Unexpectedly Strong Effect of Caffeine on the Vitality of Western Honeybees (Apis mellifera)
A. Strachecka1*, M. Krauze2, K. Olszewski1, G. Borsuk1, J. Paleolog1, M. Merska2, J. Chobotow3, M. Bajda1, and K. Grzywnowicz4
1Department of Biological Basis of Animal Production, Faculty of Biology and Animal Breeding, University of Life Sciences in Lublin, Akademicka 13, 20-950 Lublin, Poland; E-mail: aneta.strachecka@up.lublin.pl; krzysztof.olszewski@up.lublin.pl; grzegorz.borsuk@up.lublin.pl; jerzy.paleolog@up.lublin.pl; milena.bajda@wp.pl2Department of Biochemistry and Toxicology, Faculty of Biology and Animal Breeding, University of Life Sciences in Lublin, Lublin, Poland; E-mail: magdalena.krauze@up.lublin.pl; malwina.merska@interia.pl
3Department of Zoology, Museum Zoological/Laboratory, Faculty of Biology and Biotechnology, Maria Curie-Sklodowska University, Lublin, Poland; E-mail: jacek.chobotow@op.pl
4Department of Biochemistry, Faculty of Biology and Biotechnology, Maria Curie-Sklodowska University, Lublin, Poland; E-mail: grzyw@hermes.umcs.lublin.pl
* To whom correspondence should be addressed.
Received May 22, 2014; Revision received June 26, 2014
We examined the influence of caffeine on honeybee lifespan, Nosema resistance, key enzyme activities, metabolic compound concentrations, and total DNA methylation levels. Caffeine slowed age-related metabolic tendencies. Bees that consumed caffeine lived longer and were not infested with Nosema spp. Caffeine-treated workers had higher protein concentrations. The levels increased with aging but they then decreased in older bees. Caffeine increased the activities of antioxidant enzymes (SOD, GPx, CAT, GST), AST, ALT, ALP, neutral proteases, and protease inhibitors, and the concentrations of uric acid, triglycerides, cholesterol, glucose, and Ca2+. Acidic and alkaline protease activities were lower in the bees treated with caffeine. Creatinine and Mg2+ concentrations were higher in the caffeine-treated workers but only up to 14 days of age. Caffeine significantly decreased DNA methylation levels in older bees. The compound could be considered as a natural diet supplement increasing apian resistance to stress factors. Our studies will enhance possibilities of using Apis mellifera as a model organism in gerontological studies.
KEY WORDS: honeybee, caffeine, vitality, antioxidant, proteolysis, DNA methylationDOI: 10.1134/S0006297914110066
Caffeine acts as a chemical stimulant in mammals that can affect mood, alertness, and concentration. It offers great prospects for the preventive treatment of neurological disorders, e.g. Parkinson’s disease. Caffeine acts as an antagonist at A1 and A2A adenosine receptors. It releases a signal transduction cascade that involves adenylate cyclase and cAMP. It also participates in protein phosphorylation by protein kinases and in gene expression. At the neurotransmitter level, several systems might be affected including dopaminergic and cholinergic transmissions [1]. At the cellular level, adding caffeine to hippocampal slices leads to calcium release from internal stores and the development of new dendritic branches [2]. Caffeine increases the levels of circulating fatty acids, and therefore enhances body fat oxidation. It impairs glucose homeostasis through transient increases in insulin resistance [3] and is known to have antioxidant properties [4, 5]. It might protect against a variety of health problems [6]. Advantageous or disadvantageous effects of caffeine depend on its dosage and treatment duration, as well as on individual susceptibility and genotype, especially in the case of polymorphic genes associated with caffeine metabolism, i.e. cytochrome P450 – CYP1A2 and catechol-O-methyltransferases (COMT) [7].
The excitation effect of caffeine has also been observed in invertebrates. Caffeine-treated Drosophila flies are more active than control flies. The response, however, depends on the dose of the compound [8]. The survival time of fruit flies that chronically ingested caffeine was not only related with its dosage but also with the sex and the genotype of the flies [9]. Caffeine exerts influences on both developmental and cognitive processes in mature honeybees in a way that is similar to the effects reported in humans and other vertebrates [10]. In young honeybees, caffeine reduces the age at which constrained individuals are able to perform olfactory associative tasks, whereas in older forager bees, caffeine not only promotes motivation but also boosts cognitive performance in complicated learning tasks [11]. The effects of caffeine on the processes occurring in apian brains have been studied [12, 13].
Apis mellifera is used as a model organism in relation to vertebrates with regard to both epigenetics and experimental gerontology [14-16]. However, the biochemical response to caffeine administration in the case of key apian metabolic pathways has not been thoroughly explored. Therefore, further studies on specific caffeine effects on biochemical processes and DNA methylation in honeybees are needed, particularly in the context of aging, since interspecies differences exist, even though many metabolic pathways are conservative.
On the other hand, natural bioactive preparations and antioxidants that will boost apian resistance and aid body detoxification are in demand. This is particularly important at a time of intensified agriculture, honeybee colony collapse disorder (CCD), and the proliferation of such diseases as varroatosis or nosemosis [17-20]. In this situation, hopes are pinned on natural, bioactive nutrients. One of them is caffeine.
Considering that caffeine has strong antioxidative properties, we proposed the hypothesis that it lengthens apian life, reduces the level of infestation by Nosema spp., affects key biochemical processes, and slows the age-related increase in global DNA methylation level.
In this study, we decided to analyze the age-related impact of caffeine on the activities/concentrations of apian antioxidants, key enzymes/metabolic compounds, proteolytic system, and total DNA methylation levels. These parameters are of particular importance for the maintenance of homeostasis, strengthening stress resistance, and therefore for postponement of senescence. We also decided to specifically check whether caffeine might protect bees against Nosema spp. Infestation, which is now one of the major problems in worldwide apiculture [21-23].
MATERIALS AND METHODS
Two worker groups (A. mellifera) were set up each consisting of 100 wooden cages (12 × 12 × 4 cm) with 40 one-day-old workers in each. Each cage has a glass front screen as well as ventilation and feeding slots. The workers were fed with sugar syrup (1 : 1) ad libitum using inner feeders. In the first group, the syrup was supplemented with caffeine (FP VI, Pharma Cosmetic) at the concentration of 5 µg/ml, whereas in the second group, the control, caffeine was not added to the syrup. The cages were kept in an air-conditioned chamber (26°C and 65% relative humidity). In each of the groups, 30 of the 100 cages were designated for longevity tests and the remaining 70 for biochemical assays. The procedures described below were started 2 h after the start of feeding.
Procedure 1. Dead workers were removed every two days from the 30 longevity-test cages (in total, 1200 workers were tested) and frozen at –25°C for further assessment of Nosema spp. infestation [24] in each of the groups. Numbers of workers that remained alive were recorded and quartiles (75, 50, 25, and 0%) of worker lifespan distributions were determined.
Procedure 2. Following Strachecka et al. [25], on the 1st, 4th, 10th, 18th, 25th, and 32nd day, fresh hemolymph was taken 3 to 7 times from all the remaining 70 cages from 12 to 15 living workers (altogether 2800 workers). The procedure was performed in each of the groups. Because the bees fed with caffeine lived longer, analogically on the 42nd and 53rd day, 3 to 7 pooled samples were obtained that contained 100 µl of fresh hemolymph, respectively. A glass capillary was introduced between the third and fourth apian tergite and, sampled in this way, hemolymph was collected into sterile Eppendorf (Germany) tubes containing 200 µl of ice-cooled 0.6% NaCl. The tubes, each containing one pooled hemolymph sample, were immediately frozen at –25°C for further biochemical analyses. Additionally, 10 live workers were randomly captured from all the remaining 70 cages and refrigerated at –25°C to further analyze global DNA methylation levels.
Protein concentration was determined using the Lowry method as modified by Schacterle and Pollack [26]. Copper reagent (10 µl) was added to 10 µl samples for a 10 min incubation at 25°C. Subsequently, 40 µl of Folin reagent was added (1 : 17) for a 5 min incubation at 55°C. The resulting samples were analyzed spectrophotometrically to measure the absorbance at 280 nm.
Antioxidant enzyme activities. Catalase (CAT) activity was measured according to Aebi [27]. A sample of H2O2 solution (165 µl of 54 mM) was supplemented with 335 µl of 50 mM phosphate buffer (pH 7.0) at 25°C. The reaction was started with the addition of a 5 µl hemolymph sample. Decomposition of H2O2 was monitored at 240 nm. One unit of catalase was taken as the amount of the enzyme that decomposes 1 µmol of H2O2 per minute at 25°C.
Glutathione peroxidase (GPx) activity was measured according to Chance and Maehly [28]. A 25 µl sample of the assay mixture (125 µM phosphate buffer, pH 6.8, 50 µM pyrogallol, 50 µM H2O2, and 5 µl of hemolymph) was incubated for 5 min at 25°C, after which the reaction was stopped by adding 5 µl of 5% H2SO4. Absorbance was monitored at 420 nm.
Glutathione S-transferase (GST) activity was measured by the method of Warholm et al. [29]. A cuvette was prepared with 215 µl of 0.1 M sodium phosphate buffer (pH 6.5), 13 µl of 20 mM GSH, and 13 µl of 20 mM 1-chloro-2.4-dinitrobenzene in 95% ethanol. The reaction, which was carried out at 30°C, was started with the addition of a 13 µl hemolymph sample. Absorbance increase was monitored at 340 nm.
Superoxide dismutase (SOD) activity was measured according to the method of Podczasy and Wei [30]. The following substances were added to the tubes: 0.5 mM xanthine, 0.3 mM EDTA, 49 mM p-iodonitrotetrazolium, 0.92 mM Na2CO3, and a 5 µl hemolymph sample. The reaction was initiated by addition of the indicated amount of xanthine oxidase (up to 0.25 U/ml). After 15 min incubation at 25°C, the mixture was cooled. Absorbance was monitored at 505 nm.
All antioxidant enzyme activities were calculated per mg of protein.
Non-enzymatic antioxidant (albumin, uric acid, urea, creatinine) concentrations were determined by colorimetry using monotests from Cormay (Poland) according to the manufacturer’s instructions.
Activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) were measured with the kinetic method using monotests from Cormay according to the manufacturer’s instructions.
Concentrations of triglycerides, cholesterol, glucose, Mg2+, and Ca2+ were measured by colorimetry using Cormay monotests according to the manufacturer’s instructions.
Protease and protease inhibitor activities were determined as follows. Proteolytic activity in relation to substrates (gelatin, hemoglobin, ovalbumin, albumin, cytochrome c, casein) was tested according to the Anson method [31] modified by Strachecka et al. [32]. Five microliters of each sample was incubated with 10 µl of 1% substrates (each of six) in an appropriate buffer for 2 h at 37°C. The reactions were ended by adding 40 µl of cold 5% trichloroacetic acid (TCA). The supernatant was spectrophotometrically analyzed to measure the absorbance at 280 nm. Hemoglobin was changed as the optimal substrate for subsequent analyses.
Activities of acidic, neutral, and basic proteases were measured by Anson’s method [31] modified by Strachecka et al. [33]. Protease activities were assayed in three buffers: 100 mM glycine-HCl, pH 2.4; 100 mM Tris-HCl, pH 7.0, and 100 mM glycine-NaOH, pH 11.2. Next, 5 µl of each sample was incubated with 10 µl of 1% hemoglobin in an appropriate buffer for 90 min at 37°C. The reactions were ended by adding 40 µl of cold 5% TCA; the undigested proteins were precipitated and centrifuged. The supernatant was analyzed spectrophotometrically to measure the absorbance at 280 nm. One unit of enzyme activity was defined as the amount of enzyme producing a 0.001 increase in absorbance per minute.
Levels of natural inhibitors of acidic, neutral, and alkaline proteases were determined by the method of Lee and Lin [34]. When determining general levels of protease inhibitor activities, pepsin was used as a protease marker at acidic pH and trypsin as a protease marker at neutral and alkaline pH. Five microliters of pepsin or trypsin was preincubated with 5 µl of a given sample for 30 min. After this time, 25 µl of 1% hemoglobin in an appropriate buffer was added and the incubation continued for 60 min. The reactions were ended by adding 60 µl of TCA, and the supernatants were assayed as described above. Inhibitor activities were calculated according to Lee and Lin [34].
Proteolytic activities in relation to diagnostic inhibitors of proteolytic enzymes (pepstatin A, phenylmethylsulfonyl fluoride (PMSF), iodoacetamide, o-phenanthroline) were measured according to Lee and Lin [34]. Five microliters of the diagnostic inhibitors were preincubated with 5 µl of a given sample for 30 min. After this time, 25 µl of 1% hemoglobin in an appropriate buffer was added and the incubation continued for 90 min. The reactions were ended by adding 60 µl of TCA, and the supernatants was assayed as described above.
Global DNA methylation. DNA was extracted from the head and thorax of 10 live workers additionally captured from all the remaining 70 cages using the DNeasy Blood & Tissue Kit (Qiagen, Germany) following the producer’s instructions. Extracted DNA samples were stored at –25°C and subsequently modified by treatment with Capture Antibody using MDQ1-96RXN Imprint® Methylated DNA Quantification Kit following the producer’s instructions (Sigma, USA).
Statistical analysis. Caffeine effects and the significance of differences between the group averages were determined using one-way ANOVA plus the least significant analysis procedures (LSD) with SAS statistical software (SAS Institute v9.13, 2002-2003 license 86636). The Mann–Whitney U-test was used to analyze differences between the two groups in the number of Nosema spp. spores.
RESULTS
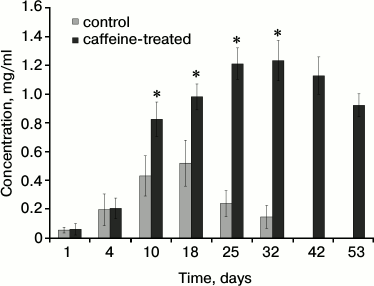
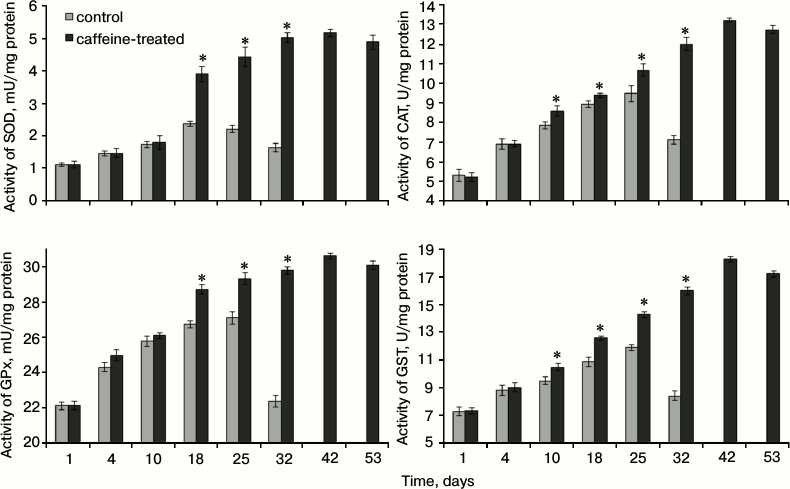
Bees that consumed caffeine lived longer and were not infested with Nosema spp. (Table 1). In particular, the percentage of long-lived workers was greater in the bee group that consumed caffeine. Caffeine-treated workers had higher concentrations of proteins (Fig. 1). The following age-related tendencies were revealed. First, protein concentrations increased during the aging/maturation process, but they then decreased in older bees. The decrease, however, began much later in the caffeine-treated group. Caffeine also increased the activities of antioxidant enzymes (SOD, GPx, CAT, GST) (Fig. 2) as well as the activities of AST, ALT, ALP (Table 2), neutral proteases (Table 3), and protease inhibitors (Table 4). The stimulating effects of caffeine were always more distinct in older workers. Age-related tendencies similar to those revealed in the case of protein concentrations were also observed.
Fig. 1. Protein concentrations in the hemolymph of workers from the control and caffeine-treated groups. * Differences are statistically significant for comparisons between the groups (control and caffeine-treated) and workers of the same age at P ≤ 0.05.
Fig. 2. Activities of antioxidant enzymes in the hemolymph of workers from the control and caffeine-treated groups. * Differences are statistically significant for comparisons between the groups (control and caffeine-treated) and workers of the same age at P ≤ 0.05. SOD, superoxide dismutase; GPx, peroxidase; CAT, catalase; GST, glutathione S-transferase.
Table 1. Longevity of caged workers from the
control and caffeine-treated groups (expressed as quartiles of their
life-span distributions) and intensity of Nosema spp.
infestation
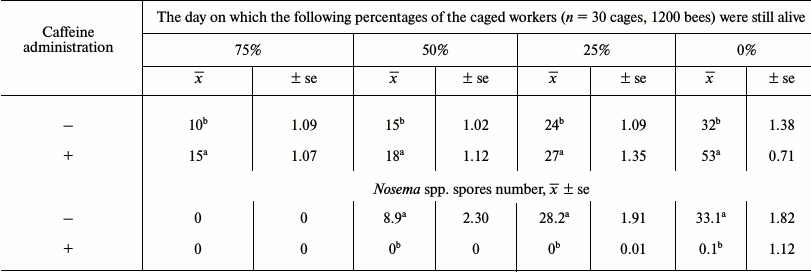
Notes: x̅, mean value; ±se, standard error;
a,b differences are statistically significant at P
≤ 0.05 for comparisons within the columns.
Table 2. Mean activities of aspartate
aminotransferase (AST), alanine aminotransferase (ALT) and alkaline
phosphatase (ALP) in workers from the control and caffeine-treated
groups
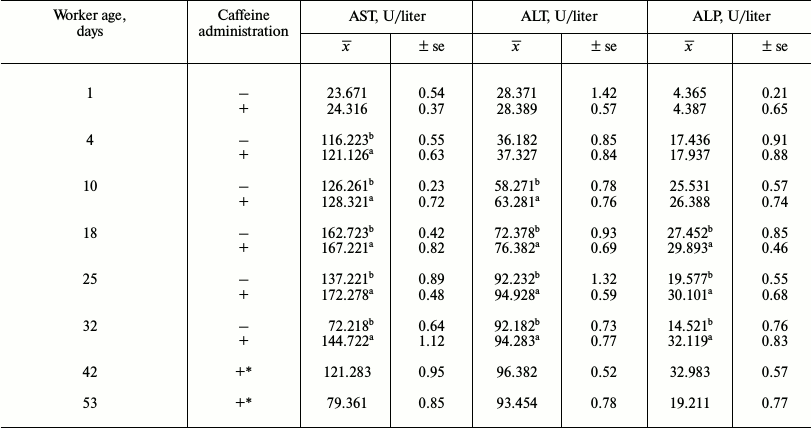
Notes: x̅, mean value; ±se, standard error;
a,b the differences between the groups are statistically
significant for comparisons made within each of the rows separately
(bees of the same age and particular enzymes) at P ≤ 0.05; *
there were no living workers in the control group.
Table 3. Mean protease activities in workers
from the control and caffeine-treated groups
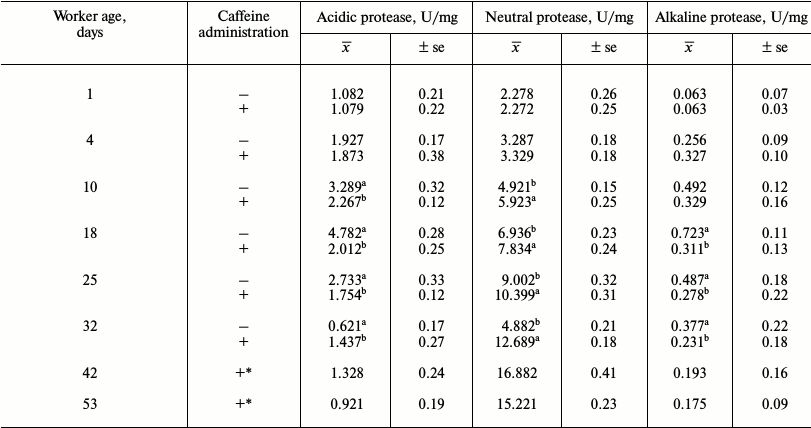
Notes: x̅, mean value; ±se, standard error;
a,b the differences between the groups are statistically
significant for comparisons made within each of the rows separately
(bees of the same age and particular enzymes) at P ≤ 0.05; *
there were no living workers in the control group.
Table 4. Mean protease inhibitor activities
in workers from the control and caffeine-treated groups
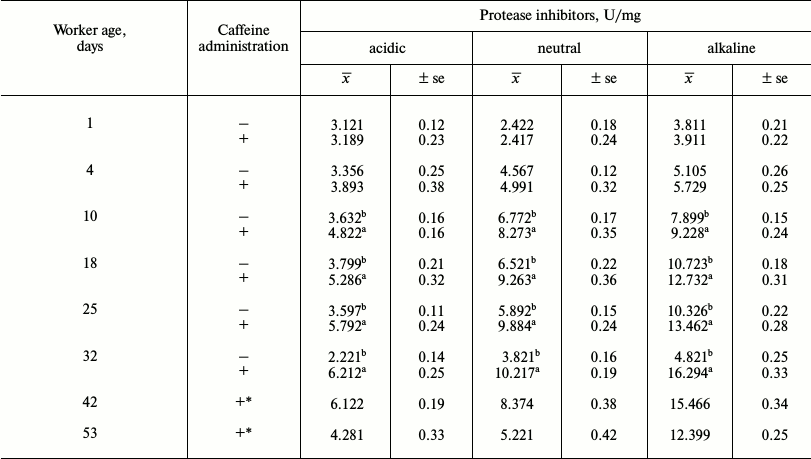
Notes: x̅, mean value; ±se, standard error;
a,b the differences between the groups are statistically
significant for comparisons made within each of the rows separately
(bees of the same age and particular enzymes) at P ≤ 0.05; *
there were no living workers in the control group.
The activities of acidic and alkaline proteases (Table 3) were lower in the bees that were administered caffeine. Both groups of bees were found to have aspartic and serine proteases (activity in relation to diagnostic inhibitors pepstatin A and PMSF). The activities of these enzymes first increased with age in young and adult workers and then decreased in older bees (Tables 3 and 4).
Caffeine-treated workers had higher concentrations of uric acid (Table 5), triglycerides, cholesterol, glucose, and Ca2+ (Table 6). Concentrations of creatinine (Table 5) and Mg2+ (Table 6) were higher in caffeine-treated workers but only up to 14 days of age. After the 15th day of age, they were higher in the control group. Concentrations of albumin and urea were higher in the control group (Table 5). In both groups the concentrations of albumin and uric acid (Table 5) decreased with age, while concentrations of urea and creatinine increased until the 14th day and then decreased. Growth tendencies in the concentration of the non-enzymatic biochemical parameters (Table 6) were observed in young and mature bees in both groups. Only the concentration of glucose in the control group decreased with age (Table 6).
Table 5. Mean concentration of non-enzymatic
antioxidants in workers from the control and caffeine-treated
groups
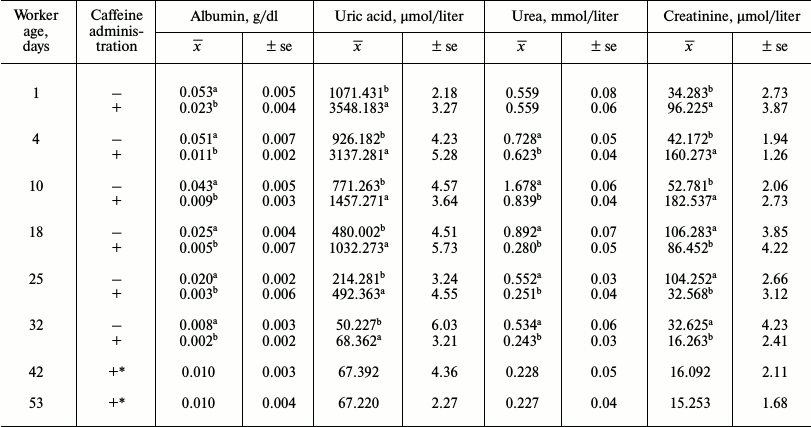
Notes: x̅, mean value; ±se, standard error;
a,b the differences between the groups are statistically
significant for comparisons made within each of the rows separately
(bees of the same age and particular enzymes) at P ≤ 0.05; *
there were no living workers in the control group.
Table 6. Mean concentrations of
triglycerides, cholesterol, glucose, Mg2+ and
Ca2+ in workers from the control and caffeine-treated
groups
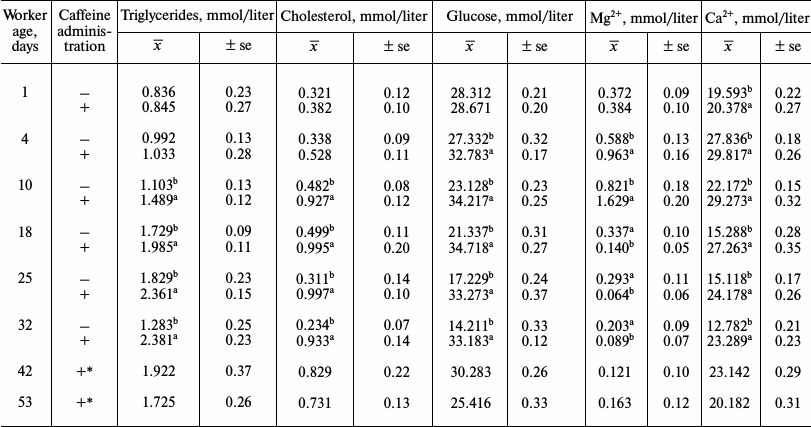
Notes: x̅, mean value; ±se, standard error;
a,b the differences between the groups are statistically
significant for comparisons made within each of the rows separately
(bees of the same age and particular enzymes) at P ≤ 0.05; *
there were no living workers in the control group.
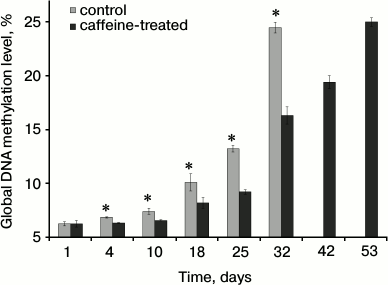
The global methylation level increased with age. The levels identified in young, just emerged workers (1- to 3-day-old) were the same in the bees that consumed caffeine as in those that did not (Fig. 3). However, caffeine significantly decreased DNA methylation levels in older bees.
Fig. 3. Mean global DNA methylation levels (%) in workers from the control and caffeine-treated groups.* Differences are statistically significant for comparisons between the groups (control and caffeine-treated) and workers of the same age at P ≤ 0.05.
DISCUSSION
Caffeine is reportedly the most popular psycho-stimulant [35] that influences general activity and, particularly, learning efficacy in honeybees as well [36-43]. Our studies showed, however, that metabolic effects of caffeine have a far greater significance in honeybees than one could expect.
Caffeine substantially extended the lifespan of caged bees (Table 1). Among other possible reasons, this might be caused by a significant reduction in Nosema spp. infection in the workers that consumed the compound. Caffeine activates the signaling pathway that produces proteolytic system agents, especially protease inhibitors, of which high activities were observed in our study (Table 4). The high activity of these compounds led to amino acid deficiency that might have suppressed Nosema growth or at least reduced the availability of amino acids essential for the production of Nosema proteins [44]. Thus, we showed in this study that caffeine can protect bees against Nosema. On the other hand, Ashihara and Crozier [45] reported that caffeine had long been considered to constitute chemical defense against biological stressors. Kim and Sano [46] suggest that this substance stimulates the production and/or translocation of protease inhibitors and signaling molecules, and therefore it enhances plant resistance against infection by viruses and bacterial pathogens. Therefore, not only increased resistance to Nosema but also improved general vitality can be found in bees that consume caffeine. Extended lifespan of our bees corresponded with the results of the biochemical analyses.
Caffeine changed the metabolism of the bees through modifications in the concentrations and activities of many other biochemical parameters (Tables 2-6 and Figs. 1 and 2). First, caffeine positively influenced the protective functions in the bees by increasing the activities of the antioxidant system (Fig. 2). Higher activities of the antioxidant enzymes, which were triggered by caffeine treatment, suggest that caffeine may promote detoxification [47, 48]. These activities may also result from a low degree of infection by Nosema spp. [49]. Moreover, caffeine modified non-enzymatic antioxidants, e.g. by decreasing albumin and urea concentrations and increasing uric acid concentration in the hemolymph of the bees over their entire lifespans. This was probably caused by the intensity of processes connected with intercepting reactive oxygen forms and binding ions [50]. Second, enzymes involved in the proteolytic system are essential components of insect resistance barriers [51] and are associated with the activity of the antioxidant system [52]. The enzymes also contribute to the amelioration of the consequences of oxidative damage [53, 54]. As mentioned above, caffeine increased proteolytic activity in our bees. Third, following caffeine administration, a rise was observed in the activity of AST, ALT, and ALP. We confirmed that, in a similar fashion to natural methylxanthines, caffeine increased the concentration of triglycerides, cholesterol, glucose, and Ca2+. Such responses to caffeine consumption have previously been observed in mammals [3, 48, 55-57]. Consequently, this study might provide new possibilities for using A. mellifera as a model animal. Particularly, it also fills the lacuna in biochemical knowledge on caffeine impact on apian vitality in the context of aging.
Biochemical changes occurring in bees with aging and in senility were another topic of this study. In senile workers, activities of antioxidant and proteolytic enzymes (Fig. 2 and Tables 3 and 4), as well as those of AST, ALT, and ALP, decreased (Table 2). At the same time, concentrations of proteins (Fig. 1) and non-enzymatic antioxidant (Table 5) and non-enzymatic biochemical compounds (Table 6) also decreased. However, all these changes were observed on the 32nd day of age in the control group, whereas only on the 53rd day in the caffeine-treated group. This is most likely due to a deceleration of senescence-related metabolic changes [58] in the workers that consumed caffeine. It should be also noted that biochemical changes that we observed in the old bees were similar to those observed in old mammals [59]. This confirms that new opportunities have emerged for the use of honeybees in experimental gerontology [14, 58].
We confirmed that global DNA methylation levels increase in bees during aging (compare [15] and [60]), but caffeine slowed this process (Fig. 3). Apparently, caffeine affects the epigenetic control of the apian genome by reducing global DNA methylation. Barres et al. [61] suggest that caffeine induces promoter hypomethylation in rat myotubes. DNA hypomethylation is a transient mechanism involved in mRNA synthesis. Such changes in the genome affect protein inhibitor synthesis and methyltransferase activity, as well as inducing changes in key biochemical pathways. Caffeine-dependent changes in DNA methylation and, consequently, in the levels of many biochemical compounds, which were observed in our study, might be connected with changes in gene expression induced in the apian brain [13].
It should be noted that, as in the case of many other compounds [62], the effects of caffeine largely depend on the dose [55, 63]. Caffeine treatment may cause either favorable or disadvantageous biochemical changes, as well as even resulting in dysfunctions of specific tissues and metabolic disorders [64] depending on its dose. The caffeine doses applied in our study were similar to those successfully and safely applied in rats and mice (5 µg/ml) [55]. They were not disadvantageous and extended apian lifespan while slowing senescence in both biochemical and epigenetic terms. It appears that honeybees respond to caffeine dosage in a similar manner to mammals.
Caffeine supplementation of the diet (5 µg/ml) influenced the worker bee lifespan, global DNA methylation levels, protein content, antioxidative and proteolytic system activities, as well as enzymatic and non-enzymatic biochemical parameters. The response seems surprisingly clear and significant.
Caffeine could be taken into consideration as a natural dietary supplement increasing apian resistance to stress conditions, e.g. diseases, infestations with microorganisms, and oxidative stress. The possibilities of using caffeine to prevent Nosema spp. infestation seem particularly promising in queen bee rearing.
This study facilitates the use of A. mellifera as a model organism in epigenetic and biochemical gerontology studies.
REFERENCES
1.Schwarzschild, M. A., Chen, J. F., and Ascherio, A.
(2002) Caffeinated clues and the promise of adenosine A(2A) antagonists
in PD, Neurology, 58, 1154-1160.
2.Korkotian, E., and Segal, M. (1999) Release of
calcium from stores alters the morphology of dendritic spines in
cultured hippocampal neurons, Proc. Natl. Acad. Sci. USA,
96, 12068-12072.
3.James, J. (2012) Coffee and mortality: urgent need
for clinical trials to assess putative benefits and harms, J.
Caffeine Res., 2, 53-54.
4.Lee, C. (2000) Antioxidant ability of caffeine and
its metabolites based on the study of oxygen radical absorbing capacity
and inhibition of LDL peroxidation, Clin. Chim. Acta,
295, 141-154.
5.Krisko, A., Kveder, M., and Pifat, G. (2005) Effect
of caffeine on oxidation susceptibility of human plasma low density
lipoproteins, Clin. Chim. Acta, 355, 47-53.
6.Len-Carmona, J., and Galano, A.
(2011) Caffeine as an antioxidant deactivates hydroxyl radical,
J. Phys. Chem. B, 115, 4538-4546.
7.Dworzanski, W., Opielak, G., and Burdan, F. (2009)
Side effects of caffeine, Pol. Merk. Lek., 161,
357-361.
8.Shaw, P. J., Cirelli, C., Greenspan, R. J., and
Tononi, G. (2000) Correlates of sleep and waking in Drosophila
melanogaster, Science, 287, 1834-1837.
9.Carillo, R., and Gibson, G. (2002) Unusual genetic
architecture of natural variation affecting drug resistance in
Drosophila melanogaster, Genet. Res., 80,
205-213.
10.Kucharski, R., and Maleszka, R. (2002) Evaluation
of differential gene expression during behavioral development in the
honeybee using microarrays and northern blots, Genome Biol.,
3, Research 0007.1-0007.9.
11.Si, A., Helliwell, P., and Maleszka, R. (2004)
Effects of NMDA receptor antagonists on olfactory learning and memory
in the honeybee (Apis mellifera), Pharm. Biochem. Behav.,
77, 91-97.
12.Si, A., Zhang S., and Maleszka, R. (2005) Effects
of caffeine on olfactory and visual learning in the honey bee (Apis
mellifera), Pharm. Biochem. Behav., 82, 664-672.
13.Kucharski, R., and Maleszka, R. (2005) Evaluation
of differential gene expression during behavioral development in the
honeybee using microarrays and northern blots, J. Mol.
Neurosci., 27, 269-276.
14.Amdam, G. V. (2011) Social context, stress, and
plasticity of ageing, Aging Cell, 10, 18-27.
15.Lyko, F., and Maleszka, R. (2011) Insects as
innovative models for functional studies of DNA methylation, Trends
Genet., 27, 127-164.
16.Strachecka, A., Borsuk, G., Olszewski, K.,
Paleolog, J., Gagos, M., Chobotow, J., Nawrocka, A., Gryzinska, M., and
Bajda, M. (2012) The effect of amphotericin B on the lifespan,
body-surface protein concentrations, and DNA methylation levels of the
honey bees (Apis mellifera), J. Apic. Sci., 56,
107-113.
17.Borsuk, G., Paleolog, J., Olszewski, K., and
Strachecka, A. (2013) Laboratory assessment of the effect of nanosilver
on longevity, sugar syrup ingestion, and infection of honeybees with
Nosema spp., Med. Weter., 69, 730-732.
18.Bak, B., Wilde, J., and Siuda, M. (2013)
Efficiency of Varroa destructor management with medications used
in Poland, Med. Weter., 69, 744-748.
19.Lopienska-Biernat, E., Dmitryjuk, M., Zaobidna,
E., Lipinski, Z., and Zoltowska, K. (2013) The body composition and
enzymes of carbohydrate metabolism of Varroa destructor, J.
Apic. Sci., 57, 95-102.
20.Ptaszynska, A., and Mulenko, W. (2013) Selected
aspects of the structure, development, taxonomy and biology of
microsporidian parasites belonging to the genus Nosema, Med.
Weter., 69, 716-725.
21.Ptaszynska, A., Borsuk, G., Anusiewicz, M., and
Mulenko, W. (2012) Location of Nosema spp. spores within the
body of the honey bee, Med. Weter., 68, 618-621.
22.Ptaszynska, A., Borsuk, G., Mulenko, W., and
Olszewski, K. (2012) Monitoring of nosemosis in the Lublin region and
preliminary morphometric studies of Nosema spp. spores, Med.
Weter., 68, 622-625.
23.Sokol, R., and Michalczyk, M. (2012) Detection of
Nosema spp. in workers bees of different ages during the flow
season, J. Apic. Sci., 56, 19-25.
24.Pohorecka, K., Bober, A., Skubida, M., and
Zdanska, D. (2011) Epizootic status of apiaries with massive losses of
bee colonies (2008-2009), J. Apic. Sci., 55, 137-150.
25.Strachecka, A., Olszewski, K., Paleolog, J.,
Borsuk, G., and Bajda, M. (2014) Coenzyme Q10 treatments influence the
lifespan and key biochemical resistance systems in the honeybee Apis
mellifera, Arch. Insect Biochem. Physiol., DOI:
10.1002/arch.21159.
26.Schacterle, G., and Pollack, R. (1973) Simplified
method for quantitative assay of small amounts of protein in biological
material, Analyt. Biochem., 51, 654-655.
27.Aebi, H. (1983) in Catalase. Methods of
Enzymatic Analysis (Bergmeyer, H. V., ed.) 3rd Edn., Verlag Chemie,
Weinheim, Germany, pp. 277-282.
28.Chance, B., and Maehly, A. (1955) in Assay of
Catalases and Peroxidases. Methods in Enzymology (Colowick, S., and
Kaplan, N., eds.) Vol. 2, Academic Press Inc., New York, pp.
764-775.
29.Warholm, M., Guthenberg, C., von Bahr, C., and
Mannervik, B. (1985) Glutathione transferases from human liver,
Methods Enzymol., 113, 499-504.
30.Podczasy, J., and Wei, R. (1988) Reduction of
iodonitrotetrazolium violet by superoxide radicals, Biochem.
Biophys. Res. Commun., 150, 1294-1301.
31.Anson, M. (1938) The estimation of pepsin,
trypsin, papain and cathepsin with hemoglobin, J. Gen. Physiol.,
22, 79-84.
32.Strachecka, A., Gryzinska, M., and Krauze, M.
(2010) The influence of environmental pollution on the protective
proteolytic barrier of the honey bee Apis mellifera, Pol. J.
Environ. Stud., 19, 855-859.
33.Strachecka, A., Gryzinska, M., Krauze, M., and
Grzywnowicz, K. (2011) Profile of the body surface proteolytic system
in Apis mellifera queens, Czech J. Anim. Sci., 56,
15-22.
34.Lee, T., and Lin, Y. (1995) Trypsin inhibitor and
trypsin – like protease activity in air – or
submergence – grown rice (Oryza sativa L.)
coleoptiles, Plant Sci., 106, 43-54.
35.Smith, A. P., Clark, R., and Gallagher, J. (1999)
Breakfast cereal and caffeinated coffee: effects on working memory,
attention, mood, and cardiovascular function, Physiol. Behav.,
67, 9-17.
36.Nehling, A., Daval, J., and Debry, G. (1992)
Caffeine and the central nervous system: mechanisms of action,
biochemical, metabolic and psychostimulant effects, Brain Res. Brain
Res. Rev., 17, 139-170.
37.Giurfa, M., Eichmann, B., and Menzel, R. (1996)
The concepts of sameness and difference in an insect, Nature,
382, 458-461.
38.Fredholm, B., Battig, K., Holmen, J., Nehlig, A.,
and Zvartau, E. (1999) Actions of caffeine in the brain with special
reference to factors that contribute to its widespread use,
Pharmacol. Rev., 51, 83-133.
39.Zhang, S. W., Lehrer, M., and Srinivasan, M. V.
(1999) Honeybee memory: navigation by associative grouping and recall
of visual stimuli, Neurobiol. Learn. Mem., 72,
180-201.
40.Giurfa, M., Zhang, S., Jenett, A., Menzel, R.,
and Srinivasan, M. V. (2001) Symmetry perception in an insect,
Nature, 410, 930-933.
41.Zhang, S. W., Bock, F., Si, A., Tautz, J., and
Srinivasan, M. V. (2005) Visual working memory in decision making by
honeybees, Proc. Natl. Acad. Sci. USA, 102,
5250-5255.
42.Zhang, S. W., Srinivasan, M. V., Zhu, H., and
Wong, J. (2004) Grouping of visual objects by honeybees, J. Exp.
Biol., 207, 3289-3298.
43.Lockett, G., Helliwell, P., and Maleszka, R.
(2010) Involvement of DNA methylation in memory processing in the honey
bee, NeuroReport, 21, 812-816.
44.Mello, M., and Silva-Filho, M. (2002)
Plant–insect interactions: an evolutionary arms race between two
distinct defense mechanisms, Braz. J. Plant Physiol., 14,
71-81.
45.Ashihara, H., and Crozier, A. (2001) Caffeine: a
well-known but little mentioned compound in plant science, Trends
Plant Sci., 6, 407-413.
46.Kim, Y., and Sano, H. (2008) Pathogen resistance
of transgenic tobacco plants producing caffeine, Phytochemistry,
69, 882-888.
47.Weirich, G., Collins, A., and Williams, V. (2002)
Antioxidant enzymes in the honey bee Apis mellifera,
Apidologie, 33, 3-14.
48.Dworzanski, W., Burdan, F., Szumilo, M.,
Jaskolska, A., and Anielska, E. (2011) Coffee and caffeine –
enemie or alliantes of a cardiologist? Kardiol. Pol., 69,
173-176.
49.Dussaubat, C., Brunet, J., Higes, M., Colbourne,
J., Lopez, J., Choi, J., Martin-Hernandez, R., Botias, C., Cousin, M.,
McDonnell, C., Bonnet, M., Belzunces, L., Moritz, R., Le Conte, Y., and
Alaux, C. (2012) Gut pathology and responses to the microsporidium
Nosema ceranae in the honey bee Apis mellifera, PLoS
One, 7, e37017.
50.Augustyniak, A., and Skrzydlewska, E. (2004)
Antioxidative abilities during aging, Post. Hig. Med. Dosw.,
58, 194-201.
51.Strachecka, A., and Paleolog, J. (2011) The body
surface proteolytic system of Apis mellifera in preserving the
health of bee colonies, Kosmos, 60, 43-51.
52.Davies, K. (1986) in Free Radicals,
Oxidative Stress, and Antioxidants: Pathological and
Physiological Significance (Ozben, T., ed.) Akdeniz University,
Antalya, pp. 253-266.
53.Harman, D. (1956) A theory based on free radical
and radiation chemistry, J. Gerontol., 11, 298-300.
54.Salmon, A., Richardson, A., and Perez, V. (2010)
Update on the oxidative stress theory of aging: does oxidative stress
play a role in aging or healthy aging? Free Rad. Biol. Med.,
48, 642-655.
55.Fredholm, B. (2010) Methylxanthines,
Springer, Stockholm, pp. 11-92.
56.Ballesteros-Yanez, I., Castillo, C., Amo-Salas,
M., Albasanz, J., and Martin, M. (2012) Differential effect of caffeine
consumption on diverse brain areas of pregnant rats, J. Caffeine
Res., 2, 90-98.
57.Tomaszewski, M., Olchowik, G., Tomaszewska, M.,
and Burdan, F. (2012) Use of X-ray microprobe to diagnose bone tissue
demineralization after caffeine administration, Folia Histochem.
Cytobiol., 50, 436-443.
58.Munch, D., Amdam, G., and Wolschin, F. (2008)
Ageing in a eusocial insect: molecular and physiological
characteristics of life span plasticity in the honey bee, Funct.
Ecol., 22, 407-421.
59.Miric, D., Kisic, D., Zoric, L., Dolicanin, Z.,
Mitic, R., and Miric, M. (2012) The impact of senile cataract maturity
on blood oxidative stress markers and glutathione-dependent
antioxidants: relations with lens variables, J. Med. Biochem.,
31, 184-192.
60.Strachecka, A., Paleolog, J., Borsuk, G.,
Olszewski, K., and Bajda, M. (2012) DNA methylation in the honey bee
(Apis mellifera) and its importance for biological research,
Med. Weter., 68, 391-396.
61.Barres, R., Yan, J., Egan, B., Treebak, J.,
Rasmussen, M., Fritz, T., Caidahl, K., Krook, A., O’Gorman, D.,
and Zierath, J. (2012) Acute exercise remodels promoter methylation in
human skeletal muscle, Cell Metab., 15, 405-411.
62.Kang, H., Benzer, S., and Min, K. (2002) Life
extension in Drosophila by feeding a drug, Proc. Natl. Acad.
Sci. USA, 99, 838-843.
63.Mustard, J., Dews, L., Brugato, A., Dey, K., and
Wright, G. (2012) Consumption of an acute dose of caffeine reduces
acquisition but not memory in the honey bee, Behav. Brain Res.,
232, 217-224.
64.Vinader-Caerols, C., Monleon, S., Carrasco, C.,
and Parra, A. (2012) Effects of alcohol, coffee, and tobacco, alone or
in combination, on physiological parameters and anxiety in a young
population, J. Caffeine Res., 2, 70-76.