) gelation temperature. It is worth mentioning that alginate gels with relatively low gelation temperature can be used as well. Nonetheless, the effect of temperature results in development of dark mutants of photobacteria having a temperature optimum for luminescence at 15-25°C (depending on species). The salt composition contained in the culture media plays an important role for marine photobacteria. Optimal concentrations for emission activity of cells are reached in 2-6% NaCl solution. Ca-alginate gels can be somewhat destabilized in such high salt concentration solutions due to the process when Ca2+ ions derived from the gel are replaced by Na+ ions. Moreover, complexation of Ca2+ and Sr2+ with phosphate and carbonate ions used as buffer solutions may occur. Therein, Sr-alginate matrices turn out to be more stable in high salt solutions. Cryoprotective properties of alginate gels play a positive role upon storing immobilized preparations at low temperature; nevertheless, for effective storage glycerol (10-30%) as an additional component must be used as well. Data on immobilizing Photobacterium phosphoreum in five different gel-forming materials are presented in [11]: agar, agarose (including low melting point agarose), polyacrylamide, and calcium (strontium) alginate. It was found that the technologies used for immobilization sharply differed in terms of their effect on stability of the photobacteria. Alginate gels used for immobilization of the cells were the most effective among the selected carriers by substantially increasing stability of cellular luminescence. Luminescence for the alginate–glycerol cell suspension upon incubation in 3% NaCl solution at 4°C lasted for up to 4 weeks (detectable level of bioluminescence for cell suspension – up to 6 weeks). Duration of luminescence for free cells (NaCl, glycerol suspension) in the same incubation conditions did not exceed 2 weeks. The cells immobilized in agar were characterized by a markedly lower stability compared to the free cells. However, the cells immobilized in agarose gel had luminescence lasting approximately 2 weeks, similarly to the free cells.
Alginate–glycerol suspension fully preserved baseline luminescence level upon storage at –80°C for 12 weeks. However, with storage at –20°C the alginate cell suspension lost emission activity similarly to that observed at 4°C. Advantages and drawbacks of the materials used for immobilization as well as technological procedures and storage conditions were thoroughly analyzed. Technological operations and conditions for conducting continuous monitoring were developed and optimized for Ca-alginate preparations as the carriers providing the most stable emission activity. After finishing a reactivation process, cells immobilized in a polyacrylamide gel lost their luminescence activity almost instantly. Stability of luminescence for cells immobilized in agar and agarose gel was much lower than for free cells (90% of activity was lost within 2 weeks). Altogether, this shows that photobacteria immobilized in these carriers were negatively impacted in cellular metabolism. Cell suspension in alginate was found to have the most stable luminescence. It is considered that the low stability of luminescence for the cells immobilized in agar and agarose is related to the sensitivity of the photobacteria to relatively high (up to 50°C) temperature used during gelation. This interpretation logically follows from the well-known temperature-dependent effects on luminescence of photobacteria that have been describe in detail. A temperature impact (30-36°C, 30-60 min) results in formation of the so-called TS, TSAS, and other dim and dark mutants. In addition, the temperature factor has especially strong impact on Photobacterium phosphoreum, most of which have the maximum bioluminescence activity at 15-18°C.
Data on luminescence kinetics during incubation of cells immobilized in alginate gel have important significance. Emission of luminescence was found to be unstable because of physical instability of Ca-alginate gels due to partial replacement of Ca2+ derived from the carrier for Na+, which seems logical and well justified. Replacement of Ca2+ by Sr2+ in the carrier enhanced the stability of preparations. Also, a quantitative discrepancy obtained in bioassays for several toxins using a standard assay (Microtox; Azur Enviromental, USA) was noted. Nevertheless, a good correlation with free bacteria was noted. It is assumed that the discrepancy between the two test systems is related to the differences in diffusion characteristics of toxic agents. An analytical procedure for discrete analysis (with Ca-, Sr-alginate gels) was tested for detecting salts of five different heavy metals – Pb(NO3)2, NaAsO2, NiCl2, CdCl2, HgCl2 – as well as SDS and pentachlorophenol (PCP). A long duration of assay (5 h) at room temperature should be noted as a negative feature of this approach. Significant differences were noted in kinetics of inhibition triggered by these toxins in free vs. immobilized cells, although the level of threshold sensitivity was very similar in the two preparations. Moreover, stimulation of emission was observed using a low concentration of PCP (<0.1 ppm) and CdCl2 (<10 ppm). Procedures designed for discrete biomonitoring were applied for continuous analysis of toxins. Conditions for continuous biomonitoring of toxins were optimized in terms of stability and efficacy (speed) of the analysis that involves the use of a system for reactor cooling. It was emphasized that interaction of toxins with cellular targets depends on optimizing hydrodynamic parameters: flow rate – 25 ml/h, lag-time after toxin administration – 37 s, duration of toxin administration – 1-2 min, stability of luminescence emission – >40 min. Pulse administration of toxicant to immobilized cells accounts for reversible kinetics of inhibition. When a toxicant in the flow is washed out, full or partial recovery of emission is observed, which allows using the same biosensor many times for biomonitoring. A “dose/time” effect shows up in the kinetic profile of emission quenching and its reversal. In this study, the main approach was to use P. phosphoreum immobilized in Sr-alginate gel to be effectively applied for conducting both discrete and continuous detection of the selected toxins. Computer software designed for this approach represents the basis for practical application of a real-time continuous monitoring in an aqueous environment [11]. Photobacterium phosphoreum immobilized in Sr-alginate gel were effectively used for continuous monitoring of toxicants [8, 11].
A study aimed at biomonitoring for toxicity of aqueous environment [31] was based on continuous cultivation of P. phosphoreum. The issue of potential development of dark mutants (after a 10-day incubation) that begin to dominate and cause rapid emission quenching was investigated. To avoid this problem, a system using a specially designed reactor (fluidized-bed reactor) containing cells immobilized in alginate gel was proposed. This system provided growth of immobilized cells accompanied by their continuous supply into the effluent. It was found that the dominance of dark mutants was significantly reduced inside the beads without observing any decrease in bioluminescence. Relatively high dilution rate prevented contamination with other microbes inside the reactor. The concentration and emission activity of the released cells in this mode were sufficient to monitor water toxicity for 4 weeks. It is crucial that photobacteria are able to multiply inside alginate beads and enter the aqueous compartment. Importantly, survival of cells inside the carrier during immobilization, storage, and application of the cells was analyzed. This was done by counting a number of colonies after destroying the matrix with Na6O18P6 (sodium hexametaphosphate). It was noted that colonies of different size and shape were developed within the entire volume of the alginate beads 24 h after the onset of the process. Electron microscopy showed that most of the cells were spread over the matrix, and only a few were attached to the surface of the carrier. In addition, it was shown that given the initial operations lasting for 40 h, the beads contained 80% of the wild type cells. Usually, during of cultivation dark mutants started to appear from day 7 and reached 100% of the cells by day 10 of cultivation. It was assumed that mutations occurred not in immobilized but in the released cells. Therefore, it can be considered that the matrix protects immobilized cells from mutagenesis.
Also, model experiments on toxicity of HgCl2 on immobilized and free cells were analyzed. It was found that immobilized cells were more sensitive to this toxin, although the differences were minor. The data are considered as promising for practical application of the system for monitoring water toxicity in real-time mode. Importantly, technological operations designed in the study significantly protected from dark mutagenesis. In this case, the analytical system can be used for more than 30 days. Also, means of protection from contamination with other microbes were proposed. The cells in the immobilized material were found to detect as little as 0.5 mg/liter of environmental HgCl2 [31]. A new technological solution for conducting complex biomonitoring was presented in [32]. In particular, a multichannel water-toxicity biomonitoring system was designed using different recombinant bacteria containing luxCDABE-operon. Each channel of the system was composed of a series of two mini-bioreactors necessary for stepwise continuous operation and contained a certain recombinant bacterial strain carrying the gene derived from photobacteria: DPD2440 (fabA:luxCDABE), DPD2794 (recA:luxCDBE), TV1061 (grpE:luxCDABE) that had varying sensitivity to membrane-, DNA-, and protein-damaging agents, respectively. The interaction of these agents induced luminescence. Along with this, bioluminescence of a constitutive strain was suppressed by toxins, thus characterizing cellular toxicity that was used as a reference strain. Model experiments were done using phenol and mitomycin C for detecting toxic agents. Procedures of biomonitoring were developed and optimized for further practical application to analyze toxic environmental chemicals. For practical analysis, water samples discharged from two power plants (nuclear and thermoelectric) were examined. Continuous biomonitoring was done by cultivating bacteria inside the bioreactors. The bacteria grew inside the first bioreactor and were then released into the second bioreactor supplied with toxin-containing water sample. Each channel showed specific bioluminescent response profiles corresponding to the toxic compounds present in the water samples. By comparing the bioluminescent response signals between the standard toxic chemical samples and discharged water samples, it was possible to estimate the equivalent toxicity of the field water. Such a multichannel continuous toxin biomonitoring system was proposed to consider as a novel strategy for protection of biological objects from environmental pollutants (an alternative system for rapid monitoring and quality control of aqueous environments). Special attention was given to production of recombinant bacterial strains as well as in channel technological operations that were developed and optimized in a model setting and used for analysis of cooling water discharged from nuclear and thermoelectric reactors. It was noted that the samples used for biomonitoring were stable for 1 week at 4°C, pH 7.0. Biotesting was performed in real-time mode using automated computer control. All examined reagents stimulated luminescence emission; however, they differed in terms of the maximum emission intensity and length of lag-phase. The analysis time was quite long – up to 500 min; herein, high concentration of toxins induced luminescence quenching from recombinant strains rather its induction. In other words, reaction specificity of recombinant strains was lacking at high toxin concentrations. Metabolism of all recombinant strains responded to high toxin concentration by luminescence quenching. In fact, it was demonstrated that a genotoxic effect was overlapped by the cytotoxic effect at certain toxin concentrations. No clear-cut quantitative data on inhibitory action on the external membrane targets were obtained for inducible recombinant strains. If such data were available, it might substantially adjust the magnitude of the bioluminescent response.
On the other hand, it should be noted that the proposed biomonitoring system is quite complex, laborious, and longstanding, although, basically, it gives positive results of toxin biotesting. Attention is drawn to the fact that the toxic effect on inhibiting luminescence is revealed in all recombinant strains. Thus, it is viewed that the proposed continuous biomonitoring system can be considered as a novel approach in identifying the nature and toxic action of agents that might be applied for detection of various industrial wastes and discharges. Moreover, it was noted that prior to the assay, samples of interest must be pretreated to remove potentially contaminating bacteria followed by filtration. Application of different recombinant strains for specific toxin biomonitoring can be considered as the major result of this study [32].
An integral water biomonitoring system (aquatic toxicology) using recombinant bacteria as the test objects was described in [33]. The system included four channels with two mini-bioreactors. Recombinant E. coli EBHJ2, DP1, DK1, and DPD279 strains were used as the test objects. These strains served three specific tasks to protect the cells from oxygen shock, namely, defense from O.–, H2O2, and DNA-damaging agents. A biodetection system based on these strains is applicable both for discrete and continuous monitoring of toxins. Mitomycin C and H2O2 injected into the second bioreactor were used as inducers. The first bioreactor was used for propagation of the bacterial strains, followed by their release into the second bioreactor. It was found that bioluminescent response was induced in a dose-dependent manner. Increase in luminescence was observed only within a small range of concentrations for the examined inducers. Both the maximum rate of response and speed of ascending luminescence were specific for each toxicant inducer that was reversed to an inhibitory effect at their high concentrations. Technological operations for conducting continuous biotesting as well as kinetic parameters of luminescence induction and quenching were discussed in detail including different types of instrumental implementation for continuous monitoring. Miniaturization of the detection system was considered as a special advantage of the developed system that comprised 1-2 ml of the reactor volume. It was assumed that a four-channel mini-monitoring system containing different recombinant strains might be a promising asset for specific analysis of certain classes of environmental toxins.
IMMOBILIZATION OF PHOTOBACTERIA IN POLYVINYL ALCOHOL
CRYOGEL
Polyvinyl alcohol (PVA) cryogels along with natural gel-forming agents have been effectively used to immobilize microorganisms [19, 34, 35]. In contrast to other carriers, polyvinyl alcohol gels have several structural and physicochemical properties that are optimal for immobilizing luminescent bacteria.
Heterophase properties of the matrix structure that develops during a freeze–thaw procedure is optimal for cell encapsulation and stabilization. Macropores (0.1-1 µm) present in the gel eliminate diffusion limitations for “elastic” structure of the formed gel to substrates, toxins, and other substances of various chemical nature. Gel thermostability has special significance. The physical characteristics for the formed PVA gels are stable over a wide range of positive temperatures up to 70-80°C, which allows a bioreactor to operate in different temperature modes. Physicochemical parameters of the matrix negligibly depend on chemical composition of the media used for gel formation, in particular, its salt composition that has crucial importance for marine halophilic bacteria. In addition, PVA gels are advantageous in terms of their high stability to biological damage caused by bacteria and fungi. It is essential that the PVA cryogel is nontoxic with respect to the embedded microorganisms.
Altogether, the abovementioned characteristics suggest good perspectives for using PVA in technology of bacterial immobilization. However, it should be noted that a PVA carrier used for immobilization of luminescent bacteria or recombinant strains containing the cloned genes of bacterial bioluminescent system have been published in a limited number of papers.
The results of immobilizing Photobacterium phosphoreum in PVA, polyurethane, polyacrylamide, Ca-alginate, and Ca-carboxymethylcellulose were analyzed in several studies [21, 36, 37]. The best data on intensity and emission duration were obtained using Ca-alginate gel. It was noted that the preparations immobilized in PVA cryogel did not luminesce immediately after finishing the immobilization procedure. Light emission was induced after keeping the preparations for a certain time at room temperature.
A technology for immobilizing Vibrio fischeri and genetically engineered Pseudomonas putida strain containing lux-operon derived from Photorhabdus luminescens inside PVA gel was accomplished for biodetection of phenol toxins present in industrial waste.
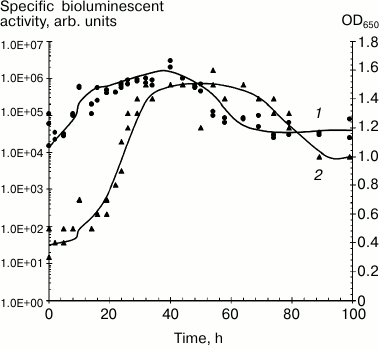
It can be assumed that intensity and stability of luminescence from immobilized preparations is determined by the type of carrier, immobilization procedure, and luminescent characteristics of the selected photobacterial strain. Psychrophilic P. phosphoreum strains have the most intense and long-lasting luminescent activity in submerged culture (Fig. 1) [20, 25].
Fig. 1. Dynamics of growth and bioluminescent activity of Photobacterium phosphoreum (strain 331 KM MGU) isolated from the White Sea during submerged cultivation, 20°C: 1) bioluminescent activity; 2) optical density.
Photon yield for the isolated cells is determined by both media composition and conditions of incubation. Apart from nutritional components, sodium chloride concentration, temperature, and pH together influence luminescent activity of the cells have significant importance for intensity and duration of bioluminescence of quiescent cells. The temperature factor has the most critical impact. Incubation at temperatures exceeding the optimal range determined for each bacterial strain is accompanied by a substantial decrease or complete loss of the biosensor luminescent activity. The isolated P. phosphoreum strain 331 KM MGU retains bioluminescent activity for 2-4 weeks depending on the incubation temperature [9, 18]. In several reports, it was noted that immobilization of photobacteria in various carriers can enhance stability of luminescence [25, 38, 39].
Data obtained in our studies provide technological approaches and scientific basis for creating PVA-containing photobiosensors. Scientific approaches underlying development of highly active biosensors with prolonged emission were based on the following premises.
1) Intensity and duration of luminescence both for growing and quiescent cell culture were primarily determined by the specific natural properties of photobacterial species and strain.
2) Luminescent reaction was controlled by combining the following elements and processes: (i) metabolic cellular potential that provides a pool of reduced equivalents; (ii) efficacy of electron transfer onto luciferase depending on activity of dehydrogenase–reductase enzymatic complexes and degree of dark leakages; (iii) physicochemical parameters (oxygen level, pH, temperature, salt composition), and presence of activators and inhibitors.
3) The pool of reduced flavin is a limitation in emission activity in the cells.
A TECHNOLOGY OF IMMOBILIZING PHOTOBACTERIA IN PVA CRYOGEL
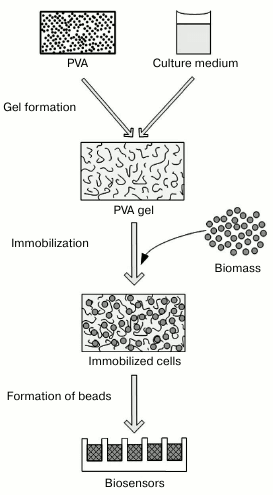
The technological procedures used to immobilize photobacteria are presented in Fig. 2. Introduction of the additional components acting as substrates and protectors into a gel-forming solution represents key differences of the proposed protocol distinguishing it from standard procedures [34, 40], when PVA is used as a synthetic matrix, and gel formation occurs in simple salt solutions.
Fig. 2. Scheme of procedure for immobilizing photobacteria in polyvinyl alcohol-containing cryogel.
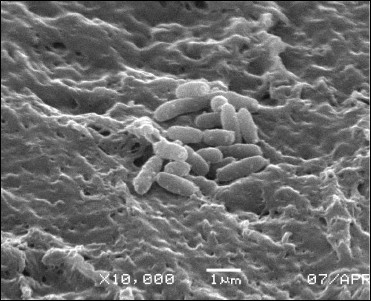
Using electron microscopy, characteristics of the binary carrier–cell system were evaluated, such as gel structure formed after a freeze–thaw procedure, appearance of cells tightly bound to the surface and within the volume of the carrier (Fig. 3).
It was found that the cells were mainly spread in pores of the PVA carrier. Long-term incubation at positive temperatures resulted in intensive development of bacterial colonies containing cells tightly bound on the surface of the carrier as well as formation of a biofilm.
Fig. 3. Electron microscopy imaging of cells on PVA carrier in 0.1 M phosphate buffer, pH 7.6.
Beads containing immobilized photobacteria were stabilized on storage at negative temperatures using preparations containing complete culture media. It was found that 100% bioluminescent activity in the beads at –80°C was preserved for 2 years. However, at –20°C residual bead activity reached only 70% of the basal level.
FACTORS STABILIZING BIOLUMINESCENCE UPON IMMOBILIZATION OF
PHOTOBACTERIA IN PVA CRYOGEL
Using immobilized photobacteria as biosensors, there are special requirements for duration and stability of luminescence of the preparations.
We found that luminescence stability for immobilized preparations is determined by specific natural characteristics of the luminescent cycle in the bacterial strain [41-46]. The media used for gel formation, storage temperature regime, and pH of the incubation media are the most critical factors affecting emission activity of the immobilized cells.
Data on the stability of luminescence for different bacterial strains upon storage of immobilized in PVA gel cells are presented in the table. It was demonstrated that the psychrophilic P. phosphoreum strain 331 KM MGU isolated from the White Sea had the most intense and long-lasting luminescent emission compared to the other bacterial strains maintained under optimal cultivation conditions.
Integral photon yield and duration of emission for P.
phosphoreum, V. harveyi, and V. fischeri bacteria
immobilized in PVA cryogel
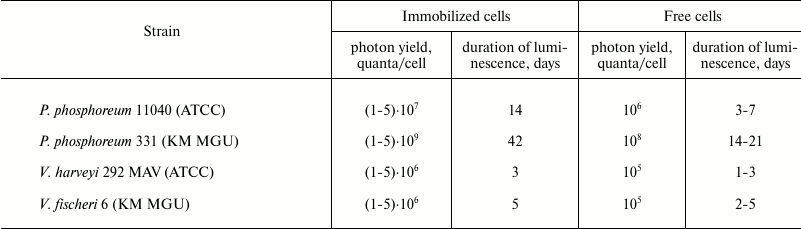
Note: Incubation was performed in 0.1 M Na-phosphate buffer with 2%
NaCl, pH 7.8, at 4°C.
The data presented in the table characterize total integral photon yield and duration of luminescence for free and immobilized photobacteria containing comparable number of cells in the preparation and buffer solution ((1-2)·107 cells/bead). It was found that the immobilized cells had more prolonged luminescence compared to free cells, as shown for all examined bacterial strains. The intensity and duration of luminescence for immobilized preparations were proportional to the differences observed for the free cells of the bacterial strains: immobilized cells of P. phosphoreum strain 331 had the longest and strongest intensity of luminescence, whereas in V. harveyi these parameters were at the lowest level. An impact of media composition used for gel formation on stability and specific activity of the cells was predicted upon low-temperature immobilization. Assuming that the media used for gel formation should influence the stability and metabolic activity of the cells inside the carrier primarily at the cryogenic gelation stage, emission activity and kinetics of luminescence decay after a freeze–thaw procedure were analyzed using three types of gel-forming media: (1) a media for submerged cultivation (CM); (2) semi-synthetic media (CM without peptone); (3) 3% NaCl solution. The analysis of specific activity of beads after their thawing to 4°C and stabilization of the cellular luminescence showed that the cultivation medium was the most effective for gel formation (not shown in the table). In this medium, the bacteria preserved virtually 100% luminescent activity after cryogenic immobilization and thawing. Decline in luminescence of the beads did not exceed more than 1.5 orders of magnitude during a 200-h incubation, and detectable luminescence level (10–6 of the baseline) was detected for more than 1 month. Removing peptone from the media used for gel formation resulted in decreasing initial emission activity by 1.0-1.5 orders of magnitude. A severe decline of luminescence (by 103-104-fold) was observed upon immobilization in gels formed in salt solution (3% NaCl solution). Thus, the composition of the media used for gel formation significantly affects intensity and duration of luminescence emission by immobilized preparations. Carrier concentration (5, 7, and 10%) had virtually no effect on the kinetics and emission parameters of the beads. A kinetic profile for the beads formed using cultivation media depended insignificantly on the composition of the incubation media. The magnitude of initial luminescence emission and rate of decay were different upon incubating the beads at 4°C in three different media: cultivation medium, 0.1 M Na-phosphate buffer containing 2% NaCl (pH 7.6), and 3% NaCl solution. It was crucial that the rate of luminescence decay for free cells was markedly higher than for the bacteria immobilized in all examined media. Upon incubation in 3% NaCl solution, luminescence decay occurred much faster than in buffered alkaline media or cultivation media having pH > 7.5 that was caused by a faster pH shift towards the acidic range. By transferring beads with lost luminescence from the salt-containing media into buffered media, it fully restored luminescence. Apparently, the rate of reduction of flavin substrate by NAD-dependent dehydrogenases represented the main limiting stage in this process. By analyzing the ATP pool in immobilized cells during incubation under shortage of energy substrates or oxygen, it was demonstrated that the amount of ATP was stable (~10–18 mol/cell) during the entire (>200 h) luminescent cycle, i.e. luminescence decay did not result from energy deficiency in the immobilized cells.
Thus, it was found that a pH shift in the media used for incubation of the beads represented one of the most crucial parameters due to acidic metabolites generated by the photobacteria. The fact that the transfer of photobacteria with decayed luminescence from the salt-containing media into buffered media having pH 8.5 resulted in full restoration of emission activity confirms this assumption [43, 46]. Special attention should be given to several crucial issues. PVA-immobilized P. phosphoreum had low stability, and restoration of luminescence required a long-term reactivation in special media at room temperature [21, 36]. Luminescence was detected for one week. Low luminescence stability of PVA-immobilized photobacteria might be due to the low concentration (3%) of PVA as well as temperature (–10°C) of gel formation. In another study, a procedure for immobilizing V. fischeri and genetically engineered Ps. putida containing lux-operon derived from P. luminescens was presented [40]. For this, a 14% PVA solution was used, and the cells were frozen to –20°C. Glycerol (20-30%) was used in both cases as an auxiliary cryoprotector added to the cryogel solution. It is noteworthy that the preparations had a high noise level, indicating low specific activity of the cells and rapid decay of their luminescence. Perhaps, low activity and unstable luminescence are related to the fact that gel formation occurred in a pure NaCl solution containing no substrates and protectors. We attempted to optimize technological operations to immobilize P. phosphoreum in PVA gel and demonstrated virtually 100% emission activity being preserved without adding any auxiliary cryoprotector and performing a procedure to activate luminescence [41, 42].
High level of endogenous substrates preserved during a freeze–thaw procedure is responsible for the high level of specific activity of immobilized cells upon their incubation in deficient media or pure salt-containing solution. Immobilization in cryogel provides a markedly prolonged emission activity of the bacteria as well. Elevated duration of the matrix-stabilized luminescence was demonstrated using various types of carriers [3, 23, 28]. However, the nature of this phenomenon has not been clarified in any of the published works. It may be assumed that increase in the total photon yield by immobilized bacteria is provided due to stabilization of the structural organization of the membrane-bound enzymatic complexes belonging to the luminescent electron-transporting system in a way similar to other cell types and enzymatic systems [19]. The data show that the specific natural emission characteristics of the particular bacterial strain as well as the media used for gel formation, freeze–thaw procedure, storage and incubation temperature, initial pH value, and buffer properties of the incubation solution represent the main components affecting intensity and stability of luminescence for immobilized photobacteria. According to the conducted studies, we concluded that the selection of psychrophilic P. phosphoreum strain 331 KM MGU for immobilization had huge importance for producing preparations having intense and long-lasting emission, with the composition of media used for gel formation playing a significant role in it. Also, it was demonstrated that protective action on emission activity by Ca-alginate-immobilized P. phosphoreum was exhibited by glucose, sorbitol, and DL-threonine along with glycerol [37].
Diffusion of the cells from the cryogel matrix into solution was virtually absent as evidenced by the low (<1%) initial activity (perhaps, related to cells being washed out from the superficial layers) that completely disappeared within 24 h upon further incubation. The temperature regime used for storage and incubation of the preparations played the most critical role for emission activity and its stability in photobacteria immobilized in the carrier. PVA was shown to effectively stabilize luminescence of the cells upon storage at negative temperature without adding auxiliary cryoprotectors. Nonetheless, the best storage temperature was –80°C. Similar results were obtained using an alginate suspension of photobacteria supplemented with glycerol: bioluminescent activity was preserved unaltered for one year at –80°C, whereas storage at –20°C was observed to significantly accelerate decay rate so that luminescence was fully lost within 4 weeks [11]. Data on incubating beads in various media have significant importance for practical application of immobilized preparations. Because the rate of luminescence decay poorly depends on composition of the bead incubation media, a biosensor may be effectively used in simple salt-containing solutions that have special importance during analysis of heavy metals.
BIOMONITORING OF TOXINS USING IMMOBILIZED PHOTOBACTERIAL
PREPARATIONS
The preparations with bacteria immobilized in PVA cryogel were used as biosensors for discrete and continuous biomonitoring of various classes of ecotoxicants.
The optimized conditions for production and storage of immobilized bacteria were the basis for the usage of luminescent beads as biosensors of toxins. To eliminate diffusion limitations for toxins, the size of the beads was minimized (1 × 4-mm disks). Beads containing immobilized photobacteria are depicted in Fig. 4. The size of the beads varied: height – 1-2 mm, diameter – up to 5 mm, weight – 50-100 mg.
Fig. 4. Luminescent beads with P. phosphoreum strain 331 KM MGU immobilized in PVA-containing cryogel.
The kinetics of luminescence inhibition caused by chlorophenol was similar for both immobilized and free cells triggered by heavy metals and chlorophenols. The expected incubation time in assay using free and immobilized cells as biosensors presumed incubation with toxins was 5, 15, 30 min [10, 21]. Optimal physical and geometric parameters of biosensors with minimal limitations for toxin diffusion were determined. It was found that the threshold sensitivity of free and immobilized cells to the selected emission quenchers (heavy metals, chlorophenols, aliphatic and aromatic carbohydrates, pesticides, and herbicides) was almost the same.
Continuous bioluminescent monitoring of toxicants was successfully done using biosensors containing PVA-immobilized psychrophilic P. phosphoreum strains having long lasting (>100 h in submerged culture) and intensive (105 quanta/s per cell) luminescence [43, 46]. All measurements were made in a specially designed chamber with flow cell. The device contained a light-detector segment, a media flow chamber (2% NaCl solution of marine water, peristaltic pump) and sample injector (a toxicant dissolved in a special solution, a dispenser). The temperature of the running media was maintained using a cooling thermostat. Flow conditions were optimized by analyzing the length of time for luminescence quenching in response to the minimum toxicant concentrations – the flow rate was maintained below the rate of luminescence quenching; flow rate was 0.5 ml/min, lag-time for a 100-µl sample – 2-5 s, volume of reactor – 1.5 ml, spherical beads.
It was found that using a flow mode, emission activity remained stable during 24 h when the temperature of the solution was kept at ≤20°C. The kinetic profile for luminescence quenching depended on the concentration of toxicant. The kinetics of emission reversal corresponded to the reversibility of the inhibitory effect related to the inhibitor removed by flow of the medium.
Thus, technological operations for immobilizing luminescent bacteria in a polyvinyl alcohol-containing cryogel were designed and optimized. The most prominent physicochemical and biochemical parameters describing stabilization of luminescence in the preparation were scientifically justified. The biosensors were effectively tested by analyzing toxic substances both during discrete and continuous biomonitoring modes. A novel type of biosensors based on using photobacteria immobilized in PVA cryogel was found to be perspective for practical application for ecological tasks of environmental biomonitoring.
REFERENCES
1.Deryabin, D. G. (2009) Bacterial
Chemiluminescence: Fundamental and Applied Aspects [in Russian],
Nauka, Moscow.
2.Danilov, V. S., and Ismailov, A. D. (1989)
Bacterial luciferase as a biosensor of biologically active compounds,
in Applied Biosensors (Wise, D., ed.) Boston, pp. 39-78.
3.Mitchell, R. J., and Gu, M. B. (2006)
Characterization and optimization of two method in the immobilization
of 12 bioluminescent strains, Biosens. Bioelectron., 22,
192-199.
4.Parvez, S., Venkataraman, C., and Mukherji, S.
(2006) A review on advantages of implementing luminescence inhibition
test (Vibrio fischeri) for acute toxicity prediction of
chemicals, Environ. Int., 32, 265-268.
5.Dizer, H., Wittekindt, E., Fischer, B., and Hansen,
P. D. (2002) The cytotoxic and genotoxic potential of surface water and
wastewater effluents as determined by bioluminescence, umu-assays and
selected biomarkers, Chemosphere, 46, 225-233.
6.Yin, J., Li, X., Zhou, C., and Zhang, Y.
(2005) Luminescent bacterial sensors made from immobilized films of
Photobacterium phosphoreum, Chem. Res. Chinese Univ.,
21, 44-47.
7.Yoo, S. K., Lee, J. H., Yun, S. S., Gu, M. B., and
Lee, J. H. (2007) Fabrication of a bio-MEMS based cell-chip for
toxicity monitoring, Biosens. Bioelectron., 22,
1586-1592.
8.Lee, J. H., Mitchell, R. J., Kim, B. C., Cullen, D.
C., and Gu, M. B. (2005) A cell array biosensor for
environmental toxicity analysis, Biosens. Bioelectron.,
21, 500-507.
9.Bulich, A. A. (1979) Use of luminescent bacteria
for determining toxicity in aquatic environments, in Aquatic
Toxicology. American Society for Testing and Materials (Markings,
L. L., and Kimerleeds, R. A., eds.) Philadelphia, p. 8.
10.Sun, T. S., and Stahr, H. M. (1993) Evaluation
and application of a bioluminescent bacterial genotoxicity test, J.
AOAC Int., 76, 893-898.
11.Verschaeve, L., Van Gompel, J., Thilemans, L.,
Regniers, L., Vanparys, P., and van der Lelie, D. (1999) VITOTOX
bacterial genotoxicity and toxicity test for the rapid screening of
chemicals, Environ. Mol. Mutagen., 33, 240-248.
12.Chun, U.-H., Simonov, N., Chen, Y., and Britzb,
M. L. (1996) Continuous pollution monitoring using Photobacterium
phosphoreum, Resour. Conserv. Recycl., 18, 25-40.
13.Park, K. S., Baumstark-Khan, Ch., Rettberg, P.,
Horneck, G., Rabbow, E., and Gu, M. B. (2005) Immobilization as a
technical possibility for long-term storage of bacterial biosensors,
Radiat. Environ. Biophys., 44, 69-71.
14.Heitzer, A., Malachowsky, K., Thonnard, J. E.,
Bienkowski, P. R., White, D. C., and Sayler, G. S. (1994) Optical
biosensor for environmental on-line monitoring of naphthalene and
salicylate bioavailability with an immobilized bioluminescent catabolic
reporter bacterium, Appl. Environ. Microbiol., 60,
1487-1494.
15.Van Dyk, T. K., Majarian, W. R., Konstantinov, K.
B., Young, R. M., Dhurjati, P. S., and Larossa, R. A. (1994) Rapid and
sensitive pollutant detection by induction of heat shock
gene-bioluminescence gene fusions, Appl. Environ. Microbiol.,
60, 1414-1420.
16.Belkin, Sh. (2003) Microbial whole-cell sensing
systems of environmental pollutants, Curr. Opin. Microbiol.,
6, 206-212.
17.Okamoto, K., Ishiura, M., Torii, T., and Aoki, S.
(2007) A compact multi-channel apparatus for automated real-time
monitoring of bioluminescence, J. Biochem. Biophys. Methods,
70, 535-538.
18.Polyak, B., Bassis, E., Novodvorets, A., Belkin,
Sh., and Marks, R. S. (2001) Bioluminescent whole cell optical fiber
sensor to genotoxicants: system optimization, Sens. Actuators B
Chem., 74, 18-26.
19.Sinitsin, A. P., Raynina, E. I., Lozinskiy, V.
I., and Spasov, S. D. (1994) Immobilized Microbial Cells [in
Russian], Moscow State University Publishers, Moscow.
20.Brodelius, P., and Vandamme, E. J. (1987)
Immobilized cells systems, in Biotechnology. A Comprehensive
Treatise (Rehm, H. J., and Reed, G., eds.) Vol. 7a, VCH Verlag,
Weinheim, pp. 405-464.
21.Makiguchi, N., Arita, M., and Asai, Y. (1980)
Immobilization of a luminous bacterium and light intensity of luminous
material, J. Ferment. Technol., 58, 17-21.
22.Arnold, M. A. (1990) Fiber-optic biosensors,
J. Biotechnol., 15, 219-228.
23.Kohler, S., Belkin, S., and Schmid, R. D. (2000)
Reporter gene bioassays in environmental analysis, Fresenius J.
Anal. Chem., 366, 769-779.
24.Yin, J., Li, X., Zhou, C., and Zhang, Y.
(2005) Luminescent bacterial sensors made from immobilized films of
Photobacterium phosphoreum, Chem. Res. Chinese Univ.,
21, 44-47.
25.Blume, L. J., Gautier, S. M., and Coulet, P. R.
(1993) Design of bioluminescence-based fiber optic sensors for
flow-injection analysis, J. Biotechnol., 31, 357-368.
26.Blume, L. J., Gautier, S. M., and Coulet, P. R.
(1989) Design of luminescence photobiosensors, J. Biolum.
Chemilum., 4, 543-550.
27.Cho, J., Park, K., Ihm, H., Park, J., Kim, S.,
Kang, I., Lee, K., Jahng, D., Lee, K., and Kim, S. (2004) A novel
continuous toxicity test system using a luminously modified freshwater
bacterium, Biosens. Bioelectron., 20, 338-344.
28.Lee, B., Lee, J., Shin, D., and Kim, E. (2006)
Statistical optimization of bioluminescence Photobacterium
phosphoreum KCTC 2852, Environ. Int., 32,
265-268.
29.Marks, R., Polyak, B., Novodvorets, A., and
Belkin, Sh. (2001) Bacterial biosensors for environmental analysis,
G. I. T. Laboratory J., 3, 122-123.
30.Premkumar, J. R., Ovadia, L., Marks, R. S.,
Polyak, B., Rosen, R., and Belkin, Sh. (2001) Antibody-based
immobilization of bioluminescent bacterial sensor cells,
Talanta, 55, 1029-1038.
31.Kim, S. K., Lee, B. S., Lee, J. G., Seo, H. J.,
and Kim, E. K. (2003) Continuous water toxicity monitoring using
immobilized Photobacterium phosphoreum, Biotechnol. Bioproc.
Eng., 8, 147-150.
32.Kim, B. Ch., and Gu, M. B. (2005) A multi-channel
continuous water toxicity monitoring system: its evaluation and
application to water discharged from a power plant, Environ. Monit.
Assess., 109, 123-133.
33.Lee, J. H., and Gu, M. B. (2005) An integrated
mini biosensor system for continuous water toxicity monitoring,
Biosens. Bioelectron., 20, 1744-1749.
34.Lozinsky, V. I., and Plieva, F. M. (1998)
Poly(vinyl alcohol) cryogels employed as matrices for cell
immobilization. Overview of recent research and developments, Enzyme
Microb. Technol., 23, 227-242.
35.Varfolomeev, S. D., Rainina, E. I., Lozinsky, V.
I., Kalyuzhnyi, S. B., Sinitsyn, A. P., Makhlis, T. A., Bachurina, G.
P., Bokova, I. G., Sklyankina, O. A., and Agafonov, E. V. (1989)
Application of poly(vinyl alcohol) cryogel for immobilization of
mesophilic and thermophilic microorganisms, in Physiology of
Immobilized Cells (de Bont, J. A. M, Visser, J., Mattiasson,
B., and Tramper, J., eds.) Elsevier, Wageningen, pp. 325-330.
36.Makiguchi, N., Arita, M., and Asai, Y. (1980)
Optimum cultural conditions for strong light production by
Photobacterium phosphoreum, J. Gen. Appl. Microbiol.,
26, 75-83.
37.Makiguchi, N., Arita, M., and Asai, Y.
(1980) Optimal conditions for frozen storage of immobilized
luminous bacteria, J. Ferment. Technol., 58, 333-337.
38.Lozinskiy, V. N. (1998) A cryotropic gelation of
polyvinyl alcohol solutions, Uspekhi Khim., 67,
641-655.
39.Bechor, O., Smulski, D. R., Van Dyk, T. K.,
LaRossa, R. A., and Belkin, S. (2002) Recombinant microorganisms as
environmental biosensors: pollutants detection by Escherichia
coli bearing fabA::lux fusions, J. Biotechnol.,
94, 125-132.
40.Philp, J. C., Balmand, S., Hajto, E., Bailey, M.
J., Wiles, S., Whiteley, A. S., Lilley, A. K., Hajto, J., and Dunbar,
S. A. (2003) Whole cell immobilization biosensors for toxicity
assessment of wastewater treatment plant treating phenolics-containing
waste, Anal. Chim. Acta, 487, 61-74.
41.Efremenko, E. N., Sen’ko, O. V.,
Kuts, V. V., Alenina, K. A., Kholstov, A. V., and Ismailov, A. D.
(2010) A luminescent biocatalyst for detection of toxicants [in
Russian], Patent No. 2394910.
42.Efremenko, E. N., Aleskerova, L. E., Alenina, K.
A., and Ismailov, A. D. (2014) Toxicological biosensors containing
luminescent Photobacterium phosphoreum bacteria immobilized in
polyvinyl alcohol-based cryogel, Prikl. Biokhim. Mikrobiol.,
5, 490-496.
43.Alenina, K. A., Aleskerova, L. E., Kascheyeva, P.
B., and Ismailov, A. D. (2012) The poly(vinyl alcohol)-immobilized
photobacteria for toxicology monitoring, Engineering, 4,
118-119.
44.Ismailov, A. D., Kutz, V. V., and Yefremenko, E.
N. (2010) Factors affecting the stability of a light emission at
PVA-immobilized cells of Photobacterium phosphoreum, J.
Luminesc., 25, 166-167.
45.Aleskerova, L. E., Alenina, K. A., Efremenko, E.
N., Mazhul’, M. M., Piskunkova, N. F., and Ismailov, A. D. (2014)
ATP pool and bioluminescence activity in psychrophilic
Photobacterium phosphoreum bacteria, Mikrobiologiya,
83, 315-321.
46.Kuts, V. V., Alenina, K. A., Sen’ko, O. V.,
Efremenko, E. N., and Ismailov, A. D. (2011) Bioluminescent analysis of
toxicants (an ecological luminometry), Voda Khim. Ekol.,
10, 47-53.