Mitochondria-Targeted Plastoquinone Antioxidant SkQR1 Has Positive Effect on Memory of Rats
E. V. Stelmashook1,2, A. V. Stavrovskaya1, N. G. Yamshchikova1, A. S. Ol’shanskii1, N. A. Kapay1,2, O. V. Popova1,2, L. G. Khaspekov1, V. G. Skrebitsky1, and N. K. Isaev1,2,3*
1Research Center of Neurology, Russian Academy of Medical Sciences, Volokolamskoe Shosse 80, 125367 Moscow, Russia; E-mail: estelmash@mail.ru2Lomonosov Moscow State University, Institute of Mitoengineering, 119991 Moscow, Russia; E-mail: info@skq-project.ru
3Lomonosov Moscow State University, Belozersky Institute of Physico-Chemical Biology, 119991 Moscow, Russia; fax: +7 (495) 939-3161; E-mail: isaev@genebee.msu.ru
* To whom correspondence should be addressed.
Received November 25, 2014
A single intraperitoneal injection to rats of the mitochondria-targeted plastoquinone antioxidant SkQR1 at dose 1 µmol/kg significantly improved reproduction by the rats of the passive avoidance conditional reflex. In vitro experiments on hippocampal slices showed that a single intraperitoneal injection of SkQR1 24 h before the preparation of the slice significantly increases the synaptic transmission efficiency of the pyramidal neurons of the CA1 field. The findings indicate that SkQR1 has a positive effect on memory processes.
KEY WORDS: memory, long-term potentiation, hippocampus, mitochondria-targeted antioxidants, SkQR1DOI: 10.1134/S0006297915050119
Abbreviations: Abeta, β-amyloid peptide 1-42; AD, Alzheimer’s disease; HFS, high-frequency stimulation; PACR, passive avoidance conditioned reflex; PS, population spike; ROS, reactive oxygen species; SkQR1, 10-(6′-plastoquinonyl)decylrhodamine 19.
Memory is the major component of personality; it connects a
human’s past with present and future and is a complex
physiological process that includes the retention, reproduction, and
accumulation of information. There is no doubt that abilities for
training depend on the memory state. Memory can weaken with age and as
a consequence of vascular diseases of the brain, its trauma-caused
damages, and also because of development of such a fatal age-related
neurodegenerative disease as Alzheimer’s disease (AD). AD is
characterized by progressing disorders in memory leading to personality
destruction and finally to death. Memory impairments in AD are thought
to be mainly associated with the β-amyloid peptide (Abeta) and the
hyperphosphorylated τ-protein involved in synaptic degeneration and
in destruction of mitochondria [1]. These disorders
can be prevented by mitochondria-targeted antioxidants [2-5]. However, a pharmacological
stimulation of memory processes is still rather an open problem, and
just this is the subject of the present work. We show that the
mitochondria-targeted antioxidant SkQR1 strengthens the long-term
potentiation of the population spike of the CA1 field pyramid neurons,
i.e. increases synaptic transmission efficiency in hippocampal slices
that is believed to underlie the training and memory processes [6] and also improves the ability of animals to
memorize a negative stimulation in the test of passive avoidance
conditioned reflex (PACR). The findings demonstrate that SkQR1 has
pronounced mnemotropic properties.
MATERIALS AND METHODS
The work was performed on male Wistar rats. The animals were kept in a vivarium under conditions of food and water ad libitum and natural changes of day illumination and darkness. Experiments were performed according to international rules of Guide for the Care and Use of Laboratory Animals and appreciated by the Ethics Committee of the Neurology Research Center, Russian Academy of Medical Sciences.
Rats at the age of 1 month and with body weight of 80-110 g were injected once intraperitoneally with SkQR1 at the dose of 1 µmol/kg. Slices of the hippocampus prepared 24 or 48 h after the SkQR1 injection were placed into a chamber to record the bioelectric activity and perfused at 29-30°C with modified Ringer solution ((mM): NaCl, 124; KCl, 3; CaCl2, 2.5; MgSO4, 2.5; Na2HPO4, 1.25; NaHCO3, 26; D-glucose, 10) continuously saturated with carbogen (95% O2 + 5% CO2). The recording started 1.5-2 h after the slice was prepared. The population response caused by stimulation of the radial layer by single rectangular impulses (0.1 ms, 1/15 s) was recorded with a glass microelectrode filled with 1.5 M NaCl (resistance of 2-5 mΩ) in the field CA1 pyramid layer. The stimulus strength was chosen to provide the peak component amplitude of the response reflecting the total population spike (PS) response of the pyramidal neurons to be about half of its maximum value. The PS was potentiated by high-frequency stimulation (HFS, 100 Hz, 1 s) of the entrance through the same electrodes and at the same strength of the stimulus. Each slice was subjected to HFS only once. The reactivity of the pyramidal neurons was evaluated by changes in the PS amplitude with respect to its average value determined by the 15-min recording of the background activity before the HFS.
In the in vivo experiments, rats at the age of 3-4 months were used. The experimental group animals were injected intraperitoneally with SkQR1 in saline at the dose of 1 µmol/kg, and 24 h later the animals were investigated using the PACR test. The control group animals were injected with saline. An experimental set Panlab (Spain) for investigation of the passive defensive behavior was a rectangular chamber with a metallic floor divided by a vertical partition into two compartments (dark and lightly illuminated ones) with an aperture near the floor between the compartments. To adapt the animals to the set, they were placed into the illuminated compartment and the time was determined of latent periods at the passages into the dark compartment without a pain-causing stimulus. After the rats were placed repeatedly into the illuminated compartment, their passage into the dark compartment was accompanied by a pain-causing stimulus with a constant current blow (2 mA, 3 s). At this time, the illuminated compartment was closed, and immediately after the current was switched off the rats were removed from the set and restored into their domestic cage. The better an animal remembered the pain obtained in the dark compartment, the longer was the time interval before its entering the “dangerous” compartment.
The behavioral reactions were recorded and the experiments were analyzed using an ANY-maze Video Tracking System (Stoelting, USA) for video registration.
Statistical analysis was performed using Student’s t-test or ANOVA with a Bonferroni post-test. Differences were considered significant at the level of p < 0.05. The results are presented as mean values ± SEM.
RESULTS
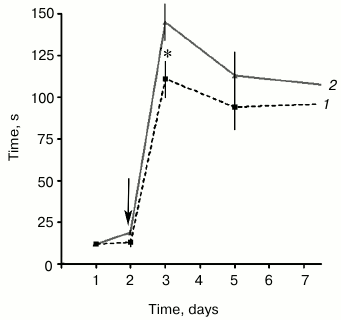
Influence of SkQR1 on PACR execution. The animals that received a single injection of SkQR1 (1 µmol/kg) 24 h before the negative stimulus demonstrated significantly more prolonged latent periods of the passage into the dark compartment than the control animals on the first day after the pain-causing stimulus. Later, by the 3rd and 7th days, the difference between the experimental and control groups was less pronounced (Fig. 1).
Fig. 1. Effect of a single injection of SkQR1 24 h before painful stimulus on the PACR test reproduction: 1) control animals; 2) animals injected with SkQR1 (1 µmol/kg). Ordinate is time of the animal’s passage into the dark compartment of the chamber. The arrow indicates the moment of painful stimulation; * p < 0.05, n = 26 (number of animals in each group).
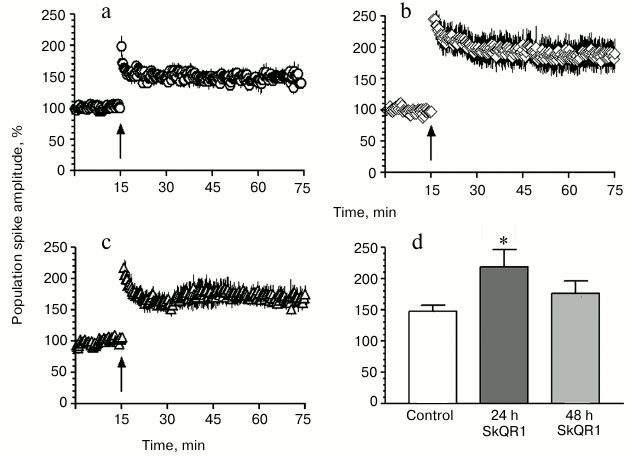
Effect of SkQR1 on PS amplitude in hippocampal slices. A standard HFS (100 Hz, 1 s) from Schaffer’s collaterals induced a long-term post-tetanic potentiation in the CA1 field of the hippocampal slices that 30 min after the HFS resulted in an increase in the PS amplitude to 147.7 ± 9.7% (n = 6; Fig. 2, a and d). In slices prepared 24 h after the injection to rats of SkQR1 (1 µmol/kg), the PS amplitude was significantly higher than in the control and reached 218.6 ± 27.6% (n = 7; Fig. 2, b and d), and 48 h later it was 176.4 ± 19.7% (n = 5; Fig. 2, c and d).
Fig. 2. SkQR1 stimulates long-term post-tetanic potentiation in hippocampal slices. a-c) Time-dependent changes in the total PS amplitude of hippocampal slices: a) control (n = 6); b) slices prepared 24 h later; c) 48 h after the injection to animals of SkQR1 (1 µmol/kg) (n = 5). n, number of slices from different animals. The arrow indicates the HFS moment. d) Mean values of the amplitude of PS recorded 30 min after the HFS; * p < 0.01 compared to control.
DISCUSSION
Hyperproduction of reactive oxygen species (ROS) has been established to play an important role in pathogeny of heart arrhythmias, myocardial and kidney infarctions, brain stroke and trauma, and Alzheimer’s disease. Using antioxidants, the mitochondria-targeted SkQ family allows researchers to purposefully and effectively prevent the negative action of ROS on modeling of these pathologic conditions [2, 7-12]. SkQ1 is one of the most promising mitochondria-targeted antioxidants of this group, and it is now already used in clinical practice [13]. Moreover, a positive influence of SkQ1 has been also shown on experimental models of normal and pathological aging. Thus, studies on the behavior in a cross-shaped labyrinth and in an open field of Wistar rats and of senescence-accelerated OXYS rats have shown that the injection of SkQ1 significantly increases their motor and exploratory activities due to prevention of age-related impairment of memory. SkQ1 also decelerates the accumulation in the brain of the hyperphosphorylated τ-protein, Abeta, and its precursor protein APP [14], which are involved in the development of degeneration in AD. Moreover, SkQ1 prevents such age-related changes in rats as decrease in vision and manifestation of retinopathy and cataract [15]. Our earlier results, as well as findings of other authors, have shown that mitochondria-targeted antioxidants can correct Abeta-induced disorders in processes underlying memory and other cognitive functions [2, 3, 5]. In the present work, we used a highly effective mitochondria-targeted rhodamine-containing analog of SkQ1 – SkQR1 [16]. This substance is not only a very effective antioxidant, but on intraperitoneal injection it also inhibits glycogen synthase kinase 3β (GSK3β) in rat brain [17]. A decrease in GSK3β activity increases both in vivo and in vitro the long-term post-tetanic potentiation of the population spike (PS) in the hippocampus [18]. Long-term post-tetanic potentiation is an increase in the efficiency of the synaptic transmission between neurons that is retained for a long time after a high-frequency stimulation of the synaptic conductibility. At present, the majority of neurophysiologists think that mechanisms of long-term potentiation underlie cellular mechanisms of memory and training. The increase in long-term potentiation that we found in slices of rat hippocampus, as well as the increase in the ability of the rats for training in the PACR test recorded 24 h after the SkQR1 injection, can be caused by both the antioxidant effect and inhibition of GSK3β. It should be noted that the short-time duration of these effects (they are lowered 48 h after the injection) seems to be associated with a gradual metabolism of SkQR1 and its elimination from the organism.
Thus, our findings have shown that the mitochondria-targeted antioxidant SkQR1 has pronounced mnemotropic properties. It can be supposed that mechanisms of memory correction by SkQR1 are associated not only with inhibition of excess production of mitochondrial ROS, but also with its ability to lower in the brain the activity of protein GSK3β [17] that contributes to the inhibition of long-term post-tetanic potentiation [19].
We are very grateful to V. P. Skulachev for his continuous attention and support of our work.
This work was financially supported by the Russian Science Foundation project 14-24-00107.
REFERENCES
1.Schmitt, K., Grimm, A., Kazmierczak, A.,
Strosznajder, J. B., Gutz, J., and Eckert, A. (2012) Insights into
mitochondrial dysfunction: aging, amyloid-β, and τ-A
deleterious trio, Antioxid. Redox Signal., 16,
1456-1466.
2.Kapay, N. A., Isaev, N. K., Stelmashook, E. V.,
Popova, O. V., Zorov, D. B., Skrebitsky, V. G., and Skulachev, V. P.
(2011) In vivo injected mitochondria-targeted plastoquinone
antioxidant SkQR1 prevents β-amyloid-induced decay of long-term
potentiation in rat hippocampal slices, Biochemistry (Moscow),
76, 1367-1370.
3.Ma, T., Hoeffer, C. A., Wong, H., Massaad, C. A.,
Zhou, P., Iadecola, C., Murphy, M. P., Pautler, R. G., and Klann, E.
(2011) Amyloid β-induced impairments in hippocampal synaptic
plasticity are rescued by decreasing mitochondrial superoxide, J.
Neurosci., 31, 5589-5595.
4.Skulachev, V. P. (2012) Mitochondria-targeted
antioxidants as promising drugs for treatment of age-related brain
diseases, J. Alzheimer’s Dis., 28, 283-289.
5.Kapay, N. A., Popova, O. V., Isaev, N. K.,
Stelmashook, E. V., Kondratenko, R. V., Zorov, D. B., Skrebitsky, V.
G., and Skulachev, V. P. (2013) Mitochondria-targeted plastoquinone
antioxidant SkQ1 prevents amyloid-β-induced impairment of
long-term potentiation in rat hippocampal slices, J.
Alzheimer’s Dis., 36, 377-383.
6.Malenka, R. C., and Nicoll, R. A. (1999) Long-term
potentiation – a decade of progress, Science,
285, 1870-1874.
7.Bakeeva, L. E., Chernyak, B. V., Erichev, V. P.,
Filenko, O. F., Kalinina, N. I., Kapelko, V. I., Kolosova, N. G.,
Kopnin, B. P., Korshunova, G. A., Lichinitser, M. R., Obukhova, L. A.,
Pasyukova, E. G., Pisarenko, O. I., Roginsky, V. A., Ruuge, E. K.,
Senin, I. I., Severina, I. I., Skulachev, M. V., Spivak, I. M.,
Tashlitsky, V. N., Tkachuk, V. A., Vyssokikh, M. Y., Yaguzhinsky, L.
S., and Zorov, D. B. (2009) An attempt to prevent senescence: a
mitochondrial approach, Biochim. Biophys. Acta, 1787,
437-461.
8.Bakeeva, L. E., Barskov, I. V., Egorov, M. V.,
Isaev, N. K., Kapelko, V. I., Kazachenko, A. V., Kirpatovsky, M. I.,
Kozlovsky, S. V., Lakomkin, V. L., Levina, S. V., Pisarenko, O. I.,
Plotnikov, E. Y., Saprunova, V. B., Serebryakova, L. I., Skulachev, M.
V., Stelmashook, E. V., Studneva, I. M., Tskitishvili, O. V.,
Vasilyeva, A. K., Viktorov, I. V., Zorov, D. B., and Skulachev, V. P.
(2008) Mitochondria-targeted plastoquinone derivatives as tools to
interrupt execution of the aging program. 2. Treatment of some ROS- and
age-related diseases (heart arrhythmia, heart infarctions, kidney
ischemia, and stroke), Biochemistry (Moscow), 73,
1317-1328.
9.Plotnikov, E. Y., Silachev, D. N., Chupyrkina, A.
A., Danshina, M. I., Yankauskas, S. S., Morosanova, M. A., Stelmashook,
E. V., Vasilyeva, A. K., Goryacheva, E. S., Pirogov, Y. A., Isaev, N.
K., and Zorov, D. B. (2010) New generation Skulachev ions exhibiting
nephroprotective and neuroprotective properties, Biochemistry
(Moscow), 75, 145-152.
10.Isaev, N. K., Novikova, S. V., Stelmashook, E.
V., Barskov, I. V., Silachev, D. N., Khaspekov, L. G., Skulachev, V.
P., and Zorov, D. B. (2012) Mitochondria-targeted plastoquinone
antioxidant SkQR1 decreases trauma-induced neurological deficit in rat,
Biochemistry (Moscow), 77, 996-1001.
11.Isaev, N. K., Stelmashook, E. V., Sharonova, I.
N., and Skrebitsky, V. G. (2013) Brain aging and mitochondria-targeted
plastoquinone antioxidants of SkQ-type, Biochemistry (Moscow),
78, 351-356.
12.Severina, I. I., Severin, F. F., Korshunova, G.
A., Sumbatyan, N. V., Ilyasova, T. M., Simonyan, R. A., Rogov, A. G.,
Trendeleva, T. A., Zvyagilskaya, R. A., Dugina, V. B., Domnina, L. V.,
Fetisova, E. K., Lyamzaev, K. G., Vyssokikh, M. Y., Chernyak, B. V.,
Skulachev, M. V., Skulachev, V. P., and Sadovnichii, V. A. (2013) In
search of novel highly active mitochondria-targeted antioxidants:
thymoquinone and its cationic derivatives, FEBS Lett.,
587, 2018-2024.
13.Skulachev, V. P. (2012) What is
“phenoptosis” and how to fight it, Biochemistry
(Moscow), 77, 689-706.
14.Stefanova, N. A., Fursova, A. Zh., and Kolosova,
N. G. (2010) Behavioral effects induced by mitochondria-targeted
antioxidant SkQ1 in Wistar and senescence-accelerated OXYS rats, J.
Alzheimer’s Dis., 21, 479-491.
15.Stefanova, N. A., Muraleva, N. A., Skulachev, V.
P., and Kolosova, N. G. (2014) Alzheimer’s disease-like pathology
in senescence-accelerated OXYS rats can be partially retarded with
mitochondria-targeted antioxidant SkQ1, J. Alzheimer’s
Dis., 38, 681-694.
16.Skulachev, V. P. (2013) Cationic antioxidants as
a powerful tool against mitochondrial oxidative stress, Biochem.
Biophys. Res. Commun., 441, 275-279.
17.Silachev, D. N., Isaev, N. K., Pevzner, I. B.,
Zorova, L. D., Stelmashook, E. V., Novikova, S. V., Plotnikov, E. Y.,
Skulachev, V. P., and Zorov, D. B. (2012) The mitochondria-targeted
antioxidants and remote kidney preconditioning ameliorate brain damage
through kidney-to-brain cross-talk, PLoS One, 7,
e51553.
18.King, M. K., Pardo, M., Cheng, Y., Downey, K.,
Jope, R. S., and Beurel, E. (2014) Glycogen synthase kinase-3
inhibitors: rescuers of cognitive impairments, Pharmacol. Ther.,
141, 1-12.
19.Takashima, A. (2012) GSK-3β and memory
formation, Front. Mol. Neurosci., 23, 5-47.