Human Myeloma Immunoglobulins of the Fourth Subclass (IgG4 MAM) Contain a Fraction with Different Properties of CH2 Domains
V. M. Tischenko
Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences, ul. Institutskaya 3, 142290 Pushchino, Moscow Region, Russia; fax: (4967) 33-0553; E-mail: tischen@vega.protres.ru
Received March 31, 2014; Revision received September 2, 2014
A long-lived metastable minor fraction has been detected and characterized in myeloma protein IgG4 MAM by hydro- and thermodynamic methods. The sedimentation constants of the minor and the major protein fractions are different. The stability of the two CH2 domains in the minor fraction varies. The unique characteristics of these IgG4 MAM conformers arise from the fact that on exchange of the heavy chains between IgG4 molecules, in some of them only one noncanonical bond Cys226–Cys229 is formed in the central part of the “hinge region” instead of two canonical interchain disulfide bonds Cys226–Cys226 and Cys229–Cys229. This leads to asymmetric structure of the IgG4 MAM molecules.
KEY WORDS: immunoglobulin IgG4, CH2-domain, stability, carbohydrate moiety, disulfide bondDOI: 10.1134/S0006297915010034
Abbreviations: BSA, bovine serum albumin; CH2(3), the constant domains forming the Fc-subunit (fragment); DSS, disuccinimidyl ester of suberic acid; DTT, dithiothreitol; FITC, fluorescein isothiocyanate; Fv subfragment, a structure formed by a pair of variable domains VL–VH; GuHCl, guanidine hydrochloride; H(L)-chains, heavy (light) chains; IgG4 MAM, human myeloma immunoglobulin of class G belonging to the fourth subclass obtained from patient MAM; SPDP, N-succinimidyl 3-(2-pyridyldithio)propionate; VH, variable domains of heavy chain; VL, variable domains of light chain.
Human immunoglobulins of class G belonging to four subclasses are highly
homological structures consisting of two identical light and two
identical heavy chains (H2L2) [1-4]. Each light chain is folded
into two globular domains, and each heavy chain is folded into four
globular domains, the latter chains having a region with unique primary
sequence in its middle part (between the CH1 and
CH2 domains). The N-terminal moieties of the H- and L-chains
form two antigen-binding Fab subunits, and the C-terminal moieties of
the H-chains form one Fc subunit [5, 6] that is responsible for several effector functions
– interaction with an C1q factor of the complement system, with A
and G proteins, as well as with numerous cellular receptors [7, 8]. In an intact molecule, the
Fab and Fc subunits are linked by the above-mentioned so-called
“hinge” region. It can be subdivided into three segments
– a rigid and highly stable core hinge [9-15] formed by double poly-L-proline helix linked by
disulfide bonds, and flexible upper and lower hinges. The last two
segments provide intramolecular mobility of Fab and Fc subunits and
flexibility of the whole Ig molecule, which was shown both by X-ray
diffraction analysis [11, 12,
16, 17] and by fluorescence
depolarization [18].
However, in the last decade it has been shown that at least some IgG subclasses are capable of yet another significantly different type of conformational mobility that is associated with rearrangements of disulfide bonds and leads to the formation of conformers. Thus, in the case of IgG2, the intramolecular rearrangement of disulfide bonds results in formation of conformers in which one and then the second Fab subunits appear to be drawn together with the Fc subunit [19-22]. As shown in our previous work [23], in the first stage of this process the asymmetric IgG2 structure is formed, which is manifested, in particular, in difference in thermodynamic properties of the CH2 domains.
According to many reports, IgG4 molecules can undergo rearrangements of S–S bonds, but of somewhat different character. During its implementation, initially the cleavage of the interchain bonds in the two heavy chains of a single molecule occurs, and then the exchange of molecule halves between two molecules (so-called heavy-chain exchange, or Fab-arm exchange) takes place [24-27].
The questions arise – what kind of structural changes in IgG4 molecules might occur in the course of such rearrangements of interchain disulfide bonds at different stages of the reaction, and what are the subsequent conformational changes that will take place in specific domains? To answer these questions, it is first necessary to obtain experimental data on the availability of intermediate stages in this process (fast kinetic or relatively long-lived metastable), and then for the affirmative answer, to characterize them. It turned out that IgG4 MAM might be in the intermediate long-lived metastable state, which allowed its use for the study of a comprehensive approach based on the use of hydrodynamic, optical, and calorimetric methods.
MATERIALS AND METHODS
Isolation and typing myeloma IgG4 and obtaining its Fab and Fc fragments and CH2 domains. Myeloma IgG4 MAM was separated from the serum of a patient with multiple myeloma according to a standard method, as in the case of rabbit and goat IgG [28-30]. All procedures were carried out in the shortest possible time at a temperature of 4°C. Using the double immunodiffusion test in a gel, IgG MAM was ascribed to the fourth subclass and its light chain to λ-type [31]. The number of interchain disulfide bonds and free SH-groups was determined with 14C-labeled iodoacetic acid [32]. The Fab and Fc fragments of these antibodies were obtained by a standard proteolysis procedure using papain [28-30] and then separated from the uncleaved material by chromatography on a Superdex column and further from each other on a Mono Q column [33, 34]. The Fv subfragments from rabbit and goat anti-Fab fragments were obtained by low-temperature pepsin proteolysis as described in our previous work [35]. The VH and VL domains of Fv were linked using the SPDP or DSS by methods described earlier [36, 37]. The Fc fragments were identified by immune electrophoresis using an antiserum to the light and heavy chains of IgG as well as to all serum proteins [38]. The N-terminal amino acid residues of the heavy and light chains were determined by the dansyl chloride method [39], and their C-terminal residues were determined using carboxypeptidase [40] as in our earlier works [41, 42]. Protein of the IgG4 MAM main fraction (see below) containing partially reduced (nonselectively) disulfide bonds between the H-chains was obtained and alkylated as described in [43] in the presence of 10 mM DTT and 20 mM iodoacetamide or 14C-labeled iodoacetic acid. Heavy and light chains were separated by gel filtration in 6 M GuHCl of the protein containing reduced disulfide bonds.
The CH2 domains were obtained from papain IgG4 Fc fragments by trypsin hydrolysis as described previously [44, 45] with some modifications. First, the incubation before proteolysis was carried out at pH 2.8 [45-47] instead of pH 2.5. Second, to stabilize the labile structure of these domains, hydrolysis was carried out in the presence of Fab fragments from rabbit antibodies against CH2 domains at CH2/Fab ratio of 1 : 1 [47-49]. After completion of the reaction, the CH2–Fab complex was dissociated at pH 4.0 (10 mM glycine buffer), and the two proteins were separated by gel filtration under the mentioned conditions. The resulting fragments were homogenous according both to SDS-electrophoresis [50] and two-dimensional immune electrophoresis using commercial mono- and polyspecific antisera by the Laurell method [51].
Preparation of antibodies against intact IgG4 MAM, its Fab and Fc fragments, and CH2 domains. Antibodies against intact IgG4 MAM, its Fab and Fc fragments, and the CH2 domains (both native and fluorescent labeled) were obtained by immunization of rabbits and goats with the corresponding proteins including labeled ones [46]. Antibodies against the fluorescence label were obtained by immunizing animals with FITC-modified BSA. Affinity chromatography was performed as described earlier [46].
Preparation of IgG4 MAM selectively labeled with a fluorescent label at the CH2 and CH3 domains as well as of fully modified protein. The proteins were selectively modified with FITC fluorescent label in 200 mM borate buffer, pH 8.9, containing 150 mM NaCl, for 12 h at 4°C and at protein/label ratio 2 : 1 [46, 47, 49, 52]. The only deviation from the original technique used further [52] consisted of a slight decrease in pH (from 9.1 to 8.9). In the case of full modification, the protein/label ratio was equal to 1 : 4 and the incubation time was increased to 24 h. The amount of the label incorporated in the Fc fragment was determined from the ratio of the solution optical densities at 495 and 470 nm [53]. Intact IgG4 MAM proteins containing fluorescently labeled CH2 or CH3 domains were isolated as described earlier [46, 47] by affinity chromatography using immobilized rabbit antibodies. Fully modified samples were obtained by affinity chromatography using antibodies against FITC-BSA. All protein samples were prepared using carefully degassed buffer solutions and stored under nitrogen.
Determination of molecular mass. The molecular masses of the intact IgG4 and its fragments were determined by high-speed (average molecular mass Mw) and low-speed (z-average molecular mass Mz) equilibrium centrifugation by the methods of Yphantis [54] and of Van Holde-Baldwin [55] using a Beckman Spinco model E high-speed analytical ultracentrifuge (Beckman Coulter, USA) and MOM ultracentrifuge (MOM, Hungary) at 20°C. Equilibrium centrifugation of immunoglobulins (in the absence and presence of 6 M GuHCl) was carried out with incompletely filled centrifuge tubes to reach equilibrium in a shorter time. (As it is known, the time needed to reach the equilibrium in a tube is proportional to the square of liquid layer height [56]). The issue of the time of centrifugation may be critical for large molecules like IgG4 when they are in a metastable state. In our experiments, equilibrium was reached within 12-14 h, and for the control samples duration of centrifugation was increased to 24 h.
Incomplete filling of centrifuge tubes increases the error of the molecular mass determination, because the part of the curve (describing dependence of shift of fringe on rotation radius) that can be used for reliable analysis is truncated. In experiments with CH2 domains, the centrifuge tubes were completely filled.
Determination of the sedimentation coefficient. Sedimentation of intact IgG4 MAM and its CH2 domains was studied using the Beckman model E ultracentrifuge with rotor speed of 24,050 and 50,150 rpm, respectively. Schlieren optics at protein concentration of 2-9 mg/ml or a double-beam UV system at wavelengths 230 and 278 nm at protein concentration of 0.05-0.3 mg/ml was used for registration. The sedimentation coefficients were determined in 1 mM buffer (phosphate or glycine) in the presence of 150 mM NaCl (if necessary supplemented with 10 mM DTT). The value of the sedimentation constant s200 was determined by extrapolating the values of sedimentation coefficients to zero concentration.
Study of conformational changes in the Fc fragments by fluorescence spectroscopy. The temperature induced conformational changes in the proteins were studied by measuring the fluorescence intensity of the solution in the 460-600-nm range with excitation of 450 nm using a Shimadzu RF-5301 PC spectrofluorimeter (Shimadzu, Japan) with thermostatted cell. Concentrations of the test solutions were in the range 0.05-0.3 mg/ml. Heating rate was varied in the range 0.25-2°C/min. Prior to each experiment, gel filtration of test samples on a column with Ultragel AcA-34 or AcA-44 (LKB, Sweden) equilibrated with the appropriate buffer solutions was performed. The temperature dependence of the fluorescence was analyzed by a method described earlier [46, 49, 52, 57].
Calorimetric measurements and calculation of thermodynamic data. All experiments were performed using a DASM-4A microcalorimeter with a gold capillary cell of 1 ml volume or a DASM-4 microcalorimeter with a platinum spiral capillary cell of 0.43 ml volume. The concentration of protein in solution was varied in the range of 1-6 mg/ml. Measurements were carried out at speeds of 0.25-2°C/min. The calorimetric curves were processed as described by Privalov and Potekhin [58].
RESULTS
Myeloma IgG4 MAM consists of two fractions with different immunological properties. Using affinity chromatography on a column with immobilized goat antibodies against IgG4 MAM equilibrated with 1 mM phosphate buffer, pH 7.0, the protein can be divided into two fractions. The major fraction (approximately 90%) eluted from the column with Fc and Fab fragments, and a minor fraction (about 10%) can be removed under harsher conditions (10 mM glycine buffer, pH 4.0). When stored for 5 days under “physiological” conditions (1 mM phosphate buffer, pH 7.0, 20°C), in both fractions a new separation into two fractions with the same proportions occurred. (Of note, trace amounts of another protein fraction differing from the first two fractions by the molecular mass can be detected after extended storage at protein concentration of 2-3 mg/ml.)
Fractions of IgG4 MAM are formed by the exchange of heavy chains. The IgG4 MAM major fraction (2 mg/ml in 1 mM phosphate buffer, pH 7.0) was supplemented with an equimolar amount of the same protein that was fully modified with FITC. (Each protein molecule contained on average three fluorescent labels.) After 5 days of incubation, over 85% of the IgG4 MAM from both major and minor fractions was sorbed on a column with immobilized anti-FITC antibodies, indicating modification by FITC (at least one label per protein molecule). Moreover, the minor fraction accumulated symbatically with increase in number of molecules containing at least one fluorescent label.
Note that the kinetics of the heavy-chain exchange in the proteins of the major fraction with partially reduced disulfide bonds has not been studied in detail. However, we conclude (using the above-described procedure) that although it is somewhat faster (up to 2-fold), it remains rather slow.
Proteins from both fractions are in monomeric state. The presence of two IgG4 MAM fractions with relatively low rate of exchange from one faction to the other allows exploring some physicochemical parameters of the protein in each of the states. As only intramolecular properties of proteins will be further investigated, it is necessary to ensure that the representatives of the two fractions, at least under certain conditions, are in the form of monomers. This approach is especially relevant because, as mentioned above [24-27], IgG4 molecules can exchange their moieties, which implies the existence of intermolecular contacts. Therefore, the molecular masses of proteins in both fractions were determined by equilibrium centrifugation, resulting in the calculated average molecular mass Mw and z-average molar mass Mz (table).
Characteristics of proteins from two IgG4 MAM fractions
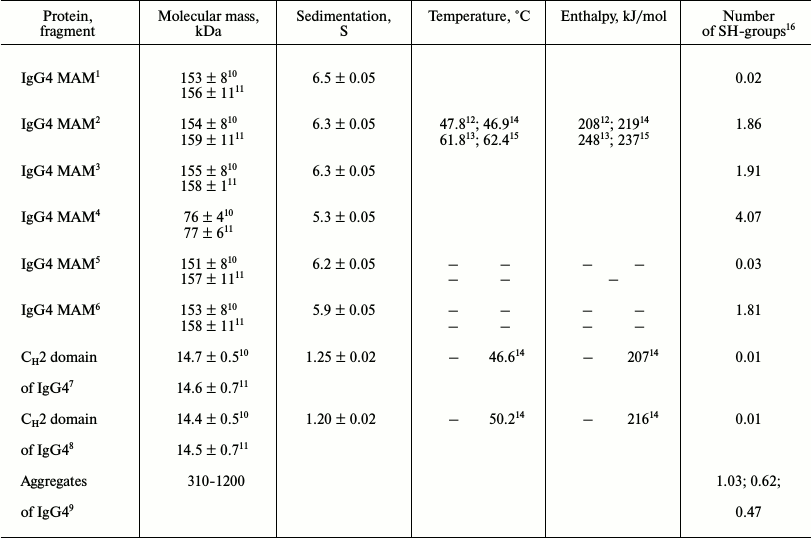
1,2 Major and minor fractions of IgG4 MAM at pH 7.0,
respectively.
3,4 Major and minor fractions of IgG4 MAM at pH 7.0 and
10 mM DTT + 6 M GuHCl, respectively.
5,6 Major and minor fractions of IgG4 MAM at pH 4.0,
respectively.
7,8 Fractions of CH2 domains with larger (smaller)
positive charge, respectively.
9 Dimer, trimer, and tetramer based on gel-filtration
data.
10,11 Average molecular mass and z-average molecular mass,
respectively.
12,13 Temperature and enthalpy for the first and second
elementary transitions, respectively, determined from the calorimetric
curves; errors ±0.5ºC and ±12 kJ/mol.
14,15 Temperature and enthalpy for the first and second
elementary transitions, respectively, and for isolated CH2
domains determined from optical curves; errors ±1.5ºC and
±21 kJ/mol.
16 Calculated per monomer of IgG (150 kDa).
It is seen that average molecular mass of protein from major fraction is Mw = 153 ± 8 kDa and z-average molecular mass is Mz = 156 ± 11 kDa. For protein from the second fraction, Mw = 154 ± 8 kDa and Mz = 159 ± 11 kDa. Since Mw and Mz are differently sensitive to the presence of aggregates, from these data one can draw the following conclusions. First, the two proteins do not differ in molecular mass; second, both are in the monomeric form, which is consistent with data previously obtained for the unfractionated IgG4 [29]. Furthermore, the previously obtained data on standard SDS-electrophoresis of unfractionated protein with reduced S–S bonds showed only two distinct bands corresponding to the heavy and light chain [30]. This indicates at least at the proximity of molecular masses of the heavy and light chains in the proteins from the two fractions, if not complete coincidence. It should be noted that the mechanism of exchange with halves of IgG4 molecules itself requires contact between two IgG4 molecules. Almost identical molecular masses at different times of centrifugation indicate that the monomer–dimer equilibrium in the two fractions is strongly shifted towards monomers (table), and the characteristic time associated with the formation of aggregates exceeds a few days.
Note that moderate concentrations of DTT do not affect the molecular mass values of proteins from either fraction, although after the only disulfide bond in IgG4 MAM of the minor fraction is reduced (see below), this protein converts into a dimer (HL + HL) without covalent bonds that is subject to dissociation in 6 M GuHCl. Under native conditions, the presence of the dimer is exclusively caused by very strong interactions between the CH3 domains of the two H-chains [59-62] and, therefore, by a very low dissociation constant of the dimer. Therefore, at the concentrations of protein that are used in the subsequent experiments, the equilibrium is strongly shifted towards the dimer.
The N-terminal portion of heavy chains in proteins from both fractions is locked. Glycine was revealed as the N-terminal amino acid residue in the light chains of both fractions. Glycine was found as the C-terminal amino acid residue in the heavy chain, while Ser was found in the light chain of proteins from both fractions [9]. These data confirm the correctness of assignment of the protein to MAM IgG4 (λ) [29, 30] and indicate that the presence of the fractions themselves is not related with partial degradation of any of the four polypeptide chains constituting the intact IgG4 MAM molecule.
One SH group per H-chain is present in minor fraction protein. The presence of free SH groups in the protein of the minor fraction was confirmed by its titration with 14C-labeled iodoacetic acid [63, 64]. According to these data, one free SH group is present in this protein, i.e. in the H-chain with stoichiometry of one group per chain. The data of the gel filtration of IgG4 MAM from the indicated fraction under denaturing conditions when disulfide bonds of the protein are reduced unequivocally show this [32, 63, 64]. It was found that the radiolabel is only in the heavy chain, but not in the light one (see table; data of gel filtration is not shown). This is also indicated by the fact that the conditions that caused only partial reduction of the interchain disulfide bonds in the major fraction proteins in proteins of minor fractions lead to disappearance of the disulfide bonds, as evidenced by the data of ultracentrifugation in 6 M GuHCl.
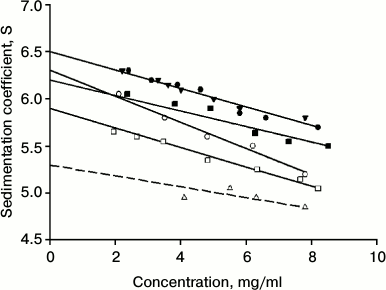
Determination of sedimentation constant of IgG4 of major and minor fractions. The long relaxation time allows determining the sedimentation coefficient of intact proteins from the two factions. Figure 1 shows the corresponding results. It is evident that they are characterized by different values of the sedimentation constant s200. For the protein that belongs to the major fraction, this value is 6.50 ± 0.05 S, which is very close to the value that was obtained earlier for IgG4 in other reports [65, 66]. It is also seen that on nonselective partial restoration of one from the two disulfide bonds between the H-chains, changes in hydrodynamic parameters in IgG4 MAM from the major fraction are not observed (Fig. 1 and table).
Fig. 1. Determination of sedimentation constant for IgG4 MAM at pH 7.0 (10 mM phosphate buffer, 150 mM NaCl): for protein of the major fraction (closed circle) and minor fraction (open circle), for protein of the major fraction with one reduced disulfide bond (closed triangle), for protein of the minor fraction with one reduced disulfide bond (open triangle); at pH 4.0 (50 mM glycine buffer, 150 mM NaCl) for protein of the major fraction (closed square) and minor fraction (open square).
For “intact” protein from the minor fraction, the s200 value slightly but quite reliably differs and is 6.3 ± 0.05 S. The reliability of the distinctions indicates not only the difference in the values of s200 for the two conformers, but also more clear distinguishes the slope of the straight line describing the dependence of the sediment coefficient on concentration (non-ideality coefficient ks [67]), as seen in Fig. 1. It is interesting to note the differences in the hydrodynamic properties of the proteins from the two factions also stored at pH 4.0 (table). This means that differences in the properties in the case of IgG4 MAM conformers are not associated with a property of the immunoglobulin under “physiological” conditions in a metastable state, after preincubation of the protein under acidic pH conditions [44, 45, 68]. As shown in our earlier reports [59, 62], this condition can be long-lived for a number of myeloma proteins.
In the case of protein of the minor fraction, the addition of the same DTT concentration leads to very significant changes in its hydrodynamic parameters (Fig. 1 and table). However, it should be noted that in this case the “sedimentation constant” s200 has no strict sense, since in this case we are dealing with a noncovalent dimer of HL + HL. In this case, the equilibrium is shifted toward dimerization even at relatively low protein concentrations due to the high affinity between the CH3 domains [7, 8, 12, 16, 46, 52].
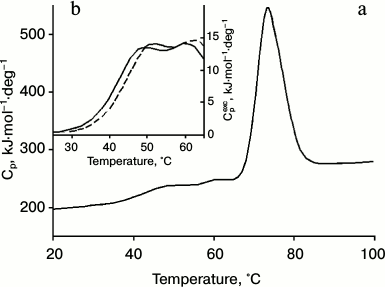
Calorimetric studies of IgG4 from the two fractions. The proteins of the two fractions also have different thermodynamic characteristics. The dependence of the molar heat capacity on temperature for IgG4 MAM of the minor fraction, which is derived from the melting curve at pH 5.8, is shown in Fig. 2. These conditions were chosen for the following reasons. On one hand, it is desirable to have results obtained under conditions as close as possible to “physiological”. On the other hand, proper thermodynamic analysis requires data to be obtained under equilibrium conditions. However, it should be emphasized that when the pH is increased to 7.0, the shape of the melting curve does not change significantly, suggesting that the data (see below) are also valid for “physiological” conditions.
Fig. 2. a) Temperature dependence of partial molar heat capacity of IgG4 MAM; b) a fragment of the curve of dependence of excess molar heat capacity on temperature in the absence of anti-CH2 Fab (Fv) fragments (solid line) and in the presence of anti-CH2 Fab (Fv) fragments in equimolar amounts with CH2 domains (dashed line). Conditions: 10 mM phosphate buffer, pH 5.8, at concentration of IgG4 equal to 20 µmol/ml (3 mg/ml).
It is noteworthy that the region of the melting curve of IgG4 MAM from the minor fraction that describes processes at relatively low temperatures has very distinctive features (Fig. 2). Earlier only one peak of heat absorption could be seen in this region [40, 69] of the heat absorption curve of IgG4 protein (which can be attributed to the major fraction), which was true for a number of other intact IgGs and their Fc fragments. Such experiments included various subclasses of human IgG as well as IgGs from different animals [29, 30, 41, 46, 70, 71]. That is, it was a quite general rule reflecting a general principle of the structural organization of Fc subunits in IgG molecules. In a similar melting curve of the minor fraction protein, in this region two peaks are clearly visible, indicating the presence in this temperature range of at least two elementary heat absorption peaks, each of which corresponds to the melting of a cooperative unit (domain).
Earlier it has been shown repeatedly for all investigated immunoglobulins that the melting process at low temperatures is entirely due to the disintegration of the cooperative structure of the two CH2 domains. In this case, almost all of the data quite unequivocally indicate melting of two identical and practically non-interacting domains. The only exception was myeloma IgG2 MAT, whose melting curves in the low-temperature region were similar to those described above for the minor fraction of IgG4 MAM [41, 47]. That is, on the melting curve of myeloma IgG2 MAT, two separate heat absorption peaks are present corresponding to the melting of two CH2 domains that are non-interacting and differing in thermal stability. It is noteworthy that according to electron microscopy the structure of IgG2 MAT has a distinct asymmetry because one of the CH2 domains is adjacent to the Fab subunit [23].
To be able to interpret the results concerning the low-temperature part of the calorimetric melting curve of IgG4 MAM directly and unambiguously, we used two independent approaches. The first approach, which has already been repeatedly tested in the study of other IgGs [46, 47, 52], is based on selective introduction of a fluorescent label in certain domains. This approach was used in the present work.
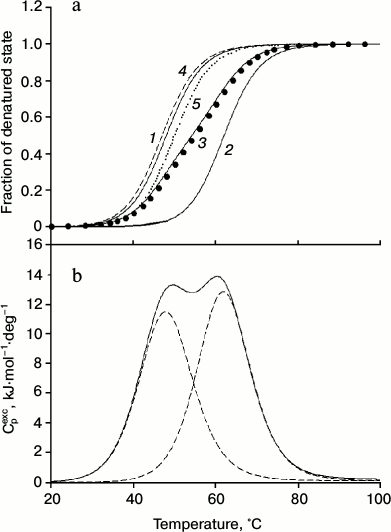
As can be seen from the data presented in Fig. 3 and the table, the results support the assumption that the low-temperature peaks in the melting curve of the minor fraction of IgG4 MAM are related to the disintegration of the structure of two CH2 domains (Fig. 2). Furthermore, the results allow satisfactory decomposition of the transition curve (change in fraction of denatured state calculated from the experimental optical melting curve) into two transitions by postulating their number (Fig. 3a, curves 1 and 2). Of course, all thermodynamic parameters for these transitions, which can be calculated from the optical curves, especially melting enthalpy, are determined with large error. However, at this stage it is extremely important that according to both optical and calorimetric data, the melting of the CH2 domains occurs in the same temperature range (Figs. 3a and 3b).
Fig. 3. a) Change in the fraction of denatured state of CH2 domains of IgG4 MAM of minor fraction determined based on fluorescence intensity of the intact protein (closed circle), and decomposition of the curve into elementary transitions corresponding to melting of each from the two CH2 domains (1, 2). Curve 3 is the sum of curves 1 and 2. Curves 4 (dashed line) and 5 (dotted line) correspond to the melting of the two insulated CH2 domains derived from IgG4 MAM of the minor fraction. b) Temperature dependence of the excess heat absorption during melting of CH2 domains (solid line) and its decomposition into elementary transitions corresponding to the melting of each from the two domains (dotted line).
The second approach that we applied for analysis of melting curves of IgG4 MAM minor fraction involves use of antibodies (more precisely, their Fab fragments or Fv subfragments) against different regions of the intact protein. The Fab fragments (Fv subfragments) against the CH2 and CH3 domains as well as against the Fab subunits were used. It follows from the Fig. 2b that changes in the low-temperature region of the calorimetric melting curve were observed only in the case when Fab fragments (Fv subfragments) against the CH2 domain were used. It can be seen that in this case there are changes in the position of the maxima of both peaks in the low-temperature range. The changes in the calorimetric melting curve at higher temperatures were only recorded when either anti-Fab (Fv) fragments against Fab subunits or against CH3 domains were used (data not shown).
Qualitative evidence that the optical melting curve of IgG4 MAM reflects decay of just two cooperative blocks formed by two independent CH2 domains are also fully supported by more rigorous thermodynamic analysis of the excess heat absorption curves [58, 72]. The results are shown in Fig. 3b and the table. It can be seen that there are two elementary transitions that correspond to the melting of two different cooperative blocks. The results do not correspond to the model of a molecule in solution, based on a set of thermodynamic and structural data for IgG (Fc fragments), according to which two CH2 domains do not interact and have identical characteristics. The latter is possible only in the case when not only the domains themselves are identical in protein composition and their carbohydrate moieties have similar composition, but the Fc subunit (fragment) also has a symmetrical structure and environment [11, 12, 73, 74].
Therefore, the results can be explained simply given that the thermal stability of the CH2 domains is determined by not only the structure of the globule itself, but also the carbohydrate moieties, which in all IgG conserved Asn297 was modified [71]. The CH2 domains were isolated from the intact proteins of the minor fraction and separated on a Mono Q column [75] into two new subfractions whose melting curves were obtained by recording change in fluorescence intensity (Fig. 3a). According to these data, the thermal stability of the CH2 domains of the subfractions is only slightly different (table).
DISCUSSION
Based on the data presented above, we can quite clearly state that conformers of IgG4 MAM molecules appear having properties differing from standard IgG4 and suggest the structure of these conformers. According to our results, which were obtained in experiments using fully modified protein, a minor fraction is formed by the exchange of heavy chains (together with the adjoining light chains) of two molecules of IgG4 MAM. The most reasonable hypothesis about the mechanism of the exchange of the heavy chains is that at the intermediate stage, the breaking of the interchain bonds Cys226–Cys226, Cys229–Cys229 and the formation of intrachain bonds Cys226–Cys229 takes place [26]. Then the original protein devoid of covalent bonds between the two its moieties dissociates and new dimerization occurs with HL dimer of the other protein formed similarly. The process is accompanied by the formation of two canonical bonds between the heavy chains. The presented findings of centrifugation of a minor fraction protein under denaturing conditions (6 M GuHCl) but without reducing the disulfide bonds reactants clear indicate that this protein keeps the tetramer form H2L2 using disulfide bonds. However, data of titration of minor fraction protein indicate formation of one free SH group in the heavy chain. This leads to the unequivocal conclusion that from two disulfide interchain bonds only one chain is retained.
A question arises – which of the two interchain disulfide bonds is formed in the protein structure? To answer this question, it is possible to obtain the corresponding peptides from the “hinge” area of the IgG4 MAM minor fraction in which these bonds are localized and to determine which Cys residues were modified by 14C-labeled iodoacetic acid.
There is, however, another possibility to make a definite conclusion concerning this. Earlier, it was shown that the partial reduction (and subsequent alkylation) of interchain disulfide bonds in myeloma IgG4 does not lead to a change in such parameter as hydrodynamic volume, which characterizes the overall configuration of protein molecules [43]. According to a more precise definition of configuration of molecules of the IgG4 MAM major fraction, both with intact and partially reduced interchain disulfide bonds, we can conclude that not only the sedimentation constant s200 for the two proteins but also the slope, which determines the concentration dependence of the sedimentation coefficients s, remain virtually unchanged. This follows from the ultracentrifugation data (table). A completely different picture is observed during centrifugation of the IgG4 MAM minor fraction. Both sedimentation constant s200 of the protein and the corresponding slope have significant differences (table).
IgG4 has only two interchain disulfide bonds between the H-chains, and the partially reduced proteins of the major fraction retain either the bond between two Cys226 or a bond between two Cys229 of heavy chains (and this does not influence hydrodynamic parameters). From this, a logical suggestion can be made that in the case of the minor fraction proteins, the only disulfide bond is formed not between the canonical pairs Cys226–Cys226 or Cys229–Cys229, but between Cys226 and Cys229 of the neighboring H-chains. This leads to a significant change in the conformation of the molecule, which is manifested, in particular, in a noticeable decrease in the sedimentation constant s200 (table).
The formation of such noncanonical bond with high probability can be accompanied also by quite noticeable changes in other characteristics of the IgG4 MAM macromolecule of the minor fraction such as mobility of Fab subunits, rate of splitting of the “hinge” area with altered conformation by action of proteolytic enzymes, and others. This is a subject for further studies.
In this study, we have shown that the probable formation of noncanonical disulfide bond in IgG4 MAM results in significant difference in thermostability between the two CH2 domains within the Fc subunit. Since a significant effect is observed only for CH2 domains of the intact minor fraction protein but not in the case of isolated CH2 domains, we can assume that it is due to the overall asymmetry of the molecule. It can occur when Fab subunits are differently arranged relative to the Fc subunit. Earlier, we observed a similar situation for IgG2 using different methods, in particular, electron microscopy [23]. The asymmetry is also shown for IgG4 molecules using the same method [69]. Existence of such an asymmetric structure (described using a number of methods) suggests that the activity of IgG4, different from the activity of immunoglobulins of the other subclasses, can be due not only to steric hindrances from Fab subunits [7, 8]. It is shown that the state of one of the CH2 domains is changed, and this may induce changes in its affinity for a variety of ligands involved in immunological reactions. Anyway, such a pattern seems to occur in case of IgG2, which we have investigated in detail [23, 29, 30, 41, 47, 76].
The author thanks S. A. Potekhin and V. V. Filimonov for discussion of the data on the thermodynamics, J. Wayne (UK) for assistance in centrifugation, and A. V. Kulikov for the help in determination of the N- and C-terminal residues.
This work was supported by the Russian Foundation for Basic Research (grants 11-04-00064a, 14-04-01682a, and 07-04-12199-ofi).
REFERENCES
1.Rutishauser, U., Cunningham, B. A., Bennett, C.,
Konigsberg, W. H., and Edelman, G. M. (1968) Amino acid sequence of the
Fc region of a human gamma G-immunoglobulin, Proc. Natl. Acad. Sci.
USA, 61, 1414-1421.
2.Edelman, G. M., Cunningham, B. A., Gall, W. E.,
Gottlieb, P. D., Rutishauser, U., and Waxdal, M. J. (1969) The covalent
structure of an entire gamma G-immunoglobulin molecule, Proc. Natl.
Acad. Sci. USA, 63, 78-85.
3.Gottlieb, P. D., Cunningham, B. A., Rutishauser,
U., and Edelman, G. M. (1970) The covalent structure of a human gamma
G-immunoglobulin. VI. Amino acid sequence of the light chain,
Biochemistry, 9, 3155-3161.
4.Edelman, G. M. (1970) The covalent structure of a
human gamma G-immunoglobulin. XI. Functional implications,
Biochemistry, 9, 3197-3205.
5.Porter, R. R. (1959) The hydrolysis of rabbit
γ-globulin and antibodies with crystalline papain, Biochem.
J., 73, 119-126.
6.Noelken, M. E., Nelson, C. A., Buckley, C. E., 3rd,
and Tanford, C. (1965) Gross conformation of rabbit 7S
gamma-immunoglobulin and its papain-cleaved fragments, J. Biol.
Chem., 240, 218-224.
7.Burton, D. R. (1985) Immunoglobulin G: functional
sites, Mol. Immunol., 22, 161-206.
8.Burton, D. R., and Woof, J. M. (1992) Human
antibody effector function, Adv. Immunol., 51, 1-84.
9.Kabat, E. A., Wu, T. T., Reid-Miller, M., Perry, H.
M., and Gottesman, K. (1987) Sequences of Proteins of Immunological
Interest, 4th Edn., National Institutes of Health, Bethesda,
MD.
10.Michaelsen, T. E., Natvig, J. B., and Sletten, K.
(1974) Isolation of a fragment, Fh, corresponding to the hinge region
of human IgG3, Scand. J. Immunol., 3, 491-498.
11.Marquart, M., Deisenhofer, J., Huber, R., and
Palm, W. (1980) Crystallographic refinement and atomic models of the
intact immunoglobulin molecule Kol and its antigen-binding fragment at
3.0 and 1.9 Å resolution, J. Mol. Biol., 141,
369-391.
12.Harris, L. J., Larson, S. B., Hasel, K. W., Day,
J., Greenwood, A., and McPherson, A. (1992) The three-dimensional
structure of an intact monoclonal antibody for canine lymphoma,
Nature, 360, 369-372.
13.Tischenko, V. M. (1999) Fh fragment from IgG3 Kuc
exist in compact and rod-like shape, in Abstr. of XIth Int. Conf. on
Small-Angle Scattering, Brookhaven National Laboratory, p. 93.
14.Tischenko, V. M. (2000) Three states of the
pFh-fragment (hinge region) from human myeloma IgG3 Kuc with
native-like secondary structure, Biochemistry (Moscow),
65, 1227-1230.
15.Tishchenko, V. M. (2011) Unusual thermodynamic
properties of a compact state of IgG3 Kuc and Sur pFh fragments (hinge
region), Mol. Biol. (Moscow), 45, 976-982.
16.Harris, L. J., Skaletsky, E., and McPherson, A.
(1998) Crystallographic structure of an intact IgG1 monoclonal
antibody, J. Mol. Biol., 275, 861-872.
17.Saphire, E. O., Stanfield, R. L., Crispin, M. D.,
Parren, P. W., Rudd, P. M., Dwek, R. A., Burton, D. R., and Wilson, I.
A. (2002) Contrasting IgG structures reveal extreme asymmetry and
flexibility, J. Mol. Biol., 319, 9-18.
18.Dangl, J. L., Wensel, T. G., Morrison, S. L.,
Stryer, L., Herzenberg, L. A., and Oi, V. T. (1988) Segmental
flexibility and complement fixation of genetically engineered chimeric
human, rabbit and mouse antibodies, EMBO J., 7,
1989-1994.
19.Wypych, J., Li, M., Guo, A., Zhang, Z., Martinez,
T., Allen, M. J., Fodor, S., Kelner, D. N., Flynn, G. C., Liu, Y. D.,
Bondarenko, P. V., Ricci, M. S., Dillon, T. M., and Balland, A. (2008)
Structural and functional characterization of disulfide isoforms of the
human IgG2 subclass, J. Biol. Chem., 283,
16194-16205.
20.Martinez, T., Guo, A., Allen, M. J., Han, M.,
Pace, D., Jones, J., Gillespie, R., Ketchem, R. R., Zhang, Y., and
Balland, A. (2008) Disulfide connectivity of human immunoglobulin G2
structural isoforms, Biochemistry, 47, 7496-7508.
21.Allen, M. J., Guo, A., Martinez, T., Han, M.,
Flynn, G. C., Wypych, J., Liu, Y. D., Shen, W. D., Dillon, T. M.,
Vezina, C., and Balland, A. (2009) Interchain disulfide bonding in
human IgG2 antibodies probed by site-directed mutagenesis,
Biochemistry, 48, 3755-3766.
22.Lightle, S., Aykent, S., Lacher, N., Mitaksov,
V., Wells, K., Zobel, J., and Oliphant, T. (2010) Mutations within a
human IgG2 antibody form distinct and homogeneous disulfide isomers but
do not affect Fc gamma receptor or C1q binding, Protein Sci.,
19, 753-762.
23.Ryazantsev, S., Tischenko, V., Nguyen, C.,
Abramov, V., and Zav’yalov, V. (2013) Three-dimensional structure
of the human myeloma IgG2, PLoS One, 8, e64076.
24.Schuurman, J., Perdok, G. J., Gorter, A. D., and
Aalberse, R. C. (2001) The inter-heavy chain disulfide bonds of IgG4
are in equilibrium with intrachain disulfide bonds, Mol.
Immunol., 38, 1-8.
25.Aalberse, R. C., and Schuurman, J. (2002) IgG4
breaking the rules, Immunology, 105, 9-19.
26.Rispens, T., Ooijevaar-de Heer, P., Bende, O.,
and Aalberse, R. C. (2011) Mechanism of immunoglobulin G4 Fab-arm
exchange, J. Am. Chem. Soc., 133, 10302-10311.
27.Sedykh, M. A., Buneva, V. N., and Nevinsky, G. A.
(2013) Polyreactivity of natural antibodies: exchange by HL-fragments,
Biochemistry (Moscow), 78, 1305-1320.
28.Frangione, B., Franklin, E. C., Fudenberg, H. H.,
and Koshland, M. E. (1966) Structural studies of human gamma-G-myeloma
proteins of different antigenic subgroups and genetic specificities,
J. Exp. Med., 124, 715-732.
29.Zav’yalov, V. P., Abramov, V. M.,
Ivannikov, A. I., Loseva, O. I., Dudich, I. V., Dudich, E. I.,
Tischenko, V. M., Khechinashvili, N. N., Franek, F., Medgyesi, G.,
Zavodszky, P., and Jaton, J. C. (1981) Correspondence between structure
and function of immunoglobulin G subclasses, Haematologia,
14, 85-94.
30.Denesiuk, A. I., Tishchenko, V. M., Abramov, V.
M., and Zav’ialov, V. P. (1983) Theoretical and experimental
studies on the conformation of the hinge regions in human
immunoglobulin G subclasses, Mol. Biol. (Moscow), 17,
1262-1271.
31.Navalkar, R. G., Norlin, M., and Ouchterlony, O.
(1965) Characterization of leprosy sera with various mycobacterial
antigens using double diffusion-in-gel analysis, Int. Arch. Allergy
Appl. Immunol., 28, 250-260.
32.Michaelsen, T., and Natvig, J. B. (1974) Unusual
molecular properties of human IgG3 proteins due to an extended hinge
region, J. Biol. Chem., 249, 2778-2785.
33.Rispens, T. L., Ooievaar-De Heer, P., Vermeulen,
E., Schuurman, J., van der Neut Kolfschoten, M., and Aalberse, R. C.
(2009) Human IgG4 binds to IgG4 and conformationally altered IgG1 via
Fc–Fc interactions, J. Immunol., 182,
4275-4281.
34.Gilhespy-Muskett, A. M., Partridge, J., Jefferis,
R., and Homans, S. W. (1994) A novel 13C isotopic labelling
strategy for probing the structure and dynamics of glycan chains in
situ on glycoproteins, Glycobiology, 4, 485-489.
35.Zav’yalov, V. P., and Tishchenko, V. M.
(1991) Mechanisms of generation of antibody diversity as a cause for
natural selection homoiothermal animals in the process of evolution,
Scand. J. Immunol., 33, 755-762.
36.Tishchenko, V. M. (2001) Effect of hinge region
state on interaction of human IgG3 with the complement system,
Biochemistry (Moscow), 66, 1352-1355.
37.Bliznyukov, O. P., Kozmin, L. D., Vysotskaya, L.
L., Golenkov, A. K., and Tishchenko, V. M. (2005) Human immunoglobulin
light chains λ form amyloid fibrils and granular aggregates in
solution, Biochemistry (Moscow), 70, 458-466.
38.Grabar, P., and Williams, C. A. (1953) Method
permitting the combined study of the electrophoretic and the
immunochemical properties of protein mixtures: application to blood
serum, Biochim. Biophys. Acta, 10, 193-194.
39.Walker, J. M. (1994) The dansyl method for
identifying N-terminal amino acids, Methods Mol. Biol.,
32, 321-328.
40.Hartley, B. S. (1970) Strategy and tactics in
protein chemistry, Biochem. J., 119, 805-812.
41.Tishchenko, V. M. (2014) Relations between macro-
and microstability of CH2 domains and human IgG2 and their
biological activity. 1. Analysis of calorimetric and optical melting
curves, Mol. Biol. (Moscow), 48, 480-490.
42.Tishchenko, V. M. (2002) Molecular
biophysics-study of the domain structure of the protein Caf1M of
Yersinia pestis: structure and role of individual domains,
Biophysics (Moscow), 47, 218-225.
43.Michaelsen, T. E., Naess, L. M., and Aase, A.
(1993) Human IgG3 is decreased and IgG1, IgG2 and IgG4 are unchanged in
molecular size by mild reduction and reoxidation without any major
change in effector functions, Mol. Immunol., 30,
35-45.
44.Ellerson, J. R., Yasmeen, D., Painter, R. H., and
Dorrington, K. J. (1972) A fragment corresponding to the C(H)2 region
of immunoglobulin G (IgG) with complement fixing activity, FEBS
Lett., 24, 318-322.
45.Ellerson, J. R., Yasmeen, D., Painter, R. H., and
Dorrington, K. J. (1976) Structure and function of immunoglobulin
domains. III. Isolation and characterization of a fragment
corresponding to the gamma2 homology region of human immunoglobulin G1,
J. Immunol., 116, 510-517.
46.Tischenko, V. M., Abramov, V. M., and
Zav’yalov, V. P. (1998) Investigation of the cooperative
structure of Fc fragments from myeloma immunoglobulin G,
Biochemistry, 37, 5576-5581.
47.Timchenko, M. A., and Tischenko, V. M. (2013)
Destabilization of CH2 domains in intact IgG2 is accompanied
by reduced ability to inhibit complement system factor C1,
Biochemistry (Moscow), 78, 667-673.
48.Tishchenko, V. M., Khristoforov, V. S., and
Blizniukov, O. P. (2009) Thermodynamic and hydrodynamic study of
Bence–Jones proteins, Mol. Biol. (Moscow), 43,
148-156.
49.Tischenko, V. M. (2000) Metastable state of the
Fc fragment, J. Therm. Anal. Cal., 62, 63-68.
50.Weber, K., and Osborn, M. (1969) The reliability
of molecular weight determinations by dodecyl sulfate polyacrylamide
gel electrophoresis, J. Biol. Chem., 244, 4406-4412.
51.Laurell, C. B. (1965) Antigen–antibody
cross-electrophoresis, Analyt. Biochem., 10, 358-361.
52.Tischenko, V. M., and Zav’yalov, V. P.
(2002) Long-term metastable conformation of human Fc gamma subunit,
Immunol. Lett., 84, 241-245.
53.Mercola, D. A., Morris, J. W., and Arquilla, E.
R. (1972) Use of resonance interaction in the study of the chain
folding of insulin in solution, Biochemistry, 11,
3860-3874.
54.Yphantis, D. A. (1964) Equilibrium
ultracentrifugation of dilute solutions, Biochemistry, 3,
297-317.
55.Van Holde, K. E., and Baldwin, R. L. (1958) New
approach for molecular weight determination, J. Phys. Chem.,
62, 734-743.
56.Bowen, T. J. (1973) Introduction to
Ultracentrifugation [Russian translation], Mir, Moscow.
57.Tishchenko, V. M. (2000) The study of domain
structure of chaperone Caf1M from Yersinia pestis, Mol.
Biol. (Moscow), 34, 116-122.
58.Privalov, P. L., and Potekhin, S. A. (1986)
Scanning microcalorimetry in studying temperature-induced changes in
proteins, Meth. Enzymol., 131, 4-51.
59.Labrijn, A. F., Rispens, T., Meesters, J., Rose,
R. J., den Bleker, T. H., Loverix, S., van den Bremer, E. T., Neijssen,
J., Vink, T., Lasters, I., Aalberse, R. C., Heck, A. J., van de Winkel,
J. G., Schuurman, J., and Parren, P. W. (2011) Species-specific
determinants in the IgG CH3 domain enable Fab-arm exchange by affecting
the noncovalent CH3–CH3 interaction strength, J. Immunol.,
187, 3238-3246.
60.Davies, A. M., Rispens, T., den Bleker, T. H.,
McDonnell, J. M., Gould, H. J., Aalberse, R. C., and Sutton, B. J.
(2013) Crystal structure of the human IgG4 C(H)3 dimer reveals the role
of Arg409 in the mechanism of Fab-arm exchange, Mol. Immunol.,
54, 1-7.
61.Davies, A. M., Rispens, T., Ooijevaar-de Heer,
P., Gould, H. J., Jefferis, R., Aalberse, R. C., and Sutton, B. J.
(2014) Structural determinants of unique properties of human IgG4-Fc,
J. Mol. Biol., 426, 630-644.
62.Rispens, T., Davies, A. M., Ooijevaar-de Heer,
P., Absalah, S., Bende, O., Sutton, B. J., Vidarsson, G., and Aalberse,
R. C. (2014) Dynamics of inter-heavy chain interactions in human
immunoglobulin G (IgG) subclasses studied by kinetic Fab arm exchange,
J. Biol. Chem., 289, 6098-6109.
63.Michaelsen, T. E., and Natvig, J. B. (1972) The
hinge region of IgG3, an extended part of the molecule, FEBS
Lett., 28, 121-124.
64.Michaelsen, T. E., Frangione, B., and Franklin,
E. C. (1977) Primary structure of the “hinge” region of
human IgG3, J. Biol. Chem., 252, 883-889.
65.Gregory, L. L., Davis, K. G., Sheth, B., Boyd,
J., Jefferis, R., Nave, C., and Burton, D. R. (1987) The solution
conformations of the subclasses of human IgG deduced from sedimentation
and small angle X-ray scattering studies, Mol. Immunol.,
24, 821-829.
66.Abe, Y., Gor, J., Bracewell, D. G., Perkins, S.
J., and Dalby, P. A. (2010) Masking of the Fc region in human IgG4 by
constrained X-ray scattering modelling: implications for antibody
function and therapy, Biochem. J., 432, 101-111.
67.Schachman, H. K. (1959) Ultracentrifugation in
Biochemistry, Academic Press Inc., New-York-London.
68.Connell, G. E., and Porter, R. R. (1971) A new
enzymic fragment (Facb) of rabbit immunoglobulin G, Biochem. J.,
124, 53P.
69.Tischenko, V. M., Ryazantsev, S. N., and
Zav’yalov, V. P. (2014) Three-dimensional structure of the human
myeloma IgG4, Immnol. Lett., in press.
70.Tischenko, V. M., Zav’yalov, V. P.,
Medgyesi, G. A., Potekhin, S. A., and Privalov, P. L. (1982) A
thermodynamic study of cooperative structures in rabbit immunoglobulin
G, Eur. J. Biochem., 126, 517-521.
71.Tishchenko, V. M., Lund, L., Goodall, M., and
Jefferis, R. (1998) Cooperative structures within glycosylated and
aglycosylated mouse IgG2b, in Biocalorimetry. Applications of
Calorimetry in the Biological Sciences (Labbury, J. E., and
Chowhry, B. Z., eds.) Wiley, London, pp. 267-275.
72.Tischenko, V. M., Ichtchenko, A. M., Andreyev, C.
V., and Kajava, A. V. (1993) Thermodynamic studies of the collagen-like
region of human subcomponent C1q. A water-containing structural model,
J. Mol. Biol., 234, 654-660.
73.Padlan, E. F. (1990) X-ray diffraction studies of
antibody constant region, in Fc Receptor and the Action of
Antibodies (Metzger, H., ed.) American Society for Microbiology,
Washington, D. C., pp. 12-30.
74.Girardi, E., Holdom, M. D., Davies, A. M.,
Sutton, B. J., and Beavil, A. J. (2009) The crystal structure of rabbit
IgG-Fc, Biochem. J., 417, 77-83.
75.Min, S., Yan, F., Zhang, Y., Ye, X.,, Zhong, M.,
Cao, J., Zou, H., and Chen, J. (2014) Characterization of a novel
hemolytic activity of human IgG fractions arising from diversity in
protein and oligosaccharide components, PLoS One, 9,
e85711.
76.Tishchenko, V. M. (2014) Relations between macro-
and microstability of CH2 domains and human IgG2 and their
biological activity. II. Calculation of thermodynamic functions
characterizing the stability domain, Mol. Biol. (Moscow),
48, 842-849.