REVIEW: Use of Intracellular Transport Processes for Targeted Drug Delivery into a Specified Cellular Compartment
A. A. Rosenkranz1,2, A. V. Ulasov1,3, T. A. Slastnikova1, Y. V. Khramtsov1, and A. S. Sobolev1,2*
1Institute of Gene Biology, Russian Academy of Sciences, ul. Vavilova 34/5, 199334 Moscow, Russia; fax: +7 (499) 135-4105; E-mail: info@genebiology.ru2Faculty of Biology, Lomonosov Moscow State University, 119234 Moscow, Russia; fax: +7 (495) 939-4309; E-mail: sobolev@igb.ac.ru; info@mail.bio.msu.ru
3Targeted Delivery of Pharmaceuticals “Translek” LLC, ul. Vavilova 34/5, 199334 Moscow, Russia; E-mail: translek@genebiology.ru
* To whom correspondence should be addressed.
Received June 15, 2014
Targeted drug delivery into the cell compartment that is the most vulnerable to effects of the corresponding drug is a challenging problem, and its successful solution can significantly increase the efficiency and reduce side effects of the delivered therapeutic agents. To accomplish this one can utilize natural mechanisms of cellular specific uptake of macromolecules by receptor-mediated endocytosis and intracellular transport between cellular compartments. A transporting construction combining the components responsible for different steps of intracellular transport is promising for creating multifunctional modular constructions capable of delivering the necessary therapeutic agent into a given compartment of type-specified cells. This review focuses on intracellular transport peculiarities along with approaches for designing such transporting constructions for new, more effective, and safer strategies for treatment of various diseases.
KEY WORDS: targeted drug delivery, intracellular transport, receptor-mediated endocytosis, transport of macromolecules, nuclear import, modular nanotransporters, cancer therapyDOI: 10.1134/S0006297914090090
Abbreviations: CPP, cell penetration peptides; HMP, hemoglobin-like protein of E. coli; MNT, modular nanotransporters; NES, nuclear export signal; NLS, nuclear localization signal; PAA, polyamidoamine; PEI, polyethyleneimine; PS, photosensitizer; ROS, reactive oxygen species; TAT, trans-activator of transcription.
The targeted delivery of therapeutic agents is now one of the most
urgent problems of modern biomedicine, pharmaceutics, and nanomedicine:
it is sufficient to note that Web of Science refers to five
journals with titles including the word combination “Drug
Delivery”, and Google Academy considers more than a dozen
journals with such titles. The development of systems for drug delivery
is one of the major lines in the innovation activities of the largest
pharmacological firms of the world. During the last decade, special
attention has been given to delivery of a drug not simply into the
target cell but also into its specified compartment (about drugs for
which just such approach is required, see below). If the drug is not
specific to the required intracellular compartment, to enter this
compartment it has either (1) to be highly stabile to get into the
required compartment due to random translocation processes without
degradation, or (2) to be given with special features allowing
directional reach of this compartment. The second variant is much more
significant if the drug has increased affinity for an undesired
compartment [1]. There have been and still are
attempts to solve this problem using both approaches.
The attempts using the first approach resulted in substances capable of getting into, e.g. acidified endocytotic compartments due to their physicochemical properties; however, the same result can be obtained using the second approach with receptor-mediated endocytosis [2, 3]. In the present review we shall focus attention just to the second line of studies and developments, which use natural intracellular traffics for targeted drug delivery. This approach simplifies the problem of combining in the drug or in the system of its delivery two apparently discordant complexes of features: providing for specific “recognition” of target cells (that can be realized based on specific features of the cell surface molecules) and the highest efficiency (that for many drugs can be realized only on penetration into the cell and its particular compartment) [4].
DRUGS REQUIRING INTRACELLULAR TARGETED DELIVERY
Despite of essential advances in the development and creation of new therapeutic approaches, there is still no effective treatment for many socially important diseases. By and large, this paradox is explained by limitations inherent in traditional therapies: low doses of drugs are not always effective, whereas high doses can often be associated with serious toxic manifestations. However, the increasing bulk of data on genes, signaling cascades, and regulatory proteins involved in pathogenesis of various diseases suggests new potential targets for therapy. This creates possibilities for development of many addressed or targeted preparations aimed to a certain types of target cells, and, as an ideal, to a particular compartment within these cells. Such addressed delivery of drugs is able not only to attach specificity to a drug, but also to significantly increase its efficiency due to its delivery into the cellular compartment where its activity will be maximal.
Considering pathways of the distribution of pharmaceuticals in the body, available drugs can be roughly divided into two large groups. The first group usually includes low molecular weight substances that are passively distributed throughout organs, tissues, cells, and intracellular space due to diffusion and convection processes. Drugs of this group are effective on sufficient saturation of the organism until the drug concentration in the action site (usually within a cell) becomes sufficient for influence on a certain biochemical or regulatory process. The second group usually includes high molecular weight substances or substances carrying a charge that prevents their penetration across membranes, and they are unable to passively diffuse into cells but can act, in particular, through interaction with some receptor on the surface of target cells. This interaction activates or, by contrast, inhibits certain biochemical and regulatory reactions either directly or through a ligand to the chosen receptor in the most complicated delivery system after the internalization and release of an active low molecular weight component.
Both these approaches have no direct influence on many regulatory processes occurring inside the cell due to interactions between macromolecules. Drugs of these two groups are usually poorly specific in their influences on such interactions. Low molecular weight compounds rather easily penetrating across membranes do not always have sufficient specificity for affecting such interactions. Moreover, as differentiated from inhibition of enzymes when there is competition with natural ligands for binding in the active site or in a protein pocket, protein–protein interactions are realized on rather large areas of the protein molecule surface (1000-3000 Å2), and the interacting surfaces are often rather flat and lack pronounced pockets available for inserting a low molecular weight compound [5, 6]. On the other hand, the activation or inhibition of receptors on the cell surface, even highly specific as they are, leads to changes in the regulation of not one regulatory process but of a whole network of processes.
However, many diseases are caused just by disorders in interactions between intracellular macromolecules. Studies intended to create a system for delivery of macromolecules capable of getting into the cell and influencing a pathological interaction (or an insufficient normal interaction) between regulatory macromolecules are very important for treatment of many diseases. This approach opens a possibility to effectively influence different diseases caused by disorders in the regulation of a definite type of cells, including malignant tumors, and to deliver cytotoxic agents based on differences of the transformed cells in both their surface receptors available for macromolecules and intracellular traffic or regulation.
Pharmaceuticals for which targeted delivery is required include various functional molecules: nucleic acids (DNA, siRNA, miRNA, shRNA, antisense-oligonucleotides) used for gene therapy, proteins and small peptides interacting with intracellular targets, “small molecules” (photosensitizers, low molecular weight inhibitors of different intracellular processes), isotopes emitting short-range particles, etc. These compounds are very different in structure and properties and are characterized by the necessity of being delivered into a specific intracellular compartment. Moreover, for the majority of them passive transport into the cell is insufficiently effective or is associated with high toxicity for non-targeted cells. Such pharmaceuticals can be divided into two groups [7]. The first group includes molecules that act in a strictly defined intracellular compartment and are unable to penetrate into it as they are or can only penetrate with low efficiency (e.g. gene therapeutic preparations). The other group includes drugs that are most efficient in a particular compartment, e.g. photosensitizers (PSs) and radionuclides emitting short-range particles.
Genetic information for therapeutic purpose can be delivered using either viruses or different synthetic constructions. Despite of some advantages, first of all high efficiency of transfection, viral vectors have some shortcomings such as a high immunogenicity and associated toxicity, a possible reversion to the wild type due to recombination or mutations, a low capacity for genetic material to be transferred, and the virus having its own cell specificity. Some viruses (retroviruses) used as vectors can incorporate into DNA of the host’s cell, which can lead to generation of tumors because of activation of oncogenes. These shortcomings make the clinical use of viral vectors rather dangerous, which was confirmed by the death of a patient upon using the adenoviral vector [8], the development of leukemia during retroviral gene therapy [9], and autoimmune reaction induced by an adeno-associated viral vector [10].
Synthetic delivery systems, such as nucleic acid complexes with cationic lipids (lipoplexes), nucleic acid complexes with cationic polymers (polyplexes), and more complicated systems based on them, are used as alternatives to viral vectors [11]. Compared to viral vectors, nonviral delivery systems of genetic material are usually characterized by the necessity to deliver a greater amount of DNA to induce a comparable effect and by a short time of exogenous DNA expression. However, lipoplexes and polyplexes have low immunogenicity and low toxicity, are not limited in the amount of transported DNA, and their production is easier and less expensive than the production of viral vectors.
Photosensitizers are molecules that under exposure to a specific wavelength of light generate reactive oxygen species (ROS) capable of damaging DNA, cell membranes, and macromolecules. Depending on their nature, PSs penetrate into different cellular structures where they can display photodynamic action [12]. To increase the sensitivity of PSs to target cells (usually cancer cells), PSs are joined to different molecules specifically interacting with the target cells. But ROS generated on illumination of PSs can overcome distances not more than several tens of nanometers, and this creates a non-optimal distribution of PSs within the cell, whereas the most sensitive target for a PS is the cell nucleus [12] and PSs injected in the free state virtually do not arrive at the nucleus. Thus, the efficiency of PSs can be significantly increased by delivering them into nuclei of target cells concurrently with decrease in their side effects due to lowering of the doses [13].
Radionuclides with short-range particles used as therapeutic agents include emitters of α-particles and emitters of Auger electrons. The cell nucleus is the most radiosensitive cellular structure because of increased probability of crossing of the nucleus by the degradation track of the particles that leads to damage to DNA. Moreover, recoil nuclei produced during α-decay has linear energy transfer about 10-fold higher than α-particles themselves and can act over short distances (<100 nm); for using this cytotoxic mechanism, a radioisotope emitting α-particles has to be immediately close to the cell nucleus or better within it [14]. Auger electron emitters are known to be inefficient outside the cell nucleus [15] and have high cytotoxicity in close vicinity of nuclear DNA due to generation of double-stranded virtually irreparable DNA breaks [16]. Because the emitted Auger electrons are short-range (some tens of nanometers), their cytotoxic effects are manifested only within the site of decay. This feature makes them potentially highly specific anticancer agents in the case of delivery of these isotopes into the target cell nuclei.
PATHWAYS OF PENETRATION INTO CELLULAR COMPARTMENTS
A pharmaceutical agent unable to penetrate across membranes can occur inside the cell nonspecifically through pinocytosis or due to joining to specially developed transporters capable either of penetrating directly across the plasma membrane or of using natural mechanisms of endocytosis and phagocytosis allowing them to specifically enter the cell. Uptake through pinocytosis cannot provide accumulation of injected drugs in concentrations higher than in the external medium, as well as their effective penetration into the hyaloplasm. To provide for direct penetration across the cell membrane, constructions have been proposed that contain cell penetration peptides (CPP). However, in addition to the not very effective direct penetration into the hyaloplasm, such constructs concurrently undergo pronounced nonspecific endocytosis accompanied by subsequent more or less effective penetration across endosome membranes [17]. This approach is nonspecific by definition and the effect can be obtained only on saturation of the whole body by the delivering construct that contains the acting agent. Therefore, it seems attractive to use active transport into the desired cells using internalized surface receptors and intracellular traffic. In turn, this makes it necessary to include into the delivering construct (transporter) a component capable of recognizing the desired cell, i.e. a module with features of an internalized ligand. The transporter taken up by endocytosis, depending on the receptor and endocytosis types, can either be returned back onto the cell surface due to natural recirculation or occur in late endosomes and then in lysosomes. Moreover, transporter with the acting agent can be targeted into the endoplasmic reticulum or the Golgi apparatus through sorting and vesicular transport. To do this, in the general case an additional component (module) with the corresponding function is required. This is not associated with transfer across the membrane, and the substance to be delivered topologically remains on the same side of the plasma membrane as the extracellular space. For transporting into other cellular compartments (hyaloplasm, nucleus, mitochondria), transfer is needed across an ordinary (release from endosomes or endoplasmic reticulum) or double membrane (entering the nucleus through a nuclear pore complex [18] and entering into the internal space of mitochondria using translocational complexes TOM and TIM23 [19]). For transport into these compartments from the outside, not only an additional component with corresponding function is needed, but for effective penetration into the hyaloplasm corresponding modules are also required. Therefore, simple inclusion of the nuclear localization signal into the transporter containing the internalized ligand will not be sufficient for efficient traffic into the cell nucleus.
USE OF RECEPTOR-MEDIATED ENDOCYTOSIS: SOME PROBLEMS ARE SOLVED
BUT OTHERS APPEAR
Therapeutic molecules considered in the present review (PSs, emitters of Auger electrons, emitters of α-particles, nucleic acids, proteins, other macromolecules, and nanoparticles) preferentially act in certain cellular compartments and need to be delivered to their intracellular targets. In the cell the target can be localized differently for different therapeutic agents, e.g. the nucleus for DNA, lysosomes for lysosomal enzymes, mitochondria for proapoptotic enzymes. A necessary step of delivery into the corresponding cellular compartment is the required penetration into the cell. The majority of drugs used in clinical practice can penetrate by themselves across the cell membrane due to passive diffusion [20] or using membrane transporters. However, a significant number of drugs proposed for use as targeted ones cannot penetrate across the cell membrane as they are because of their size or charge, or they can penetrate only with low efficiency. As a result, the targeted delivery of such drugs has to include not only the recognizing of target cells, but also penetration into them, usually by receptor-mediated endocytosis or, less frequently, due to inclusion into the transporter of membrane-active peptides capable of producing pores in the cell membrane.
The idea of using internalized receptors for delivery of drugs into target cells is already several decades old. Active studies were started in the 1980s when studies on tumor cell biology revealed the existence of various receptors overexpressed on tumor cells but in an ideal case absent or represented more poorly on the surface of normal cells. Receptors overexpressed on the target cell surface are markers that can be recognized using receptor-specific ligands, antibodies, or aptamers [21]. If the receptors not only are abundant on the target cells but also are internalized in them, these molecules can be used for delivery of drugs not only to the surface of the target cells but also into these cells through receptor-mediated endocytosis [22]. Receptor-mediated endocytosis can also be used for treatment of other diseases, because the expression of many receptors significantly varies in different normal cells of the organism. Ligands most frequently used for development of targeted drug delivery (mainly into cancer cells) are exemplified by ligands to transferrin receptors [23] for delivery of doxorubicin [24], hydroxycamptothecin [25], and oxaliplatin [26]; epidermal growth factor for delivery of cisplatin [27], indium-111 [28], PSs [29], iodine-125 [30], and astatine-211 [31]; folate for delivery of doxorubicin [32], paclitaxel [33], vinblastin derivative [34], and siRNA [35]; vascular endothelium growth factor for docetaxel delivery [36]; first type melanocortin receptor for PS delivery [29, 37], and therapeutic genes [38]; somatostatin for delivery of α-emitter bismuth-213 [39], paclitaxel [40], combretastatin and doxorubicin [41], hydroxycamptothecin [42], and irinotecan [43]; αvβ3-integrin for delivery of doxorubicin [44], PS HPPH [44], siRNA [45], and therapeutic genes [46].
The use of receptor-mediated endocytosis for delivery into the cell can markedly improve the effect of many therapeutic agents: more sensitive intracellular targets become available for PSs and emitters of particles with high linear energy transfer and the probability increases of reaching the targeted compartment for low molecular weight particles delivered within liposomes or nanoparticles. Nevertheless, the intracellular distribution after endocytosis remains not optimal even for those drugs, which act in the hyaloplasm, nucleus, and mitochondria. Moreover, natural pathways bring the substances delivered by endocytosis into the recirculatory endocytotic compartment from where many of them are eliminated from the cell. Another usual pathway of endocytosis brings into lysosomes the contents which undergo the action of hydrolytic enzymes. To abandon the “routine pathways” of endocytosis, the delivering construct has to include additional components with corresponding features.
NECESSITY TO LEAVE THE ENDOCYTOSIS PATHWAY: WHY AND HOW?
As mentioned above, the transporter molecules upon penetration into the cell due to various endocytosis or phagocytosis actions appear in endosomes. One of the major pathways of molecular trafficking results in acidification of the contents of early endosomes due to activities of membrane ATPase proton pumps (the pH value decreases from 6.0-6.5 to 5.0), and they become late endosomes and finally fuse with lysosomes (pH < 5). There are some drugs that are delivered just into lysosomes [47]. In particular, these are lysosomal enzymes, which are to be delivered into lysosomes on insufficiency of these enzymes in some diseases [48]. They can be delivered using liposomes with lysosome-tropic octadecyl-rhodamine B on their surface [49].
It is also known that the release of lysosomal enzymes results in apoptosis of the cell [47]. Therefore, approaches for treatment of different types of cancer are often developed that include targeted delivery into the cancer cell lysosomes of different substances affecting the integrity of the lysosomal membranes [50]. Such substances can be detergents (e.g. sphingosine), lysosome-tropic toxins, PSs, and also substances that can be accumulated in lysosomes and affect their integrity at concentrations above a critical value [47]. For this purpose various chemotherapeuticals can also be used; thus, the delivery of ceramide into lysosomes by transferrin-modified liposomes noticeably increased apoptosis of cancer cells both in vitro and in vivo [51]. Moreover, to keep radioisotopes within cancer cells, they are often delivered into endosomes/lysosomes where they can be retained by joining to a charged group [52].
However, for targeted delivery of drugs into hyaloplasm, nucleus, or mitochondria, the transporter for this delivery has to be capable of leaving endosomes before getting into the lysosomes to prevent the degradation of the transported drug under the influence of lysosomal hydrolases (proteases, lipases, phosphatases, glycosidases, and sulfatases). The system of compartments interconnected through the transport of vesicles (endosomes, lysosomes, endoplasmic reticulum, Golgi apparatus) where the transporter is delivered due to endocytosis has a natural retrograde pathway into the hyaloplasm, the so-called ERAD (endoplasmic reticulum-associated degradation) [53], and this pathway is normally used for utilization of proteins with damaged structure. Some toxins, such as the pertussis, cholera, and Shiga toxin [54], and also ricin [55], upon getting into endosomes can be at least partially transported along the retrograde pathway across the sorting endosomes into the Golgi apparatus and further into the endoplasmic reticulum, which allows these molecules to be released into the hyaloplasm. It is thought that for movement along the retrograde pathway molecules are bound with particular receptor proteins on the cell surface [54]. Thus, Shiga toxin can use GPP130 or Gb3 as a receptor [54, 56]. Shiga toxin and similar toxins consist of one A-subunit and five B-subunits [54]. The B-subunit is responsible for the traffic along the retrograde pathway, and in the Shiga toxin case the B-subunit binds with the Gb3 receptor [54, 57]. It should be noted that the Gb3 receptors are overexpressed on some type cancer cells and also on dendritic cells, and this is a reason for attempts to use transporters of drugs containing the toxin B-subunit for therapy of cancer and infectious diseases [57]. In particular, the toxin B-subunit was supplemented with such cytotoxic compounds as the topoisomerase I inhibitor SN38 for therapy of colorectal carcinoma [58], a proapoptotic ligand of the peripheral benzodiazepine receptor RO5-4864 [59], and such PSs as a porphyrin derivative TPP(p-O-β-GluOH)3 [60] and chlorin e6 [61]. Because Gb3 receptors are overexpressed on antigen-presenting dendritic cells, conjugates of cancer and viral antigens with the Shiga toxin B-subunit can be included into vaccines against different tumors and infectious diseases [62, 63]. However, the retrograde pathway is now seldom used for development of targeted drug delivery because its functions seem to be closely associated with unfolding of proteins and subsequent utilization by proteasomes, and this markedly narrows the number of transporters and transported substances.
It is not surprising that attempts to use the medium acidification occurring during endocytosis are more frequent for delivery of endocytosed substances into the hyaloplasm. For the viral delivery of genes the transport mechanisms of viruses are used, which allow them to transfer their contents into the hyaloplasm [64, 65]. Adenoviruses, which are the most efficient transporters of genetic material into cells, can leave endosomes due to changes in the conformation of the capsid components under the influence of acidification of the endosomal medium [66]. This ability of adenoviruses to induce pH-dependent destruction of the lipid bilayer was repeatedly successfully tested for delivery into the desired cellular compartment of toxins [67] or plasmids brought into cells due to receptor-mediated endocytosis [68].
Artificial systems of delivery into the hyaloplasm, nucleus, or mitochondria also have to contain components capable of transporting a pharmaceutical agent across a membrane, in particular, agents affecting the membrane integrity. Consider this mechanism for transporters using the lysosomal pathway for the diphteria toxin translocation domain [69]. This mechanism based on the pH-sensitive change in the conformation of a protein allows it to acquire affinity for membranes, incorporate into them, and affect their integrity, e.g. by formation of channels. The incorporation mechanism is known to include a number of intermediate steps [69]. The diphtheria toxin translocation T-domain consists of nine α-helices, eight of which completely surround the most hydrophobic α-helix (TH8). At pH > 7.5 the T-domain is in the W-state, which lacks the affinity for membranes. Changes in the T-domain conformation are caused by protonation of histidine amino acid residues, among which His257 is the key one. This protonation on decrease in pH results in the transition of the T-domain into the W+-state when hydrophobic α-helix begins to contact with water and the T-domain acquires affinity for membranes. Transition to the W+-state does not occur at pH > 7.5 due to the fact that His223, which is more easily protonated than His257, causes a shift in pKa of His257 by 1.5 units to the more acidic region, while being in the protonated state [70]. In the W+-state the protein rapidly binds to the lipid bilayer surface producing the membrane-bound I-state. Further decrease in pH can be accompanied by incorporation into the lipid bilayer of α-helical hairpin TH8-9 and production of the I+-state. This incorporation is promoted by the presence in the lipid bilayer of negatively charged lipids. Then, depending on pH and transmembrane potential, a number of transmembrane (TM) states can be generated that are responsible for translocation of the diphtheria toxin catalytic domain. The structure of these states is not known in detail, but it seems that channels can be produced in the lipid membrane [71, 72]. The C-terminal histidine residues 322, 323, and 372 are crucial for production of the open channel state [73].
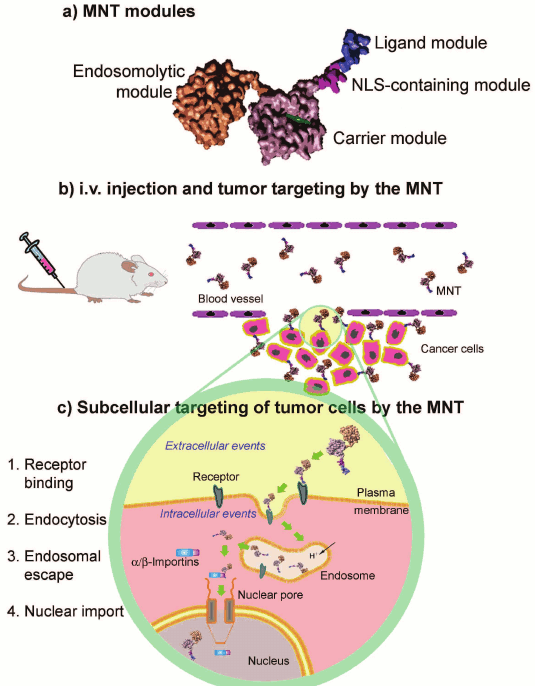
The diphtheria toxin T-domain is responsible for traffic across the endosomal membrane of the N-terminal catalytic domain, which in the cytosol is detached from the T-domain by a special site [74]. Not all delivery tools that include the diphtheria toxin T-domain can leave endosomes using this mechanism. However, this mechanism of translocation domain functioning seems to be not the only one, because the limiting size of molecules capable of penetrating across the pores produced by diphtheria toxin depends on its concentration [75], and the diphtheria toxin can form oligomers on the membrane at decreased pH [76]. Moreover, polypeptide-transporting constructs (modular nanotransporters (MNT)) with the T-domain on the N-terminus of the molecule could successfully leave endosomes [29, 77-79] and ensure the delivery of the majority of the endocytosed molecules into the nucleus [30]. A decrease in pH from 7.5 to 5.5 caused in 5-15 min in these nanotransporters formation of circular structures with average diameter of 43.1 ± 1.2 nm in the initially free of defects lecithin bilayer on mica [77-79]. Every structure consisted of 11 ± 2 molecules of MNT [29]. As judged by a large increase in the conductivity recorded in experiments with bilayer lipid membranes, these structures were pores in the membrane [78]. After 40-60 min, at pH 5.5 in lipid bilayers on mica fluctuating pores began to appear with diameter of 50-200 nm and depth corresponding to the lipid bilayer thickness [29, 77-79]. Such pores were produced in both neutral and negatively charged lipid bilayers [78]. If similar defects were produced in endosomes, their size was quite sufficient to allow MNT molecules not bound with the lipid membrane to leave the endosomes for the cytosol. The exit of MNT molecules from endosomes is confirmed by experimental determination of pH of the local microenvironment of MNT molecules endocytosed by Cloudmann melanoma S91 cells in culture. As was shown by image ratio video intensification microscopy, a part of MNT molecules lacking the T-domain occurred in an acidic environment specific for late endosomes and lysosomes. In contrast, MNT molecules containing the T-domain were not found in the late endosomes and lysosomes [37, 80].
Not all membrane effects observed for these MNT can be ascribed to the T-domain. Thus, although the separate T-domain could aggregate in the lipid bilayer or on its surface, it did not induce the generation of similar circular structures [79]. In contrast, the T-domain-deprived MNT could produce circular structures at pH 5.5. Moreover, at pH 5.5 large fluctuating pores were not detected for either MNT deprived of the T-domain or the separate T-domain. The observed effects seemed to be due to a combined action of the T-domain and another membrane-active MNT domain, the hemoglobin-like protein of E. coli (HMP), acting in MNT as a carrier module [79]. It is reasonable to take into account such combined action of different domains when drug transporters are being developed.
To deliver different transporters into the cytosol, cell penetration peptides (CPP) are often included into them [81]. Active studies on these peptides began after type I human immunodeficiency virus transcription transactivator (TAT) fragments were found to penetrate into cells [82]. Usually CPP are short peptide molecules (10-30 amino acids) positively charged in neutral medium [83]. Such CPP also include transportan, penetratin, and oligoarginines. Rather frequently these peptides are amphiphilic. CPP are widely used for delivery into the cell of nucleic acids, proteins, fluorophores, and various drugs [81]. It seems that these peptides in high concentrations can directly penetrate across plasma membranes both separately and as components of small transporters; however, the major pathway for their penetration into the cell is endocytosis, and the efficiency of the CPP-containing transporter release from endosomes into the cytosol is low [81]. The mechanism of the CPP release from the endosomes is not known in detail. It is supposed to be associated with an uneven distribution of negatively charged lipid inside and outside the endosomes, with apparently higher contents in the external monolayer [84]. Electrostatic interactions between positively charged CPP and these lipids can lead to destabilization of the bilayer and production of defects in it. At the stage of late endosomes, the negative surface charge of the external lipid monolayer of the endosomes significantly increases. Therefore, it is not surprising that many CPP including the TAT-peptide leave for the cytosol during the stage of late endosomes [81]. If the transporter carries proteins, this can cause their partial degradation and loss of functional activity because of action of hydrolases. To increase the efficiency of releasing the CPP-containing transporters from the endosomes, delivery systems were created containing several copies of CPP. This multivalence allowed the local concentration of CPP to be significantly increased and more effectively destabilize the bilayer due to improved interaction with lipids. The multivalent CPP can leave the endosomes significantly better than usual CPP [81, 85]. Thus, fluorescently labeled TAT-peptide trimer leaves endosomes at concentrations 5-10 times lower than the TAT-peptide monomer or dimer [81, 85]. However, too many copies of CPP can cause an inverse effect because of strong summary interaction of these peptides with the endosomes [81]. A disadvantage of this approach also is increased immunogenicity of multivalent CPP in vivo.
The pH-dependent exit from endosomes can be also obtained by using amphiphilic negatively charged peptides [86, 87]. Similarly to the above-described diphtheria toxin T-domain, these peptides become membrane active with a decrease in pH. These peptides contain residues of both hydrophobic and glutamic and aspartic amino acids, which are protonated at low pH values that increases the hydrophobicity of the molecule and leads to its ordered, most frequently α-helical, conformation and interaction with the lipid bilayer [81]. Such peptides are exemplified by the HA2-peptide (the fusion peptide from the influenza virus hemagglutinin) [88] and the GALA-peptide [87]. The combined use of such peptides and CPP within the same transporter can significantly increase the efficiency of the transporter getting into the cytosol [81]. Thus, the HA2-peptide combined with the tumor suppressor protein p53 containing the polyarginine sequence R11 (HA2–p53–R11) suppressed the growth of tumor cells more effectively than the p53–R11 conjugate separately [89]. However, these anionic peptides can be anchored in the endosomal membrane; therefore, to ensure the effective exit of the transporter from the endosomes the anionic peptide seems to need to be joined to the transporter with a bond hydrolyzed in the cytosol, e.g. with a disulfide bond [81].
The exit from endosomes of transporters including CPP-containing ones can be obtained by incorporation into them of a PS and by illumination with the corresponding wavelength [90, 91]. The illumination of a PS results in generation of ROS, which oxidize lipids and can lead to production of defects in lipid membranes. Because ROS are short-range, it is necessary to place PSs immediately close to the endosomal membrane that can be realized due to the interaction of CPP with the lipid bilayer. The destruction of endosomes mediated by ROS often leads to cell death. Moreover, using this approach in vivo is principally limited by the light penetration depth into the organism’s tissue [81].
To allow some transporters to leave endosomes, for these transporters it is not obligatory to contain a separate membrane-active module. It was supposed for some nonviral systems of DNA delivery that protonated polymers [92] commonly used as a modular carrier and packager of DNA could induce swelling and osmotic lysis of endosomes according to the “proton sponge” theory. This mechanism was proposed for explanation of the exit of some polymers such as polyethyleneimine (PEI) and polyamidoamine (PAA). These polymers are characterized by the presence in their structure of numerous secondary and tertiary amino groups with pKa values of 5-7. On entering endosomes, these polymers can create a buffer effect that increases the activity of H+-ATPase and, consequently, a greater accumulation of protons in the endosomes. And this, in turn, due to activities of H+/Cl–-exchangers, results in accumulation in the endosomes of chlorine ions (from 40 to 115 mM) [93]. A sharp increase in the osmotic pressure leads to increase in the volume (by 140%) and to lysis of the endosomes, and the authors of this hypothesis believe that it allows transporters containing PEI or PAA to release into the hyaloplasm. However, there are some data that contradict the “proton sponge” hypothesis or cannot be explained by it [94-98]. It should be added that both PEI and PAA as well as other similar cationic polymers are able to directly destabilize the endosomal membrane [99, 100]. It is difficult to evaluate the contribution of this mechanism to trafficking from endosomes because such intracellular membrane compartments as endosomes, endoplasmic reticulum, and Golgi apparatus represent a dynamic system possessing constant incoming and outgoing flows of lipids realized by transport vesicles. The shape of endosomes is often far from spherical [101] that also has to prevent their rupture. Although PEI and some other protonated polycations are significantly more effective than the majority of other polymers used for the same purpose, the efficiency of their leaving the endosomal pathway is insufficient as shown by an increase in transfection on addition into polyplexes of the diphtheria toxin translocation domain specifically affecting the membrane structure in a weakly acidic medium [102, 103].
Some lipid nanoparticles containing cationic lipids also can release their contents from endosomes into the hyaloplasm. The presence of positively charged phospholipids in the structure of such particles as lipoplexes induce an electrostatic interaction with negatively charged phospholipids accompanied by the flip-flop transition into the internal monolayer of the endosomal membrane, and this results in membrane destabilization and release of contents from the endosomes [104]. The release of nucleic acids from endosomes can also be increased by inclusion into the structure of lipoplexes of dioleoyl phosphatidylethanolamine (DOPE) due to the pH-dependent phase transition of this lipid with production of a hexagonal phase that leads to destabilization of the endosomal membrane during its interaction with lipoplexes [105].
Thus, by now a real arsenal of tools has been developed to ensure the trafficking of drugs across membranes into the target cell hyaloplasm, and these tools can be used as a component of multifunctional transporting constructs.
CHANGE IN PATHWAYS LEADING INTO THE NUCLEUS
To obtain the maximal effect of the majority of anticancer cytotoxic agents, it is insufficient only to deliver them into the cancer cells because one of the most sensitive cellular compartments is the nucleus [106]. Thus, the cell nucleus is the most sensitive to the damaging action of ROS (the origin of cytotoxicity of PSs [107-110]), emitters of α-particles [111], and especially of emitters of Auger electrons, which are virtually ineffective beyond the cell nucleus [112]. But neither of the above-listed antitumor agents has an ability to preferentially accumulate in the cell nucleus.
Moreover, many widely used in clinical practice anticancer drugs of the so-called “first line therapy”, i.e. those which are used first of all in the treatment of the corresponding disease, act directly on DNA or on DNA-associated enzymes [113]. Thus, cisplatin, camptothecin, doxorubicin, and actinomycin D [114] are to be delivered into the nucleus to obtain their maximal efficiency, especially in resistant cases when these preparations are actively pumped out from the cell cytoplasm [113]. Finally, DNA used for gene therapy displays its effect only upon getting into the target cell nucleus. In this case, the delivery of DNA into the nucleus using transport systems is especially important for non-dividing or slowly dividing cells in which the nuclear membrane is not disassembled at all or is rarely disassembled during mitosis.
Thus, targeted delivery into the nucleus is extremely desirable and often simply necessary for all these different pharmaceuticals.
The successful release from endosomes (see the previous chapter) into the cytoplasm is the necessary but not sufficient condition for the subsequent entry into the cell nucleus of a delivered therapeutic agent. To understand and successfully use mechanisms of accumulation in the nucleus of the preparation under delivery, it is necessary to take into account the organization of traffic of molecules in the cytoplasm (especially the active traffic from the cytoplasm into the cell nucleus). The cell cytoplasm is an organized structure consisting of the cytoskeleton and organelles and subdivided into separate regions [115, 116], which creates some obstacles for free diffusion of macromolecules [117, 118]. Therefore, the targeted trafficking of endogenous proteins and organelles in the cell is active and is realized along a kind of cytoskeleton “rails” – microtubules (preferentially) and actin filaments [117, 119, 120]. These components of the cytoskeleton are involved in both “anchoring” in the cytoplasm of proteins imported into the nucleus [121, 122] and their active delivery to the nucleus [123-127]. The targeted intracellular traffic along the system of microtubules is realized under the influence of special motor proteins, which transfer macromolecules towards the nucleus (dynein) or outward from the nucleus (kinesin) [120]. Thus, the next step in therapeutic agent under delivery on its pathway toward the nucleus upon its successful leaving the endosomes can be an interaction with the retrograde transport system along microtubules (dynein/microtubules) for its arrival close to the nuclear pore complex (see below) [118, 128]. Based on this, some authors use sequences for interaction with the light chain of dynein [129] or even the light chain itself [128] to ensure the more effective delivery of the acting agent (e.g. DNA) into the nucleus. Thus, the use of a modular construction consisting of the light chain of dynein Rp3, the N-terminal DNA-binding domain, and the C-terminal membrane-active TAT-peptide for delivery of DNA allowed researchers to obtain a rather effective and lowly toxic nonviral vector for transfection [128].
The double nuclear envelope containing pores is the major barrier for transporting macromolecules from the cytosol into the cell nucleus. It is thought that only small molecules (with molecular weight lower than 40 kDa and size not more than 9 nm), e.g. nucleotides, ions, and water can pass across the nuclear membrane complex due to passive diffusion [130]. Macromolecules with weight more than 45 kDa are transported into and out of the nucleus under the influence of special transport proteins, transportins (importins and exportins) [131] that “recognize” specific signals in the structure of transported macromolecules – nuclear localization signals (NLS) and nuclear export signals (NES) [131-135]. By now many different NLS are characterized. Proteins containing the best-studied “classic” NLS (the large T-antigen of SV-40 virus, nucleoplasmin) are delivered into the nucleus as a component of a complex with a heterodimer produced by importins α and β [136]. Six classes of “classic” NLSs are described: five classes of one-part and one class of two-part NLSs [137] consisting, respectively, of one or two clusters of basic amino acids (separated by a spacer of 10-12 amino acids) [132]. In addition to “classic” sequences of the nuclear import, there are numerous “noncanonical” NLSs recognized only by importin-β and its homologs [134], dimers of importin-β with its homologs [138], or a dimer of importin-β with another adaptor transportin, snurportin-1 [134]. Because only a few NLSs have been characterized, many different methods have been developed for searching for and predicting potential NLSs in a given sequence [129, 135, 139, 140].
Nucleocytoplasmic transport is regulated through different mechanisms that play central roles in such crucial cellular processes as differentiation and oncogenesis [133]. Changes in nucleocytoplasmic transport often occur in cancer cells. In particular, an increased expression of various transportins has been observed in transformed cells (SV40 virus, human papilloma virus type 16) and in culture of cancer cells and also in the tumor material taken from patients (carcinomas of urinary bladder, esophagus, uterus neck, ovaries, large intestine, liver, pancreas, and osteosarcoma [141, 142]). Moreover, an increased transport into and from the nucleus of a fluorescent protein with NLS/NES combination was shown in transformed cells as compared to their normal analogs [141].
From proteins with different behavior in cancer and normal cells, those proteins attract special interest that are accumulated in the nuclei of cancer cells. Such features are displayed by the human α-lactalbumin derivative HAMLET [143-145] and the protein of chicken anemia apoptin [133, 146-148].
HAMLET is a complex of α-lactalbumin and oleic acid [143]. HAMLET can be accumulated in the cytoplasm and rapidly translocated into the cancer cell nuclei (75% within 1 h) [149], wherease in normal cells it is detected only in the cytoplasm and in significantly lower amounts [144]. But the mechanisms of its release from endosomes and entry into the nucleus are still unclear. The protein itself has diverse proapoptotic features: it disturbs the potential on mitochondrial membranes, induces the release of cytochrome c from mitochondria, affects the activity of proteasomes, and in the nucleus binds with high affinity to chromatin and causes its condensation [145].
Apoptin is a small protein consisting of 121 amino acids of the chicken anemia virus; it is capable of selective accumulation in nuclei of cancer and transformed cells and without accumulation in normal cells [146, 150]. Although there are many works concerning, in particular, mechanisms of selective accumulation of apoptin in cancer cell nuclei [151-153], up to now there is no clear idea about this mechanism. Some hypotheses have been proposed mainly based on the tumor-specific inhibition of NES within the apoptin molecule along with retention of the NLS functions in the sequence of this protein [147].
To realize targeted delivery of an agent into a target cell nucleus, into constructions (nanoparticles, polymers, micelles, proteins, peptides, antibodies) transporting the drug itself different NLS are often included [154-156]. And for this the best-studied “classic” NLS are used preferentially. Table 1 lists NLSs used for the targeted delivery of cytotoxic agents and DNA into cellular nuclei.
Table 1. Examples of nuclear localization
signals used for targeted delivery of cytotoxic agents and DNA into
nuclei of target cells
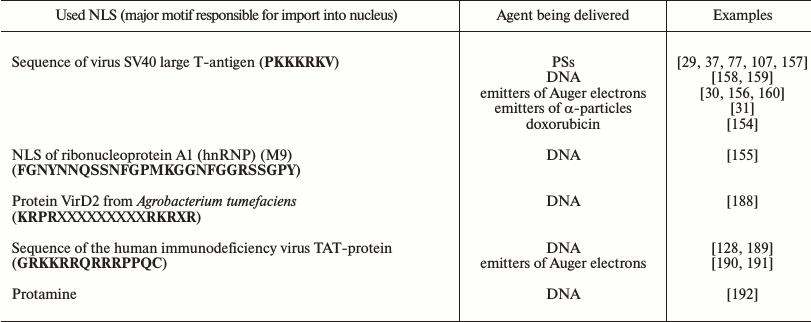
Thus, the introduction of a sequence of the virus SV40 large T-antigen into constructs for delivery of therapeutic agents (DNA, PSs, radionuclides, doxorubicin) resulted in their significant accumulation in the target cell nuclei and an increase in their efficiency [29-31, 37, 77, 107, 154, 156-160].
Considering that such agents as emitters of Auger electrons are virtually ineffective outside of the cell nucleus, some researchers use internalized antibodies or peptides conjugated with NLS for targeted delivery of emitters of Auger electrons (e.g. 111In, 99mTc, 125I, 67Ga) into cancer cell nuclei [29, 156, 160-162]. As mentioned, to interact with the system of nuclear import localized in the cytoplasm, NLS-containing macromolecules are absorbed by the cells through receptor-mediated endocytosis and have to leave the endosomes into the cytoplasm. To realize this, modular nanotransporters (MNT) were developed, i.e. transporting constructs that included modules responsible for different functions: receptor-mediated endocytosis, release from endosomes, and delivery into the nucleus [80, 106]. Such constructs ensure rapid accumulation of the delivered radionuclide in the target cell nuclei (up to 60% of the radionuclide internalized upon 1 h of incubation) [30]. The attachment to the transporting construct of one to four similar NLSs provides the delivery into the nuclei of no more than 25-30% of the internalized radioactivity [156, 161]. The attachment of a greater amount of NLS can increase the delivery into the nucleus up to 2/3 of the internalized radioactivity [161]. It is unclear what is responsible for the increase in accumulation in the nucleus: it seems that the joining of such amount of positively charged sequences begins to influence the penetration of the delivering construct across the endosomal membranes.
CHANGE IN PATHWAYS INTO OTHER INTRACELLULAR COMPARTMENTS
In addition to the nucleus, mitochondria represent a compartment available for the delivered drug only through the hyaloplasm. Numerous approaches have been proposed for delivery into mitochondria. Macromolecules are proposed to be delivered using mitochondrial targeting sequences, which are usually N-terminal fragments of proteins synthesized in the nucleus but functioning in mitochondria [163]. Lipophilic cationic peptides capable of transporting small negatively charged or zwitterionic molecules are promising for delivery into mitochondria [164]. Among other peptide sequences, the proapoptotic peptide D-(KLAKLAK)2 destroying the mitochondrial membrane and short synthetic Szeto-Schiller peptides (SS-peptides) are accumulated in mitochondria [165]. Nucleic acids can be delivered into mitochondria through a voltage-dependent anion channel (VDAC) as has been shown for mRNA [166], or in the case of tRNA through TIM/TOM transporters upon binding with the pre-mitochondrial lysyl-tRNA synthetase [167]. Moreover, delocalized lipophilic cations, sulfonyl urea derivatives, dicationic derivatives of quinoline, and liposomal MITO-porter able to fuse with the mitochondrial membrane are also considered as tools for delivery into mitochondria [47, 165, 168].
If lysosomes are the targeted compartment, delivery can be realized using different kinds of endocytosis [47, 168]. The mannose-6-phosphate pathway is specific for delivery from the cell surface into lysosomes [169]. The delivery into the endoplasmic reticulum from lysosomes is realized using transport vesicles. A number of peptide sequences are known that are signals for delivery into this compartment [170].
ACHIEVEMENTS, UNSOLVED PROBLEMS, AND FUTURE PROSPECTS
The analysis of available data shows that incorporation into the transporting construct of components with different functions, or in other words a modular principle of designing the system for delivery of a pharmaceutical agent into a target compartment using a set of modules providing successive use of different mechanisms of intracellular traffic to reach an intracellular target (e.g. the nucleus) seems to be promising for targeted drug delivery (figure).
A possible scheme of the structure and action of a modular construct for
delivery of therapeutic agents into target cell nuclei (after
Slastnikova et al. [29])
By now a great number of various systems for delivery of different drugs into a desired compartment of the target cell have been developed and tested under in vitro and in vivo (for some drugs) conditions. Thus, transfection efficiency in gene therapy can be significantly increased providing an effective (but desirably selective) accumulation in the target cells of the DNA complex with the transporting vector [171] and also active traffic of the complex to the nucleus [128]. Thus, the incorporation into polyplexes of a peptide responsible for specific binding with internalized melanocortin I type receptors, which are frequently hyperexpressed on melanoma cells, significantly increased the efficiency of suicidal therapy of the melanoma under the influence of polyplexes carrying the gene of thymidine kinase from the human Herpes simplex virus [38]. Using a modular protein consisting of the light chain of dynein Rp3, N-terminal DNA-binding domain, and C-terminal membrane-active TAT-peptide for delivery of DNA into the perinuclear space of the cell due to the active transport through the system of microtubules resulted in highly efficient transfection, only slightly weaker than Lipofectamine (Table 2), but with significantly lower toxicity of the new system of delivery [128]. The targeted delivery of doxorubicin, which is a widely used antitumor agent, into the nuclei of mammary gland cancer cells using NLS-modified nanoparticles made its efficiency an order of magnitude higher than that of free doxorubicin [172].
Table 2. Examples of targeted intracellular
delivery of therapeutic agents
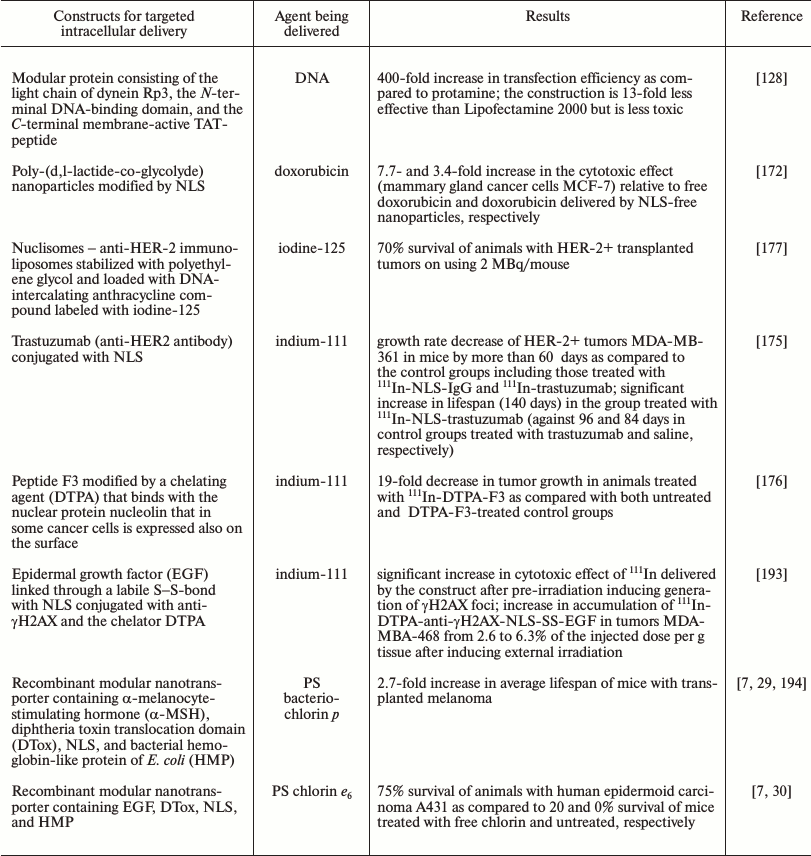
The efficiency significantly increases on targeted delivery of PSs into the nuclei of cancer cells, which are the most sensitive compartment to the action of ROS mediating effects of photodynamic therapy. Even a simple joining of a NLS to a PS [173, 174] increases its efficiency several-fold compared to the free PS. However, in the majority of cases [173, 174] the delivered PS can be detected in lysosomes and the endoplasmic reticulum but not in the nucleus, which most likely is associated with the inability of the systems under considration to effectively leave the endosomes and lysosomes for the cytoplasm and with insufficient sensitivity of the detection methods. In this connection it seems reasonable that the targeted delivery of PSs by modular nanotransporters containing in addition to NLS (and also the ligand module) an endosomolytic module significantly increased (hundreds- and thousands-fold) the cytotoxic effect of the delivered agent, and the trasporters were detected in the cancer cell nuclei in vitro and in vivo [13, 29, 31]. And the targeted delivery of PSs by modular nanotransporters into the nuclei of cancer cells caused a significant therapeutic effect in vivo (up to 75% survival by the end of the observation period) (Table 2) [29].
Targeted intracellular delivery of such promising agents for endoradiotherapy as emitters of Auger electrons, which as a rule are highly effective only within the cell nucleus because of an extremely short run of these particles, also seems promising.
In the overwhelming majority of cases the penetration into the cell alone is insufficient for effective work of such radionuclides; therefore, some groups of researchers have successfully used antibodies or peptides conjugated with NLS for targeted delivery of Auger electrons (111In, 99mTc) into the nuclei of cancer cells. Thus, the growth rate of HER-2+ tumors MDA-MB-361 in mice was slowed by more than 60 days compared to the control groups, and lifespan was significantly increased (Table 2) on the delivery of 111In by NLS-conjugated trastuzumab, an antibody to HER-2 frequently overexpressed in mammary gland carcinoma [175]. The tumor growth in the animals was 19-fold slower than in the control groups when 111In was delivered using the peptide F3 bound to nucleolin, the nuclear protein expressed on the surface of various cancer cells (Table 2) [176].
As differentiated from NLS-conjugated antibodies or peptides, MNT contain an additional endosomolytic module for release from the endosomes into the hyaloplasm, which includes a system mediating nuclear import and are also promising for targeted delivery of emitters of Auger electrons (67Ga and 125I). The cytotoxic effect of such emitters of Auger electrons as 67Ga and 125I on MNT-mediated delivery was increased two and three orders of magnitude, respectively [30, 160]. Such encouraging in vitro results allow us to hope that systems of delivery of Auger electrons have significant therapeutic potential [160].
Systems containing compounds able to directly bind with DNA can also be used for delivery of emitters of Auger electrons to the most sensitive intracellular target. Thus, using nuclisomes (anti-HER-2 immunoliposomes stabilized under the influence of polyethylene glycol and loaded with a DNA-intercalating anthracycline compound supplemented with 125I) resulted in 70% survival of animals with HER-2+ grafted tumors (Table 2) [177].
The encouraging results obtained in creating systems of targeted delivery of drugs into a desired compartment of a target cell (Table 2) allow us to think that such constructs are promising as potential tools in clinical practice. However, many problems preventing the effective development of such systems are still unsolved.
One of these problems is the scarcity of knowledge about numerous biological barriers arising on the pathway of a drug to be delivered [178, 179] from the place of introduction to the final target (tumor cells or a definite compartment in the target cell). Another important problem is the necessity to take into account all known factors influencing the delivery of a drug into the tumor, from the interactions with blood proteins and lipoproteins [180], resistance in biological fluids, biodegradability, features of intratumoral acidity [181, 182], blood and lymph supply [182], distribution of the interstitial pressure [183, 184], the ability to induce the immune response, to be phagocytized by macrophages and neutrophils – to heterogeneity of tumoral cells in the expression of target receptors, resistance to the delivered drugs, etc. Moreover, because in many cases a combination of several agents seems more promising [185-187], it is important to consider possible interactions of the target delivery system and the agent delivered with the drugs traditionally used for treatment of a particular cancer.
The more complete and versatile use of specific features (including those determining intracellular traffic) of a particular type of cells, mainly of cancer cells, seems hopeful for further increase in the efficiency of targeted delivery of anticancer drugs. This can be exemplified by the use of tumor-specific signals of nuclear localization [147], tumor-specific intracellular targets, the concurrent use of several different targets and/or intracellular delivery pathways in connection with high heterogeneity of cancer cells, and the combination of such systems with other approaches for treatment (e.g. immunotherapy).
Based on technologies under development and data on functioning of different types of cells of a given patient, it is possible to create approaches for correcting pathological processes in the desired organs and tissues of the given patient (personalized medicine).
This work was supported by the Russian Scientific Foundation (project No. 14-14-00874).
REFERENCES
1.D’Souza, G. G., and Weissig, V. (2009)
Subcellular targeting: a new frontier for drug-loaded pharmaceutical
nanocarriers and the concept of the magic bullet, Expert. Opin. Drug
Deliv., 6, 1135-1148.
2.Rajendran, L., Knolker, H. J., and Simons, K.
(2010) Subcellular targeting strategies for drug design and delivery,
Nat. Rev. Drug Discov., 9, 29-42.
3.Bareford, L. M., and Swaan, P. W. (2007) Endocytic
mechanisms for targeted drug delivery, Adv. Drug Deliv. Rev.,
59, 748-758.
4.Sobolev, A. S. (2009) Novel modular transporters
delivering anticancer drugs and foreign DNA to the nuclei of target
cancer cells, J. BUON, 14, Suppl. 1, S33-S42.
5.Chen, J., Sawyer, N., and Regan, L. (2013)
Protein–protein interactions: general trends in the relationship
between binding affinity and interfacial buried surface area,
Protein Sci., 22, 510-515.
6.Lo Conte, L., Chothia, C., and Janin, J. (1999) The
atomic structure of protein–protein recognition sites, J. Mol.
Biol., 285, 2177-2198.
7.Sobolev, A. S. (2013) Modular nanocarriers as a
multipurposed platform for delivery of anticancer drugs, Vestn. Ros.
Akad. Nauk, 83, 685-697.
8.Raper, S. E., Haskal, Z. J., Ye, X., Pugh, C.,
Furth, E. E., Gao, G. P., and Wilson, J. M. (1998) Selective gene
transfer into the liver of non-human primates with E1-deleted,
E2A-defective, or E1-E4 deleted recombinant adenoviruses, Hum. Gene
Ther., 9, 671-679.
9.Howe, S. J., Mansour, M. R., Schwarzwaelder, K.,
Bartholomae, C., Hubank, M., Kempski, H., Brugman, M. H., Pike-Overzet,
K., Chatters, S. J., de Ridder, D., Gilmour, K. C., Adams, S.,
Thornhill, S. I., Parsley, K. L., Staal, F. J., Gale, R. E., Linch, D.
C., Bayford, J., Brown, L., Quaye, M., Kinnon, C., Ancliff, P., Webb,
D. K., Schmidt, M., von Kalle, C., Gaspar, H. B., and Thrasher, A. J.
(2008) Insertional mutagenesis combined with acquired somatic mutations
causes leukemogenesis following gene therapy of SCID-X1 patients, J.
Clin. Invest., 118, 3143-3150.
10.Manno, C. S., Pierce, G. F., Arruda, V. R.,
Glader, B., Ragni, M., Rasko, J. J., Ozelo, M. C., Hoots, K., Blatt,
P., Konkle, B., Dake, M., Kaye, R., Razavi, M., Zajko, A., Zehnder, J.,
Rustagi, P. K., Nakai, H., Chew, A., Leonard, D., Wright, J. F.,
Lessard, R. R., Sommer, J. M., Tigges, M., Sabatino, D., Luk, A.,
Jiang, H., Mingozzi, F., Couto, L., Ertl, H. C., High, K. A., and Kay,
M. A. (2006) Successful transduction of liver in hemophilia by
AAV-Factor IX and limitations imposed by the host immune response,
Nat. Med., 12, 342-347.
11.Ogris, M. (2006) Nucleic acid based therapeutics
for tumor therapy, Anticancer Agents Med. Chem., 6,
563-570.
12.Sobolev, A. S., Rosenkranz, A. A., and Gilyazova,
D. G. (2004) Approaches for targeted intracellular delivery of
photosensitizers for increasing their efficiency and lending cell
specificity, Biofizika, 49, 351-379.
13.Gilyazova, D. G., Rosenkranz, A. A., Gulak, P.
V., Lunin, V. G., Sergienko, O. V., Khramtsov, Y. V., Timofeyev, K. N.,
Grin, M. A., Mironov, A. F., Rubin, A. B., Georgiev, G. P., and
Sobolev, A. S. (2006) Targeting cancer cells by novel engineered
modular transporters, Cancer Res., 66, 10534-10540.
14.Roessler, K., and Eich, G. (1989) Nuclear recoils
from 211-At decay, Radiochim. Acta, 47, 87-89.
15.Boswell, C. A., and Brechbiel, M. W. (2005) Auger
electrons: lethal, low energy, and coming soon to a tumor cell nucleus
near you, J. Nucl. Med., 46, 1946-1947.
16.Buchegger, F., Perillo-Adamer, F., Dupertuis, Y.
M., and Delaloye, A. B. (2006) Auger radiation targeted into DNA: a
therapy perspective, Eur. J. Nucl. Med. Mol. Imaging,
33, 1352-1363.
17.Hoyer, J., and Neundorf, I. (2012) Peptide
vectors for the nonviral delivery of nucleic acids, Acc. Chem.
Res., 45, 1048-1056.
18.Alber, F., Dokudovskaya, S., Veenhoff, L. M.,
Zhang, W., Kipper, J., Devos, D., Suprapto, A., Karni-Schmidt, O.,
Williams, R., Chait, B. T., Sali, A., and Rout, M. P. (2007) The
molecular architecture of the nuclear pore complex, Nature,
450, 695-701.
19.Becker, T., Bottinger, L., and Pfanner, N. (2012)
Mitochondrial protein import: from transport pathways to an integrated
network, Trends Biochem. Sci., 37, 85-91.
20.Allen, T. M., and Cullis, P. R. (2013) Liposomal
drug delivery systems: from concept to clinical applications, Adv.
Drug Deliv. Rev., 65, 36-48.
21.Byrne, J. D., Betancourt, T., and Brannon-Peppas,
L. (2008) Active targeting schemes for nanoparticle systems in cancer
therapeutics, Adv. Drug Deliv. Rev., 60, 1615-1626.
22.Muro, S. (2012) Challenges in design and
characterization of ligand-targeted drug delivery systems, J.
Control Release, 164, 125-137.
23.Tros de Ilarduya, C., and Duzgunes, N. (2013)
Delivery of therapeutic nucleic acids via transferrin and transferrin
receptors: lipoplexes and other carriers, Expert Opin. Drug
Deliv., 10, 1583-1591.
24.Golla, K., Bhaskar, C., Ahmed, F., and Kondapi,
A. K. (2013) A target-specific oral formulation of
doxorubicin–protein nanoparticles: efficacy and safety in
hepatocellular cancer, J. Cancer, 4, 644-652.
25.Hong, M., Zhu, S., Jiang, Y., Tang, G., Sun, C.,
Fang, C., Shi, B., and Pei, Y. (2010) Novel anti-tumor strategy:
PEG-hydroxycamptothecin conjugate loaded transferrin-PEG-nanoparticles,
J. Control Release, 141, 22-29.
26.Suzuki, R., Takizawa, T., Kuwata, Y., Mutoh, M.,
Ishiguro, N., Utoguchi, N., Shinohara, A., Eriguchi, M., Yanagie, H.,
and Maruyama, K. (2008) Effective anti-tumor activity of oxaliplatin
encapsulated in transferrin-PEG-liposome, Int. J. Pharm.,
346, 143-150.
27.Wang, Y., Zhou, J., Qiu, L., Wang, X., Chen, L.,
Liu, T., and Di, W. (2014) Cisplatin–alginate conjugate liposomes
for targeted delivery to EGFR-positive ovarian cancer cells,
Biomaterials, 35, 4297-4309.
28.Razumienko, E., Dryden, L., Scollard, D., and
Reilly, R. M. (2013) MicroSPECT/CT imaging of co-expressed HER2 and
EGFR on subcutaneous human tumor xenografts in athymic mice using
111In-labeled bispecific radioimmunoconjugates, Breast
Cancer Res. Treat., 138, 709-718.
29.Slastnikova, T. A., Rosenkranz, A. A., Gulak, P.
V., Schiffelers, R. M., Lupanova, T. N., Khramtsov, Y. V., Zalutsky, M.
R., and Sobolev, A. S. (2012) Modular nanotransporters: a multipurpose
in vivo working platform for targeted drug delivery, Int. J.
Nanomed., 7, 467-482.
30.Slastnikova, T. A., Koumarianou, E., Rosenkranz,
A. A., Vaidyanathan, G., Lupanova, T. N., Sobolev, A. S., and Zalutsky,
M. R. (2012) Modular nanotransporters: a versatile approach for
enhancing nuclear delivery and cytotoxicity of Auger electron-emitting
125I, EJNMMI Res., 2, 59.
31.Rosenkranz, A. A., Vaidyanathan, G., Pozzi, O.
R., Lunin, V. G., Zalutsky, M. R., and Sobolev, A. S. (2008) Engineered
modular recombinant transporters: application of new platform for
targeted radiotherapeutic agents to alpha-particle emitting
211At, Int. J. Radiat. Oncol. Biol. Phys., 72,
193-200.
32.Watanabe, K., Kaneko, M., and Maitani, Y. (2012)
Functional coating of liposomes using a folate-polymer conjugate to
target folate receptors, Int. J. Nanomed., 7,
3679-3688.
33.Stevens, P. J., Sekido, M., and Lee, R. J. (2004)
A folate receptor-targeted lipid nanoparticle formulation for a
lipophilic paclitaxel prodrug, Pharm. Res., 21,
2153-2157.
34.Naumann, R. W., Coleman, R. L., Burger, R. A.,
Sausville, E. A., Kutarska, E., Ghamande, S. A., Gabrail, N. Y.,
DePasquale, S. E., Nowara, E., and Gilbert, L. (2013) Precedent: a
randomized phase II trial comparing vintafolide (EC145) and pegylated
liposomal doxorubicin (PLD) in combination versus PLD alone in patients
with platinum-resistant ovarian cancer, J. Clin. Oncol.,
31, 4400-4406.
35.Dong, D. W., Xiang, B., Gao, W., Yang, Z. Z., Li,
J. Q., and Qi, X. R. (2013) pH-responsive complexes using
prefunctionalized polymers for synchronous delivery of doxorubicin and
siRNA to cancer cells, Biomaterials, 34, 4849-4859.
36.Liu, D., Liu, F., Liu, Z., Wang, L., and Zhang,
N. (2011) Tumor specific delivery and therapy by double-targeted
nanostructured lipid carriers with anti-VEGFR-2 antibody, Mol.
Pharmaceutics, 8, 2291-2301.
37.Rosenkranz, A. A., Lunin, V. G., Gulak, P. V.,
Sergienko, O. V., Shumiantseva, M. A., Voronina, O. L., Gilyazova, D.
G., John, A. P., Kofner, A. A., Mironov, A. F., Jans, D. A., and
Sobolev, A. S. (2003) Recombinant modular transporters for
cell-specific nuclear delivery of locally acting drugs enhance
photosensitizer activity, FASEB J., 17, 1121-1123.
38.Durymanov, M. O., Beletkaia, E. A., Ulasov, A.
V., Khramtsov, Y. V., Trusov, G. A., Rodichenko, N. S., Slastnikova, T.
A., Vinogradova, T. V., Uspenskaya, N. Y., Kopantsev, E. P.,
Rosenkranz, A. A., Sverdlov, E. D., and Sobolev, A. S. (2012)
Subcellular trafficking and transfection efficacy of
polyethylenimine–polyethylene glycol polyplex nanoparticles with
a ligand to melanocortin receptor-1, J. Control Release,
163, 211-219.
39.Nayak, T. K., Atcher, R. W., Prossnitz, E. R.,
and Norenberg, J. P. (2008) Somatostatin-receptor-targeted
alpha-emitting 213Bi is therapeutically more effective than
beta(-)-emitting 177Lu in human pancreatic adenocarcinoma
cells, Nuclear Med. Biol., 35, 673-678.
40.Shen, H., Hu, D., Du, J., Wang, X., Liu, Y.,
Wang, Y., Wei, J. M., Ma, D., Wang, P., and Li, L. (2008)
Paclitaxel–octreotide conjugates in tumor growth inhibition of
A549 human non-small cell lung cancer xenografted into nude mice,
Eur. J. Pharmacol., 601, 23-29.
41.Dai, W., Jin, W., Zhang, J., Wang, X., Wang, J.,
Zhang, X., Wan, Y., and Zhang, Q. (2012) Spatiotemporally controlled
co-delivery of anti-vasculature agent and cytotoxic drug by
octreotide-modified stealth liposomes, Pharmac. Res., 29,
2902-2911.
42.Su, Z., Shi, Y., Xiao, Y., Sun, M., Ping, Q.,
Zong, L., Li, S., Niu, J., Huang, A., and You, W. (2013) Effect of
octreotide surface density on receptor-mediated endocytosis in
vitro and anticancer efficacy of modified nanocarrier in
vivo after optimization, Int. J. Pharmaceutics, 447,
281-292.
43.Iwase, Y., and Maitani, Y. (2012) Dual functional
octreotide GAP modified liposomal irinotecan leads to high therapeutic
efficacy for medullary thyroid carcinoma xenografts, Cancer
Sci., 103, 310-316.
44.Amin, M., Badiee, A., and Jaafari, M. R. (2013)
Improvement of pharmacokinetic and antitumor activity of PEGylated
liposomal doxorubicin by targeting with N-methylated cyclic RGD peptide
in mice bearing C-26 colon carcinomas, Int. J. Pharmaceutics,
458, 324-333.
45.Schiffelers, R. M., Ansari, A., Xu, J., Zhou, Q.,
Tang, Q., Storm, G., Molema, G., Lu, P. Y., Scaria, P. V., and Woodle,
M. C. (2004) Cancer siRNA therapy by tumor selective delivery with
ligand-targeted sterically stabilized nanoparticle, Nucleic Acids
Res., 32, e149-e149.
46.Hemminki, A., Belousova, N., Zinn, K. R., Liu,
B., Wang, M., Chaudhuri, T. R., Rogers, B. E., Buchsbaum, D. J.,
Siegal, G. P., and Barnes, M. N. (2001) An adenovirus with enhanced
infectivity mediates molecular chemotherapy of ovarian cancer cells and
allows imaging of gene expression, Mol. Ther., 4,
223-231.
47.Biswas, S., and Torchilin, V. P. (2014)
Nanopreparations for organelle-specific delivery in cancer, Adv.
Drug Deliv. Rev., 66, 26-41.
48.Torchilin, V. P. (2006) Recent approaches to
intracellular delivery of drugs and DNA and organelle targeting,
Annu. Rev. Biomed. Eng., 8, 343-375.
49.Koshkaryev, A., Thekkedath, R., Pagano, C.,
Meerovich, I., and Torchilin, V. P. (2011) Targeting of lysosomes by
liposomes modified with octadecyl-rhodamine B, J. Drug Target,
19, 606-614.
50.Kurz, T., Terman, A., Gustafsson, B., and Brunk,
U. T. (2008) Lysosomes and oxidative stress in aging and apoptosis,
Biochim. Biophys. Acta, 1780, 1291-1303.
51.Koshkaryev, A., Piroyan, A., and Torchilin, V. P.
(2012) Increased apoptosis in cancer cells in vitro and in
vivo by ceramides in transferrin-modified liposomes, Cancer
Biol. Ther., 13, 50-60.
52.Vaidyanathan, G., Affleck, D. J., Li, J., Welsh,
P., and Zalutsky, M. R. (2001) A polar substituent-containing acylation
agent for the radioiodination of internalizing monoclonal antibodies:
N-succinimidyl 4-guanidinomethyl-3-[131I]iodobenzoate
([131I]SGMIB), Bioconj. Chem., 12,
428-438.
53.Olzmann, J. A., Kopito, R. R., and Christianson,
J. C. (2013) The mammalian endoplasmic reticulum-associated degradation
system, Cold Spring Harb. Perspect. Biol., 5,
a013185.
54.Mukhopadhyay, S., and Linstedt, A. D. (2013)
Retrograde trafficking of AB(5) toxins: mechanisms to therapeutics,
J. Mol. Med. (Berl.), 91, 1131-1141.
55.Wesche, J., Rapak, A., and Olsnes, S. (1999)
Dependence of ricin toxicity on translocation of the toxin A-chain from
the endoplasmic reticulum to the cytosol, J. Biol. Chem.,
274, 34443-34449.
56.Mukhopadhyay, S., and Linstedt, A. D. (2012)
Manganese blocks intracellular trafficking of Shiga toxin and protects
against Shiga toxicosis, Science, 335, 332-335.
57.Johannes, L., and Romer, W. (2010) Shiga toxins
— from cell biology to biomedical applications, Nat. Rev.
Microbiol., 8, 105-116.
58.El, A. A., Schmidt, F., Amessou, M., Sarr, M.,
Decaudin, D., Florent, J. C., and Johannes, L. (2007) Shiga
toxin-mediated retrograde delivery of a topoisomerase I inhibitor
prodrug, Angew. Chem. Int. Ed. Engl., 46,
6469-6472.
59.El, A. A., Schmidt, F., Sarr, M., Decaudin, D.,
Florent, J. C., and Johannes, L. (2008) Synthesis and properties of a
mitochondrial peripheral benzodiazepine receptor conjugate, Chem.
Med. Chem., 3, 1687-1695.
60.Amessou, M., Carrez, D., Patin, D., Sarr, M.,
Grierson, D. S., Croisy, A., Tedesco, A. C., Maillard, P., and
Johannes, L. (2008) Retrograde delivery of photosensitizer
(TPPp-O-beta-GluOH)3 selectively potentiates its
photodynamic activity, Bioconjug. Chem., 19,
532-538.
61.Tarrago-Trani, M. T., Jiang, S., Harich, K. C.,
and Storrie, B. (2006) Shiga-like toxin subunit B (SLTB)-enhanced
delivery of chlorin e6 (Ce6) improves cell killing,
Photochem. Photobiol., 82, 527-537.
62.Vingert, B., Adotevi, O., Patin, D., Jung, S.,
Shrikant, P., Freyburger, L., Eppolito, C., Sapoznikov, A., Amessou,
M., Quintin-Colonna, F., Fridman, W. H., Johannes, L., and Tartour, E.
(2006) The Shiga toxin B-subunit targets antigen in vivo to
dendritic cells and elicits anti-tumor immunity, Eur. J.
Immunol., 36, 1124-1135.
63.Adotevi, O., Vingert, B., Freyburger, L.,
Shrikant, P., Lone, Y. C., Quintin-Colonna, F., Haicheur, N., Amessou,
M., Herbelin, A., Langlade-Demoyen, P., Fridman, W. H., Lemonnier, F.,
Johannes, L., and Tartour, E. (2007) B subunit of Shiga toxin-based
vaccines synergize with alpha-galactosylceramide to break tolerance
against self antigen and elicit antiviral immunity, J. Immunol.,
179, 3371-3379.
64.Beatty, M. S., and Curiel, D. T. (2012) Chapter
two — adenovirus strategies for tissue-specific targeting,
Adv. Cancer Res., 115, 39-67.
65.Boisvert, M., and Tijssen, P. (2012) Endocytosis
of non-enveloped DNA viruses, in Molecular Regulation of
Endocytosis, Chap. 17 (Ceresa, B., ed.) InTech; http://dx.doi.org/10.5772/45821.
66.Meier, O., and Greber, U. F. (2004) Adenovirus
endocytosis, J. Gene Med., 6, Suppl. 1, S152-S163.
67.FitzGerald, D. J., Padmanabhan, R., Pastan, I.,
and Willingham, M. C. (1983) Adenovirus-induced release of epidermal
growth factor and pseudomonas toxin into the cytosol of KB cells during
receptor-mediated endocytosis, Cell, 32, 607-617.
68.Michael, S. I., and Curiel, D. T. (1994)
Strategies to achieve targeted gene delivery via the receptor-mediated
endocytosis pathway, Gene Ther., 1, 223-232.
69.Ladokhin, A. S. (2013) pH-Triggered
conformational switching along the membrane insertion pathway of the
diphtheria toxin T-domain, Toxins (Basel), 5,
1362-1380.
70.Kurnikov, I. V., Kyrychenko, A., Flores-Canales,
J. C., Rodnin, M. V., Simakov, N., Vargas-Uribe, M., Posokhov, Y. O.,
Kurnikova, M., and Ladokhin, A. S. (2013) pH-Triggered conformational
switching of the diphtheria toxin T-domain: the roles of N-terminal
histidines, J. Mol. Biol., 425, 2752-2764.
71.Senzel, L., Gordon, M., Blaustein, R. O., Oh, K.
J., Collier, R. J., and Finkelstein, A. (2000) Topography of diphtheria
toxin’s T domain in the open channel state, J. Gen.
Physiol., 115, 421-434.
72.Huynh, P. D., Cui, C., Zhan, H., Oh, K. J.,
Collier, R. J., and Finkelstein, A. (1997) Probing the structure of the
diphtheria toxin channel. Reactivity in planar lipid bilayer membranes
of cysteine-substituted mutant channels with methanethiosulfonate
derivatives, J. Gen. Physiol., 110, 229-242.
73.Vargas-Uribe, M., Rodnin, M. V., Kienker, P.,
Finkelstein, A., and Ladokhin, A. S. (2013) Crucial role of H322 in
folding of the diphtheria toxin T-domain into the open-channel state,
Biochemistry, 52, 3457-3463.
74.Murphy, J. R. (2011) Mechanism of diphtheria
toxin catalytic domain delivery to the eukaryotic cell cytosol and the
cellular factors that directly participate in the process, Toxins
(Basel), 3, 294-308.
75.Sharpe, J. C., and London, E. (1999) Diphtheria
toxin forms pores of different sizes depending on its concentration in
membranes: probable relationship to oligomerization, J. Membr.
Biol., 171, 209-221.
76.Kent, M. S., Yim, H., Murton, J. K., Satija, S.,
Majewski, J., and Kuzmenko, I. (2008) Oligomerization of membrane-bound
diphtheria toxin (CRM197) facilitates a transition to the open form and
deep insertion, Biophys. J., 94, 2115-2127.
77.Gilyazova, D. G., Rosenkranz, A. A., Gulak, P.
V., Lunin, V. G., Sergienko, O. V., Khramtsov, Y. V., Timofeyev, K. N.,
Grin, M. A., Mironov, A. F., Rubin, A. B., Georgiev, G. P., and
Sobolev, A. S. (2006) Targeting cancer cells by novel engineered
modular transporters, Cancer Res., 66, 10534-10540.
78.Khramtsov, Y. V., Rokitskaya, T. I., Rosenkranz,
A. A., Trusov, G. A., Gnuchev, N. V., Antonenko, Y. N., and Sobolev, A.
S. (2008) Modular drug transporters with diphtheria toxin translocation
domain form edged holes in lipid membranes, J. Control Release,
128, 241-247.
79.Rosenkranz, A. A., Khramtsov, Y. V., Trusov, G.
A., Gnuchev, N. V., and Sobolev, A. S. (2008) Studies on the pore
formation in lipid layers by modular transporters containing the
translocational domain of the diphtheria toxin, Dokl. Ros. Akad.
Nauk, 421, 385-387.
80.Sobolev, A. S. (2008) Modular transporters for
subcellular cell-specific targeting of anti-tumor drugs,
Bioessays, 30, 278-287.
81.Erazo-Oliveras, A., Muthukrishnan, N., Baker, R.,
Wang, T. Y., and Pellois, J. P. (2012) Improving the endosomal escape
of cell-penetrating peptides and their cargos: strategies and
challenges, Pharmaceuticals (Basel), 5, 1177-1209.
82.Green, M., and Loewenstein, P. M. (1988)
Autonomous functional domains of chemically synthesized human
immunodeficiency virus tat trans-activator protein, Cell,
55, 1179-1188.
83.Madani, F., Abdo, R., Lindberg, S., Hirose, H.,
Futaki, S., Langel, U., and Graslund, A. (2013) Modeling the endosomal
escape of cell-penetrating peptides using a transmembrane pH gradient,
Biochim. Biophys. Acta, 1828, 1198-1204.
84.Cahill, K. (2009) Molecular electroporation and
the transduction of oligoarginines, Phys. Biol., 7,
16001.
85.Angeles-Boza, A. M., Erazo-Oliveras, A., Lee, Y.
J., and Pellois, J. P. (2010) Generation of endosomolytic reagents by
branching of cell-penetrating peptides: tools for the delivery of
bioactive compounds to live cells in cis or trans, Bioconjug.
Chem., 21, 2164-2167.
86.Yessine, M. A., and Leroux, J. C. (2004)
Membrane-destabilizing polyanions: interaction with lipid bilayers and
endosomal escape of biomacromolecules, Adv. Drug Deliv. Rev.,
56, 999-1021.
87.Li, W., Nicol, F., and Szoka, F. C., Jr. (2004)
GALA: a designed synthetic pH-responsive amphipathic peptide with
applications in drug and gene delivery, Adv. Drug Deliv. Rev.,
56, 967-985.
88.Wharton, S. A., Martin, S. R., Ruigrok, R. W.,
Skehel, J. J., and Wiley, D. C. (1988) Membrane fusion by peptide
analogues of influenza virus haemagglutinin, J. Gen. Virol.,
69, 1847-1857.
89.Michiue, H., Tomizawa, K., Wei, F. Y.,
Matsushita, M., Lu, Y. F., Ichikawa, T., Tamiya, T., Date, I., and
Matsui, H. (2005) The NH2 terminus of influenza virus
hemagglutinin-2 subunit peptides enhances the antitumor potency of
polyarginine-mediated p53 protein transduction, J. Biol. Chem.,
280, 8285-8289.
90.Berg, K., Selbo, P. K., Prasmickaite, L., Tjelle,
T. E., Sandvig, K., Moan, J., Gaudernack, G., Fodstad, O., Kjolsrud,
S., Anholt, H., Rodal, G. H., Rodal, S. K., and Hogset, A. (1999)
Photochemical internalization: a novel technology for delivery of
macromolecules into cytosol, Cancer Res., 59,
1180-1183.
91.Berg, K., Weyergang, A., Prasmickaite, L.,
Bonsted, A., Hogset, A., Strand, M. T., Wagner, E., and Selbo, P. K.
(2010) Photochemical internalization (PCI): a technology for drug
delivery, Methods Mol. Biol., 635, 133-145.
92.Boussif, O., Lezoualch, F., Zanta, M. A., Mergny,
M. D., Scherman, D., Demeneix, B., and Behr, J. P. (1995) A versatile
vector for gene and oligonucleotide transfer into cells in culture and
in vivo: polyethylenimine, Proc. Natl. Acad. Sci. USA,
92, 7297-7301.
93.Sonawane, N. D., Szoka, F. C., Jr., and Verkman,
A. S. (2003) Chloride accumulation and swelling in endosomes enhances
DNA transfer by polyamine–DNA polyplexes, J. Biol. Chem.,
278, 44826-44831.
94.Benjaminsen, R. V., Mattebjerg, M. A., Henriksen,
J. R., Moghimi, S. M., and Andresen, T. L. (2013) The possible
“proton sponge” effect of polyethylenimine (PEI) does not
include change in lysosomal pH, Mol. Ther., 21,
149-157.
95.Forrest, M. L., Meister, G. E., Koerber, J. T.,
and Pack, D. W. (2004) Partial acetylation of polyethylenimine enhances
in vitro gene delivery, Pharm. Res., 21,
365-371.
96.Funhoff, A. M., van Nostrum, C. F., Koning, G.
A., Schuurmans-Nieuwenbroek, N. M., Crommelin, D. J., and Hennink, W.
E. (2004) Endosomal escape of polymeric gene delivery complexes is not
always enhanced by polymers buffering at low pH,
Biomacromolecules, 5, 32-39.
97.Gabrielson, N. P., and Pack, D. W. (2006)
Acetylation of polyethylenimine enhances gene delivery via weakened
polymer/DNA interactions, Biomacromolecules, 7,
2427-2435.
98.Richardson, S. C., Pattrick, N. G., Lavignac, N.,
Ferruti, P., and Duncan, R. (2010) Intracellular fate of bioresponsive
poly(amidoamine)s in vitro and in vivo, J. Control
Release, 142, 78-88.
99.Zhang, Z. Y., and Smith, B. D. (2000)
High-generation polycationic dendrimers are unusually effective at
disrupting anionic vesicles: membrane bending model, Bioconjug.
Chem., 11, 805-814.
100.Klemm, A. R., Young, D., and Lloyd, J. B.
(1998) Effects of polyethyleneimine on endocytosis and lysosome
stability, Biochem. Pharmacol., 56, 41-46.
101.Helmuth, J. A., Burckhardt, C. J., Greber, U.
F., and Sbalzarini, I. F. (2009) Shape reconstruction of subcellular
structures from live cell fluorescence microscopy images, J. Struct.
Biol., 167, 1-10.
102.Kakimoto, S., Hamada, T., Komatsu, Y., Takagi,
M., Tanabe, T., Azuma, H., Shinkai, S., and Nagasaki, T. (2009) The
conjugation of diphtheria toxin T domain to poly(ethylenimine) based
vectors for enhanced endosomal escape during gene transfection,
Biomaterials, 30, 402-408.
103.Kakimoto, S., Tanabe, T., Azuma, H., and
Nagasaki, T. (2010) Enhanced internalization and endosomal escape of
dual-functionalized poly(ethyleneimine)s polyplex with diphtheria toxin
T and R domains, Biomed. Pharmacother., 64, 296-301.
104.Xu, Y., and Szoka, F. C., Jr. (1996) Mechanism
of DNA release from cationic liposome/DNA complexes used in cell
transfection, Biochemistry, 35, 5616-5623.
105.Zuhorn, I. S., Bakowsky, U., Polushkin, E.,
Visser, W. H., Stuart, M. C., Engberts, J. B., and Hoekstra, D. (2005)
Nonbilayer phase of lipoplex-membrane mixture determines endosomal
escape of genetic cargo and transfection efficiency, Mol. Ther.,
11, 801-810.
106.Sobolev, A. S. (2009) Modular nanotransporters
of anticancer drugs attaching them cellular specificity and increased
efficiency, Usp. Biol. Khim., 49, 389-404.
107.Akhlynina, T. V., Jans, D. A., Rosenkranz, A.
A., Statsyuk, N. V., Balashova, I. Y., Toth, G., Pavo, I., Rubin, A.
B., and Sobolev, A. S. (1997) Nuclear targeting of chlorin e6 enhances
its photosensitizing activity, J. Biol. Chem., 272,
20328-20331.
108.Liang, H., Shin, D. S., Lee, Y. E., Nguyen, D.
C., Kasravi, S., Aurasteh, P., and Berns, M. W. (2000) Subcellular
phototoxicity of photofrin-II and lutetium texaphyrin in cells in
vitro, Lasers Med. Sci., 15, 109-122.
109.Liang, H., Do, T., Kasravi, S., Aurasteh, P.,
Nguyen, A., Huang, A., Wang, Z., and Berns, M. W. (2000) Chromosomes
are target sites for photodynamic therapy as demonstrated by
subcellular laser microirradiation, J. Photochem. Photobiol. B,
54, 175-184.
110.Ling, D., Bae, B. C., Park, W., and Na, K.
(2012) Photodynamic efficacy of photosensitizers under an attenuated
light dose via lipid nanocarrier-mediated nuclear targeting,
Biomaterials, 33, 5478-5486.
111.Vaidyanathan, G., and Zalutsky, M. R. (2011)
Applications of 211At and 223Ra in targeted
alpha-particle radiotherapy, Curr. Radiopharm., 4,
283-294.
112.Jackson, M. R., Falzone, N., and Vallis, K. A.
(2013) Advances in anticancer radiopharmaceuticals, Clin. Oncol. (R.
Coll. Radiol.), 25, 604-609.
113.Sui, M., Liu, W., and Shen, Y. (2011) Nuclear
drug delivery for cancer chemotherapy, J. Control Release,
155, 227-236.
114.Jang, H., Ryoo, S. R., Kostarelos, K., Han, S.
W., and Min, D. H. (2013) The effective nuclear delivery of doxorubicin
from dextran-coated gold nanoparticles larger than nuclear pores,
Biomaterials, 34, 3503-3510.
115.Luby-Phelps, K. (2000) Cytoarchitecture and
physical properties of cytoplasm: volume, viscosity, diffusion,
intracellular surface area, Int. Rev. Cytol., 192,
189-221.
116.Luby-Phelps, K. (2013) The physical chemistry
of cytoplasm and its influence on cell function: an update, Mol.
Biol. Cell, 24, 2593-2596.
117.Campbell, E. M., and Hope, T. J. (2003) Role of
the cytoskeleton in nuclear import, Adv. Drug Deliv. Rev.,
55, 761-771.
118.Glover, D. J. (2012) Artificial viruses:
exploiting viral trafficking for therapeutics, Infect. Disord. Drug
Targets, 12, 68-80.
119.Rogers, S. L., and Gelfand, V. I. (2000)
Membrane trafficking, organelle transport, and the cytoskeleton,
Curr. Opin. Cell Biol., 12, 57-62.
120.Lakadamyali, M. (2014) Navigating the cell: how
motors overcome roadblocks and traffic jams to efficiently transport
cargo, Phys. Chem. Chem. Phys., 16, 5907-5916.
121.Luscher, B., and Eisenman, R. N. (1992)
Mitosis-specific phosphorylation of the nuclear oncoproteins Myc and
Myb, J. Cell Biol., 118, 775-784.
122.Dong, C., Li, Z., Alvarez, R., Jr., Feng, X.
H., and Goldschmidt-Clermont, P. J. (2000) Microtubule binding to Smads
may regulate TGF beta activity, Mol. Cell, 5, 27-34.
123.Giannakakou, P., Sackett, D. L., Ward, Y.,
Webster, K. R., Blagosklonny, M. V., and Fojo, T. (2000) p53 is
associated with cellular microtubules and is transported to the nucleus
by dynein, Nat. Cell Biol., 2, 709-717.
124.Lam, M. H., Thomas, R. J., Loveland, K. L.,
Schilders, S., Gu, M., Martin, T. J., Gillespie, M. T., and Jans, D. A.
(2002) Nuclear transport of parathyroid hormone (PTH)-related protein
is dependent on microtubules, Mol. Endocrinol., 16,
390-401.
125.Lopez-Perez, M., and Salazar, E. P. (2006) A
role for the cytoskeleton in STAT5 activation in MCF7 human breast
cancer cells stimulated with EGF, Int. J. Biochem. Cell Biol.,
38, 1716-1728.
126.Roth, D. M., Moseley, G. W., Pouton, C. W., and
Jans, D. A. (2011) Mechanism of microtubule-facilitated “fast
track” nuclear import, J. Biol. Chem., 286,
14335-14351.
127.Roth, D. M., Moseley, G. W., Glover, D.,
Pouton, C. W., and Jans, D. A. (2007) A microtubule-facilitated nuclear
import pathway for cancer regulatory proteins, Traffic,
8, 673-686.
128.Favaro, M. T., de Toledo, M. A., Alves, R. F.,
Santos, C. A., Beloti, L. L., Janissen, R., de la Torre, L. G., Souza,
A. P., and Azzoni, A. R. (2014) Development of a non-viral gene
delivery vector based on the dynein light chain Rp3 and the TAT
peptide, J. Biotechnol., 173, 10-18.
129.Moseley, G. W., Leyton, D. L., Glover, D. J.,
Filmer, R. P., and Jans, D. A. (2010) Enhancement of protein
transduction-mediated nuclear delivery by interaction with
dynein/microtubules, J. Biotechnol., 145, 222-225.
130.Stewart, M. (2007) Molecular mechanism of the
nuclear protein import cycle, Nat. Rev. Mol. Cell Biol.,
8, 195-208.
131.Chook, Y. M., and Suel, K. E. (2011) Nuclear
import by karyopherin-betas: recognition and inhibition, Biochim.
Biophys. Acta, 1813, 1593-1606.
132.Xu, D., Farmer, A., and Chook, Y. M. (2010)
Recognition of nuclear targeting signals by karyopherin-beta proteins,
Curr. Opin. Struct. Biol., 20, 782-790.
133.Poon, I. K., Oro, C., Dias, M. M., Zhang, J.
P., and Jans, D. A. (2005) A tumor cell-specific nuclear targeting
signal within chicken anemia virus VP3/apoptin, J. Virol.,
79, 1339-1341.
134.Marfori, M., Mynott, A., Ellis, J. J., Mehdi,
A. M., Saunders, N. F., Curmi, P. M., Forwood, J. K., Boden, M., and
Kobe, B. (2011) Molecular basis for specificity of nuclear import and
prediction of nuclear localization, Biochim. Biophys. Acta,
1813, 1562-1577.
135.Lin, J. R., and Hu, J. (2013) SeqNLS: nuclear
localization signal prediction based on frequent pattern mining and
linear motif scoring, PLoS One, 8, e76864.
136.Lott, K., and Cingolani, G. (2011) The importin
beta binding domain as a master regulator of nucleocytoplasmic
transport, Biochim. Biophys. Acta, 1813, 1578-1592.
137.Kosugi, S., Hasebe, M., Matsumura, N.,
Takashima, H., Miyamoto-Sato, E., Tomita, M., and Yanagawa, H. (2009)
Six classes of nuclear localization signals specific to different
binding grooves of importin alpha, J. Biol. Chem., 284,
478-485.
138.Flores, K., and Seger, R. (2013) Stimulated
nuclear import by beta-like importins, F1000Prime. Rep.,
5, 41.
139.Kosugi, S., Hasebe, M., Tomita, M., and
Yanagawa, H. (2009) Systematic identification of cell cycle-dependent
yeast nucleocytoplasmic shuttling proteins by prediction of composite
motifs, Proc. Natl. Acad. Sci. USA, 106, 10171-10176.
140.Nguyen Ba, A. N., Pogoutse, A., Provart, N.,
and Moses, A. M. (2009) NLStradamus: a simple hidden Markov model for
nuclear localization signal prediction, BMC Bioinformatics,
10, 202.
141.Kuusisto, H. V., Wagstaff, K. M., Alvisi, G.,
Roth, D. M., and Jans, D. A. (2012) Global enhancement of nuclear
localization-dependent nuclear transport in transformed cells, FASEB
J., 26, 1181-1193.
142.Turner, J. G., Dawson, J., and Sullivan, D. M.
(2012) Nuclear export of proteins and drug resistance in cancer,
Biochem. Pharmacol., 83, 1021-1032.
143.Fast, J., Mossberg, A. K., Nilsson, H.,
Svanborg, C., Akke, M., and Linse, S. (2005) Compact oleic acid in
HAMLET, FEBS Lett., 579, 6095-6100.
144.Gustafsson, L., Hallgren, O., Mossberg, A. K.,
Pettersson, J., Fischer, W., Aronsson, A., and Svanborg, C. (2005)
HAMLET kills tumor cells by apoptosis: structure, cellular mechanisms,
and therapy, J. Nutr., 135, 1299-1303.
145.Hallgren, O., Aits, S., Brest, P., Gustafsson,
L., Mossberg, A. K., Wullt, B., and Svanborg, C. (2008) Apoptosis and
tumor cell death in response to HAMLET (human alpha-lactalbumin made
lethal to tumor cells), Adv. Exp. Med. Biol., 606,
217-240.
146.Maddika, S., Mendoza, F. J., Hauff, K., Zamzow,
C. R., Paranjothy, T., and Los, M. (2006) Cancer-selective therapy of
the future: apoptin and its mechanism of action, Cancer Biol.
Ther., 5, 10-19.
147.Kuusisto, H. V., Wagstaff, K. M., Alvisi, G.,
and Jans, D. A. (2008) The C-terminus of apoptin represents a unique
tumor cell-enhanced nuclear targeting module, Int. J. Cancer,
123, 2965-2969.
148.Los, M., Panigrahi, S., Rashedi, I., Mandal,
S., Stetefeld, J., Essmann, F., and Schulze-Osthoff, K. (2009) Apoptin,
a tumor-selective killer, Biochim. Biophys. Acta, 1793,
1335-1342.
149.Ho, C. S. J., Rydstrom, A., Trulsson, M.,
Balfors, J., Storm, P., Puthia, M., Nadeem, A., and Svanborg, C. (2012)
HAMLET: functional properties and therapeutic potential, Future
Oncol., 8, 1301-1313.
150.Backendorf, C., Visser, A. E., de Boer, A. G.,
Zimmerman, R., Visser, M., Voskamp, P., Zhang, Y. H., and Noteborn, M.
(2008) Apoptin: therapeutic potential of an early sensor of
carcinogenic transformation, Annu. Rev. Pharmacol. Toxicol.,
48, 143-169.
151.Heilman, D. W., Teodoro, J. G., and Green, M.
R. (2006) Apoptin nucleocytoplasmic shuttling is required for cell
type-specific localization, apoptosis, and recruitment of the
anaphase-promoting complex/cyclosome to PML bodies, J. Virol.,
80, 7535-7545.
152.Kucharski, T. J., Gamache, I., Gjoerup, O., and
Teodoro, J. G. (2011) DNA damage response signaling triggers nuclear
localization of the chicken anemia virus protein Apoptin, J.
Virol., 85, 12638-12649.
153.Lee, Y. H., Cheng, C. M., Chang, Y. F., Wang,
T. Y., and Yuo, C. Y. (2007) Apoptin T108 phosphorylation is not
required for its tumor-specific nuclear localization but partially
affects its apoptotic activity, Biochem. Biophys. Res. Commun.,
354, 391-395.
154.Yu, J., Xie, X., Zheng, M., Yu, L., Zhang, L.,
Zhao, J., Jiang, D., and Che, X. (2012) Fabrication and
characterization of nuclear localization signal-conjugated glycol
chitosan micelles for improving the nuclear delivery of doxorubicin,
Int. J. Nanomed., 7, 5079-5090.
155.Subramanian, A., Ranganathan, P., and Diamond,
S. L. (1999) Nuclear targeting peptide scaffolds for lipofection of
nondividing mammalian cells, Nat. Biotechnol., 17,
873-877.
156.Chan, C., Cai, Z., Su, R., and Reilly, R. M.
(2010) 111In- or 99mTc-labeled recombinant VEGF
bioconjugates: in vitro evaluation of their cytotoxicity on
porcine aortic endothelial cells overexpressing Flt-1 receptors,
Nucl. Med. Biol., 37, 105-115.
157.Bisland, S. K., Singh, D., and Gariepy, J.
(1999) Potentiation of chlorin e6 photodynamic activity in vitro
with peptide-based intracellular vehicles, Bioconjug. Chem.,
10, 982-992.
158.Chan, C. K., Hubner, S., Hu, W., and Jans, D.
A. (1998) Mutual exclusivity of DNA binding and nuclear localization
signal recognition by the yeast transcription factor GAL4: implications
for nonviral DNA delivery, Gene Ther., 5, 1204-1212.
159.Kim, B. K., Kang, H., Doh, K. O., Lee, S. H.,
Park, J. W., Lee, S. J., and Lee, T. J. (2012) Homodimeric SV40 NLS
peptide formed by disulfide bond as enhancer for gene delivery,
Bioorg. Med. Chem. Lett., 22, 5415-5418.
160.Koumarianou, E., Slastnikova, T. A.,
Pruszynski, M., Rosenkranz, A. A., Vaidyanathan, G., Sobolev, A. S.,
and Zalutsky, M. R. (2014) Radiolabeling and in vitro evaluation
of Ga-NOTA-modular nanotransporter – a potential Auger electron
emitting EGFR-targeted radiotherapeutic, Nucl. Med. Biol.,
41, 441-449.
161.Chen, P., Wang, J., Hope, K., Jin, L., Dick,
J., Cameron, R., Brandwein, J., Minden, M., and Reilly, R. M. (2006)
Nuclear localizing sequences promote nuclear translocation and enhance
the radiotoxicity of the anti-CD33 monoclonal antibody HuM195 labeled
with 111In in human myeloid leukemia cells, J. Nucl.
Med., 47, 827-836.
162.Cornelissen, B., and Vallis, K. A. (2010)
Targeting the nucleus: an overview of Auger-electron radionuclide
therapy, Curr. Drug Discov. Technol., 7, 263-279.
163.Yousif, L. F., Stewart, K. M., and Kelley, S.
O. (2009) Targeting mitochondria with organelle-specific compounds:
strategies and applications, Chembiochem., 10,
1939-1950.
164.Yousif, L. F., Stewart, K. M., Horton, K. L.,
and Kelley, S. O. (2009) Mitochondria-penetrating peptides: sequence
effects and model cargo transport, Chembiochem., 10,
2081-2088.
165.Frantz, M. C., and Wipf, P. (2010) Mitochondria
as a target in treatment, Environ. Mol. Mutagen., 51,
462-475.
166.Michaud, M., Ubrig, E., Filleur, S., Erhardt,
M., Ephritikhine, G., Marechal-Drouard, L., and Duchene, A. M. (2014)
Differential targeting of VDAC3 mRNA isoforms influences mitochondria
morphology, Proc. Natl. Acad. Sci. USA, 111,
8991-8996.
167.Wang, G., Shimada, E., Koehler, C. M., and
Teitell, M. A. (2012) PNPase and RNA trafficking into mitochondria,
Biochim. Biophys. Acta, 1819, 998-1007.
168.Sakhrani, N. M., and Padh, H. (2013) Organelle
targeting: third level of drug targeting, Drug Design Devel.
Therap., 7, 585-599.
169.Gary-Bobo, M., Nirde, P., Jeanjean, A., Morere,
A., and Garcia, M. (2007) Mannose 6-phosphate receptor targeting and
its applications in human diseases, Curr. Med. Chem., 14,
2945-2953.
170.Mossalam, M., Dixon, A. S., and Lim, C. S.
(2010) Controlling subcellular delivery to optimize therapeutic effect,
Ther. Deliv., 1, 169-193.
171.Durymanov, M. O., Slastnikova, T. A., Kuzmich,
A. I., Khramtsov, Y. V., Ulasov, A. V., Rosenkranz, A. A., Egorov, S.
Y., Sverdlov, E. D., and Sobolev, A. S. (2013) Microdistribution of
MC1R-targeted polyplexes in murine melanoma tumor tissue,
Biomaterials, 34, 10209-10216.
172.Misra, R., and Sahoo, S. K. (2010)
Intracellular trafficking of nuclear localization signal conjugated
nanoparticles for cancer therapy, Eur. J. Pharm. Sci.,
39, 152-163.
173.Ke, M. R., Yeung, S. L., Fong, W. P., Ng, D.
K., and Lo, P. C. (2012) A phthalocyanine–peptide conjugate with
high in vitro photodynamic activity and enhanced in vivo
tumor-retention property, Chemistry, 18, 4225-4233.
174.Verwilst, P., David, C. C., Leen, V., Hofkens,
J., de Witte, P. A., and de Borggraeve, W. M. (2013) Synthesis and
in vitro evaluation of a PDT active BODIPY—NLS conjugate,
Bioorg. Med. Chem. Lett., 23, 3204-3207.
175.Costantini, D. L., McLarty, K., Lee, H., Done,
S. J., Vallis, K. A., and Reilly, R. M. (2010) Antitumor effects and
normal-tissue toxicity of 111In-nuclear localization
sequence-trastuzumab in athymic mice bearing HER-positive human breast
cancer xenografts, J. Nucl. Med., 51, 1084-1091.
176.Cornelissen, B., Waller, A., Target, C.,
Kersemans, V., Smart, S., and Vallis, K. A. (2012)
111In-BnDTPA-F3: an Auger electron-emitting radiotherapeutic
agent that targets nucleolin, EJNMMI Res., 2, 9.
177.Gedda, L., Fondell, A., Lundqvist, H., Park, J.
W., and Edwards, K. (2012) Experimental radionuclide therapy of
HER2-expressing xenografts using two-step targeting nuclisome
particles, J. Nucl. Med., 53, 480-487.
178.Labhasetwar, V. (2005) Nanotechnology for drug
and gene therapy: the importance of understanding molecular mechanisms
of delivery, Curr. Opin. Biotechnol., 16, 674-680.
179.Sui, M., Liu, W., and Shen, Y. (2011) Nuclear
drug delivery for cancer chemotherapy, J. Control Release,
155, 227-236.
180.Opanasopit, P., Nishikawa, M., and Hashida, M.
(2002) Factors affecting drug and gene delivery: effects of interaction
with blood components, Crit. Rev. Ther. Drug Carrier Syst.,
19, 191-233.
181.Jain, R. K. (1994) Barriers to drug delivery in
solid tumors, Sci. Am., 271, 58-65.
182.Jain, R. K. (1999) Understanding barriers to
drug delivery: high resolution in vivo imaging is key, Clin.
Cancer Res., 5, 1605-1606.
183.Li, Y., Wang, J., Zhu, X., Feng, Q., Li, X.,
and Feng, X. (2012) Urinary protein markers predict the severity of
renal histological lesions in children with mesangial proliferative
glomerulonephritis, BMC Nephrol., 13, 29.
184.Minchinton, A. I., and Tannock, I. F. (2006)
Drug penetration in solid tumours, Nat. Rev. Cancer, 6,
583-592.
185.Blackwell, K. L., Burstein, H. J., Storniolo,
A. M., Rugo, H. S., Sledge, G., Aktan, G., Ellis, C., Florance, A.,
Vukelja, S., Bischoff, J., Baselga, J., and O’Shaughnessy, J.
(2012) Overall survival benefit with lapatinib in combination with
trastuzumab for patients with human epidermal growth factor receptor
2-positive metastatic breast cancer: final results from the EGF104900
study, J. Clin. Oncol., 30, 2585-2592.
186.LoRusso, P. M., Canetta, R., Wagner, J. A.,
Balogh, E. P., Nass, S. J., Boerner, S. A., and Hohneker, J. (2012)
Accelerating cancer therapy development: the importance of combination
strategies and collaboration. Summary of an Institute of Medicine
workshop, Clin. Cancer Res., 18, 6101-6109.
187.Bozic, I., Reiter, J. G., Allen, B., Antal, T.,
Chatterjee, K., Shah, P., Moon, Y. S., Yaqubie, A., Kelly, N., Le, D.
T., Lipson, E. J., Chapman, P. B., Diaz, L. A., Jr., Vogelstein, B.,
and Nowak, M. A. (2013) Evolutionary dynamics of cancer in response to
targeted combination therapy, Elife, 2, e00747.
188.Ziemienowicz, A., Gorlich, D., Lanka, E., Hohn,
B., and Rossi, L. (1999) Import of DNA into mammalian nuclei by
proteins originating from a plant pathogenic bacterium, Proc. Natl.
Acad. Sci. USA, 96, 3729-3733.
189.Rudolph, C., Plank, C., Lausier, J.,
Schillinger, U., Muller, R. H., and Rosenecker, J. (2003) Oligomers of
the arginine-rich motif of the HIV-1 TAT protein are capable of
transferring plasmid DNA into cells, J. Biol. Chem., 278,
11411-11418.
190.Cornelissen, B., Hu, M., McLarty, K.,
Costantini, D., and Reilly, R. M. (2007) Cellular penetration and
nuclear importation properties of 111In-labeled and
123I-labeled HIV-1 TAT-peptide immunoconjugates in BT-474
human breast cancer cells, Nucl. Med. Biol., 34,
37-46.
191.Cornelissen, B., Darbar, S., Kersemans, V.,
Allen, D., Falzone, N., Barbeau, J., Smart, S., and Vallis, K. A.
(2012) Amplification of DNA damage by a gammaH2AX-targeted
radiopharmaceutical, Nucl. Med. Biol., 39, 1142-1151.
192.Masuda, T., Akita, H., and Harashima, H. (2005)
Evaluation of nuclear transfer and transcription of plasmid DNA
condensed with protamine by microinjection: the use of a nuclear
transfer score, FEBS Lett., 579, 2143-2148.
193.Cornelissen, B., Waller, A., Able, S., and
Vallis, K. A. (2013) Molecular radiotherapy using cleavable
radioimmunoconjugates that target EGFR and gammaH2AX, Mol. Cancer
Ther., 12, 2472-2482.
194.Slastnikova, T. A., Rosenkranz, A. A.,
Lupanova, T. N., Gulak, P. V., Gnuchev, N. V., and Sobolev, A. S.
(2012) Study of efficiency of the modular nanotransporter for targeted
delivery of photosensitizers to melanoma cell nuclei in vivo,
Dokl. Biochem. Biophys., 446, 235-237.