REVIEW: Structural and Functional Studies of Multiheme Cytochromes c Involved in Extracellular Electron Transport in Bacterial Dissimilatory Metal Reduction
T. V. Tikhonova* and V. O. Popov
Bach Institute of Biochemistry, Russian Academy of Sciences, Leninsky pr. 33, 119071 Moscow, Russia; E-mail: ttikhonova@inbi.ras.ru* To whom correspondence should be addressed.
Received July 18, 2014
Bacteria utilizing insoluble mineral forms of metal oxides as electron acceptors in respiratory processes are widespread in the nature. The electron transfer from a pool of reduced quinones in the cytoplasmic membrane across the periplasm to the bacterial outer membrane and then to an extracellular acceptor is a key step in bacterial dissimilatory metal reduction. Multiheme cytochromes c play a crucial role in the extracellular electron transfer. The bacterium Shewanella oneidensis MR-1 was used as a model organism to study the mechanism of extracellular electron transport. In this review, we discuss recent data on the composition, structures, and functions of multiheme cytochromes c and their functional complexes responsible for extracellular electron transport in Shewanella oneidensis.
KEY WORDS: bacterial dissimilatory metal reduction, extracellular electron transfer, multiheme cytochrome cDOI: 10.1134/S0006297914130094
Abbreviations: BDMR, bacterial dissimilatory metal reduction; ETC, electron transport chain; Fe-NTA, iron nitrilotriacetate; OMC, outer-membrane cytochrome c; rmsd, root-mean-square deviation.
Under anaerobic conditions, some bacteria utilize mineral (insoluble)
forms of variable-valence metal compounds as electron acceptors. The
ability of microorganisms to perform obligatory iron reduction (iron
breathing) under anaerobic conditions was shown for the first time by
Balashova and Zavarzin in 1979 [1]. In their
experiments, a bacterium identified as Pseudomonas sp. was found
to be responsible for the reduction of iron hydroxide and ferrihydrite
by molecular hydrogen. In the late 1980s, Lovely et al. found that
Fe(III) and Mn(IV) serve as the final electron acceptors in the growth
of microorganisms on non-fermentable organic compounds [2, 3] and showed that these
organisms are widespread in the nature [4-9]. The bacteria that were isolated in these studies
and assigned to the Geobacter and Shewanella genera were
later used as model species for investigation of biochemical and
physiological aspects of microbial metal reduction. This phenomenon,
referred to as bacterial dissimilatory metal reduction (BDMR), plays a
great role in transformations of such abundant and vitally important
elements as iron and manganese [10, 11]. In some marine, freshwater, and soil ecosystems,
dissimilatory iron(III) reduction by microorganisms is a key process
coupled to the oxidation of organic matter [9, 12]. The microbial reduction of Fe(III) might have
played an even more important role in the ancient biosphere, where
Fe(III) was apparently the first and, for a certain period of time, the
main oxidizing agent for organic carbon [13]. It
was hypothesized that dissimilatory Fe(III) reduction might have been
the first type of metabolism [14]. Now, bacterial
metal reduction is considered as a process having great
biotechnological potential [11, 15] for the manufacturing of fuel cells [16-19], microbial electron
transfer chains [20], and bioremediation of soils
contaminated with toxic metals, including radioactive ones, by the
reduction and transformations of these elements into less soluble forms
[15] (for example, of U(VI) into U(IV) [21] and Tc(VII) into Tc(V) [22]). Recently, the possibility of bacterial
extracellular reduction of graphene oxide has been reported, this
process being considered as an approach to the preparation of graphene
[23].
One of the main questions that has been raised in studies of BDMR using insoluble metal compounds as terminal electron acceptors – what is the mechanism of electron transfer from the cytoplasmic membrane, in which reduced quinones serve as the electron source, across the periplasm and the bacterial outer membrane to the insoluble substrate localized outside the cell? It should be noted that all microorganisms for which this mechanism has been studied are gram-negative bacteria. Consequently, all known mechanisms of extracellular electron transport take into account the structural features of the cells and cell membranes of gram-negative organisms. Investigations of BDMR in gram-positive bacteria are in their infancy [24-29].
KEY MECHANISMS OF EXTRACELLULAR ELECTRON TRANSPORT
The bacteria Shewanella oneidensis MR-1 and Geobacter sulfurreducens (the strains DL-1 and KN400 were studied in most detail [15]) are the best-known model organisms used for investigation of the mechanisms of extracellular electron transport to insoluble and soluble substrates. The results obtained for these two organisms and then tested using other gram-negative metal-reducing bacteria [30-35] suggest several underlying mechanisms of extracellular electron transport [4, 11, 14, 15, 36-38], which can take place simultaneously complementing each other under natural conditions.
The first mechanism involves multiheme cytochromes c whose functional complexes form extended electron transport chains responsible for electron transfer from reduced quinones in the cytoplasmic membrane to redox-active proteins anchored at the extracellular side of the bacterial outer membrane and then to extracellular soluble and insoluble substrates ([11, 15, 39-44] and references therein). Evidently, this mechanism can take place only if there is a close contact (shorter than 15-20 Å) between the outer surface of the bacterial cell containing terminal reductases and an extracellular electron acceptor. In this case, direct electron transfer can occur between the solvent-exposed heme of extracellular c-type cytochrome serving as a terminal reductase and the electron acceptor [45]. For this electron transfer to occur, cells of dissimilatory metal-reducing bacteria are equipped with mechanisms for detection of substrates, taxis to the substrate, and interactions with the surface of the insoluble substrate [46-48]. The latter step depends on the ability of the cell to synthesize outer-membrane cytochromes c. Mutants of S. oneidensis, which do not produce outer-membrane cytochromes, cannot interact with the surface of insoluble substrates [48].
The second mechanism that supplements and extends the possibilities of the first mechanism is associated with the synthesis of soluble low molecular weight redox mediators (e.g. flavins) secreted by the cell into the environment [11, 15, 49-54]. This mechanism was studied in most detail in the bacterium S. oneidensis MR-1 [49, 51-56]. The level of riboflavin and FMN secreted by the bacterium S. oneidensis MR-1 depends on the nature of the electron acceptor and is increased in cell growth on iron(III) compounds [56]. It is hypothesized that the reduction of mediators occurs at the same multiheme cytochromes c anchored to the outer membrane (hereinafter, referred to as outer-membrane c-type cytochromes (OMC)) as those that mediate direct electron transfer to insoluble acceptors by the first mechanism [54, 55]. This is why flavins do not reduce insoluble substrates in the absence of OMC. The contribution of flavins to the overall rate of electron transfer to insoluble iron oxide is 75-80% [52, 53] or, according to other data, in the presence of micromolar concentrations of flavins, the rate of electron transfer by the cytochrome MtrC to an electrode increased by a factor of ten [54]. The rate of reduction of soluble extracellular substrates remains unchanged in the presence of flavins [52, 54].
The third mechanism, which has been extensively discussed in recent years, is associated with the formation of electrically conductive pili in some metal-reducing bacteria. This concept was considered in most detail for the bacterium G. sulfurreducens [15, 57-59]. According to this concept, electrically conductive pili (the length of one pilus is 10-20 µm) make the major contribution to the electron transport to insoluble acceptors in G. sulfurreducens. These pili are formed during the growth of bacteria on insoluble Fe(III) oxide [60, 61]. Deletion mutants of G. sulfurreducens that do not produce pili cannot efficiently reduce iron oxide. According to the data reported by D. Lovley [15, 57-59], the pili have metallic-like conductivity, which increases as the temperature is decreased. The protein pilin (PilA) makes the major contribution to the conductivity of pili [57-59]. It is suggested that the metallic-like conductivity is possible due to the overlap of π orbitals of aromatic residues and electron delocalization in PilA [57, 59]. This type of conductivity in proteins was found here for the first time. In biofilms produced on electrodes, pili mediate electron transport over distances of up to 1 cm (so-called long-range electron transfer) [57, 58].
Multiheme cytochromes c have no effect on the electron transfer through pili [15, 58], but they are apparently essential for the electron transfer from pili to acceptors [58], i.e. they serve as terminal reductases. The key role was assigned to the octaheme cytochrome OmcS specifically associated with pili [58, 62, 63]. It also cannot be ruled out that multiheme cytochromes are involved in electron transfer from the cytoplasmic membrane to the pili [58].
In addition, multiheme cytochromes c can serve the function of accumulating and storing electrons, thus providing electron transfer from the inner membrane and proton gradient formation in the absence of acceptors [58, 62, 64].
Even this cursory analysis of the three mechanisms under consideration shows that multiheme cytochromes play a key role in electron transfer from the cytoplasmic membrane to the external surface of the outer membrane, where they are utilized for different purposes. The present review discusses available data on the composition, structures, and functions of multiheme cytochromes c and their functional complexes involved in extracellular electron transport.
EXTRACELLULAR ELECTRON TRANSPORT IN THE BACTERIUM Shewanella
oneidensis MR-1
The scheme of extracellular electron transport proposed for the gram-negative facultatively anaerobic bacterium S. oneidensis MR-1 has been studied in most detail. S. oneidensis MR-1 can couple the oxidation of different organic substrates to the reduction of a broad range of terminal electron acceptors. The acceptors can be either soluble or insoluble. The reduction of the former, such as O2, fumarate, nitrate, nitrite, thiosulfate, and sulfite occurs in the periplasm, whereas the reduction of the latter takes place outside the cell and requires electron transport to the surface of the outer membrane and then to the acceptor. Among the latter acceptors are Fe(III) and Mn(IV) oxides, including those present in minerals. Amorphous Fe(III) hydroxides, weakly crystalline iron oxides (e.g. ferrihydrite 5Fe2O3·9H2O), and highly crystalline iron oxides (e.g. hematite α-Fe2O3, magnetite Fe3O4, and goethite α-FeOOH) [65] are the most widespread forms of iron subjected to microbial reduction. The reduction of such soluble electron acceptors as dimethyl sulfoxide (DMSO) and iron citrate, as well as compounds of chromium(VI), uranium(VI), and technetium(VII), also occurs extracellularly [42].
The respiratory flexibility of S. oneidensis MR-1 is provided by the presence of a multicomponent and branched electron transport chain (ETC). This chain includes cytochromes c as the main components, which are encoded by 42 genes identified in the genome of this bacterium [66]; about 30 cytochromes c contain two or more c-type hemes. The inactivation of the target genes and the subsequent phylogenetic analysis of mutants for the ability to reduce soluble and insoluble iron compounds resulted in the identification of four multiheme cytochromes c (CymA, MtrA, MtrC, and OmcA) and the transmembrane protein MtrB as the minimum set of proteins necessary for extracellular electron transport to insoluble acceptors (mtr, metal reducing; omc, outer-membrane cytochrome) [32, 67-70].
The genes of four proteins − mtrC, mtrB, mtrA, and omcA − are arranged in the genome of S. oneidensis MR-1 in one mtrCAB–omcA–mtrDEF gene cluster, which includes also a set of mtrDEF genes paralogous to mtrCAB genes (the genes are listed in the order of their localization in the cluster) [43]. The expression of the omcA, mtrCAB, and mtrDEF genes is regulated by different promoters. This fact suggests that the proteins encoded by these genes perform different functions in the cell. It was hypothesized that the MtrCBA complex is synthesized in individually living cells, whereas the MtrDEF complex is synthesized during the cell growth in biofilms [41]. In addition, it was shown that MtrCBA and OmcA have an enhanced level of expression under anaerobic growth conditions, whereas MtrDEF exhibits an enhanced level of expression under aerobic conditions [44].
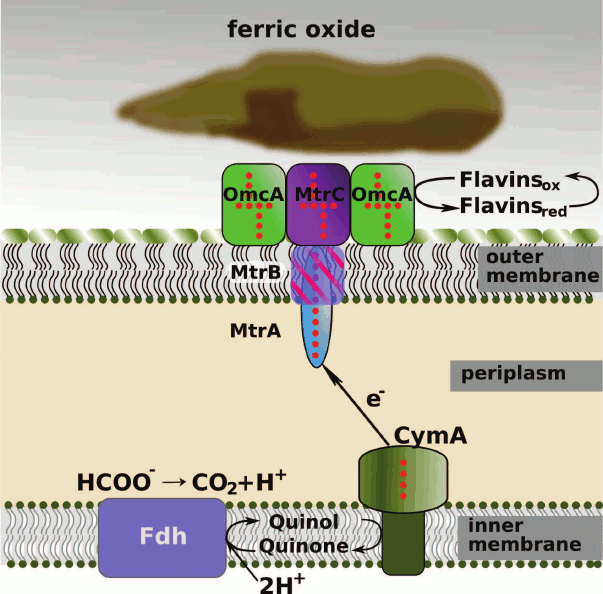
These proteins together form a chain providing electron transfer: (i) from the pool of reduced quinones in the cytoplasmic membrane to the periplasm; (ii) across the periplasm to the extracellular surface of the outer membrane; (iii) from the outer membrane to the surface of the insoluble acceptor (iron oxide in Fig. 1).
Fig. 1. Scheme of electron transport in the bacterium S. oneidensis MR-1: from the pool of reduced quinones in the cytoplasmic membrane to extracellular electron acceptors (e.g. iron(III) oxide). The hemes in c-type cytochrome molecules are indicated by red points. Formate is shown as the electron donor, Fdh is formate dehydrogenase. Soluble periplasmic cytochromes FccA, STC, and NrfA, as well as the soluble fraction of MtrA, can be involved in the electron transfer from CymA to MtrA.
Electron transfer from cytoplasmic membrane to periplasm. The protein CymA is the first protein in the extracellular electron transport chain. This is a tetraheme cytochrome c belonging to the NapC/NirT family of QH2-quinol dehydrogenases [42, 71]. Magnetic circular dichroism study showed that three c-type hemes of CymA are coordinated by histidine residues in the proximal and distal positions, whereas the fourth, high-spin, heme is coordinated by a water molecule in the distal position. The presence of a high-spin heme in the CymA molecule was confirmed by EPR spectroscopy [72]. Spectropotentiometric titration showed that the redox potentials of the low-spin His/His hemes are –110, –190, and –265 mV, and the potential of the high-spin His/H2O heme is –240 mV [73]. The spectropotentiometric titration data were confirmed by cyclic voltammetry of CymA on gold and graphite electrodes [74].
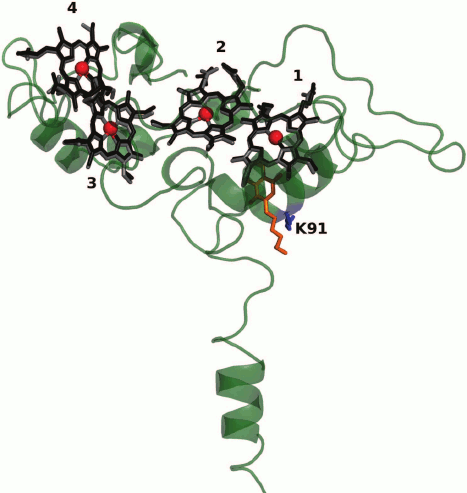
The three-dimensional structure of CymA is unknown. A model of CymA was built based on the known structure of tetraheme quinol dehydrogenase NrfH from the bacterium Desulfovibrio vulgaris that is homologous to CymA. According to this model, the protein consists of one transmembrane α-helix, through which the protein is attached to the cytoplasmic membrane, and a globular periplasmic domain containing four c-type hemes (Fig. 2) [42, 71, 75]. By analogy with D. vulgaris NrfH [76], it was suggested that the high-spin heme 1 located at the interface between the periplasm and the cytoplasmic membrane is involved in the quinol-oxidizing site [42] and serves as the entry site for electrons into the ETC of CymA. Reduced form of menaquinone-7 (MQ-7, Em ~ –80 mV), which is the main quinone of the cytoplasmic membrane under anaerobic growth conditions, serves as the electron donor for CymA [74]. However, protein film voltammetry (PFV) data suggest that MQ-7 is not only a substrate but also an addition cofactor for CymA that is necessary for the redox process to occur. It was hypothesized [74] that CymA has two quinone-binding sites. One has high affinity for MQ-7 and binds quinone as the cofactor, whereas the other binding site characterized by low affinity binds reduced quinones, including those other than MQ-7, as the substrates (electron donors). Residue Lys91 conserved in the NapC/NirT family of QH2-quinol dehydrogenases located near heme 1 is involved in the binding and oxidation of menaquinol [75].
Fig. 2. Model of CymA [75] constructed using the Swiss Modeler server based on the three-dimensional structure of tetraheme quinol dehydrogenase NrfH from the bacterium D. vulgaris (PDB ID: 2VR0) [76] homologous to CymA. The superimposition of the hemes in CymA (in black) and D. vulgaris NrfH (in gray) is shown. The hemes are numbered according to the position of the heme-binding motifs CxxCH in the amino acid sequence. Heme 1 is the possible cofactor/substrate MQ-7-binding site. A redox-inactive analog of the substrate – 2-heptyl-4-hydroxyquinoline-N-oxide – in the structure of D. vulgaris NrfH [76] is shown in orange. Residue Lys91 presumably involved in the binding and oxidation of the substrate [75] is indicated.
Comparison of the redox potentials of the hemes of CymA and primarily of the high-spin heme 1, which is the presumed acceptor of electrons (–240 mV) and menaquinone MQ-7 (–80 mV), shows that the in vivo observed electron transfer from reduced MQ-7 to CymA is thermodynamically hindered. This is confirmed by in vitro experiments in which the reverse electron transfer from reduced CymA to MQ-7 takes place [74]. The direction of the process in the cell is probably determined by the potential of the NAD/NADH couple (–320 mV), the oxidation of which as the formate dehydrogenase cofactor is accompanied by the formation of reduced quinones. It was also hypothesized that the binding of MQ-7 as the cofactor can influence the potential of the c-type hemes of CymA [77]. Thus, the degree of reduction of non-purified CymA with the redox-active menaquinol analog, 2,3-dimethoxy-1,4-naphthoquinol (Em = –75 mV) in membrane fractions of Shewanella sp. strain ANA-3 cells is 25% of the corresponding value achieved using dithionite (Em = –660 mV) [75].
The formation of a transmembrane proton gradient at the cytoplasmic membrane associated with ATP synthesis apparently takes place in the step of feeding electrons into a quinone pool [41, 71]. The subsequent electron transfer to CymA and then to other electron acceptors is not coupled with the energy accumulation in the cell.
The unique role of CymA in redox processes occurring in the periplasm is that this protein can transfer electrons to a broad range of electron acceptors, thus activating enzymatic redox systems utilizing different terminal electron acceptors. It is supposed that, in addition to the reduction of soluble and insoluble metal compounds, CymA is involved in the reduction of O2, DMSO, fumarate, nitrite, and nitrate in the bacterium S. oneidensis MR-1 [49, 67, 78-81], as well as for the reduction of arsenate in Shewanella sp. strain ANA-3 [75] and S. putrefaciens [82]. In these processes, the decaheme c-type cytochrome MtrA and its paralog MtrF responsible for the metal reduction, the decaheme c-type cytochrome DmsE involved in the extracellular reduction of DMSO, fumarate reductase FccA [83], and nitrite reductase NrfA [42, 84] serve as electron acceptors from CymA.
Periplasmic electron transport. In extracellular metal reduction processes, the periplasmic decaheme protein MtrA serves as the electron acceptor from CymA. The mtrA gene-deleted S. oneidensis MR-1 cells do not reduce iron(III) citrate but reduce other electron acceptors, such as nitrate, nitrite, etc. [85]. Recombinant MtrA was expressed in S. oneidensis and E. coli and was then isolated, purified, and characterized [70]. The molecular weight of MtrA is 32 kDa. This protein contains 10 low-spin bis-histidine coordinated c-type hemes having redox potentials in the range from –100 to –400 mV [70]. The reduced CymA can completely reduce in vitro MtrA, which is then oxidized by soluble Fe(III) complexes [86].
The three-dimensional structure of MtrA is unknown. However, it was shown that the polypeptide chain of MtrA can be divided into two pentaheme domains, each domain being homologous to the pentaheme c-type cytochrome NrfB responsible for the electron transport in the nitrite reductase complex NrfAB [42]. The three-dimensional structure of NrfB from E. coli was determined [87]. NrfB is characterized by a linear heme arrangement typical of a diverse family of multiheme cytochromes c [88]. This heme arrangement consists of alternating parallel and perpendicular di-heme motifs. The distances between the adjacent hemes in the NrfB molecule are not larger than 6 Å, thus providing fast electron exchange between the hemes that form an ETC. It was hypothesized [42] that the hemes in the MtrA molecule are also closely spaced, thus enabling efficient electron transfer. This conclusion is confirmed by the very small number of amino acid residues per c-type heme in the MtrA molecule (34 residues); this number in multiheme proteins is, on the average, 60-70 residues per heme [77]. The length of the five-heme ETC in NrfB is 40 Å [87]. Therefore, in the case of the linear arrangement of the domains, the length of the 10-heme chain in MtrA is estimated as 80 Å [89].
In the cell, MtrA is involved in the ternary complex MtrCAB, which also contains the transmembrane porin-like protein MtrB and the decaheme c-type cytochrome MtrC anchored to the external side of the outer membrane. The ternary complex is so stable that it was isolated and characterized [90]. It is assumed that MtrA and MtrC are inserted inside the MtrB pore from the opposite sides in such a way as to enable the direct electron transfer from the solvent-exposed heme of one protein to the nearest heme of another one (Fig. 1) [89]. In addition to the electron transport, MtrA is responsible for the stabilization of MtrB by protecting it from nonspecific proteolysis with the periplasmic protein DegP [91]. The mechanism of protection is unknown, but it is apparently associated with the ability of MtrA to form a stable binary complex with MtrB in solution (in the periplasm). According to an alternative hypothesis, MtrA can act as a chaperone, by binding with the unstructured form of MtrB in the periplasm and assisting the MtrB folding in the complex with MtrA [91]. This hypothesis is supported by the fact that MtrB was not inserted into the outer membrane in the case of the disturbance of MtrA expression [40, 91]. The protein MtrC does not form a binary complex with MtrB.
Small-angle X-ray scattering (SAXS) study showed that the MtrA molecule has an elongated shape with size of 104 × 20 × 50 Å [92]. This is in agreement with the assumption of the linear 10-heme ETC about 80 Å in length [89]. The degree of penetration of MtrA into the MtrB pore is unknown. Amino acid sequence analysis of MtrB using the PRED-TMBB server predicted the three-dimensional organization of MtrB [91]. The MtrB molecule comprises 28 β-strands forming a transmembrane β-barrel. The diameter of the pore inside the barrel is 30-40 Å. The β-strands are linked by 14 long and 13 short solvent-exposed loops on both sides of the lipid membrane. The long chains are directed toward the periplasm and are involved in interactions between MtrB and MtrA [93]. A comparison of the lateral sizes of MtrA and the diameter of the MtrB pore suggests that MtrA can rather deeply penetrate into the MtrB pore (Fig. 1). This may be facilitated by the above-mentioned fact that the MtrB folding can take place in the complex with MtrA. The deep penetration of MtrA into the MtrB pore to provide contact with MtrC is necessary because MtrC having lateral sizes of 70 × 30 Å (see below) cannot penetrate inside the MtrB pore and binds on the surface of the MtrAB complex localized on the extracellular side of the membrane through interactions with the short loops of the β-barrel of MtrB [93].
The thickness of the outer membrane in the bacterium S. oneidensis is 70-80 Å [42, 94]. Therefore, the length of MtrA (104 Å) is sufficient for crossing the membrane to come into contact with MtrC, but this length is insufficient for the simultaneous direct contact with CymA serving as the electron donor for MtrA, because the average thickness of the periplasm varies, according to different estimates, from 150 [94] to 235 Å [42], whereas the length of the ETC formed by four hemes of CymA is about 40 Å [75]. Soluble periplasmic proteins that could be involved in electron transfer between Cym and MtrA are not clearly known. The STC (small tetraheme cytochrome c) and fumarate reductase FccA (flavocytochrome c3) were suggested as possible candidates. The level of expression of both proteins increases during growth of S. oneidensis on Fe(III) oxide [86]. Moreover, MtrA and FccA are the most abundant periplasmic c-type cytochromes in S. oneidensis during its growth on Fe(III) oxide.
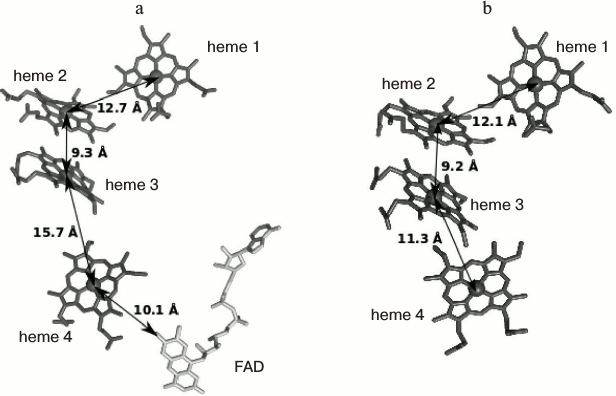
The structure of FccA from Shewanella frigidimarina was determined (PDBID 1QJD) [95] (Fig. 3). FccA is a soluble monomeric protein consisting of the following two domains: the N-terminal domain, which contains four c-type hemes and is responsible for the electron transport, and the C-terminal catalytic domain containing noncovalently bound FAD [96, 97]. In vitro experiments confirmed that FccA can undergo rapid electron exchange with both MtrA [86] and CymA [90], thus acting as the electron shuttle between the two proteins. Additionally, FccA can act as a transient electron storage protein in the periplasm of S. oneidensis cells [86].
Fig. 3. Arrangement of four c-type hemes forming an electron transport chain in (a) FccA (PDB ID 1D4C) (the FAD molecule present in the catalytic site is additionally shown), and (b) STC (PDB ID 1M1P). The arrows indicate the distances between the heme iron ions.
The structure of STC from S. oneidensis was determined (PDB ID 1M1P [98]). It can be seen that the arrangement of hemes and the overall fold of the heme-binding domain in STC are similar to those in FccA [44]. The hemes are arranged in a linear ETC consisting of hemes 2 and 3 in a parallel orientation and hemes 1 and 4 located perpendicular to the former two hemes. The mutual orientation of the hemes and the distance between them are favorable for efficient electron transfer through the ETC [88]. However, the construction of organisms with knockout genes encoding both proteins did not lead to a decrease in the rate of metal reduction and, therefore, did not confirm the involvement of either protein in this process [42, 86, 89]. Numerous electron transfer pathways and the interchangeability of proteins in certain stages can be regarded as one of the possible explanations of this fact [43, 89]. Recent analysis of interactions of the three main components of the fraction containing the periplasmic cytochromes FccA, STC, and ScyA (monoheme cytochrome c5) with CymA and MtrA by NMR spectroscopy showed [99] that the interactions of FccA with CymA and MtrA are characterized by dissociation constants of 398 and 35 µM, respectively. The corresponding dissociation constants for the interactions of STC are 250 and 572 µM. In addition, the authors of the cited study confirmed that both FccA and STC can accept electrons from the reduced forms of CymA and MtrA, thus providing electron transport in both directions. The proteins FccA and STC do not interact with each other, despite their high concentrations in the periplasm and the overlapping regions of the redox potentials (from –102 to –238 mV for FccA and from –125 to –215 mV for STC) [97, 100]. The formation of labile complexes of both proteins with CymA is consistent with the broad substrate specificity of the latter. The hemes responsible for the electron transfer to the molecule of the redox partner were identified for the first time by NMR spectroscopy. Thus, FccA interacts with CymA and MtrA through heme 2, whereas STC interacts through the most solvent-exposed heme 4. In both molecules (FccA and STC), the surface of the protein globule at the supposed contact site with the redox partner has a high negative charge. Therefore, the absence of the interaction between STC and FccA can be attributed to mutual repulsion. Since the three-dimensional structures of CymA and MtrA are unknown, the sites of interaction with FccA and STC cannot be identified. Instability of the resulting complexes and the interactions of FccA and STC with the electron donor and acceptor through the same heme provide evidence that the extracellular electron transport is not accompanied by the formation of stable multicomponent electron transport protein chains crossing the periplasm [99].
ScyA oxidizes CymA but does not interact with MtrA; diheme cytochrome c peroxidase is a possible electron acceptor for this protein [99, 101].
Pentaheme nitrite reductase NrfA, which is also expressed under these conditions, is regarded as another possible electron shuttle between CymA and MtrA [42]. It is known that cytochromes NrfB or NrfH, whose genes are arranged in a single cluster with the nrfA gene, act as electron donors for the well-characterized NrfA from different organisms. However, the genes of these proteins are absent in the S. oneidensis genome. It was speculated that the protein CymA homologous to NrfH acts as the electron donor for NrfA in nitrite reduction [84]. It was also shown that NrfA from E. coli can exchange electrons with MtrA heterologically expressed in E. coli [70]. Therefore, NrfA can transfer electrons from CymA to MtrA in the absence of nitrite.
The second MtrA molecule can also act as the soluble periplasmic cytochrome c, which transfers electrons to MtrA bound to the membrane through the formation of a complex with MtrB. It was shown [86] that, contrary to the earlier assumption, only some MtrA molecules are present in the membrane fraction, whereas 46% of the total amount of MtrA in the cell is located in the periplasm.
Outer-membrane multiheme c-type cytochromes. Electron transport to an extracellular acceptor. The key step in the BDMR process is the electron transfer to the extracellular terminal electron acceptor (soluble or insoluble). In the bacterium S. oneidensis, this process is performed by the decaheme c-type cytochromes OmcA and MtrC localized on the extracellular side of the outer membrane, as well as by MtrF encoded by the mtrF gene paralogous to mtrC. The cytochromes MtrC and MtrF involved in the transmembrane electron transfer complexes MtrCAB or MtrDEF accept electrons from MtrA (MtrE). Then MtrC or MtrF can transfer electrons either directly onto the external electron acceptor or to the other extracellular decaheme cytochrome OmcA [55, 102, 103]. The key role of MtrC and OmcA in BDMR was confirmed by several experimental data sets. First, it was shown that the mtrC and omcA gene-deleted mutants are characterized by substantially lower rate of reduction of insoluble iron and manganese compounds [104]. The double mutant reduced ferrihydrite at a rate of 14% of the activity of the wild-type cells [105].
Second, it was shown that the individual genetically engineered specimens MtrC and OmcA reduce soluble iron compounds at rates comparable to the rates of their reduction by whole cells or a membrane fraction of these cells [102]. In addition to the reduction of soluble substrates, MtrC and OmcA can transfer electrons to a graphite electrode serving as the insoluble substrate and reduce insoluble forms of iron oxide, such as hematite and goethite [82, 102, 105, 106], the reduction of the latter occurring at much lower rates, which can be attributed to both a non-optimal orientation of cytochromes on the mineral surface and the experimental conditions used [89].
Finally, atomic force microscopy study showed that recombinant MtrC and OmcA are bound to the hematite surface [107] to form single layers. The binding depends on pH and ionic strength [108]. The interaction of OmcA with the hematite surface is twice as strong as that of MtrC [107]. The binding of OmcA to hematite surface was confirmed by dynamic light scattering and fluorescence methods [109]. These data support the ability of both cytochromes to directly interact with the mineral surface. The Ser/Thr-Pro-Ser/Thr motif at the C-terminus of the amino acid sequences of MtrC and OmcA was found to be responsible for the binding of the proteins to the surface of hematite (Fe2O3) [110], thus providing hydrogen bonding through serine or threonine hydroxyl groups.
Properties and structures of MtrC and OmcA. The proteins MtrC and OmcA each contain 10 low-spin bis-histidine-coordinated c-type hemes [82, 106] that form an ETC. The spectropotentiometric titration of MtrC in solution and the study of this protein by PFV showed that MtrC is reversibly titrated between the completely oxidized and completely reduced states in the potential range from +100 to –400 mV [106]. The EPR spectra of MtrC show signals of both isolated and magnetically coupled c-type hemes [106]. The hemes in OmcA are redox-active in the potential range from –180 to –400 mV. The potentiometric titration curve is characterized by the Em values of –243 and –324 mV for two sets of approximately isopotential hemes [82]. A wide range of redox transitions of c-type hemes in the MtrC and OmcA molecules (from +100 to –400 mV) confirms that these proteins are thermodynamically able to reduce most Fe(III) compounds, including such low-potential minerals as hematite (Fe2O3, Em = –230 mV) [89]. It was impossible to determine the potentials of individual hemes by the above-mentioned methods because of the similar structures of the hemes and the overlap of the redox potentials.
Scanning electron microscopy study showed that MtrC and OmcA differ in the ability to transfer electrons, and it was concluded that these proteins play different physiological functions [111]. The protein MtrC is copurified during isolation with OmcA to form a 1 : 2 complex. The dissociation constant for this complex is 0.5-1 µM depending on the ionic strength of the solution. The MtrC–(OmcA)2 complex was shown to be more active in the reduction of Fe(III)-NTA compared to each of these individual proteins, which is indicative of an important role of this complex in electron transport in S. oneidensis MR-1 [112].
The three-dimensional structures of MtrF homologous to MtrC (30% identity in the amino acid sequence) and OmcA were determined by X-ray crystallography at 3.2 Å [105] and 2.7 Å [113] resolution, respectively. The two structures are very similar. The three-dimensional model of MtrC was constructed relying on the conserved structure of extracellular multiheme cytochromes of the MtrC/OmcA family [114]. According to the structural data, MtrF has an ellipsoid shape with size of 85 × 70 × 30 Å. As mentioned above, this fact excludes the possibility of the deep penetration of MtrF or its homolog MtrC inside the pore of the transmembrane protein MtrD/MtrB.
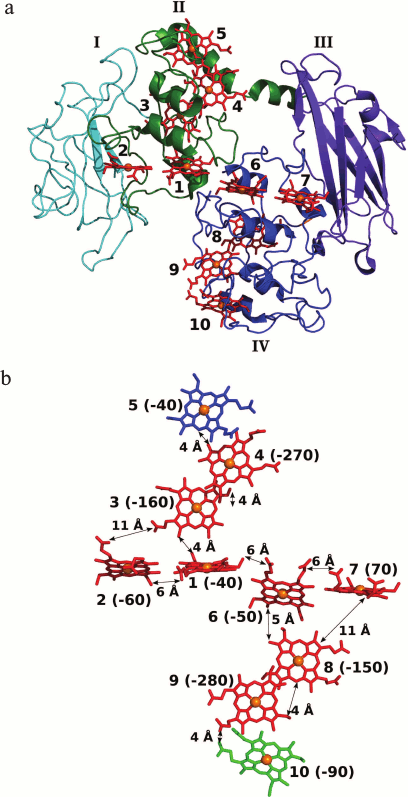
The MtrF molecule consists of four domains (Fig. 4). Domains I and III have a Greek key split β-barrel structure. Domains II and IV are composed mainly of α-helices and contain five c-type hemes each. Domains I and II are superimposed onto domains III and IV with rmsd of 2.8 Å, which suggests that the four-domain decaheme structure arose from the duplication of the pentaheme protomer. The ten hemes in the MtrF molecule are arranged to form a staggered cross [105] (Fig. 4). The staggered 65-Å chain (the longer crossbeam) comprising hemes 5, 4, 3, 1, 6, 8, 9, and 10 transects the length of the protein and is crossed by the 45-Å chain (the shorter crossbeam) composed of hemes 2, 1, 6, and 7. The staggered cross arrangement of hemes found in outer-membrane multiheme c-type cytochromes is unique among other classes of multiheme cytochromes and is apparently related to the unique function of these proteins responsible for electron transport to insoluble acceptors. The heme arrangement consists of three structural motifs [118]. First, this is the so-called heme stacking motif, in which the planes of the hemes are parallel to each other, thus providing the maximum overlap of the π-orbitals of the hemes and the highest rate of electron transfer between the hemes [118]. This motif includes hemes 5, 4, 3 and 8, 9, 10, for which the distance between the edges of the hemes is not larger than 4 Å. The second heme motif found in outer-membrane c-type cytochromes is T-shaped, in which the planes of the hemes are perpendicular to each other. This arrangement is observed for hemes 3 and 1 (the distance between the edges of the hemes is 4 Å) and hemes 8 and 6 (5 Å). The third motif is a chain of hemes lying almost in one plane. This arrangement is observed for hemes 2, 1, 6, and 7; the distances between the edges of the hemes are not larger than 6 Å, which also implies the possibility of efficient direct electron transfer (tunneling) from each heme to the adjacent one [115]. Direct electron transfer is also possible between hemes 2-3 and 7-8, which are located at a distance of 11 Å [105].
Fig. 4. a) Three-dimensional structure of S. oneidensis MtrF (PDB ID 3PMQ). Domains I and III having a Greek key split β-barrel structure are shown in cyan and violet, respectively; domains I and IV containing 10 hemes (the hemes are shown in red, the heme iron atoms are represented by orange spheres) are shown in green and blue, respectively. b) Arrangement of hemes in the MtrF molecule; the orientation corresponds to the orientation of the molecule in Fig. 4a. Terminal hemes 5 and 10 are shown in blue and green, respectively. The distances between the edges of the c-type hemes [105] are given. The potentials of hemes in mV are given in parentheses [120].
All of the hemes are solvent-exposed. The average solvent-accessible surface area is 173 ± 90 Å2. The most solvent-exposed terminal hemes 5 and 10 (the solvent-accessible surface area is about 300 Å2) are localized at the opposite ends of the longer crossbeam of the staggered cross and can serve as the entry and exit sites for electron into/from the cytochrome ETC. A detailed analysis of the MtrF structure, including the search for sites responsible for protein–protein interactions and the analysis of conserved residues at the possible protein–protein interfaces, showed that domains I and IV can be involved in protein–protein contacts. A cluster of highly conserved residues, which can be responsible for the binding to MtrDE, was found near the region of heme 10 on the surface of domain IV. Based on these data, it was concluded that heme 10 can serve as the entry site for electrons into the ETC of MtrF from the terminal heme of MtrE [105]. In his case, heme 5 of MtrF is the terminal heme responsible for the electron transfer to the acceptor: OmcA or variable-valence metal compounds. A high degree of exposure to solvent of the hemes creates a negative charge on the surface of MtrF due to the presence of 20 heme propionate side chains. This charge is compensated by the binding of about 18 Ca2+ ions to each MtrF molecule.
The Greek key split β-barrel structure found for domains I and III is characteristic of flavin-binding domains. Therefore, it was hypothesized that domains I and III might be responsible for the binding of extracellular flavins followed by the reduction of the latter with the participation of one or two adjacent hemes. Hemes 2 and 7 located at the distance of 14 Å from the centers of domains I and III can perform electron transfer to the flavins bound to these domains [105]. If this is the case, OmcA and MtrC/MtrF can play the following two roles in the respiratory process: perform direct electron transfer to the acceptor, and cyclic reduction of low molecular weight mediators (flavins in the case of S. oneidensis MR-1), which also transfer electrons to the terminal acceptor [89].
As mentioned above, OmcA is structurally very similar to MtrF. The same molecular organization and the ETC structure were found in yet another extracellular c-type cytochrome, the undecaheme cytochrome UndA [116], which probably performs functions analogous to OmcA in Shewanella sp. strain HRCR-6 [117]. All three structures are characterized by a four-domain structure, with hemes being arranged in a unique staggered cross motif. An extra eleventh heme in UndA (heme 7) is involved in the shorter crossbeam of the staggered cross immediately next to heme 8 (heme 7 in OmcA and MtfA) and is perpendicular to the latter (Fig. 5). The arrangement and orientation of hemes 5 in the three structures are the least conserved, which supports the hypothesis that these hemes are responsible for the interactions of the c-type cytochromes under consideration with electron acceptors of different nature [113, 116].
Fig. 5. Arrangement of hemes in UndA molecules [116]. The hemes in UndA are shown in black; the iron atoms are represented by spheres. The hemes are numbered according to the position of the heme-binding motifs CxxCH in the amino acid sequence. For the sake of comparison, the superimposition of the hemes in UndA and MtrF (shown in gray) is presented. The numbering of the hemes in MtrF is given in parentheses.
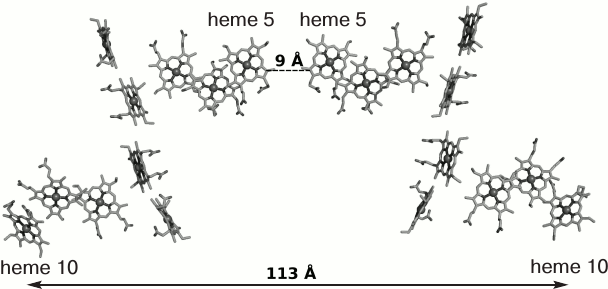
Despite the similarity of the three-dimensional structures, each extracellular multiheme cytochrome has a number of distinguishing features associated apparently with different functions of these proteins in the cell. The 3-D structure-based amino acid sequence alignment of MtrF, UndA, and OmcA revealed insertions and deletions in each protein, which shield the heme propionate groups, thus influencing the electronegativity of the surface and, as a consequence, the interaction of the c-type cytochrome with the substrate surface [113]. A change in the nearest environment of the hemes leads also to a change in their redox properties, which accounts for the different electrical conductivity of the decaheme cytochromes MtrC and OmcA noted earlier [111]. Besides, it was shown that OmcA exists as a dimer in crystals (Fig. 6). Despite the small interface surface area between the monomers in the OmcA dimer (about 500 Å2) and low stability of the dimer, the formation of the dimer can play a substantial role in the formation of the ETC. The formation of the OmcA dimer results in contact between terminal hemes 5 of different monomers and the formation of a 113-Å 20-heme chain. The possibility of the existence of this dimer on the cell surface was confirmed by the isolation of the MtrC–(OmcA)2 complex from S. oneidensis cells [112]. In the OmcA dimer, only hemes 10 remain free and can form contacts both with MtrC by accepting electrons and with the surface of the electron acceptor (e.g. hematite). The above-mentioned Thr-Pro-Ser motif responsible for the binding to the hematite surface is present in the OmcA structure just between hemes 9 and 10 [113], thus providing a short contact between heme 10 and Fe(III) ions of the acceptor.
Fig. 6. Arrangement of hemes in the OmcA dimer. The distance between hemes 5 of different monomers is 9 Å; the length of the possible electron-transport chain is 113 Å (the distance between the iron atoms of hemes 10) [113].
It should be noted that UndA, which presumably performs the same functions in the cell as the protein OmcA, exists in the crystal as a monomer [116]. Complexes of UndA with soluble substrates – Fe(III) citrate and Fe-NTA – were obtained by soaking crystals of free UndA in a solution of the corresponding ligand, and their three-dimensional structures were determined [116]. In both complexes, the acceptor molecules are bound near solvent-exposed heme 7. In the resulting complex, the distance between the porphyrin ring of heme 7 and Fe(III) ions was not larger than 8.5 Å, which implies the possibility of direct electron transfer from the heme to the acceptor. The solvent accessibility of the heme (the solvent accessible surface area of the heme is 123 Å2) suggests that this interaction is nonspecific [116].
Attempts to obtain crystals of the binary complexes of UndA and MtrF with soluble electron shuttles, such as FMN and riboflavin, by soaking and co-crystallization failed [105, 116], despite the fact that UndA, MtrF, and MtrC reduce flavins at rates comparable to or higher than those of the reduction of soluble Fe(III) compounds [102, 105, 116], and the saturation of MtrF with FMN was achieved already at 10 µM FMN concentration [105].
Energetics of electron transport in the MtrF molecule. To describe the electron transfer through the heme-containing ETC of OMC by molecular dynamics, the redox potentials of the individual hemes of MtrF were calculated (Fig. 4b) [118-120]. The calculated redox potentials of the hemes of MtrF vary within 350 mV (from 70 to –280 mV), which correlates with the potentiometric titration data for MtrF in solution (from 0 to –260 mV) [105]. The potential difference between hemes 10 and 5 (the electron entry and exit sites) does not exceeds 50 mV, which suggests the possibility of electron transport in both directions. Hemes 4 and 9 have the lowest potentials (–270 and –280 mV, respectively) [120]. The non-compensated negative charges of the nearby propionate groups of adjacent hemes 3 and 8 make a substantial contribution to a decrease in the potential of these hemes [118]. To understand the role of low-potential hemes and estimate their effect on the overall rate of electron transport, the energy profile for electron transfer through the 10-heme ETC of MtrF was constructed [118-120]. The energy profile for the main ETC (10 → 9 → 8 → 6 → 1 → 3 → 4 → 5) is characterized by the presence of two symmetrically located maxima corresponding to thermodynamic barriers with a height of about 0.2 eV localized at low-potential hemes 4 and 9. In this case, the electron transfer involves two steps accompanied by an increase in the free energy of the system (uphill steps 10 → 9 and 3 → 4), which should lead to a substantial decrease in the rate of electron transport. Analysis of the rates of electron transfer between adjacent hemes [118] shows that low-potential hemes 4 and 9 are involved in stacking motifs, which are characterized by the maximum electron transfer rate. The presence of low-potential hemes in these packing motifs leads to a decrease in the electron transport rate, due to which this rate approximates the rate of electron transfer through other elements of the ETC. As a result, the maximum rate of electron transfer through the main ETC 10 → 9 → 8 → 6 → 1 → 3 → 4 → 5 approaches 104-105 s–1, which is higher than the maximum measured rate of electron transfer to the insoluble electron acceptor mediated by the MtrCBA complex (8500 s–1) [93]. Therefore, the last extracellular electron transport step involving the electron transfer to the acceptor is the rate-limiting step, and a decrease in the electron transfer rate due to uphill steps has no effect on the overall rate of the process.
On the other hand, the presence of hemes 4 and 9 with redox potentials in the range from –270 to –280 mV in the main ETC of MtrF gives the possibility of reducing low-potential electron acceptors, for example flavins (Em of about –200 mV), at these sites [19]. The possibility of this process is confirmed by high solvent accessibility of hemes 4 and 9 (the solvent accessible surface area is 226 Å2 for heme 4 and 231 Å2 for heme 9 [116]).
Heme 2 (E = –60 mV) and particularly heme 7 (E = 70 mV) located near the potential flavin-binding domains I and III [120] proved to be too high-potential to be involved in the reduction of flavins, as expected in [105]. However, it was shown [121] that the binding of FMN to MtrC leads to a 100 mV shift toward positive potentials of the one-electron reduction of FMN to semiquinone, resulting in that the reduction of FMN at hemes 2 and 7 is thermodynamically more feasible.
Therefore, the branched ETC and the presence of low-potential hemes in this chain underlie the structural basis of the polyfunctionality of proteins of the MtrC/OmcA family, which has been observed earlier in biochemical experiments.
The determination of the three-dimensional structures of OMC made it possible to estimate the possible length of the extracellular part of the ETC, which includes the MtrC protein (65 Å) and the OmcA dimer linked to the former protein through hemes 10 (113 Å). The length of this ETC may be as great as 200 Å.
WIDESPREAD OCCURRENCE AND UNIVERSALITY OF THE MECHANISM OF
EXTRACELLULAR ELECTRON TRANSPORT SHOWN FOR THE BACTERIUM S.
oneidensis
An analysis of 19 complete Shewanella genomes made it possible to annotate gene clusters responsible for the metal reduction in bacteria of this genus [117, 122]. It was shown that (i) the mtrCAB-omcA gene cluster is present in all published genomes of the Shewanella genus; (ii) in some species, such as S. putrefaciens and S. baltica, the omcA gene is replaced with undA in the mtrCAB-undA gene cluster; (iii) the mtrCAB-omcA-mtrDEF gene cluster is present only in closely related strains of S. oneidensis; (iv) in some species (e.g. S. halifaxensis), the cluster includes genes of nine proteins: mtrD-mtrF-mtrE-omcA-undA-omcA-mtrC-mtrB-mtrA.
In addition to bacteria of the Shewanella genus, mtrCAB homologs were found in the genomes of the Fe(III)-oxidizing bacteria Aeromonas hydrophila, Ferrimonas balearica, and Rhodoferax ferrireducens [122]. As in the case of the Shewanella bacteria, the mtrCAB genes in these organisms are grouped in a common cluster, in which they are arranged in the same sequence mtrC-mtrA-mtrB. In the bacterium A. hydrophila, the cluster includes only the mtrCAB genes, whereas the omcA or undA genes and their homologs are absent. This indicates that the proteins encoded by these genes are not necessary for BDMR. In the bacteria F. balearica and R. ferrireducens, a gene cluster contains mtrCAB together with two copies of the cymA gene, which serves as the primary electron acceptor from the pool of reduced quinones. In S. oneidensis, cymA is not involved in the mtr gene cluster.
The mtrCAB gene cluster was found also in six marine bacteria of the Vibrio genus, in which metal reduction was not observed earlier. It was speculated [122] that the presence of mtrCAB is evidence that these bacteria can utilize Fe(III) in the respiratory process in the absence of other electron acceptors.
The mtrCAB genes were also identified in the genomes of the Fe(II)-oxidizing bacteria Rhodopseudomonas palustris and Sideroxydans lithotrophicus ES-1, which utilize FeCO3 or FeS as electron donors, which attests to the reversibility of the electron transport performed by the MtrCAB complex [89, 122].
Despite the fact that the mechanism of extracellular electron transport discussed for bacteria of the Geobacter genus [15, 16] differs from that for Shewanella, homologs of the mtrAB gene cluster were found in the Geobacter genomes [40, 123]. However, it was noted [122] that the mtrC gene is absent in this cluster in the bacterium Geobacter sp. M21 containing mtrAB homologous genes. Therefore, proteins produced by this cluster cannot be unambiguously related to the metal reduction.
The above-considered data show that the mtrCAB gene cluster is widespread in bacteria performing respiratory metal reduction, being responsible for the reversible electron exchange between the bacterial cell and the extracellular electron acceptor/donor. However, this pathway is apparently not universal, and other mechanisms of extracellular electron transport are possible too.
A part of this cluster, to be exact, genes homologous to mtrAB, are even more widespread in genomes of gram-negative microorganisms. The MtrAB protein complex, which is the transmembrane porin-like protein MtrB with the inserted soluble periplasmic electron-transfer protein MtrA encoded by these genes, is regarded as a prototype of the universal protein module for electron transfer across bacterial outer membranes [40, 89]. This transfer may be not directly related to metal reduction, the electron transfer from the external side of the outer membrane to other extracellular acceptors can be mediated by specific reductases. Thus, it was shown that the extracellular reduction of DMSO in the bacteria Shewanella is provided by the electron-transport module DmsEF homologous to MtrAB [124].
Homologs of the MtrAB complex were found in genomes of Geobacter bacteria [123], in which they are probably involved in electron transfer to pili.
CoNCLUSION
Extracellular electron transport is a key step in bacterial dissimilatory metal reduction. This process is studied in most detail in the bacterium S. oneidensis MR-1. Multiheme c-type cytochromes play a decisive role in this process in S. oneidensis. The extracellular electron transport involves the following three main steps: (i) the oxidation of reduced quinones in the cytoplasmic membrane and the electron transfer to the periplasm; (ii) electron transfer from the periplasm across the outer membrane to outer-membrane c-type cytochromes; (iii) electron transfer from the surface of the bacterial cell to the extracellular acceptor. The first step is performed with the participation of the universal quinol dehydrogenase CymA and a number of nonspecific periplasmic c-type cytochromes. The second step is performed with the participation of the transmembrane complex MtrCAB. The latter contains the periplasmic decaheme c-type cytochrome MtrA inserted into the transmembrane porin-like protein MtrB to form the MtrAB protein module responsible for electron transfer across the cell membrane to the outer-membrane c-type cytochrome MtrC. The structures of the MtrAB complex and the proteins involved in this complex have not been determined. Taking into account the widespread occurrence of proteins homologous to MtrA and MtrB in genomes of bacteria performing extracellular electron transport to different extracellular acceptors, this complex can be considered as a prototype of the universal protein module for electron transfer across bacterial outer membranes. The outer-membrane decaheme c-type cytochrome MtrC accepts electrons from MtrAB, thus playing a key role in electron transfer to an extracellular acceptor. The S. oneidensis genome contains yet another set of genes paralogous to mtrCAB – mtrDEF. The proteins MtrC/MtrF can transfer electrons both directly to the terminal acceptor and to another decaheme c-type cytochrome OmcA or its analog, the undecaheme c-type cytochrome UndA. The determination of the structures of three outer-membrane multiheme c-type cytochromes from bacteria of the Shewanella genus became a key issue in the understanding of the mechanism of extracellular electron transport. The proteins MtrF, UndA, and OmcA have similar three-dimensional structures consisting of four domains. The structures of two domains are typical of flavin-binding sites. The other two domains contain 10(11) hemes arranged in a unique staggered cross motif, which has no analogs in other multiheme c-type cytochromes. The unique structure of the branched ETC formed by hemes provides the possibility of simultaneous involvement of S. oneidensis OMC in several processes, including electron transfer from the outer cell membrane to an insoluble acceptor through the main chain and the reduction of soluble electron shuttles (e.g. flavins) involving hemes of the additional chain located near the flavin-binding domains.
Genomic analysis of gram-negative bacteria showed that proteins homologous to MtrC, MtrA, and MtrB are widespread in bacteria of the Shewanella genus, like in other gram-negative dissimilatory metal-reducing bacteria. This is evidence of the widespread occurrence of the mechanism of extracellular electron transport formulated for the bacterium S. oneidensis. However, alternative mechanisms are also possible. One of these mechanisms, apparently, takes place in bacteria of the Geobacter genus. Extension of the range of studied organisms, the inclusion of archaea and gram-positive bacteria using dissimilatory metal reduction, will provide deeper understanding of the mechanisms of bacterial dissimilatory metal reduction.
We thank E. M. Osipov, research worker of the Laboratory of Enzyme Engineering of A. N. Bach Institute of Biochemistry of the Russian Academy of Sciences, for help in preparing figures.
This review was written with financial support of the Russian Foundation for Basic Research (project No. 13-04-40207-H).
REFERENCES
1.Balashova, V. V., and Zavarzin, G. A. (1980)
Anaerobic reduction of ferric iron by hydrogen bacteria,
Microbiology, 48, 635-639.
2.Lovley, D. R., and Phillips, E. J. (1987)
Competitive mechanisms for inhibition of sulfate reduction and methane
production in the zone of ferric iron reduction in sediments, Appl.
Environ. Microbiol., 53, 2636-2641.
3.Lovley, D. R., and Phillips, E. J. (1988) Novel
mode of microbial energy metabolism: organic carbon oxidation coupled
to dissimilatory reduction of iron or manganese, Appl. Environ.
Microbiol., 54, 1472-1480.
4.Gralnick, J. A., and Newman, D. K. (2007)
Extracellular respiration, Mol. Microbiol., 65, 1-11.
5.Chiorse, W. C. (1984) Biology of iron-depositing
and manganese-depositing bacteria, Ann. Rev. Microbiol.,
38, 515-550.
6.Myers, C. R., and Nealson, K. H. (1988) Bacterial
manganese reduction and growth with manganese oxide as the sole
electron acceptor, Science, 240, 1319-1321.
7.Lovley, D. R. (1991) Dissimilatory Fe(III) and
Mn(IV) reduction, Microbiol. Rev., 55, 259-287.
8.Lovley, D. R. (1993) Dissimilatory
metalloreduction, Ann. Rev. Microbiol., 47, 263-290.
9.Nealson, K. H., and Saffarini, D. (1994) Iron and
manganese in anaerobic respiration – environmental
significance, physiology, and regulation, Ann. Rev. Microbiol.,
48, 311-343.
10.Weber, K. A., Achenbach, L. A., and Coates, J. D.
(2006) Microorganisms pumping iron: anaerobic microbial iron oxidation
and reduction, Nature Rev. Microbiol., 4, 752-764.
11.Richardson, D. J., Fredrickson, J. K., and
Zachara, J. M. (2012) Electron transport at the microbe–mineral
interface: a synthesis of current research challenges, Biochem. Soc.
Transact., 40, 1163-1166.
12.Thamdrup, B., Rossello-Mora, R., and Amann, R.
(2000) Microbial manganese and sulfate reduction in Black Sea shelf
sediments, Appl. Environ. Microbiol., 66, 2888-2897.
13.Walker, J. C. (1987) Was the Archaean biosphere
upside down? Nature, 329, 710-712.
14.Lovley, D. R., Holmes, D. E., and Nevin, K. P.
(2004) Dissimilatory Fe(III) and Mn(IV) reduction, Adv. Microb.
Physiol., 49, 219-286.
15.Lovley, D. R., Ueki, T., Zhang, T., Malvankar, N.
S., Shrestha, P. M., Flanagan, K. A., Aklujkar, M., Butler, J. E.,
Giloteaux, L., Rotaru, A. E., Holmes, D. E., Franks, A. E., Orellana,
R., Risso, C., and Nevin, K. P. (2011) Geobacter: the microbe
electric’s physiology, ecology, and practical applications,
Adv. Microb. Physiol., 59, 1-100.
16.Lovley, D. R. (2012) Electromicrobiology, Ann.
Rev. Microbiol., 66, 391-409.
17.Logan, B. E. (2009) Exoelectrogenic bacteria that
power microbial fuel cells, Nature Rev. Microbiol., 7,
375-381.
18.Logan, B. E., and Regan, J. M. (2006)
Electricity-producing bacterial communities in microbial fuel cells,
Trends Microbiol., 14, 512-518.
19.Logan, B. E., and Regan, J. M. (2006) Microbial
fuel cells – challenges and applications, Environ. Sci.
Technol., 40, 5172-5180.
20.Malvankar, N. S., and Lovley, D. R. (2014)
Microbial nanowires for bioenergy applications, Curr. Opin.
Biotechnol., 27, 88-95.
21.Williams, K. H., Bargar, J. R., Lloyd, J. R., and
Lovley, D. R. (2013) Bioremediation of uranium-contaminated
groundwater: a systems approach to subsurface biogeochemistry, Curr.
Opin. Biotechnol., 24, 489-497.
22.Cutting, R. S., Coker, V. S., Telling, N. D.,
Kimber, R. L., Pearce, C. I., Ellis, B. L., Lawson, R. S., van der
Laan, G., Pattrick, R. A., Vaughan, D. J., Arenholz, E., and Lloyd, J.
R. (2010) Optimizing Cr(VI) and Tc(VII) remediation through nanoscale
biomineral engineering, Environ. Sci. Technol., 44,
2577-2584.
23.Jiao, Y., Qian, F., Li, Y., Wang, G., Saltikov,
C. W., and Gralnick, J. A. (2011) Deciphering the electron transport
pathway for graphene oxide reduction by Shewanella oneidensis
MR-1, J. Bacteriol., 193, 3662-3665.
24.Fredrickson, J. K., Kostandarithes, H. M., Li, S.
W., Plymale, A. E., and Daly, M. J. (2000) Reduction of Fe(III),
Cr(VI), U(VI), and Tc(VII) by Deinococcus radiodurans R1,
Appl. Environ. Microbiol., 66, 2006-2011.
25.Slobodkin, A. I. (2005) Thermophilic microbial
metal reduction, Mikrobiologiya, 74, 581-595.
26.Wrighton, K. C., Agbo, P., Warnecke, F., Weber,
K. A., Brodie, E. L., DeSantis, T. Z., Hugenholtz, P., Andersen, G. L.,
and Coates, J. D. (2008) A novel ecological role of the
Firmicutes identified in thermophilic microbial fuel
cells, ISME J., 2, 1146-1156.
27.Iavarone, A. T., Gorur, A., Yeo, B. S., Tran, R.,
Melnyk, R. A., Mathies, R. A., Auer, M., Coates, J. D., and
Carlson, H. K. (2012) Surface multiheme c-type cytochromes from
Thermincola potens and implications for respiratory metal
reduction by Gram-positive bacteria, Proc. Natl. Acad. Sci. USA,
109, 1702-1707.
28.Ibrahim, A. S., El-Tayeb, M. A., Elbadawi, Y. B.,
Al-Salamah, A. A., and Antranikian, G. (2012) Hexavalent chromate
reduction by alkaliphilic Amphibacillus sp. KSUCr3 is mediated
by copper-dependent membrane-associated Cr(VI) reductase,
Extremophiles, 16, 659-668.
29.Gavrilov, S. N., Lloyd, J. R., Kostrikina, N. A.,
and Slobodkin, A. I. (2012) Fe(III) oxide reduction by a gram-positive
thermophile: physiological mechanisms for dissimilatory reduction of
poorly crystalline Fe(III) oxide by a thermophilic gram-positive
bacterium Carboxydothermus ferrireducens, Geomicrobiol.
J., 29, 804-819.
30.Nevin, K. P., and Lovley, D. R. (2002) Mechanisms
for accessing insoluble Fe(III) oxide during dissimilatory Fe(III)
reduction by Geothrix fermentans, Appl. Environ.
Microbiol., 68, 2294-2299.
31.Myers, J. M., and Myers, C. R. (2001) Role for
outer membrane cytochromes OmcA and OmcB of Shewanella putrefaciens
MR-1 in reduction of manganese dioxide, Appl. Environ.
Microbiol., 67, 260-269.
32.Beliaev, A. S., and Saffarini, D. A. (1998)
Shewanella putrefaciens mtrB encodes an outer membrane protein
required for Fe(III) and Mn(IV) reduction, J. Bacteriol.,
180, 6292-6297.
33.Yang, Y., Chen, J., Qiu, D., and Zhou, J. (2013)
Roles of UndA and MtrC of Shewanella putrefaciens W3-18-1 in
iron reduction, BMC Microbiol., 13, 267; doi:
10.1186/1471-2180-13-267.
34.Smith, J. A., Lovley, D. R., and Tremblay, P. L.
(2013) Outer cell surface components essential for Fe(III) oxide
reduction by Geobacter metallireducens, Appl. Environ.
Microbiol., 79, 901-907.
35.Nissen, S., Liu, X., Chourey, K., Hettich, R. L.,
Wagner, D. D., Pfiffner, S. M., and Loffler, F. E. (2012) Comparative
c-type cytochrome expression analysis in Shewanella
oneidensis strain MR-1 and Anaeromyxobacter dehalogenans
strain 2CP-C grown with soluble and insoluble oxidized metal
electron acceptors, Biochem. Soc. Transact., 40,
1204-1210.
36.Fredrickson, J. K., and Zachara, J. M. (2008)
Electron transfer at the microbe–mineral interface: a grand
challenge in biogeochemistry, Geobiology, 6, 245-253.
37.Richter, K., Schicklberger, M., and Gescher, J.
(2012) Dissimilatory reduction of extracellular electron acceptors in
anaerobic respiration, Appl. Environ. Microbiol., 78,
913-921.
38.Coursolle, D., and Gralnick, J. A. (2012)
Reconstruction of extracellular respiratory pathways for iron(III)
reduction in Shewanella oneidensis strain MR-1, Front.
Microbiol., 3, 56; doi: 10.3389/fmicb.2012.00056.
39.Shi, L., Squier, T. C., Zachara, J. M., and
Fredrickson, J. K. (2007) Respiration of metal (hydr)oxides by
Shewanella and Geobacter: a key role for multiheme
c-type cytochromes, Mol. Microbiol., 65,
12-20.
40.Hartshorne, R. S., Reardon, C. L., Ross, D.,
Nuester, J., Clarke, T. A., Gates, A. J., Mills, P. C., Fredrickson, J.
K., Zachara, J. M., Shi, L., Beliaev, A. S., Marshall, M. J.,
Tien, M., Brantley, S., Butt, J. N., and Richardson, D. J. (2009)
Characterization of an electron conduit between bacteria and the
extracellular environment, Proc. Natl. Acad. Sci. USA,
106, 22169-22174.
41.Richardson, D. J., Edwards, M. J., White, G. F.,
Baiden, N., Hartshorne, R. S., Fredrickson, J., Shi, L., Zachara, J.,
Gates, A. J., Butt, J. N., and Clarke, T. A. (2012) Exploring the
biochemistry at the extracellular redox frontier of bacterial mineral
Fe(III) respiration, Biochem. Soc. Transact., 40,
493-500.
42.Shi, L., Rosso, K. M., Clarke, T. A.,
Richardson, D. J., Zachara, J. M., and Fredrickson, J. K. (2012)
Molecular underpinnings of Fe(III) oxide reduction by Shewanella
oneidensis MR-1, Front. Microbiol., 3, 50; doi:
10.3389/fmicb.2012.00050.
43.Coursolle, D., and Gralnick, J. A. (2010)
Modularity of the Mtr respiratory pathway of Shewanella
oneidensis strain MR-1, Mol. Microbiol., 77,
995-1008.
44.Paquete, C. M., and Louro, R. O. (2010) Molecular
details of multielectron transfer: the case of multiheme cytochromes
from metal respiring organisms, Dalton Transact., 39,
4259-4266.
45.Moser, C. C., Chobot, S. E., Page, C. C., and
Dutton, P. L. (2008) Distance metrics for heme protein electron
tunneling, Biochim. Biophys. Acta, 1777, 1032-1037.
46.Childers, S. E., Ciufo, S., and Lovley, D. R.
(2002) Geobacter metallireducens accesses insoluble Fe(III)
oxide by chemotaxis, Nature, 416, 767-769.
47.Harris, H. W., El-Naggar, M. Y., Bretschger, O.,
Ward, M. J., Romine, M. F., Obraztsova, A. Y., and Nealson, K. H.
(2010) Electrokinesis is a microbial behavior that requires
extracellular electron transport, Proc. Natl. Acad. Sci. USA,
107, 326-331.
48.Harris, H. W., El-Naggar, M. Y., and Nealson, K.
H. (2012) Shewanella oneidensis MR-1 chemotaxis proteins and
electron-transport chain components essential for congregation near
insoluble electron acceptors, Biochem. Soc. Transact.,
40, 1167-1177.
49.Lies, D. P., Hernandez, M. E., Kappler, A.,
Mielke, R. E., Gralnick, J. A., and Newman, D. K. (2005) Shewanella
oneidensis MR-1 uses overlapping pathways for iron reduction at a
distance and by direct contact under conditions relevant for biofilms,
Appl. Environ. Microbiol., 71, 4414-4426.
50.Newman, D. K., and Kolter, R. (2000) A role for
excreted quinones in extracellular electron transfer, Nature,
405, 93-97.
51.Von Canstein, H., Ogawa, J., Shimizu, S., and
Lloyd, J. R. (2008) Secretion of flavins by Shewanella species
and their role in extracellular electron transfer, Appl. Environ.
Microbiol., 74, 615-623.
52.Marsili, E., Baron, D. B., Shikhare, I. D.,
Coursolle, D., Gralnick, J. A., and Bond, D. R. (2008)
Shewanella secretes flavins that mediate extracellular
electron transfer, Proc. Natl. Acad. Sci. USA, 105,
3968-3973.
53.Kotloski, N. J., and Gralnick, J. A. (2013)
Flavin electron shuttles dominate extracellular electron transfer by
Shewanella oneidensis, mBio, 4, e00553-12; doi:
10.1128/mBio.00553-12.
54.Coursolle, D., Baron, D. B., Bond, D. R., and
Gralnick, J. A. (2010) The Mtr respiratory pathway is essential for
reducing flavins and electrodes in Shewanella oneidensis, J.
Bacteriol., 192, 467-474.
55.Ross, D. E., Brantley, S. L., and Tien, M. (2009)
Kinetic characterization of OmcA and MtrC, terminal reductases involved
in respiratory electron transfer for dissimilatory iron reduction in
Shewanella oneidensis MR-1, Appl. Environ. Microbiol.,
75, 5218-5226.
56.Wu, C., Cheng, Y. Y., Li, B. B., Li, W. W., Li,
D. B., and Yu, H. Q. (2013) Electron acceptor dependence of electron
shuttle secretion and extracellular electron transfer by Shewanella
oneidensis MR-1, Bioresource Technol., 136,
711-714.
57.Malvankar, N., Vargas, M., Nevin, K. P., Franks,
A. E., Leang, C., Kim, B.-C., Inoue, K., Mester, T., Covalla, S. F.,
Johnson, J. P., Rotello, V. M., Tuominen, M. T., and Lovley, D. R.
(2011) Tunable metallic-like conductivity in nanostructured biofilms
comprised of microbial nanowires, Nature Nanotechnol.,
6, 573-579.
58.Lovley, D. R. (2012) Long-range electron
transport to Fe(III) oxide via pili with metallic-like conductivity,
Biochem. Soc. Transact., 40, 1186-1190.
59.Malvankar, N. S., and Lovley, D. R. (2012)
Microbial nanowires: a new paradigm for biological electron transfer
and bioelectronics, ChemSusChem, 5, 1039-1046.
60.Reguera, G., McCarthy, K. D., Mehta, T., Nicoll,
J. S., Tuominen, M. T., and Lovley, D. R. (2005) Extracellular electron
transfer via microbial nanowires, Nature, 435,
1098-1101.
61.Lovley, D. R. (2008) Extracellular electron
transfer: wires, capacitors, iron lungs, and more, Geobiology,
6, 225-231.
62.Leang, C., Qian, X., Mester, T., and Lovley, D.
R. (2010) Alignment of the c-type cytochrome OmcS along pili of
Geobacter sulfurreducens, Appl. Environ. Microbiol.,
76, 4080-4084.
63.Lovley, D. R. (2011) Live wires: direct
extracellular electron exchange for bioenergy and the bioremediation of
energy-related contamination, Energy Environ. Sci., 4,
4896-4906.
64.Esteve-Nunez, A., Sosnik, J., Visconti, P., and
Lovley, D. R. (2008) Fluorescent properties of c-type
cytochromes reveal their potential role as an extracytoplasmic electron
sink in Geobacter sulfurreducens, Environ. Microbiol.,
10, 497-505.
65.Mitchell, A. C., Peterson, L., Reardon, C. L.,
Reed, S. B., Culley, D. E., Romine, M. R., and Geesey, G. G. (2012)
Role of outer membrane c-type cytochromes MtrC and OmcA in
Shewanella oneidensis MR-1 cell production, accumulation, and
detachment during respiration on hematite, Geobiology,
10, 355-370.
66.Meyer, T. E., Tsapin, A. I., Vandenberghe, I., de
Smet, L., Frishman, D., Nealson, K. H., Cusanovich, M. A., and van
Beeumen, J. J. (2004) Identification of 42 possible cytochrome c
genes in the Shewanella oneidensis genome and
characterization of six soluble cytochromes, Omics, 8,
57-77.
67.Myers, C. R., and Myers, J. M. (1997) Cloning and
sequence of cymA, a gene encoding a tetraheme cytochrome c
required for reduction of iron(III), fumarate, and nitrate by
Shewanella putrefaciens MR-1, J. Bacteriol.,
179, 1143-1152.
68.Myers, C. R., and Myers, J. M. (2002) MtrB is
required for proper incorporation of the cytochromes OmcA and OmcB into
the outer membrane of Shewanella putrefaciens MR-1, Appl.
Environ. Microbiol., 68, 5585-5594.
69.Beliaev, A. S., Saffarini, D. A., McLaughlin, J.
L., and Hunnicutt, D. (2001) MtrC, an outer membrane decaheme c
cytochrome required for metal reduction in Shewanella
putrefaciens MR-1, Mol. Microbiol., 39,
722-730.
70.Pitts, K. E., Dobbin, P. S., Reyes-Ramirez, F.,
Thomson, A. J., Richardson, D. J., and Seward, H. E. (2003)
Characterization of the Shewanella oneidensis MR-1 decaheme
cytochrome MtrA: expression in Escherichia coli confers the
ability to reduce soluble Fe(III) chelates, J. Biol. Chem.,
278, 27758-27765.
71.Marritt, S. J., McMillan, D. G. G., Shi, L.,
Fredrickson, J. K., Zachara, J. M., Richardson, D. J., Jeuken, L. J.
C., and Butt, J. N. (2012) The roles of CymA in support of the
respiratory flexibility of Shewanella oneidensis MR-1,
Biochem. Soc. Transact., 40, 1217-1221.
72.Marritt, S. J., Lowe, T. G., Bye, J., McMillan,
D. G. G., Shi, L., Fredrickson, J., Zachara, J., Richardson, D. J.,
Cheesman, M. R., Jeuken, L. J. C., and Butt, J. N. (2012) A functional
description of CymA, an electron-transfer hub supporting anaerobic
respiratory flexibility in Shewanella, Biochem. J.,
444, 465-474.
73.Louro, R. O., and Paquete, C. M. (2012) The quest
to achieve the detailed structural and functional characterization of
CymA, Biochem. Soc. Transact., 40, 1291-1294.
74.McMillan, D. G. G., Marritt, S. J., Butt, J. N.,
and Jeuken, L. J. C. (2012) Menaquinone-7 is specific cofactor in
tetraheme quinol dehydrogenase CymA, J. Biol. Chem., 287,
14215-14225.
75.Zargar, K., and Saltikov, C. W. (2009) Lysine-91
of the tetraheme c-type cytochrome CymA is essential for quinone
interaction and arsenate respiration in Shewanella sp. strain
ANA-3, Arch. Microbiol., 191, 797-806.
76.Rodrigues, M. L., Scott, K. A., Sansom, M. S. P.,
Pereira, I. A. C., and Archer, M. (2008) Quinol oxidation by
c-type cytochromes: structural characterization of the
menaquinol binding site of NrfHA, J. Mol. Biol., 381,
341-350.
77.Bewley, K. D., Ellis, K. E., Firer-Sherwood, M.
A., and Elliott, S. J. (2013) Multi-heme proteins: nature’s
electronic multi-purpose tool, Biochim. Biophys. Acta,
1827, 938-948.
78.Myers, J. M., and Myers, C. R. (2000) Role of the
tetraheme cytochrome CymA in anaerobic electron transport in cells of
Shewanella putrefaciens MR-1 with normal levels of menaquinone,
J. Bacteriol., 182, 67-75.
79.Schwalb, C., Chapman, S. K., and Reid, G. A.
(2003) The tetraheme cytochrome CymA is required for anaerobic
respiration with dimethyl sulfoxide and nitrite in Shewanella
oneidensis, Biochemistry, 42, 9491-9497.
80.Gao, H. C., Yang, Z. K., Barua, S., Reed, S. B.,
Romine, M. F., Nealson, K. H., Fredrickson, J. K., Tiedje, J. M., and
Zhou, J. Z. (2009) Reduction of nitrate in Shewanella oneidensis
depends on atypical NAP and NRF systems with NapB as a preferred
electron transport protein from CymA to NapA, ISME J., 3,
966-976.
81.Gao, H., Barua, S., Liang, Y., Wu, L., Dong, Y.,
Reed, S., Chen, J., Culley, D., Kennedy, D., Yang, Y., He, Z., Nealson,
K. H., Fredrickson, J. K., Tiedje, J. M., Romine, M., and Zhou, J.
(2010) Impacts of Shewanella oneidensis c-type
cytochromes on aerobic and anaerobic respiration, Microb.
Biotechnol., 3, 455-466.
82.Field, S. J., Dobbin, P. S., Cheesman, M. R.,
Watmough, N. J., Thomson, A. J., and Richardson, D. J. (2000)
Purification and magneto-optical spectroscopic characterization of
cytoplasmic membrane and outer membrane multiheme c-type
cytochromes from Shewanella frigidimarina NCIMB400, J. Biol.
Chem., 275, 8515-8522.
83.Schwalb, C., Chapman, S. K., and Reid, G. A.
(2002) The membrane-bound tetraheme c-type cytochrome CymA
interacts directly with the soluble fumarate reductase in
Shewanella, Biochem. Soc. Transact., 30,
658-662.
84.Youngblut, M., Judd, E. T., Srajer, V., Sayyed,
B., Goelzer, T., Elliott, S. J., Schmidt, M., and Pacheco, A. A. (2012)
Laue crystal structure of Shewanella oneidensis cytochrome
c nitrite reductase from a high-yield expression system, J.
Biol. Inorg. Chem., 17, 647-662.
85.Bretschger, O., Obraztsova, A., Sturm, C. A.,
Chang, I. S., Gorby, Y. A., Reed, S. B., Culley, D. E., Reardon, C. L.,
Barua, S., Romine, M. F., Zhou, J., Beliaev, A. S., Bouhenni, R.,
Saffarini, D., Mansfeld, F., Kim, B. H., Fredrickson, J. K., and
Nealson, K. H. (2007) Current production and metal oxide reduction by
Shewanella oneidensis MR-1 wild type and mutants, Appl.
Environ. Microbiol., 73, 7003-7012.
86.Schuetz, B., Schicklberger, M., Kuermann, J.,
Spormann, A. M., and Gescher, J. (2009) Periplasmic electron transfer
via the c-type cytochromes MtrA and FccA of Shewanella
oneidensis MR-1, Appl. Environ. Microbiol., 75,
7789-7796.
87.Clarke, T. A., Cole, J. A., Richardson, D. J.,
and Hemmings, A. M. (2007) The crystal structure of the pentaheme
c-type cytochrome NrfB and characterization of its
solution-state interaction with the pentaheme nitrite reductase NrfA,
Biochem. J., 406, 19-30.
88.Mowat, C. G., and Chapman, S. K. (2005)
Multi-heme cytochromes – new structures, new chemistry, Dalton
Transact., 21, 3381-3389.
89.Richardson, D. J., Butt, J. N., Fredrickson, J.
K., Zachara, J. M., Shi, L., Edwards, M. J., White, G., Baiden, N.,
Gates, A. J., Marritt, S. J., and Clarke, T. A. (2012) The
“porin–cytochrome” model for microbe-to-mineral
electron transfer, Mol. Microbiol., 85, 201-212.
90.Ross, D. E., Ruebush, S. S., Brantley, S. L.,
Hartshorne, R. S., Clarke, T. A., Richardson, D. J., and Tien, M.
(2007) Characterization of protein–protein interactions involved
in iron reduction by Shewanella oneidensis MR-1, Appl.
Environ. Microbiol., 73, 5797-5808.
91.Schicklberger, M., Bucking, C., Schuetz, B.,
Heide, H., and Gescher, J. (2011) Involvement of the Shewanella
oneidensis decaheme cytochrome MtrA in the periplasmic stability of
the barrel protein MtrB, Appl. Environ. Microbiol.,
77, 1520-1523.
92.Firer-Sherwood, M. A., Ando, N., Drennan, C. L.,
and Elliott, S. J. (2011) Solution-based structural analysis of the
decaheme cytochrome, MtrA, by small-angle X-ray scattering and
analytical ultracentrifugation, J. Phys. Chem. B, 115,
1208-1214.
93.White, G. F., Shi, Z., Shi, L., Wang, Z.,
Dohnalkova, A. C., Marshall, M. J., Fredrickson, J. K., Zachara,
J. M., Butt, J. N., Richardson, D. J., and Clarke, T. A. (2013)
Rapid electron exchange between surface-exposed bacterial cytochromes
and Fe(III) minerals, Proc. Natl. Acad. Sci. USA, 110,
6346-6351.
94.Gralnick, J. A. (2012) On conducting electron
traffic across the periplasm, Biochem. Soc. Transact.,
40, 1178-1180.
95.Taylor, P., Pealing, S. L., Reid, G. A., Chapman,
S. K., and Walkinshaw, M. D. (1999) Structural and mechanistic mapping
of a unique fumarate reductase, Nature Struct. Biol., 6,
1108-1112.
96.Leys, D., Tsapin, A. S., Nealson, K. H., Meyer,
T. E., Cusanovich, M. A., and Van Beeumen, J. J. (1999) Structure and
mechanism of the flavocytochrome c fumarate reductase of
Shewanella putrefaciens MR-1, Nature Struct. Biol.,
6, 1113-1117.
97.Pessanha, M., Rothery, E. L., Miles, C. S., Reid,
G. A., Chapman, S. K., Louro, R. O., Turner, D. L., Salgueiro, C. A.,
and Xavier, A. V. (2009) Tuning of functional heme reduction potentials
in Shewanella fumarate reductases, Biochim. Biophys.
Acta, 1787, 113-120.
98.Leys, D., Meyer, T. E., Tsapin, A. I., Nealson,
K. H., Cusanovich, M. A., and Van Beeumen, J. J. (2002) Crystal
structures at atomic resolution reveal the novel concept of
“electron-harvesting” as a role for the small tetraheme
cytochrome c, J. Biol. Chem., 277,
35703-35711.
99.Fonseca, B. M., Paquete, C. M., Neto, S. E.,
Pacheco, I., Soares, C. M., and Louro, R. O. (2013) Mind the gap:
cytochrome interactions reveal electron pathways across the periplasm
of Shewanella oneidensis MR-1, Biochem. J., 449,
101-108.
100.Firer-Sherwood, M., Pulcu, G. S., and Elliott,
S. J. (2008) Electrochemical interrogations of the Mtr cytochromes from
Shewanella: opening a potential window, J. Biol. Inorg.
Chem., 13, 849-854.
101.Schutz, B., Seidel, J., Sturm, G., Einsle, O.,
and Gescher, J. (2011) Investigation of the electron transport chain to
and the catalytic activity of the diheme cytochrome c peroxidase
CcpA of Shewanella oneidensis, Appl. Environ. Microbiol.,
77, 6172-6180.
102.Shi, L., Chen, B., Wang, Z., Elias, D. A.,
Mayer, M. U., Gorby, Y. A., Ni, S., Lower, B. H., Kennedy, D. W.,
Wunschel, D. S., et al. (2006) Isolation of a high-affinity functional
protein complex between OmcA and MtrC: two outer membrane decaheme
c-type cytochromes of Shewanella oneidensis MR-1, J.
Bacteriol., 188, 4705-4714.
103.Bucking, C., Popp, F., Kerzenmacher, S., and
Gescher, J. (2010) Involvement and specificity of Shewanella
oneidensis outer membrane cytochromes in the reduction of soluble
and solid-phase terminal electron acceptors, FEMS Microbiol.
Lett., 306, 144-151.
104.Myers, J. M., and Myers, C. R. (2001) Role
for outer membrane cytochromes OmcA and OmcB of Shewanella
putrefaciens MR-1 in reduction of manganese dioxide, Appl.
Environ. Microbiol., 67, 260-269.
105.Clarke, T. A., Edwards, M. J., Gates, A. J.,
Hall, A., White, G. F., Bradley, J., Reardon, C. L., Shi, L., Beliaev,
A. S., Marshall, M. J., Wang, Z., Watmough, N. J., Fredrickson, J. K.,
Zachara, J. M., Butt, J. N., and Richardson, D. J. (2011)
Structure of a bacterial cell surface decaheme electron conduit,
Proc. Natl. Acad. Sci. USA, 108, 9384-9389.
106.Hartshorne, R. S., Jepson, B. N., Clarke, T.
A., Field, S. J., Fredrickson, J., Zachara, J., Shi, L., Butt, J. N.,
and Richardson, D. J. (2007) Characterization of Shewanella
oneidensis MtrC: a cell-surface decaheme cytochrome involved in
respiratory electron transport to extracellular electron acceptors,
J. Biol. Inorg. Chem., 12, 1083-1094.
107.Lower, B. H., Shi, L., Yongsunthon, R.,
Droubay, T. C., McCready, D. E., and Lower, S. K. (2007) Specific
bonds between an iron oxide surface and outer membrane cytochromes MtrC
and OmcA from Shewanella oneidensis MR-1, J. Bacteriol.,
189, 4944-4952.
108.Eggleston, C. M., Voros, J., Shi, L., Lower, B.
H., Droubay, T. C., and Colberg, P. J. S. (2008) Binding and direct
electrochemistry of OmcA, an outer membrane cytochrome from an iron
reducing bacterium, with oxide electrodes: a candidate biofuel cell
system, Inorg. Chim. Acta, 361, 769-777.
109.Xiong, Y., Shi, L., Chen, B., Mayer, M. U.,
Lower, B. H., Londer, Y., Bose, S., Hochella, M. F., Fredrickson, J.
K., and Squier, T. C. (2006) High-affinity binding and direct electron
transfer to solid metals by the Shewanella oneidensis MR-1 outer
membrane c-type cytochrome OmcA, J. Am. Chem. Soc.,
128, 13978-13979.
110.Lower, B. H., Lins, R. D., Oestreicher, Z.,
Straatsma, T. P., Hochella, M. F., Jr., Shi, L., and Lower, S. K.
(2008) In vitro evolution of a peptide with a hematite binding
motif that may constitute a natural metal-oxide binding archetype,
Environ. Sci. Technol., 42, 3821-3827.
111.Wigginton, N. S., Rosso, K. M., and
Hochella, M. F. (2007) Mechanisms of electron transfer in two decaheme
cytochromes from a metal-reducing bacterium, J. Phys. Chem. B,
111, 12857-12864.
112.Shi, L., Chen, B. W., Wang, Z. M., Elias, D.
A., Mayer, M. U., Gorby, Y. A., Ni, S., Lower, B. H., Kennedy, D. W.,
Wunschel, D. S., et al. (2006) Isolation of a high-affinity functional
protein complex between OmcA and MtrC: two outer membrane decaheme
c-type cytochromes of Shewanella oneidensis MR-1, J.
Bacteriol., 188, 4705-4714.
113.Edwards, M. J., Baiden, N. A., Johs, A.,
Tomanicek, S. J., Liang, L., Shi, L., Fredrickson, J. K., Zachara, J.
M., Gates, A. J., Butt, J. N., Richardson, D. J., and Clarke, T. A.
(2014) The X-ray crystal structure of Shewanella oneidensis OmcA
reveals new insight at the microbe-mineral interface, FEBS
Lett., 588, 1886-1890.
114.Edwards, M. J., Fredrickson, J. K., Zachara, J.
M., Richardson, D. J., and Clarke, T. A. (2012) Analysis of structural
MtrC models based on homology with the crystal structure of MtrF,
Biochem. Soc. Transact., 40, 1181-1185.
115.Moser, C. C., Chobot, S. E., Page, C. C., and
Dutton, P. L. (2008) Distance metrics for heme protein electron
tunneling, Biochim. Biophys. Acta, 1777, 1032-1037.
116.Edwards, M. J., Hall, A., Shi, L., Fredrickson,
J. K., Zachara, J. M., Butt, J. N., Richardson, D. J., and Clarke, T.
A. (2012) The crystal structure of the extracellular 11-heme cytochrome
UndA reveals a conserved 10-heme motif and defined binding site for
soluble iron chelates, Structure, 20, 1275-1284.
117.Yang, Y., Chen, J., Qiu, D., and Zhou, J.
(2013) Roles of UndA and MtrC of Shewanella putrefaciens
W3-18-1 in iron reduction, BMC Microbiol., 13, 267.
118.Breuer, M., Rosso, K. M., and Blumberger, J.
(2014) Electron flow in multiheme bacterial cytochromes is a balancing
act between heme electronic interaction and redox potentials, Proc.
Natl. Acad. Sci. USA, 111, 611-616.
119.Breuer, M., Zarzycki, P., Blumberger, J., and
Rosso, K. M. (2012) Thermodynamics of electron flow in the bacterial
deca-heme cytochrome MtrF, J. Am. Chem. Soc., 134,
9868-9871.
120.Breuer, M., Zarzycki, P., Shi, L., Clarke, T.
A., Edwards, M. J., Butt, J. N., Richardson, D. J., Fredrickson, J. K.,
Zachara, J. M., Blumberger, J., and Rosso, K. M. (2012) Molecular
structure and free energy landscape for electron transport in the
decaheme cytochrome MtrF, Biochem. Soc. Transact., 40,
1198-1203.
121.Okamoto, A., Hashimoto, K., Nealson, K. H., and
Nakamura, R. (2013) Rate enhancement of bacterial extracellular
electron transport involves bound flavin semiquinones, Proc. Natl.
Acad. Sci. USA, 110, 7856-7861.
122.Shi, L., Rosso, K. M., Zachara,
J. M., and Fredrickson, J. K. (2012) Mtr extracellular
electron-transfer pathways in Fe(III)-reducing or Fe(II)-oxidizing
bacteria: a genomic perspective, Biochem. Soc. Transact.,
40, 1261-1267.
123.Bucking, C., Piepenbrock, A., Kappler, A., and
Gescher, J. (2012) Outer-membrane cytochrome-independent reduction of
extracellular electron acceptors in Shewanella oneidensis,
Microbiology, 158, 2144-2157.
124.Bewley, K. D., Firer-Sherwood, M. A., Mock, J.
Y., Ando, N., Drennan, C. L., and Elliott, S. J. (2012) Mind the gap:
diversity and reactivity relationships among multiheme cytochromes of
the MtrA/DmsE family, Biochem. Soc. Transact., 40,
1268-1273.