Novel Recombinant Anti-HER2/neu Immunotoxin: Design and Antitumor Efficiency
E. A. Sokolova1,2*, T. A. Zdobnova1,2, O. A. Stremovskiy2, I. V. Balalaeva1, and S. M. Deyev1,2
1Lobachevsky State University of Nizhny Novgorod, pr. Gagarina 23, 603950 Nizhny Novgorod, Russia; fax: +7 (831) 462-3085; E-mail: unn@unn.ru; malehanova@mail.ru2Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, ul. Miklukho-Maklaya 16/10, 117997 Moscow, Russia; fax: +7 (495) 335-0812; E-mail: office@ibch.ru
* To whom correspondence should be addressed.
Received July 7, 2014
The novel HER2/neu-specific recombinant immunotoxin 4D5scFv-PE40 consisting of 4D5scFv antibody (targeting module) and Pseudomonas exotoxin A fragment (effector module) combined in a single polypeptide chain via a flexible linker has been expressed and purified. This immunotoxin conserves specificity and affinity that are characteristics of the parental antibody 4D5scFv and exhibits selective and strong cytotoxic effect against cancer cells overexpressing HER2/neu receptor. The results of the experiments both in vitro (in cell cultures) and in vivo (in tumor-bearing animals) demonstrate high potential of 4D5scFv-PE40 for targeted therapy of tumors overexpressing HER2/neu.
KEY WORDS: recombinant immunotoxin, 4D5scFv, Pseudomonas exotoxin A, receptor HER2/neu, targeted therapyDOI: 10.1134/S0006297914120128
In recent decades, an increasing role in the treatment of malignant tumors is played by targeted therapy. This approach is based on the use of targeted agents that selectively bind with and destroy tumor cells.
The development of targeted therapy is tightly related with progress in molecular oncology, and in particular with identification of molecular markers typical of tumor cells of certain types and development of improved methods for tumor molecular profiling. An ideal variant of such targets are proteins expressed on the plasma membrane surface and capable of internalization. Among perspective targets are membrane glycoproteins (for example, mesothelin) and membrane receptors (for example, transferrin and growth factors receptors) [1].
Transmembrane receptor HER2/neu is one of the best-studied cancer markers successfully used for targeted therapy [2]. Full-length humanized monoclonal anti-HER2/neu 4D5 antibody, also known as trastuzumab, is widely used in clinical practice with the trade name Herceptin and is regarded as the gold standard for the targeted therapy of solid tumors [3]. Besides, in 2012 another HER2/neu-specific drug Perjeta based on antibody against another epitope of this cancer marker was introduced into clinical practice.
Efforts to increase the antitumor efficacy of the antibodies lead to the appearance of the next generation of targeted therapeutics – immunotoxins consisting of two structurally and functionally independent modules: the targeting one, specifically binding with targets, and the effector (toxic) one. If both of the structural–functional modules are proteins, they can be combined into a single polypeptide chain by genetic engineering. Such recombinant immunotoxins feature a number of advantages: structural homogeneity, simplicity of production in bacterial systems, and preservation of the functional properties of the modules [4].
In this work, the new recombinant immunotoxin 4D5scFv-PE40 based on HER2/neu-specific scFv antibody and a fragment of Pseudomonas exotoxin A (PE40) has been created, and its antitumor efficacy in vitro and in vivo has been estimated.
MATERIALS AND METHODS
Expression, isolation, and purification of the immunotoxin. Plasmid pSD-4D5scFv-PE40 encoding the recombinant immunotoxin 4D5scFv-PE40 was obtained based on the pSD-4D5-barnase plasmid [5]. The DNA fragment encoding PE40 was amplified from plasmid pIG6-4D5MOCB-ETA [6] using primers 5′-actacGGCGCGCCGGAGTTCCCGAAACCGTCCAC and 5′-tgcgtAAGCTTCTACAGTTCGTCTTTATGGTG. The amplified fragment was treated with AscI and HindIII restriction endonucleases (Fermentas, USA) and cloned into pSD-4D5-barnase plasmid instead of the barnase gene.
For production of the recombinant immunotoxin 4D5scFv-PE40 in bacterial producers, Escherichia coli strain BL21(DE3) cells were transformed with pSD-4D5scFv-PE40 plasmid containing the gene of the immunotoxin under the control of the lac-promoter. Freshly transformed bacteria were cultured in YTPS medium (yeast extract, 1%; tryptone, 1%; NaCl, 0.5%; K2HPO4, 80 mM, and KH2PO4, 20 mM) at 28°C to optical density of 0.8 at 550 nm; then the lac-promoter was induced by the addition of 0.5 mM isopropyl-1-thio-β-D-galactoside. The bacteria were further cultured with intensive stirring at 28°C for 12 h and harvested by centrifugation.
The bacterial pellet containing the protein of interest was resuspended in lysis buffer (Tris-HCl, 20 mM; KH2PO4, 10 mM; EDTA, 10 mM, and NaCl, 100 mM; pH 8). The cells were sonicated using a Labsonic P Sonifier (Sartorius, Germany), and the lysate was centrifuged at 18,500g for 10 min at 4°C.
The supernatant containing the water-soluble form of 4D5scFv-PE40 was purified on a 1-ml HisTrapFF column (GE Healthcare, USA) under native conditions according to the manufacturer’s instructions. The protein immobilized on the column was eluted using a linear gradient from 0 to 200 mM imidazole. The collected fractions containing the protein of interest were then further purified by ion-exchange chromatography on a 1-ml QSepharoseFF column (GE Healthcare) according to the manufacturer’s instructions. The protein of interest was eluted using a linear gradient from 25 to 500 mM NaCl. Fractions containing the protein were analyzed by electrophoresis in 12% polyacrylamide gels under denaturing conditions according to a standard protocol.
Determination of the dissociation constant of the immunotoxin. The dissociation constant of the complex formed by immunotoxin 4D5scFv-PE40 with the HER2/neu receptor was determined by the surface plasmon resonance method using a BIAcore 3000 analyzer (GE Healthcare). Recombinant protein p185HER2-ECD (Sino Biological, Inc., China) – an extracellular domain of the HER2/neu receptor – was covalently immobilized on the surface of a carboxymethyl-dextran CM5 chip according to the manufacturer’s instructions. The receptor density on the chip was 4500 RU.
The immunotoxin was dissolved in HBS-PE buffer (HEPES, 0.1 M, pH 7.4; NaCl, 0.15 M; EDTA, 3 mM; Tween-20, 0.005%) and was used at four concentrations (1 μM and 330, 110, and 37 nM). The sensograms were obtained at a flow rate of 5 μl/min at 25°C. The dissociation phase lasted for 20 min. The results were processed with the BIAevaluation Software.
Estimation of cytotoxicity of the immunotoxin. Modified cells of human ovary adenocarcinoma SKOV-3 (ATCC catalog No. HTB-77) cell line SKOV-kat overexpressing HER2/neu receptor, as well as HER2/neu-negative Chinese hamster ovary cells CHO (ATCC catalog No. CCL-61) were used.
Cells were cultured in RPMI-1640 medium (HyClone, USA) with 10% fetal calf serum (HyClone) and 2 mM glutamine (PanEco, Russia) at 37°C in an atmosphere with 5% CO2. To prevent the enzymatic removal of surface receptors during culturing, the cells were suspended with Versene solution (PanEco) without trypsin.
For the analysis of the immunotoxin cytotoxicity, cell suspension was seeded in 96-well plates at a density of 4·103 (SKOV-kat) or 6·103 (CHO) per well and cultured overnight. The culture medium was removed, and 4D5scFv-PE40 immunotoxin or one of its modules (4D5scFv or PE40) in different concentrations (from 1 pM to 1 nM) in phosphate-buffered saline (PBS) was added to the wells. After incubation at 4°C for 40 min, the cells were washed with PBS to remove the unbound protein, fresh culture medium was added, and the cells were cultured for 48 h.
Cell viability after the treatment with the immunotoxin was evaluated by a standard MTT assay [7]. The relative viability of the cells was calculated as a percentage ratio of the optical density of treated cells to the optical density of untreated cells. Data processing and calculation of IC50 (the immunotoxin concentration required to halve the cell viability relative to the control level) was carried out using the GraphPad Prism 6 software (GraphPad Software).
Estimation of antitumor effect of the immunotoxin in vivo. To create a model of human tumor with overexpression of HER2/neu receptor, immunodeficient BALB/c-Nude mice (female, 17-23 g, 4-6 weeks) obtained from the Animal Breeding Center of the Pushchino Branch of the Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences (Pushchino, Russia) were used. To create xenograft tumors, the suspension of SKOV-kat cells in PBS was mixed with Matrigel growth substrate (BD Biosciences, USA) at ratio 1 : 1 (v/v) and injected into the mice subcutaneously in the subscapular region (2·106 cells per animal).
Overexpression of HER2/neu receptor in the xenograft tumor tissue was confirmed by immunohistochemical analysis using the HercepTest kit (Dako, USA).
Animals were divided into four groups containing five animals each. Twenty-four hours after the inoculation of tumor cells animals of group 1 (control) and group 2 were intraperitoneally injected with 0.1 ml PBS and 4D5scFv-PE40 (58 pmol/animal) dissolved in 0.1 ml PBS, respectively. Animals of group 3 received three injections (on day 3, 5, and 7 after the tumor cell inoculation) of the chemotherapy drug cisplatin (single dose, 0.06 mg/animal in 0.1 ml PBS). Animals of group 4 were injected with 4D5scFv-PE40 and cisplatin according to the described protocols.
Starting from day 6 after the tumor cell inoculation, tumor nodes were measured twice a week using a Vernier caliper. The tumor node volume was calculated according to the Schrek formula: V(mm3) = a(mm) × b2(mm2)/2, where a and b is tumor length and width, respectively.
The data were statistically processed by one-way ANOVA and Dunnett’s test (GraphPad Prism 6). The difference between groups was regarded statistically significant at p < 0.05.
To quantify the effect of the antitumor agents, the coefficient of the tumor growth inhibition (TGI) was calculated according to the formula: TGI(%) = [(Vcontr – Vexp)/Vcontr] × 100%, where Vcontr and Vexp are mean tumor volumes of the control and experimental groups, respectively.
RESULTS
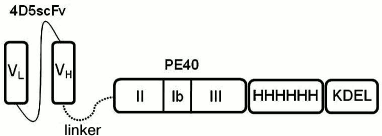
The novel anti-HER2/neu immunotoxin 4D5scFv-PE40 has been created. It is a recombinant protein in which a targeting module (responsible for the binding with a corresponding target antigen) and an effector (toxic) module are combined into one polypeptide chain (Fig. 1).
Fig. 1. Scheme of 4D5scFv-PE40 immunotoxin. VL and VH, variable domains of light and heavy chains of 4D5 antibody, respectively; PE40, fragment of Pseudomonas exotoxin A (252-613 a.a.); HHHHHH, oligohistidine tag; KDEL, amino acid sequence ensuring translocation to EPR.
The targeting module 4D5scFv is a derivate of the anti-HER2/neu antibody 4D5 [8], in which fragments of the light and heavy chains of natural immunoglobulin are connected with a flexible peptide linker, thus making one polypeptide chain (Fig. 1).
Effector module (PE40) of the immunotoxin is a fragment (252-613 a.a.) of Pseudomonas exotoxin A. This fragment contains translocation domain II, domain Ib with unknown functions, and domain III responsible for ADP ribosylation of elongation factor 2 (eET2) in eukaryotes and accounting for the cytotoxic effect (Fig. 1).
The targeting and effector modules are connected with a flexible hydrophilic 16-a.a. linker (EFPKPSTPPGSSGGAP) from the hinge region of mouse immunoglobulin IgG [9]. In stretched conformation, this linker can be as long as 2.5-2.7 nm, which releases the connected parts of the protein molecule from steric hindrances and thus ensures the retention of their functional properties.
The oligohistidine tag located at the C-terminus of the molecule makes it possible to purify the immunotoxin by means of metal-affinity chromatography. The C-terminus also contains the KDEL signal sequence that ensures translocation of the immunotoxin effector module from Golgi apparatus to the endoplasmic reticulum; it is known that in the absence of this sequence the activity of Pseudomonas exotoxin A is 1000 times lower than in its presence [10].
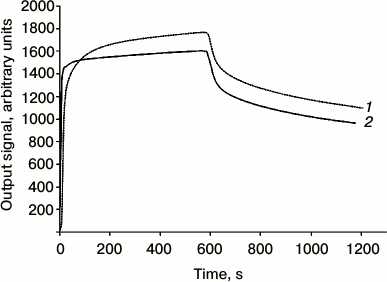
The immunotoxin exhibits a high affinity to HER2/neu receptor: the dissociation constant of the complex of 4D5scFv-PE40 with the HER2/neu receptor extracellular domain (p185HER2-ECD), estimated by surface plasmon resonance method, is 6.8 nM (Kon = 2.29·104 M–1·s–1; Koff = 1.56·10–4 s–1), which is comparable with the dissociation constant of the free antibody 4D5scFv (5.2 nM) determined by the same method (Fig. 2).
Fig. 2. Analysis of the affinity of 4D5scFv-PE40 immunotoxin. Dissociation kinetics of 4D5scFv (2) and 4D5scFv-PE40 (1) obtained by means of the surface plasmon resonance method at protein concentration 3 µM.
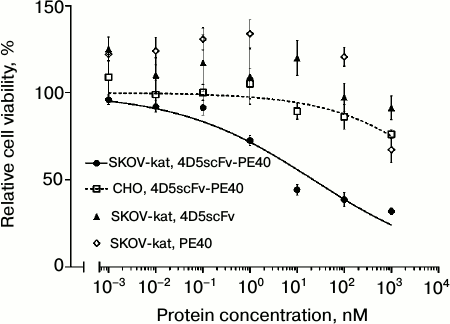
Immunotoxin 4D5scFv-PE40 considerably lowered viability of SKOV-kat cells overexpressing the HER2/neu receptor; the IC50 for this cell line was 22 nM (Fig. 3, circles and solid line). As concerns HER2/neu-negative CHO cells, no significant cytotoxic effect was observed at immunotoxin concentrations of up to 1 µM (Fig. 3, squares and dashed line). Free proteins 4D5scFv and PE40 in equimolar concentrations did not affect the viability of SKOV-kat cells (Fig. 3, triangles and diamonds, respectively).
Fig. 3. Analysis of cytotoxicity of 4D5scFv-PE40 in vitro. Relative viability of HER2/neu-positive cells SKOV-kat after their treatment with 4D5scFv-PE40 immunotoxin, 4D5scFv antibody, or PE40 toxin, and relative viability of HER2/neu-negative CHO cells after their treatment with 4D5scFv-PE40 immunotoxin. Error bars show SEM.
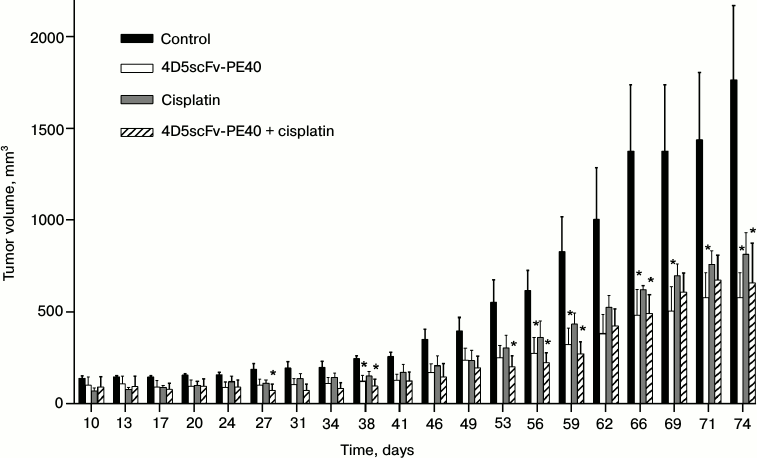
The antitumor effect of immunotoxin 4D5scFv-PE40 in vivo was estimated on subcutaneous human ovarian carcinoma xenografts based on the SKOV-kat cell line. Immunohistological analysis of the developed tumor tissue confirmed the overexpression of HER2/neu receptor (data not shown). A single injection of 4D5scFv-PE40 considerably suppressed the tumor growth with TGI of ~67% (Fig. 4). A comparable effect was shown for cisplatin – a widely used chemotherapeutic drug (TGI ~54%). Combined administration of both agents did not potentiate the effect of the immunotoxin.
Fig. 4. Tumor progression in different groups of animals. Tumor volume after the injection of PBS (control, black bars), 4D5scFv-PE40 (white bars), cisplatin (gray bars), and after combined injection of 4D5scFv-PE40 and cisplatin (hatched bars). Day 0 is the day of subcutaneous inoculation of SKOV-kat cells. The data are represented as mean ± SEM; * indicates significant (p < 0.05) difference between treatment and control groups using Dunnett’s test, n = 4-5.
DISCUSSION
Targeted therapy is now widely used for the treatment of various types of oncohematological diseases, while for the treatment of solid tumors only a few drugs have been approved. In the last decade a great number of new therapeutic targets for solid tumors have been discovered; these targets belong to signaling cascades of the HER family receptors playing an important role in malignant transformation of cells of epithelial origin [2]. A number of kinase inhibitors have been generated, both with broad spectrum of action and highly specific, affecting the proteins of the HER signaling network at almost all levels of the signal transduction. Most of the efforts, however, were concentrated on the search for ways to block the HER signaling system just at the signal input, that is, at the receptor level. Such blockade can be achieved by means of agents based on receptor-specific antibodies.
To increase the efficiency of antibodies, they are conjugated with other effector molecules: radioisotopes, toxins, interleukins, enzymes, etc. [11, 12]. In these immunoconjugates, the antibodies usually play the role of the targeting component delivering the effector agent. To attach low molecular weight agents, radioisotopes for example, chemical conjugation methods are commonly applied [13].
Of the great number of antibody-based targeted agents currently undergoing clinical trials, 44% are immunotoxins. Only two drugs are approved for clinical use: Kadcyla (trastuzumab-emtansine, or T-DM1), the humanized anti-HER2/neu antibody trastuzumab conjugated with a toxin of the maytansinoid class inhibiting mitosis, and Adcetris (brentuximab vedotin), an immunoconjugate of the antimitotic agent of the same group and a chimeric immunoglobulin specific to protein CD30 expressed on the surface of the tumor cells of Hodgkin lymphoma. This disproportion is due to objective difficulties emerging in the course of immunotoxin development.
Chemical conjugation as a method for immunotoxin production features a number of technical and scientific problems: insufficient reproducibility and variability of the conjugate composition, possible decrease in the antibody affinity or toxin efficacy, as well as admixture of non-conjugated antibody and toxin. An alternative approach is the creation the recombinant proteins. This approach has been successfully applied for generation of immunotoxins, including immunotoxins specific to HER2/neu [14-17].
In this work, a new recombinant immunotoxin based on the anti-HER2/neu antibody (4D5scFv) and a fragment of the Pseudomonas exotoxin A (PE40) has been created.
Since the efficacy of a therapeutic drug directly depends on the toxicity of the effector module, it is expedient to use toxins with IC50 ranging from pM to nM. Pseudomonas exotoxin A is among the toxins meeting this requirement [18]. We used the PE40 fragment of this toxin, devoid of a natural directing domain, for the construction of immunotoxin 4D5scFv-PE40.
Antibody fragment 4D5scFv, used in this work, is most stable, steady in a wide range of conditions, and high-affinity of all currently available anti-HER2/neu antibody fragments [19, 20]. Another feature of this antibody ensuring high efficacy of the related immunotoxins is its internalization into a cell after binding with the target receptor. Based on this antibody, we earlier generated a number of efficient antitumor agents with different mechanisms of action – immuno-RNases [21, 22] and immunophotosensitizers [23, 24]. The IC50 values of these immunotoxins for human carcinoma cells lay in the range from tens to hundreds nanomolar concentrations when the cells are treated for 72 h. The IC50 of immunotoxin 4D5scFv-PE40 is 22 nM at 1-h treatment of human ovary adenocarcinoma.
The data suggest a high selective cytotoxicity of 4D5scFv-PE40 directed against tumor cells overexpressing cancer marker HER2/neu both in vitro in cell culture and in vivo in tumor-bearing animals.
Although for a comprehensive characterization of the created immunotoxin as an antitumor agent additional studies of its pharmacological properties (including possible systemic toxicity) are necessary, as well as optimization of doses and administration protocols, our results are indicative of high potential of 4D5scFv-PE40 for targeted therapy of tumors with overexpression of HER2/neu receptor.
This work was supported by the Russian Scientific Foundation (project No. 14-14-00813).
REFERENCES
1.Kreitman, R. J. (2006) Immunotoxins for targeted
cancer therapy, AAPS J., 8, 532-551.
2.Polanovski, O. L., Lebedenko, E. N., and Deyev, S.
M. (2012) ERBB oncogene proteins as targets for monoclonal antibodies,
Biochemistry (Moscow), 77, 227-245.
3.English, D. P., Roque, D. M., and Santin, A. D.
(2013) HER2 expression beyond breast cancer: therapeutic implications
for gynecologic malignancies, Mol. Diagn. Ther., 17,
85-99.
4.Choudhary, S., Mathew, M., and Verma, R. S. (2011)
Therapeutic potential of anticancer immunotoxins, Drug Discov.
Today, 16, 495-503.
5.Deyev, S. M., Waibel, R., Lebedenko, E. N.,
Schubiger, A. P., and Pluckthun, A. (2003) Design of multivalent
complexes using the barnase*barstar module, Nat. Biotechnol.,
21, 1486-1492.
6.Di Paolo, C., Willuda, J., Kubetzko, S., Lauffer,
I., Tschudi, D., Waibel, R., Pluckthun, A., Stahel, R. A., and
Zangemeister-Wittke, U. (2003) A recombinant immunotoxin derived from a
humanized epithelial cell adhesion molecule-specific single-chain
antibody fragment has potent and selective antitumor activity, Clin.
Cancer Res., 9, 2837-2848.
7.Mosmann, T. (1983) Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays, J. Immunol. Methods, 65, 55-63.
8.Eigenbrot, C., Randal, M., Presta, L., Carter, P.,
and Kossiakoff, A. A. (1993) X-ray structures of the antigen-binding
domains from three variants of humanized anti-p185HER2 antibody 4D5 and
comparison with molecular modeling, J. Mol. Biol., 229,
969-995.
9.Muller, K. M., Arndt, K. M., Strittmatter, W., and
Pluckthun, A. (1998) The first constant domain (C(H)1 and C(L)) of an
antibody used as heterodimerization domain for bispecific
miniantibodies, FEBS Lett., 422, 259-264.
10.Seetharam, S., Chaudhary, V. K., FitzGerald, D.,
and Pastan, I. (1991) Increased cytotoxic activity of Pseudomonas
exotoxin and two chimeric toxins ending in KDEL, J. Biol.
Chem., 266, 17376-17381.
11.Carter, P. J., and Senter, P. D. (2008)
Antibody–drug conjugates for cancer therapy, Cancer J.,
14, 154-169.
12.Deyev, S. M., and Lebedenko, E. N. (2009) Modern
technologies for creating synthetic antibodies for clinical
application, Acta Naturae, 1, 32-50.
13.Ducry, L., and Stump, B. (2010)
Antibody–drug conjugates: linking cytotoxic payloads to
monoclonal antibodies, Bioconj. Chem., 21, 5-13.
14.Wang, L., Liu B., Schmidt, M., Lu, Y., Wels, W.,
and Fan, Z. (2001) Antitumor effect of an HER2-specific
antibody–toxin fusion protein on human prostate cancer cells,
Prostate, 47, 21-28.
15.Batra, J. K., Kasprzyk, P. G., Bird, R. E.,
Pastan, I., and King, C. R. (1992) Recombinant anti-erbB2 immunotoxins
containing Pseudomonas exotoxin, Proc. Natl. Acad. Sci.
USA, 89, 5867-5871.
16.Cao, Y., Marks, J. W., Liu, Z., Cheung, L. H.,
Hittelman, W. N., and Rosenblum, M. G. (2013) Design optimization and
characterization of Her2/neu-targeted immunotoxins: comparative in
vitro and in vivo efficacy studies, Oncogene,
33, 429-439.
17.Zielinski, R., Lyakhov, I., Jacobs, A., Chertov,
O., Kramer-Marek, G., Francella, N., Stephen, A., Fisher, R.,
Blumenthal, R., and Capala, J. (2009) Affitoxin – a novel
recombinant, HER2-specific, anticancer agent for targeted therapy of
HER2-positive tumors, J. Immunother., 32, 817-825.
18.Pastan, I., Hassan, R., FitzGerald, D. J., and
Kreitman, R. J. (2007) Immunotoxin treatment of cancer, Annu. Rev.
Med., 58, 221-237.
19.Jung, S., and Pluckthun, A. (1997) Improving
in vivo folding and stability of a single-chain Fv antibody
fragment by loop grafting, Protein Eng., 10, 959-966.
20.Willuda, J., Honegger, A., Waibel, R., Schubiger,
P. A., Stahel, R., Zangemeister-Wittke, U., and Pluckthun, A. (1999)
High thermal stability is essential for tumor targeting of antibody
fragments: engineering of a humanized anti-epithelial glycoprotein-2
(epithelial cell adhesion molecule) single-chain Fv fragment, Cancer
Res., 59, 5758-5767.
21.Edelweiss, E., Balandin, T. G., Ivanova, J. L.,
Lutsenko, G. V., Leonova, O. G., Popenko, V. I., Sapozhnikov, A. M.,
and Deyev, S. M. (2008) Barnase as a new therapeutic agent triggering
apoptosis in human cancer cells, PLoS ONE, 3, e2434.
22.Balandin, T. G., Edelweiss, E., Andronova, N. V.,
Treshalina, E. M., Sapozhnikov, A. M., and Deyev, S. M. (2011)
Antitumor activity and toxicity of anti-HER2 immuno-RNase scFv
4D5-dibarnase in mice bearing human breast cancer xenografts,
Invest. New Drugs, 29, 22-32.
23.Serebrovskaya, E. O., Edelweiss, E. F.,
Stremovskiy, O. A., Lukyanov, K. A., Chudakov, D. M., and Deyev, S. M.
(2009) Targeting cancer cells by using an antireceptor
antibody–photosensitizer fusion protein, Proc. Natl. Acad.
Sci. USA, 106, 9221-9225.
24.Mironova, K. E., Proshkina, G. M., Ryabova, A.
V., Stremovskiy, O. A., Lukyanov, S. A., Petrov, R. V., and Deyev, S.
M. (2013) Genetically encoded immunophotosensitizer 4D5scFv-miniSOG is
a highly selective agent for targeted photokilling of tumor cells in
vitro, Theranostics, 3, 831-840.