REVIEW: Role of Microtubule Cytoskeleton in Regulation of Endothelial Barrier Function
I. B. Alieva
Lomonosov Moscow State University, Belozersky Institute of Physico-Chemical Biology, 119992 Moscow, Russia; fax: +7 (495) 939-3181; E-mail: irina_alieva@belozersky.msu.ru
Received May 23, 2014; Revision received May 28, 2014
Cytoplasmic microtubules are an obligatory component of the cytoskeleton of all types of cells. Microtubules are involved in many cellular processes including directed transport of vesicles and signaling molecules and changes in cell shape during its spreading, polarization, and movement. The intracellular organization of the system of microtubules and their dynamic properties are different in different types of cells because they play a key role in the implementation of a variety of cell and tissue functions, including the regulation of the endothelial barrier function. This review presents an overview of current studies on the properties of endothelial microtubules, their interaction with other components of the cytoskeleton and cell adhesion structures, and the role of microtubules in the regulation of the endothelial barrier function.
KEY WORDS: endothelial barrier function, cytoskeleton, microtubules, dynamics of microtubulesDOI: 10.1134/S0006297914090119
INTRACELLULAR ORGANIZATION OF THE MICROTUBULE SYSTEM – ARCHITECTURE IS DETERMINED BY FUNCTION
Cytoplasmic microtubules, which are highly dynamic polar biopolymeric structures, are an obligatory component of the cytoskeleton of all types of cells. Microtubules are multifunctional structures involved in various cellular processes, including directed cytoplasmic transport of vesicles and signaling molecules and changes in the shape of cells and their spreading, polarization, movement of individual cells, and their division. For all these processes it is crucially important to produce and maintain in the intracellular space a system of microtubules specific for the given type of cells (or the cell cycle phase).
The radial model of organization of the intracellular microtubule system is generally known due to electron microscopic works [1] and is canonical. According to this model, microtubules are derived from the centrosome located in the cell center towards the cell periphery, and minus-ends of the microtubules are fastened on the centrosome and protected against disassembly [2-5]. Such organization of the microtubule system when the centrosome is the only organizing beginning for microtubules is called “autoritaire” [6]. The described model is true for a number of cells that have microtubules connected only with the centrosome, e.g. CHO and NRK [7].
The centrosome located in the geometrical center of the cell is believed to determine the radial organization of the microtubule system by binding their minus-ends and allowing a microtubule to grow up to the cell boundary due to polymerization from the plus-end. The radial model of the microtubule system organization is highly effective from the standpoint of the intracellular transport in small or disk-shaped cells, such as keratocytes and melanophores [8, 9]. In these cells, the general organization of the microtubule system is determined only by dynamics of the plus-ends of centrosomal microtubules. However, in addition to the centrosome-attached microtubules, the majority of cells also have in the cytoplasm free microtubules with specific features of dynamics and organization [10, 11]. According to the literature, the most significant numbers of free microtubules are found in muscle cells and neurons and in epithelial cells of different organs: kidneys [12], liver [13], intestine [14], and in other highly specialized cells [15-20]. In such cells free microtubules are strictly ordered and localized along the long axis of the cell and ensure the transport of neurotransmitters in neurons, effective distribution of membrane components, transduction of mechanical vibrations by internal cells of the organ of Corti [21], and vesicle translocation in polar epithelium cells [14]. The structure of the microtubule network can fundamentally change depending on the functional activity of the cell; thus, in polar epithelium cells the radial system of microtubules connected by the minus-ends with the centrosome can convert into the basoapical system [22].
Microtubules are critically required for some processes, e.g. for mitosis and meiosis, but their presence in the cell during interphase is not obligatory because the cell can live in the absence of microtubules. In this case, intracellular processes including those specific for executing functions of the given type cells will be disturbed. Thus, the radially organized microtubule system determines the redistribution of pigment cells (melanophores) in response to the action of humoral factors [8, 9]. In the above-mentioned neurons, parallel bundles of microtubules are responsible for the effective transport of neurotransmitters, whereas a change or destruction of such a system inevitably leads to disorders in the traffic and to pathologic processes not only in the tissue or organ, but also in the organism as a whole. Similarly, disorders in the normal organization of basoapically-oriented microtubules on the polar epithelium cells will lead to dysfunction of the organ of Corti [21].
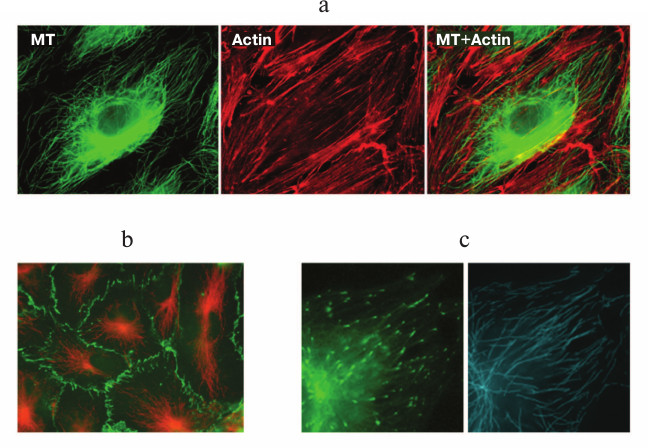
In endothelial cells in vitro, the microtubule system is organized radially (figure, panel (a)), and the majority of microtubules are attached to the centrosome [23, 24]. The density of microtubules is the highest in the inner cytoplasm and gradually decreases towards the cellular boundary where VE-cadherin contacts are settled (figure, panel (b)) and plus-ends of the microtubules are preferentially localized (figure, panel (c)) [24]. This position seems to be functionally essential for endotheliocytes because an effective interaction of the plus-ends of microtubules with cellular cortex structures is fundamentally important for the maintenance and even the recovery of the barrier function [25]. The population of microtubules in endothelial cells is not uniform – a certain part of it is represented by less dynamic and therefore more resistant to exposure, stable acetylated microtubules [24, 26].
a) Double immunofluorescent staining of endothelial cells of human pulmonary artery with antibodies against β-tubulin (green) for detecting microtubules and with antibodies against actin (red) for detecting actin stress-fibers. The scale section represents 20 μm. b) Double immunofluorescent staining of endothelial cells of the human pulmonary artery with antibodies against β-tubulin (red) and VE-cadherin (green). The scale section represents 20 μm. c) Transfection of cells with the microtubular plus-end EB1 protein fused with GFP allows identification of the position of growing plus-ends of the microtubules and study of the growth dynamics (left, EB1-GFP-labeled plus-ends of microtubules (green); right, tracks of EB1-GFP comets characterizing the length of the microtubule processive growth)
CYTOSKELETON OF ENDOTHELIOCYTES – INTERACTION OF
COMPONENTS PERFORMING THE BARRIER FUNCTION
Endothelial cells (endotheliocytes) are immediately involved in performing functions not only of tissues, but also even of organs. The main function of endothelial cells closely bordering one another and spreading on the inner surface of vessels regulates vascular tonus and permeability of the vascular wall, which ensures the exchange between the blood circulating inside the vessels and tissue fluid of the organs containing these vessels. Thus, the eye corneal endothelium is responsible for the normal permeability and exchange of the retina with the connective tissue stroma [27], whereas the endothelium of pulmonary vessels regulates the movement of fluids, macromolecules, and leukocytes into the interstice and aerial space of alveoli. Therefore, the integrity of the endothelial monolayer of pulmonary vessels is crucial for maintaining lung function. The endothelial barrier is dynamic and very sensitive to various physiological and pathological stimuli. Disorders in the barrier function of the endothelial cells of pulmonary vessels (endothelial dysfunction) leads to penetration of fluid from the vessels into surrounding tissues and/or into alveoli, which significantly weakens gas exchange. The endothelial barrier is disturbed during inflammatory diseases, e.g. an acute lung injury and more seriously – acute respiratory distress syndrome leading to pulmonary edema associated with 30-40% lethality [28].
The cytoskeleton of endothelial cells plays the main role in the barrier function. Changes in the normal architecture of the cytoskeleton lead to changes in the shape of endotheliocytes and, as a result, to increase in vascular permeability characteristics not only for the above-mentioned diseases, but also for various pathological conditions such as bronchial asthma or sepsis [29-34].
The cytoskeleton of endotheliocytes, similarly to cytoskeletons of other types of cells, is represented by three types of filaments. In addition to the aforementioned microtubules, the cytoskeleton of endotheliocytes includes actin filaments and intermediate filaments. The actin filaments are responsible for changes in cell shape and mobility, whereas the intermediate filaments are mainly responsible for cell shape. Reorganization of the endotheliocyte cytoskeleton consisting of actin filaments, microtubules, and intermediate filaments and the subsequent change in the cell shape creates a structural basis for increase in vascular permeability.
It historically occurred that contributions of different cytoskeletal components to changes in the shape of endotheliocytes during barrier function development were studied differently. In addition, up to now the role of intermediate filaments has been studied extremely poorly. The obvious role of actin filaments has been studied rather actively [29-31], whereas the involvement of the microtubule system in this process was revealed significantly later [35, 36]. The main achievement of recent years is the understanding that all fibrillar components of the cell cytoskeleton are functioning with interrelations and their interactions are coordinated on different levels of the regulation. Moreover, all fibrillar components are more or less interacting with adhesive structures of the cell. Moreover, just this complicated and finely regulated interaction allows the cells to realize the great variety of cellular, tissue, and organ functions.
In the present review, we shall analyze interactions of the cytoskeletal elements during changes in the endothelial permeability and emphasize the role of microtubules in regulation of the barrier function of the endothelium.
ENDOTHELIAL PERMEABILITY AND INTERACTION OF ENDOTHELIOCYTE
CYTOSKELETAL COMPONENTS
On analyzing interactions of the cytoskeletal structures of the cell, it should be noted at once that the contribution of intermediate elements is now studied the least not only in endotheliocytes, but also in other types of cells.
Vimentin filaments are the main intermediate filaments of endothelial cells [37, 38]. Vimentin filaments are rather stable structures, and their network remains virtually unchanged in vitro during monolayer formation [38]. It is supposed that vimentin filaments might be involved in cytoskeletal interactions indirectly: they might be responsible for centrosome positioning [39] and thus influence the architecture of the microtubule system.
It seems that actin and vimentin filaments can interact structurally because they are connected on the biochemical level [40, 41]. Thus, the tail domain of vimentin is shown to interact directly with actin filaments [41], but up to now no vimentin-binding domain of fibrillar actin has been found. Data on interactions of microtubules and intermediate filaments are still fragmentary; it is likely that kinesin [42] and some other proteins [43, 44] can act as connecting proteins between vimentin and microtubules. This insufficient attention given to intermediate filaments can be easily explained, because actin filaments are generally assumed to be the major player responsible for normal endothelial permeability.
Actin polymerization in endothelial cells is very dynamic. This dynamicity allows actin structures to rapidly rearrange and change the static phenotype characterized by a massive cortical actin ring and minimal number of stress-fibers to the so-called activated phenotype characterized by a poor layer of cortical actin (up to its complete absence) and by numerous stress-fibers compressing the cell. Moreover, just this explains why it was initially thought that the system of actin filaments should be the main cytoskeletal component responsible for functioning of the endothelium and development of its dysfunction [33]. An important role of actin filaments in barrier dysfunction development was shown in many works. In particular, treatment of cultured cells with cytochalasin D destroying the actin cytoskeleton induced a sharp increase in the endothelial monolayer permeability [45], whereas treatment with phalloidin inhibiting actin depolymerization prevented appearance of the barrier dysfunction [46]. The interaction of actin with many binding proteins (filamin, vinculin, etc.) and adhesive structures responsible for the barrier function also seem to evidence its key role in the development of endothelial dysfunction [33].
Actin microfilaments are highly dynamic, the time of their exchange on the cellular boundary being only some seconds [47]; nevertheless, they effectively regulate the cell shape and under in vitro conditions maintain a prostrated state of the cell. Adhesive structures of the cell, focal [48, 49] and intercellular contacts [35, 36, 50, 51], are also able to rapidly, during a few minutes, assemble and disassemble. In fact, actin filaments are actively involved in interactions with adhesive structures of the cell [52, 53]. It is generally assumed that the formation of intercellular gaps is regulated just by balance of competing compressing forces, which curtail the cell towards its center, and forces generated by adhesive intercellular junctions and focal cell–substrate adhesions jointly regulating the cell shape [54]. The two competing forces in this model are related through actin fibers, which interact with the numerous adhesive molecules of the focal adhesions and intercellular adherent junctions of membranes.
However, recent works have shown that actin filaments are not immediately involved in development of endothelial barrier dysfunction, while microtubules are just the structure responsible for initial stages of this process [25, 55]. Thus, thrombin, which is produced on the surface of damaged cells from prothrombin circulating in blood and inducing blood coagulation, can disturb the barrier function of the endothelium both in vitro and in vivo. Thrombin is shown to increase the permeability of the endothelium that is associated with a rapid decrease in the number of microtubules on the cell periphery and reorganization of the microtubule system in the inner cytoplasm of endotheliocytes during the first minutes of exposure. Actin stress-fibers are formed step-by-step, and the maximal effect is observed only 30 min after treatment with thrombin [25, 51]. Thus, the development of microtubule response was faster than the reorganization of the actin filament system responsible for the subsequent changes in the shape of the cells during the development of barrier dysfunction. A direct depolymerization of microtubules under the influence of nocodazole also induced an increase in the permeability of the endothelial cell monolayer and triggered a molecular cascade leading to barrier dysfunction [25, 56]. The increase in the endothelial barrier permeability under treatment with nocodazole is directly associated with the degree of depolymerization of peripheral microtubules. The normal permeability of the endothelial barrier can be disturbed even on minimal disruption of peripheral microtubules when the system of actin filaments remains intact and no morphological changes are observed [25]. The contraction of the cell body in the absence of stress-fibers is surprising, but not unique fact, as already described earlier [54]. In the great majority of cases barrier dysfunction is accompanied by formation of stress-fibers not depending on factors responsible for disturbance of the endothelial permeability, either pharmacological preparations [35, 36, 57] or neurohumoral factors [32, 35]. Thus, the disruption of peripheral microtubules was shown to be necessary and sufficient for arising of endothelial barrier dysfunction [25, 55].
Microtubules, the main focus of our review, are involved in the most active dynamic interaction with both actin filaments and adhesive structures of different types of cells. During recent decades, these interactions were actively studied initially on mobile cells, but later cells that are not normally characterized as motile also became subjects of study. The interaction of microtubules with actin filaments was found to be important for functional activities of cells of different tissues [54, 58], including endotheliocytes [59, 60], and dynamic properties of microtubules are especially important for such interactions [25, 61].
Similarly to microfilaments, microtubules are highly dynamic – they are continuously growing or shortening even in cases when no visual changes can be observed in the cytoplasm region they occupy. The ends of individual microtubules are growing or disassembling at distances of several microns [62, 63], and the whole system is continuously exchanging with the pool of tubulin dissolved in the cytoplasm with exchange time of 5-20 min [64, 65]. Such behavior was named “dynamic instability” [62, 63], because each microtubule, as discriminated from the system as a whole, is not in a stationary state. However, notwithstanding the dynamic behavior of individual microtubules, changes in the whole microtubule system occur rather slowly even in motile cells, whereas in immotile or poorly motile cells the microtubule system is maintained virtually unchanged in space and time.
The assembly of microtubules is characterized by enrichment of newly polymerized fragments of microtubules with GTP-β-tubulin. Because β-tubulin gradually hydrolyzes its GTP upon incorporation into the microtubule, GDP-β-tubulin dominates in the remaining part of microtubules. The so-called plus-end proteins specifically bind to the GTP-enriched fragments of microtubules [66, 67]. These proteins represent a large group, and the most specific role in the polymerization of microtubules belongs to proteins EB1 and EB3 from the family of End-Binding (EB) proteins of this group, which immediately interact with tubulin. Then to the EB proteins, structural proteins CLIP 115 and CLIP 170 bind (of the Cytoplasmic Linker Proteins family), and these proteins in turn bind with structural proteins of the CLASP family (CLIP Associated Proteins). The complex of plus-end proteins plays an important role in regulation of intracellular traffics and is also directly involved in the interaction of microtubules with the cell boundary and the structures localized in this region [67-69].
At present, it is thought that just the dynamic instability of the ends of microtubules and their ability to frequently change the assembly/disassembly stages allow the microtubules to locally modulate the dynamics of cell contacts – in moving fibroblasts through a direct targeted interaction of plus-ends of microtubules with the focal cell–substrate adhesions (so-called “targeting” of cell–substrate adhesions) [48, 49, 70, 71] — and also to regulate the dynamics of intercellular junctions [49, 60, 72, 73]. Moreover, microtubules can control the organization of the cell actin skeleton inducing local changes in the actomyosin contractibility on the ends of stress-fibers [74]. Later it was found in the laboratory of I. Kaverina that changes in the dynamics of microtubules in the zone of contacts depend on the presence of paxillin [75, 76], and a model that connected the asymmetric distribution of focal adhesions and the asymmetric distribution of phases of catastrophes (i.e. transitions to the assembly) of microtubules in the region of adhesion sites was proposed [77-79].
The leading role in the interaction of microtubules and actin cytoskeleton in development of the barrier dysfunction of the pulmonary endothelium is played by Rho-dependent mechanisms [35, 36, 51, 56].
Rho-DEPENDENT MECHANISMS OF INTERACTION OF MICROTUBULES AND ACTIN
FILAMENTS IN REGULATION OF ENDOTHELIAL CELL BARRIER FUNCTION
Two pathways of barrier dysfunction development are known: the so-called MLC-dependent pathway and the MLC-independent pathway, and at least the MLC-dependent pathway includes Rho-dependent mechanisms [80]. The phosphorylation level of MLC is regulated by two main intracellular factors, the calcium level and activity of Rho proteins, which are common regulatory proteins for microtubules and actin filaments. Plus-end proteins of microtubules and also proteins of the cell cortex bound to them are involved in the regulation of the barrier function through Rho protein-mediated mechanisms of interaction between microtubules and actin filaments.
As mentioned, the main achievement of recent years in studies on cytoskeletal structures is the comprehension that the dynamic morphology of the cell as a whole is associated with the dynamics of its cytoskeletal and adhesive structures that function with interrelations in any manifestation of cell activity. Moreover, a key problem for the modern biology is to understand the mechanism of these interactions fully and in detail, because it is of essential importance not only for fundamental biology but also for practical studies in embryology, medicine, and pharmacology.
Microtubules and actin filaments are connected on the structural [54, 67, 81-83], functional and regulatory levels [25, 35, 36, 55, 84-86]. In the works by A. Bershadsky, a general scheme of the regulation of cell mobility was proposed [87, 88]. According to this scheme, external signals, including those from adhesion receptors, do not directly influence the effectors (motor and adhesion elements) but are transmitted initially into a special integrating system consisting of small GTPases and G-proteins of the Rho family as main components [84-86]. Microtubules and actin filaments are two main effector systems controlled by them, and their functioning is coordinated on several levels. The structural role is played by cross-linker proteins responsible for a direct connection of microtubules and actin filaments [88-90]. However, to more flexibly coordinate in time and space the dynamics of cytoskeletal fibers, another system of regulation is needed, but not their simple mechanical connection. The most important is the regulation mediated by molecular cascades – Rho GTPases can regulate the dynamics and organization of both actin and microtubule system [91-97] and also of focal adhesions and intercellular junctions [97, 98]. One of the mechanisms underlying such parallel regulation seems to be associated with features of an immediate effector of the Rho protein, mDia [96, 99, 100], which was shown to mediate the effect of Rho also on adhesive structures [69, 88, 101]. Active mDia stimulates actin polymerization and concurrently has an independent influence on the dynamics of microtubules [102] due to concentrating their plus-ends closely to growing focal adhesions. Although microtubules suppress myosin II-dependent contractibility, their accumulation near the contacts prevents the growth of contacts and even induces their disassembly and promotes cell movement [103]. Thus, there is a system with negative control regulating the growth of focal adhesions. The effect of mDia on microtubules can be a result of its interaction with molecular complexes directly regulating microtubule dynamics, e.g. with proteins such as EB1, CLIP 170, and APC, which are associated with the ends of microtubules [69, 88, 96, 104-110]. Both EB1 and APC bind with mDia, which seems to indicate an involvement of Rho in capping microtubules [103]. Thus, mDia (a direct effector of Rho proteins) is a factor regulating the system of microtubules, actin filaments, and cell contacts. The protein mDia is not the only such factor, the protein HDAC6 also participates in the interaction of fibrillar and adhesive structures of the cell [20, 111, 112].
It is also likely that mDia modulates the dynamics of microtubules not directly but through a signaling cascade, and some data suggest that mDia can activate Rac [113], in particular, through a pathway depending on the Rac effector IQGAP, which interacts with CLIP 170 [95, 114] and binds with APC [81]. Recent data have shown that CLIPs interact with IQGAP1, possibly acting as a linker between the plus-ends of microtubules and the cortical actin network and being “downstream” for Rac1 and Cdc42 [95, 115]. On the other hand, CLIP 170 can participate in the regulation of contacts due to interaction with IQGAP [74, 114]. CLIPs and APC are involved also in the interaction of microtubules with intercellular junctions [98, 116], whereas IQGAP can bind with β-catenin of intercellular junctions [68, 114, 116, 117] and thus present a factor participating in the regulation of all cytoskeletal and adhesive structures of the cell. Numerous cross pathways have also been described for regulation of microtubules and microfilaments, which are induced by factors related with the plasma membrane [118-123] and with some other proteins. Thus, microtubules and actin filaments are connected on both structural and functional levels.
Because GTPases of the Rho family and their regulatory proteins are associated with polymerized tubulin [59], it is not surprising that the actin skeleton status regulated by Rho directly depends on the ratio between polymerized and depolymerized tubulin. The activation of Rho and Rho-dependent destruction of the endothelial barrier occurs in response to the action of agents inducing the disassembly of microtubules [25, 57, 124]. An increased permeability of the endothelium under the influence of thrombin and TGFβ is associated with both dissociation of peripheral microtubules and activation of Rho [25, 35, 125]. The mechanism of microtubule-dependent activation of Rho is likely to include the factor GEF-H1 released on the disruption of microtubules [126]. Another small GTPase, GEF, is now believed to be associated with the regulation of the status of microtubules. Rap1 GEF Epac is a cAMP-dependent protein responsible for the PKA-independent regulation of the organization of the cytoskeleton. Epac is colocalized with microtubules; its specific activation by an analog of cAMP leads to increase in vascular permeability caused by TNFα and TGFβ [127].
Many studies have shown the leading role of microtubules in disorders of the endothelial barrier [25, 27, 128, 129]. Microtubules seem to be the first structural target in the chain of reactions resulting in endothelial barrier dysfunction [25]. The available data indicate that dynamic microtubules play a significant role in barrier function in vitro; depolymerization of peripheral microtubules is the necessary and sufficient condition for development of endothelial dysfunction [25, 129]. This conclusion is extremely important for practical pharmacology because it allows researchers to determine the lines of searching for substances capable of lowering the increased permeability of vascular endothelium. The strategy must be based on inhibition of signaling pathways that depend on depolymerization of microtubules, including the stabilization of microtubules with substances decreasing their dynamic properties [25, 80].
Searches for such substances are rather intensive. Thus, a case of pulmonary edema development in pneumonia was described (as a consequence of endothelial dysfunction) resulting because of a massive ejection of pneumolysin after lysis of bacteria caused by high doses of the antibiotic [129]. This was associated with depolymerization of microtubules on the cell periphery, and not only dynamic but also stable acetylated microtubules were depolymerized. A TNP derivative, TIP-peptide, could protect the endothelial barrier integrity damaged by pneumolysin. This was associated with full recovery of the system of peripheral microtubules, and their number reached the level of the control [129]. Adenosine pronouncedly protected the endothelial permeability against damages and promoted its recovery [130]. Adenosine induced a rapid dose-dependent strengthening of the barrier function of endothelial cells and, thus, is a natural protector of the barrier function of endotheliocytes. The adenosine-induced strengthening of the endothelial barrier is accompanied by an increase in the amount of VE-cadherin on the cell surface and reorganization of the actin cytoskeleton of endotheliocytes [130].
ENDOTHELIOCYTES EXPRESSING EXOGENOUS MICROTUBULE PLUS-END PROTEIN
FUSED WITH GFP AS A MODEL FOR STUDIES ON MICROTUBULE DYNAMICS AND ITS
ROLE IN REGULATION OF ENDOTHELIUM BARRIER FUNCTION
Cultured endothelial cells expressing fluorochrome-fused exogenous microtubule plus-end protein are an excellent functional model for studies on involvement of the microtubule system in the maintenance and protection of endothelial barrier function [24, 25, 61]. In our studies, we used the plus-end protein EB1 fused with GFP (figure, panel (c)). The model allowed us not only to study the microtubule system organization in a living endotheliocyte, but we were the first to characterize the dynamics of endothelial cell microtubules [24] and analyze changes in their dynamic properties specific for barrier dysfunction [25].
Absolute values of the growth rate of microtubule plus-ends in endotheliocytes were shown to be comparable to the growth rates of microtubules in fibroblasts and in functionally active regions of the cytoplasm of polarized and moving cells, i.e. the microtubules of endotheliocytes are highly dynamic [24]. We were the first to find that the growth rates of plus-ends of microtubules were different: in the cell center region the growth rate of plus-ends was higher than on the periphery of cytoplasm [24]. In addition to the regional differences in the growth rates of microtubules, we also found that the growth rate of the plus-ends depended on the presence or absence of intercellular junctions. In individual endotheliocytes, the growth rate was higher than in the cells contacting neighbors. Moreover, individual and contact-free endotheliocytes contained a subpopulation of rapidly growing microtubules, with the growth rates fourfold higher than normal [24]. It seems that such microtubules were analogs of “pioneer” microtubules observed on the leading edge of moving cells [131]. A subsequent careful analysis of dynamic properties of microtubules of endotheliocytes resulted in their subdivision into four classes: 1) fast growing and long-living; 2) slowly growing and long-living; 3) fast growing and short-living; and 4) slowly growing and short-living [61]. Thus, dynamics of the microtubules of endotheliocytes has specific features: the growth rate of plus-ends of the endothelial microtubules is different in different regions of the cell and decreases with formation of the endothelial monolayer. Summarizing these data, we suppose that endotheliocytes possess special mechanisms for local regulation of growth of the microtubule plus-ends.
Microtubules of endotheliocytes are mainly responsible for the cortical dynamics, similarly to other types of microtubules they can differently interact with the cellular cortex. In particular, there is an interaction of microtubules with plus-ends oriented towards the cell periphery [68, 132, 133]. The dynamic instability allows these plus-ends to grow towards the periphery and potentially explore peripheral structures including integrin focal adhesions [74, 133, 134] and also to organize vesicle transport towards the cell surface [135] and deliver regulatory molecules to the cell cortex [90, 133].
We think that the high dynamics of endotheliocytes and regional differences in the growth rate of microtubules determine the specific function of the microtubules of endotheliocytes: they rapidly deliver molecular signals to the cell cortex (into the region of VE-cadherin contacts) that is important for their effective and rapid local regulation in response to external and internal signals. The high growth rate allows a microtubule born on the centrosome to rapidly lengthen and reach the cell border within a very short time. In the endothelial cell monolayer with the produced intercellular VE-cadherin junctions, microtubules can interact with them, and this interaction can lead to stabilization of microtubules in the junction region. It was shown that the intercellular VE-cadherin junctions in the endothelial cells of lungs could stabilize the dynamic behavior of plus-ends of the microtubules [73, 136-138]. Cytoplasmic microtubules were also shown to be stabilized locally on the cell border due to concentrating in this region of plus-end proteins of the CLASP and EB families [115, 139-144] and also due to interaction with the protein APC [115, 145]. We have shown that, along with other regulatory mechanisms, dynamic microtubules of endotheliocytes can also regulate the existent contacts and determine endothelial permeability [24]. Note also that the dynamics of microtubules is fundamentally important for the morphogenesis of endothelium [146, 147].
WHAT IS THE PHYSIOLOGICAL ROLE OF DYNAMIC INSTABILITY OF
MICROTUBULES IN THE ENDOTHELIAL CELL?
Just the discovery of the dynamic instability of microtubules [62, 63] provoked a question of its biological expediency. The “Search-and-Capture” model [148] suggests that, during transitions between the growth and shortening of the plus-ends, microtubules rapidly explore the three-dimensional intracellular space and search for targets to interact with or capture. Our results and the literature data have shown that the dynamics of microtubules play a key role in the in vitro behavior of the endothelial cell monolayer. The majority of microtubules in the human artery endothelium cells are highly dynamic [24, 61], and these dynamic microtubules can regulate the existent contacts and, consequently, the endothelium permeability [24]. We have found that the barrier dysfunction is accompanied by disappearance of peripheral microtubules and reorganization of the microtubule system in the inner cytoplasm – the microtubules derived from the centrosome become significantly shorter [35, 36, 149]. Such shortening can occur if microtubules do not reach the cell periphery because of a decrease in the growth rate of their plus-ends or a decrease in processive growth duration. This can also occur if the microtubules are rapidly shortening on reaching the cell periphery because of the absence of pauses and increase in the frequency of catastrophes on the cell periphery. Analysis of the growth rates revealed that at least one of these explanations was correct: for barrier function disfunction development under the influence of nanomolar concentrations of nocodazole the growth rate of the microtubule plus-end noticeably decreased. As a result, the plus-ends seemed unable to reach the cell periphery. We supposed earlier that dynamic plus-ends of individual microtubules could act as tools for regulation of endothelial permeability – to maintain normal permeability a certain number of plus-ends of microtubules had to be present near the cell cortex in the zone of VE-cadherin contacts. If the number of individual microtubules was lower a critical value, a cascade of events was triggered leading to the barrier dysfunction [25]. This conclusion is promising for a strategy for searching for protectors against endothelial dysfunction in clinical medicine; such substances have to stabilize the microtubules and prevent their disassembly. In fact, the effect of known protectors of barrier permeability is associated with changes in the dynamics of microtubules and recovery of the number of their plus-ends in the zone of VE-cadherin intercellular junctions [129, 150].
Thus, we conclude that regulation of the endothelial monolayer permeability might be a physiological role of the dynamic instability of microtubules in cells during interphase.
This work was supported by the Russian Foundation for Basic Research (project 12-04-00488).
REFERENCES
1.Porter, K. R. (1966) Cytoplasmic microtubules and
their function, Ciba Found. Symp., 8, 308-356.
2.McIntosh, J. R., and Euteneuer, U. (1984) Tubulin
hooks as probes for microtubule polarity: an analysis of the method and
an evaluation of data on microtubule polarity in the mitotic spindle,
J. Cell Biol., 98, 525-533.
3.Keating, T. J., and Borisy, G. G. (1999)
Centrosomal and non-centrosomal microtubules, Biol. Cell,
91, 321-329.
4Moritz, M., and Agard, D. (2001) Gamma-tubulin complexes and
microtubule nucleation, Curr. Opin. Struct. Biol., 11,
174-181.
5.Howard, J., and Hyman, A. A. (2003) Dynamics and
mechanics of the microtubule plus end, Nature, 422,
753-758.
6.Burakov, A. V., and Nadezhdina, E. S. (2006) Dynein
and dynactin as systems of cellular microtubules, Ontogenez,
37, 1-17.
7.Komarova, Y. A., Vorobjev, I. A., and Borisy, G. G.
(2002) Life cycle of MTs: persistent growth in the cell interior;
asymmetric transition frequencies and effects of cell boundary, J.
Cell Sci., 115, 3517-3539.
8.Rodionov, V. I., Lim, S., Gelfand, V. I., and
Borisy, G. G. (1994) Microtubule dynamics in fish melanophores, J.
Cell Biol., 126, 1455-1464.
9.Rodionov, V. I., Hope, A. J., Svitkina, T. M., and
Borisy, G. G. (1998) Functional coordination of microtubule-based and
actin-based motility in melanophores, Curr. Biol., 8,
165-168.
10Vorobjev, I. A., Alieva, I. B., Grigoriev, I. S., and Borisy, G. G.
(2003) Microtubule dynamics in living cells: direct analysis in the
internal cytoplasm, Cell Biol. Int., 27, 293-294.
11.Alieva, I. B., Borisy, G. G., and Vorobjev, I. A.
(2008) Spatial organization of centrosome-linked and free microtubules
in the cytoplasm of fibroblasts 3T3, Tsitologiya, 50,
936-946.
12.Bacallao, R., Antony, C., Dotti, C., Karsenti,
E., Stelzer, E. H., and Simons, K. (1989) The subcellular organization
of Madin-Darby canine kidney cells during the formation of a polarized
epithelium, J. Cell Biol., 109, 2817-2832.
13.Durand-Schneider, A. M., Bouanga, J. C.,
Feldmann, G., and Maurice, M. (1991) Microtubule disruption interferes
with the structural and functional integrity of the apical pole in
primary cultures of rat hepatocytes, Eur. J. Cell Biol.,
56, 260-268.
14.Gilbert, T., and Rodriguez-Boulan, E. (1991)
Induction of vacuolar apical compartments in the Caco-2 intestinal
epithelial cell line, J. Cell Sci., 100, 451-458.
15.Tassin, A. M., Maro, B., and Bornens, M. (1985)
Fate of microtubule-organizing centers during myogenesis in
vitro, J. Cell Biol., 100, 35-46.
16.Yu, W., Ahmad, F. J., and Baas, P. W. (1994)
Microtubule fragmentation and partitioning in the axon during
collateral branch formation, J. Neurosci., 14,
5872-5884.
17.Mogensen, M. M., Tucker, J. B., Mackie, J. B.,
Prescott, A. R., and Nathke, I. S. (2002) The adenomatous polyposis
coli protein unambiguously localizes to microtubule plus ends and is
involved in establishing parallel arrays of microtubule bundles in
highly polarized epithelial cells, J. Cell Biol., 157,
1041-1048.
18.Mogensen, M. M. (2004) Microtubule organizing
centers in polarized epithelial cells, in Centrosomes in Development
and Disease, Wiley-VCH Verlag GmbH & Co., KGaA, Weinheim, pp.
299-319.
19.Bugnard, E., Zaal, K. J., and Ralston, E. (2005)
Reorganization of microtubule nucleation during muscle differentiation,
Cell Motil. Cytoskeleton, 60, 1-13.
20.Bartolini, F., and Gundersen, G. G. (2006)
Generation of noncentrosomal microtubule arrays, J. Cell Sci.,
119, 4155-4163.
21.Patuzzi, R. (1998) Exponential onset and recovery
of temporary threshold shift after loud sound: evidence for long-term
inactivation of mechano-electrical transduction channels, Hear
Res., 125, 17-38.
22.Moss, D. K., Bellett, G., Carter, J. M., Liovic,
M., Keynton, J., Prescott, A. R., Lane, E. B., and Mogensen, M. M.
(2007) Ninein is released from the centrosome and moves
bi-directionally along microtubules, J. Cell Sci.,
120, 3064-3074.
23.Smurova, K. M., Birukova, A. A., Verin, A. D.,
and Alieva, I. B. (2008) Microtubule system at the barrier dysfunction
of endothelium: depolarization of the cell border in the inner
cytoplasm, Tsitologiya, 50, 49-55.
24.Alieva, I. B., Zemskov, E. A., Kireev, I. I.,
Gorshkov, B. A., Wiseman, D. A., Black, S. M., and Verin, A. D. (2010)
Dynamic microtubules are involved in human endothelial cells barrier
function, J. Biomed. Biotech., 2010, 671536.
25.Alieva, I. B., Zemskov, E. A., Smurova, K. M.,
Kaverina, I. N., and Verin, A. D. (2013) The leading role of
microtubules in endothelial barrier dysfunction: disassembly of
peripheral microtubules leaves behind the cytoskeletal reorganization,
J. Cell Biochem., 114, 2258-2272.
26.Smurova, K. M., Birukova, A. A., Verin, A. D.,
and Alieva, I. B. (2008) Dose-dependent effect of nocodazole on the
cytoskeleton of endothelial cells, Biol. Membr., 25,
181-190.
27.Jalimarada, S. S., Shivanna, M., Kini, V., Mehta,
D., and Srinivas, S. P. (2009) Microtubule disassembly breaks down the
barrier integrity of corneal endothelium, Exp. Eye Res.,
89, 333-343.
28.Ware, L. B., and Matthay, M. A. (2000) The acute
respiratory distress syndrome, N. Engl. J. Med., 342,
1334-1349.
29.Garcia, J. G., Davis, H. W., and Patterson, C. E.
(1995) Regulation of endothelial cell gap formation and barrier
dysfunction: role of myosin light chain phosphorylation, J. Cell.
Physiol., 163, 510-522.
30.Garcia, J. G., Verin, A. D., and Schaphorst, K.
L. (1996) Regulation of thrombin-mediated endothelial cell contraction
and permeability, Semin. Thromb. Hemost., 22,
309-315.
31.Lum, H., and Malik, A. B. (1996) Mechanisms of
increased endothelial permeability, Can. J. Physiol. Pharmacol.,
74, 787-800.
32.Van Nieuw Amerongen, G. P., Vermeer, M. A., and
van Hinsbergh, V. W. (2000) Role of RhoA and Rho kinase in
lysophosphatidic acid-induced endothelial barrier dysfunction,
Arterioscler. Thromb. Vasc. Biol., 20, e127-e133.
33.Dudek, S. M., and Garcia, J. G. (2001)
Cytoskeletal regulation of pulmonary vascular permeability, J. Appl.
Physiol., 91, 1487-1500.
34.Groeneveld, A. B. (2002) Vascular pharmacology of
acute lung injury and acute respiratory distress syndrome, Vasc.
Pharmacol., 39, 247-256.
35.Birukova, A., Birukov, K., Smurova, K., Kaibuchi,
K., Alieva, I., Garcia, J. G., and Verin, A. (2004) Novel role of
microtubules in thrombin-induced endothelial barrier dysfunction,
FASEB J., 18, 1879-1890.
36.Birukova, A. A, Smurova, K. M., Birukov, K. G.,
Kaibuchi, K., Garcia, J. G., and Verin, A. D. (2004) Role of Rho
GTPases in thrombin-induced lung vascular endothelial cells barrier
dysfunction, Microvasc. Res., 67, 64-77.
37.Bruneel, A., Labas, V., Mailloux, A., Sharma, S.,
Vinh, J., Vaubourdolle, M., and Baudin, B. (2003) Proteomic study of
human umbilical vein endothelial cells in culture, Proteomics,
3, 714-723.
38.Shakhov, A. V., Alieva, I. B., and Verin, A. D.
(2014) Reorganization of cytoskeleton of endothelial cells on formation
of the functional monolayer in vitro, Tsitologiya,
56, 36-47.
39.Morgan, J. T., Pfeiffer, E. R., Thirkill, T. L.,
Kumar, P., Peng, G., Fridolfsson, H. N., Douglas, G. C., Starr, D. A.,
and Barakat, A. I. (2011) Nesprin-3 regulates endothelial cell
morphology, perinuclear cytoskeletal architecture, and flow-induced
polarization, Mol. Biol. Cell, 22, 4324-4334.
40.Cary, R. B., Klymkowsky, M. W., Evans, R. M.,
Domingo, A., Dent, J. A., and Backhus, L. E. (1994) Vimentin’s
tail interacts with actin-containing structures in vivo, J.
Cell Sci., 107, 1609-1622.
41.Esue, O., Carson, A. A., Tseng, Y., and Wirtz, D.
(2006) A direct interaction between actin and vimentin filaments
mediated by the tail domain of vimentin, J. Biol. Chem.,
281, 30393-30399.
42.Kreitzer, G., Liao, G., and Gundersen, G. G.
(1999) Detyrosination of tubulin regulates the interaction of
intermediate filaments with microtubules in vivo via a
kinesin-dependent mechanism, Mol. Biol. Cell, 10,
1105-1118.
43.Draberova, E., and Draber, P. (1993) A
microtubule-interacting protein involved in co-alignment of vimentin
intermediate filaments with microtubules, J. Cell Sci.,
106, 1263-1273.
44.Liao, G., and Gundersen, G. G. (1998) Kinesin is
a candidate for cross-bridging microtubules and intermediate filaments.
Selective binding of kinesin to detyrosinated tubulin and vimentin,
J. Biol. Chem., 273, 9797-9803.
45.Shasby, D. M., Shasby, S. S., Sullivan, J. M.,
and Peach, M. J. (1982) Role of endothelial cell cytoskeleton in
control of endothelial permeability, Circ. Res., 51,
657-661.
46.Phillips, P. G., Lum, H., Malik, A. B., and Tsan,
M. F. (1989) Phallacidin prevents thrombin-induced increases in
endothelial permeability to albumin, Am. J. Physiol.,
257, 562-567.
47.Amann, K. J., and Pollard, T. D. (2000) Cellular
regulation of actin network assembly, Curr. Biol., 10,
728-730.
48.Kaverina, I., Krylyshkina, O., and Small, J. V.
(1999) Microtubule targeting of substrate contacts promotes their
relaxation, J. Cell. Biol., 146, 1033-1043.
49.Stehbens, S., and Wittmann, T. (2012) Targeting
and transport: how microtubules control focal adhesion dynamics, J.
Cell Biol., 198, 481-489.
50.Adams, C. L., Chen, Y. T., Smith, S. J., and
Nelson, W. J. (1998) Mechanisms of epithelial cell–cell adhesion
and cell compaction revealed by high-resolution tracking of
E-cadherin-green fluorescent protein, J. Cell Biol., 142,
1105-1119.
51.Smurova, K. M., Birukova, A. A., Verin, A. D.,
and Alieva, I. B. (2008) Microtubule system in endothelial barrier
dysfunction: disassembly of peripheral microtubules and microtubule
reorganization in internal cytoplasm, Cell Tissue Biol.,
2, 45-52.
52.Dejana, E., Bazzoni, G., and Lampugnani, M. G.
(1999) Vascular endothelial (VE)-cadherin: only an intercellular glue,
Exp. Cell Res., 252, 13-19.
53.Bazzoni, G., and Dejana, E. (2004) Endothelial
cell-to-cell junctions: molecular organization and role in vascular
homeostasis, Physiol. Rev., 84, 869-901.
54.Prasain, N., and Stevens, T. (2009) The actin
cytoskeleton in endothelial cell phenotypes, Microvasc. Res.,
77, 53-63.
55.Smurova, K. M., Verin, A. D., and Alieva, I. B.
(2011) Effect of Rho-kinase inhibition at the barrier dysfunction
depends on the nature of factors changing the endothelium permeability,
Tsitologiya, 53, 359-366.
56.Smurova, K. M., Birukova, A. A., Verin, A. D.,
and Alieva, I. B. (2008) Dose-dependent effect of nocodazole on
endothelial cell cytoskeleton, Biochemistry (Moscow),
Suppl. Ser. A: Membrane and Cell Biology, 2,
119-127.
57.Verin, A. D., Birukova, A., Wang, P., Liu, F.,
Becker, P., Birukov, K., and Garcia, J. G. (2001) Microtubule
disassembly increases endothelial cell barrier dysfunction: role of MLC
phosphorylation, Am. J. Physiol., 281,
565-574.
58.Meng, W., Mushika, Y., Ichii, T., and Takeichi,
M. (2008) Anchorage of microtubule minus ends to adherens junctions
regulates epithelial cell–cell contacts, Cell, 135,
948-959.
59.Lee, T. Y., and Gotlieb, A. I. (2003)
Microfilaments and microtubules maintain endothelial integrity,
Microsc. Res. Tech., 60, 115-127.
60.Brieher, W. M., and Yap, A. S. (2013) Cadherin
junctions and their cytoskeleton(s), Curr. Opin. Cell Biol.,
25, 39-46.
61.Komarova, Y. A., Huang, F., Geyer, M., Daneshjou,
N., Garcia, A., Idalino, L., Kreutz, B., Mehta, D., and Malik, A. B.
(2012) VE-cadherin signaling induces EB3 phosphorylation to suppress
microtubule growth and assembly adherens junctions, Mol. Cell,
48, 914-925.
62.Mitchison, T., and Kirschner, M. (1984) Dynamic
instability of microtubule growth, Nature, 312,
237-242.
63.Mitchison, T., and Kirschner, M. (1984)
Microtubule assembly nucleated by isolated centrosomes, Nature,
312, 232-237.
64.Vorobjev, I. A., Rodionov, V. I., Maly, I. V.,
and Borisy, G. G. (1999) Contribution of plus and minus end pathways to
microtubule turnover, J. Cell Sci., 112, 2277-2289.
65.Vorobjev, I., Malikov, V., and Rodionov, V.
(2001) Self-organization of a radial microtubule array by
dynein-dependent nucleation of microtubules, Proc. Natl. Acad. Sci.
USA, 98, 10160-10165.
66.Galjart, N. (2010) Plus-end-tracking proteins and
their interactions at microtubule ends, Curr. Biol., 20,
R528-R537.
67.Akhmanova, A., and Steinmetz, M. O. (2010)
Microtubule + TIPs at a glance, J. Cell Sci., 123,
3415-3419.
68.Gundersen, G. G., Gomes, E. R., and Wen, Y.
(2004) Cortical control of microtubule stability and polarization,
Curr. Opin. Cell Biol., 16, 106-112.
69.Carramusa, L., Ballestrem, C., Zilberman, Y., and
Bershadsky, A. D. (2007) Mammalian diaphanous-related formin Dia1
controls the organization of E-cadherin-mediated cell–cell
junctions, J. Cell Sci., 120, 3870-3882.
70.Efimov, A., Kharitonov, A., Efimova, N.,
Loncarek, J., Miller, P. M., Andreyeva, N., Gleeson, P., Galjart, N.,
Maia, A. R. R., McLeod, I. X., Yates, J. R., III, Maiato, H.,
Khodjakov, A., Akhmanova, A., and Kaverina, I. (2007) Asymmetric
CLASP-dependent nucleation of noncentrosomal microtubules at the
trans-Golgi network, Dev. Cell, 12, 917-930.
71.Kaverina, I., and Straube, A. (2011) Regulation
of cell migration by dynamic microtubules, Semin. Cell Dev.
Biol., 22, 968-974.
72.Shewan, A. M., Maddugoda, M., Kraemer, A.,
Stehbens, S. J., Verma, S., Kovacs, E. M., and Yap, A. S. (2005) Myosin
2 is a key Rho kinase target necessary for the local concentration of
E-cadherin at cell–cell contacts, Mol. Biol. Cell,
16, 4531-4542.
73.Stehbens, S. J., Paterson, A. D., Crampton, M.
S., Shewan, A. M., Ferguson, C., Akhmanova, A., Parton, R. G., and Yap,
A. S. (2006) Dynamic microtubules regulate the local concentration of
E-cadherin at cell–cell contacts, J. Cell Sci.,
1199, 1801-1811.
74.Small, J. V., and Kaverina, I. (2003)
Microtubules meet substrate adhesions to arrange cell polarity,
Curr. Opin. Cell Biol., 15, 40-47.
75.Efimov, A., Schiefermeier, N., Grigoriev, I.,
Ohi, R., Brown, M. C., Turner, C. E., Small, J. V., and Kaverina, I.
(2008) Paxillin-dependent stimulation of microtubule catastrophes at
focal adhesion sites, J. Cell Sci., 121, 196-204.
76.Broussard, J. A., Webb, D. J., and Kaverina, I.
(2008) Asymmetric focal adhesion disassembly in motile cells, Curr.
Opin. Cell Biol., 20, 85-90.
77.Efimov, A., and Kaverina, I. (2009) Significance
of microtubule catastrophes at focal adhesion sites, Cell Adh.
Migr., 3, 285-287.
78.Meenderink, L. M., Ryzhova, L. M., Donato, D. M.,
Gochberg, D. F., Kaverina, I., and Hanks, S. K. (2010) P130Cas
Src-binding and substrate domains have distinct roles in sustaining
focal adhesion disassembly and promoting cell migration, PLoS
One, 5, e13412.
79.Zhu, X., and Kaverina, I. (2013) Golgi as an
MTOC: making microtubules for its own good, Histochem. Cell
Biol., 140, 361-367.
80.Alieva, I. B., and Verin, A. D. (2013) The
functional role of the microtubule/microfilament cytoskeleton in the
regulation of pulmonary vascular endothelial barrier, in Endothelial
Cytoskeleton (Rosado, J. A., and Redondo, P. C., eds.) Science
Publishers, N. Y., pp. 116-145.
81.Watanabe, T., Wang, S., Noritake, J., Sato, K.,
Fukata, M., Takefuji, M., Nakagawa, M., Izumi, N., Akiyama, T., and
Kaibuchi, K. (2004) Interaction with IQGAP1 links APC to Rac1, Cdc42,
and actin filaments during cell polarization and migration, Dev.
Cell, 7, 871-883.
82.Applewhite, D. A., Grode, K. D., Keller, D.,
Zadeh, A. D., Slep, K. C., and Rogers, S. L. (2010) The spectraplakin
short stop is an actin – microtubule cross-linker that
contributes to organization of the microtubule network, Mol. Biol.
Cell, 21, 1714-1724.
83.Preciado Lopez, M., Huber, F., Grigoriev, I.,
Steinmetz, M. O., Akhmanova, A., Dogterom, M., and Koenderink, G. H.
(2014) In vitro reconstitution of dynamic microtubules
interacting with actin filament networks, Methods Enzymol.,
540, 301-320.
84.Kjoller, L., and Hall, A. (1999) Signaling to Rho
GTPases, Exp. Cell Res., 253, 166-179.
85.Ridley, A. J. (2001) Rho family proteins:
coordinating cell responses, Trends Cell Biol., 11,
471-477.
86.Schmidt, A., and Hall, A. (2002) Guanine
nucleotide exchange factors for Rho GTPases: turning on the switch,
Genes Dev., 16, 1587-1609.
87.Bershadsky, A. D., Balaban, N. Q., and Geiger, B.
(2003) Adhesion-dependent cell mechanosensitivity, Annu. Rev. Cell
Dev. Biol., 19, 677-695.
88.Bershadsky, A. D., Ballestrem, C., Carramusa, L.,
Zilberman, Y., Gilquin, B., Khochbin, S., Alexandrova, A. Y.,
Verkhovsky, A. B., Shemesh, T., and Kozlov, M. M. (2006) Assembly and
mechanosensory function of focal adhesions: experiments and models,
Eur. J. Cell Biol., 85, 165-173.
89.Fuchs, E., and Karakesisoglou, I. (2001) Bridging
cytoskeletal intersections, Genes Dev., 15, 1-14.
90.Rodriguez, O. C., Schaefer, A. W., Mandato, C.
A., Forscher, P., Bement, W. M., and Waterman-Storer, C. M. (2003)
Conserved microtubule–actin interactions in cell movement and
morphogenesis, Nat. Cell Biol., 5, 599-609.
91.Cook, T. A., Nagasaki, T., and Gundersen, G. G.
(1998) Rho guanosine triphosphatase mediates the selective
stabilization of microtubules induced by lysophosphatidic acid, J.
Cell Biol., 141, 175-185.
92.Daub, H., Gevaert, K., Vandekerckhove, J., Sobel,
A., and Hall, A. (2001) Rac/Cdc42 and p65PAK regulate the
microtubule-destabilizing protein stathmin through phosphorylation at
serine 16, J. Biol. Chem., 276, 1677-1680.
93.Ishizaki, T., Morishima, Y., Okamoto, M.,
Furuyashiki, T., Kato, T., and Narumiya, S. (2001) Coordination of
microtubules and the actin cytoskeleton by the Rho effector mDia 1,
Nat. Cell Biol., 3, 8-14.
94.Palazzo, A. F., Cook, T. A., Alberts, A. S., and
Gundersen, G. G. (2001) mDia mediates Rho-regulated formation and
orientation of stable microtubules, Nat. Cell Biol., 3,
723-729.
95.Fukata, M., Watanabe, T., Noritake, J., Nakagawa,
M., Yamaga, M., Kuroda, S., Matsuura, Y., Iwamatsu, A., Perez, F., and
Kaibuchi, K. (2002) Rac1 and Cdc42 capture microtubules through IQGAP1
and CLIP-170, Cell, 109, 873-885.
96.Jaffe, A. B., and Hall, A. (2005) Rho GTPases:
biochemistry and biology, Annu. Rev. Cell Dev. Biol., 21,
247-269.
97.Morris, E. J., Nader, G. P., Ramalingam, N.,
Bartolini, F., and Gundersen, G. G. (2014) Kif4 interacts with EB1 and
stabilizes microtubules downstream of Rho-mDia in migrating
fibroblasts, PLoS One, 9, e91568.
98.Mehta, D., and Malik, A. B. (2006) Signaling
mechanisms regulating endothelial permeability, Physiol. Rev.,
86, 279-367.
99.Alberts, A. S. (2002) Diaphanous-related formin
homology proteins, Curr. Biol., 12, R796.
100.Wallar, B. J., and Alberts, A. S. (2003) The
formins: active scaffolds that remodel the cytoskeleton, Trends Cell
Biol., 13, 435-446.
101.Watanabe, N., Kato, T., Fujita, A., Ishizaki,
T., and Narumiya, S. (1999) Cooperation between mDia1 and ROCK in
Rho-induced actin reorganization, Nat. Cell Biol., 1,
136-143.
102.Bartolini, F., Moseley, J. B., Schmoranzer, J.,
Cassimeris, L., Goode, B. L., and Gundersen, G. G. (2008) The formin
mDia2 stabilizes microtubules independently of its actin nucleation
activity, J. Cell Biol., 181, 523-536.
103.Wen, Y., Eng, C. H., Schmoranzer, J.,
Cabrera-Poch, N., Morris, E. J., Chen, M., Wallar, B. J., Alberts, A.
S., and Gundersen, G. G. (2004) EB1 and APC bind to mDia to stabilize
microtubules downstream of Rho and promote cell migration, Nat. Cell
Biol., 6, 820-830.
104.Heald, R., and Nogales, E. (2002) Microtubule
dynamics, J. Cell Sci., 115, 3-4.
105.Zilberman, Y., Ballestrem, C., Carramusa, L.,
Mazitschek, R., Khochbin, S., and Bershadsky, A. (2009) Regulation of
microtubule dynamics by inhibition of the tubulin deacetylase HDAC6,
J. Cell Sci., 122, 3531-3541.
106.Shemesh, T., Verkhovsky, A. B., Svitkina, T.
M., Bershadsky, A. D., and Kozlov, M. M. (2009) Role of focal adhesions
and mechanical stresses in the formation and progression of the
lamellipodium–lamellum interface, Biophys. J., 97,
1254-1264.
107.Zhang, T., Zaal, K. J., Sheridan, J., Mehta,
A., Gundersen, G. G., and Ralston, E. (2009) Microtubule plus-end
binding protein EB1 is necessary for muscle cell differentiation,
elongation and fusion, J. Cell Sci., 122, 1401-1409.
108.Okada, K., Bartolini, F., Deaconescu, A. M.,
Moseley, J. B., Dogic, Z., Grigorieff, N., Gundersen, G. G., and Goode,
B. L. (2010) Adenomatous polyposis coli protein nucleates actin
assembly and synergizes with the formin mDia1, J. Cell Biol.,
189, 1087-1096.
109.Sudhaharan, T., Goh, W. I., Sem, K. P., Lim, K.
B., Bu, W., and Ahmed, S. (2011) Rho GTPase Cdc42 is a direct
interacting partner of Adenomatous Polyposis Coli protein and can alter
its cellular localization, PLoS One, 6, e16603.
110.Zilberman, Y., Alieva, N. O., Miserey-Lenkei,
S., Lichtenstein, A., Kam, Z., Sabanay, H., and Bershadsky, A. (2011)
Involvement of the Rho-mDia1 pathway in the regulation of Golgi complex
architecture and dynamics, Mol. Biol. Cell, 22,
2900-2911.
111.Joo, E. E., and Yamada, K. M. (2014) MYPT1
regulates contractility and microtubule acetylation to modulate
integrin adhesions and matrix assembly, Nat. Commun., 5,
3510.
112.Bartolini, F., and Gundersen, G. G. (2010)
Formins and microtubules, Biochim. Biophys. Acta, 1803,
164-173.
113.Tsuji, T., Ishizaki, T., Okamoto, M.,
Higashida, C., Kimura, K., Furuyashiki, T., Arakawa, Y., Birge, R. B.,
Nakamoto, T., Hirai, H., and Narumiya, S. (2002) ROCK and mDia1
antagonize in Rho-dependent Rac activation in Swiss 3T3 fibroblasts,
J. Cell Biol., 157, 819-830.
114.Gundersen, G. G. (2002) Microtubule capture:
IQGAP and CLIP-170 expand the repertoire, Curr. Biol.,
12, 645-647.
115.Lansbergen, G., and Akhmanova, A. (2006)
Microtubule plus end: a hub of cellular activities, Traffic,
7, 499-507.
116.Komarova, Y. A., Mehta, D., and Malik, A. B.
(2007) Dual regulation of endothelial junctional permeability, Sci.
STKE, 412, re8.
117.Akiyama, T., and Kawasaki, Y. (2006) Wnt
signaling and the actin cytoskeleton, Oncogene, 25,
7538-7544.
118.Etienne-Manneville, S., and Hall, A. (2002) Rho
GTPases in cell biology, Nature, 420, 629-635.
119.Krendel, M., Zenke, F. T., and Bokoch, G. M.
(2002) Nucleotide exchange factor GEF-H1 mediates cross-talk between
microtubules and the actin cytoskeleton, Nat. Cell Biol.,
4, 294-301.
120.Krendel, M., and Mooseker, M. S. (2005)
Myosins: tails (and heads) of functional diversity, Physiology
(Bethesda), 20, 239-251.
121.Lim, Y., Lim, S. T., Tomar, A., Gardel, M.,
Bernard-Trifilo, J. A., Chen, X. L., Uryu, S. A., Canete-Soler, R.,
Zhai, J., Lin, H., Schlaepfer, W. W., Nalbant, P., Bokoch, G., Ilic,
D., Waterman-Storer, C., and Schlaepfer, D. D. (2008) PyK2 and
FAK connections to p190Rho guanine nucleotide exchange factor regulate
RhoA activity, focal adhesion formation, and cell motility, J. Cell
Biol., 180, 187-203.
122.Hotta, A., Kawakatsu, T., Nakatani, T., Sato,
T., Matsui, C., Sukezane, T., Akagi, T., Hamaji, T., Grigoriev, I.,
Akhmanova, A., Takai, Y., and Mimori-Kiyosue, Y. (2010) Laminin-based
cell adhesion anchors microtubule plus ends to the epithelial cell
basal cortex through LL5alpha/beta, J. Cell Biol., 189,
901-917.
123.Kovacs, E. M., Verma, S., Ali, R. G., Ratheesh,
A., Hamilton, N. A., Akhmanova, A., and Yap, A. S. (2011) N-WASP
regulates the epithelial junctional actin cytoskeleton through a
non-canonical post-nucleation pathway, Nat. Cell Biol.,
13, 934-943.
124.Bogatcheva, N. V., Adyshev, D., Mambetsariev,
B., Moldobaeva, N., and Verin, A. D. (2007) Involvement of
microtubules, p38, and Rho kinases pathway in
2-methoxyestradiol-induced lung vascular barrier dysfunction, Am. J.
Physiol. Lung Cell. Mol. Physiol., 292, 487-499.
125.Birukova, A. A., Birukov, K. G., Adyshev, D.,
Usatyuk, P., Natarajan, V., Garcia, J. G., and Verin, A. D. (2005)
Involvement of microtubules and Rho pathway in TGF-beta1-induced lung
vascular barrier dysfunction, J. Cell Physiol.,
204, 934-947.
126.Birukova, A. A., Adyshev, D., Gorshkov, B.,
Bokoch, G. M., Birukov, K. G., and Verin, A. D. (2006) GEF-H1 is
involved in agonist-induced human pulmonary endothelial barrier
dysfunction, Am. J. Physiol. Lung Cell Mol. Physiol.,
290, 540-548.
127.Sehrawat, S., Cullere, X., Patel, S., Italiano,
J., Jr., and Mayadas, T. N. (2008) Role of Epac1, an exchange factor
for Rap GTPases, in endothelial microtubule dynamics and barrier
function, Mol. Biol. Cell, 19, 1261-1270.
128.Tian, X., Tian, Y., Sarich, N., Wu, T., and
Birukova, A. A. (2012) Novel role of stathmin in microtubule-dependent
control of endothelial permeability, FASEB J., 26,
3862-3874.
129.Lucas, R., Yang, G., Gorshkov, B. A., Zemskov,
E. A., Sridhar, S., Umapathy, N. S., Jezierska-Drutel, A., Alieva, I.
B., Leustik, M., Hossain, H., Fischer, B., Catravas, J. D., Verin, A.
D., Pittet, J. F., Caldwell, R. B., Mitchell, T. J., Cederbaum, S. D.,
Fulton, D. J., Matthay, M. A., Caldwell, R. W., Romero, M. J., and
Chakraborty, T. (2012) Protein kinase C-α and arginase I mediate
pneumolysin-induced pulmonary endothelial hyperpermeability, Am. J.
Respir. Cell Mol. Biol., 47, 445-453.
130.Umapathy, N. S., Fan, Z., Zemskov, E. A.,
Alieva, I. B., Black, S. M., and Verin, A. D. (2010) Molecular
mechanisms involved in adenosine-induced endothelial cell barrier
enhancement, Vascul. Pharmacol., 52, 199-206.
131.Waterman-Storer, C. M., and Salmon, E. D.
(1999) Positive feedback interactions between microtubule and actin
dynamics during cell motility, Curr. Opin. Cell Biol.,
11, 61-67.
132.Akhmanova, A., and Hoogenraad, C. C. (2005)
Microtubule plus-end-tracking proteins: mechanisms and functions,
Curr. Opin. Cell Biol., 17, 47-54.
133.Akhmanova, A., Stehbens, S. J., and Yap, A. S.
(2009) Touch, grasp, deliver and control: functional cross-talk between
microtubules and cell adhesions, Traffic, 10,
268-274.
134.Schober, J. M., Komarova, Y. A., Chaga, O. Y.,
Akhmanova, A., and Borisy, G. G. (2007) Microtubule-targeting-dependent
reorganization of filopodia, J. Cell Sci., 120,
1235-1244.
135.Watanabe, T., Noritake, J., and Kaibuchi, K.
(2005) Roles of IQGAP1 in cell polarization and migration, Novartis
Found Symp., 269, 92-101.
136.Waterman-Storer, C. M., Salmon, W. C., and
Salmon, E. D. (2000) Feedback interactions between cell–cell
adherens junctions and cytoskeletal dynamics in newt lung epithelial
cells, Mol. Biol. Cell, 11, 2471-2483.
137.Shahbazi, M. N., Megias, D., Epifano, C.,
Akhmanova, A., Gundersen, G. G., Fuchs, E., and Perez-Moreno, M. (2013)
CLASP2 interacts with p120-catenin and governs microtubule dynamics at
adherens junctions, J. Cell Biol., 203, 1043-1061.
138.Van der Vaart, B., van Riel, W. E., Doodhi, H.,
Kevenaar, J. T., Katrukha, E. A., Gumy, L., Bouchet, B. P., Grigoriev,
I., Spangler, S. A., Yu, K. L., Wulf, P. S., Wu, J., Lansbergen, G.,
van Battum, E. Y., Pasterkamp, R. J., Mimori-Kiyosue, Y., Demmers, J.,
Olieric, N., Maly, I. V., Hoogenraad, C. C., and Akhmanova, A. (2013)
CFEOM1-associated kinesin KIF21A is a cortical microtubule growth
inhibitor, Dev. Cell, 27, 145-160.
139.Akhmanova, A., Hoogenraad, C. C., Drabek, K.,
Stepanova, T., Dortland, B., Verkerk, T., Vermeulen, W., Burgering, B.
M., De Zeeuw, C. I., Grosveld, F., and Galjart, N. (2001) Clasps are
CLIP-115 and -170 associating proteins involved in the regional
regulation of microtubule dynamics in motile fibroblasts,
Cell, 104, 923-935.
140.Mimori-Kiyosue, Y., Grigoriev, I., Lansbergen,
G., Sasaki, H., Matsui, C., Severin, F., Galjart, N., Grosveld, F.,
Vorobiev, I., Tsukita, S., and Akhmanova, A. (2005) CLASP1 and CLASP2
bind to EB1 and regulate microtubule plus-end dynamics at the cell
cortex, J. Cell Biol., 68, 141-153.
141.Tsvetkov, A. S., Samsonov, A., Akhmanova, A.,
Galjart, N., and Popov, S. V. (2007) Microtubule-binding proteins
CLASP1 and CLASP2 interact with actin filaments, Cell Motil.
Cytoskeleton, 64, 519-530.
142.Tanenbaum, M. E., Macurek, L., van der Vaart,
B., Galli, M., Akhmanova, A., and Medema, R. H. (2011) A complex of
Kif18b and MCAK promotes microtubule depolymerization and is negatively
regulated by Aurora kinases, Curr. Biol., 21,
1356-1365.
143.Nakaya, Y., Sukowati, E. W., and Sheng, G.
(2013) Epiblast integrity requires CLASP and dystroglycan-mediated
microtubule anchoring to the basal cortex, J. Cell Biol.,
202, 637-651.
144.Akhmanova, A., and Steinmetz, M. O. (2011)
Microtubule end binding: EBs sense the guanine nucleotide state,
Curr. Biol., 21, 283-285.
145.Kita, K., Wittmann, T., Nathke, I. S., and
Waterman-Storer, C. M. (2006) Adenomatous polyposis coli on microtubule
plus ends in cell extensions can promote microtubule net growth with or
without EB1, Mol. Biol. Cell, 17, 2331-2345.
146.Myers, K. A., Applegate, K. T., Danuser, G.,
Fischer, R. S., and Waterman, C. M. (2011) Distinct ECM mechanosensing
pathways regulate microtubule dynamics to control endothelial cell
branching morphogenesis, J. Cell Biol., 192, 321-334.
147.Lyle, K. S., Corleto, J. A., and Wittmann, T.
(2012) Microtubule dynamics regulation contributes to endothelial
morphogenesis, Bioarchitecture, 2, 220-227.
148.Kirschner, M. W., and Mitchison, T. (1986)
Microtubule dynamics, Nature, 324, 621.
149.Smurova, K. M., Birukova, A. A., Garsia, G.,
Vorobjev, I. A., Alieva, I. B., and Verin, A. D. (2004) Reorganization
of the microtubule system in the pulmonary endothelium cells in
response to treatment with thrombin, Tsitologiya, 46,
695-703.
150.Czikora, I., Sridhar, S., Gorshkov, B., Alieva,
I. B., Kasa, A., Gonzales, J., Potapenko, O., Umapathy, N. S., Pillich,
H., Rick, F. G., Block, N. L., Verin, A. D., Chakraborty, T., Matthay,
M. A., Schally, A. V., and Lucas, R. (2014) Protective effect of growth
hormone-releasing hormone agonist in bacterial toxin-induced pulmonary
barrier dysfunction, Front. Physiol., 5, 1-10.