REVIEW: Specific Organization of Golgi Apparatus in Plant Cells
M. S. Vildanova1, W. Wang2, and E. A. Smirnova1*
1Biological Faculty, Lomonosov Moscow State University, 119234 Moscow, Russia; fax: +7 (495) 939-4309; E-mail: vch41048@mail.ru; kinggobi@yandex.ru2China Rehabilitation Research Center, No. 10, Jiaomenbeilu, Fengtaiqu, Beijing 100068, China; E-mail: wzhwang@163.com
* To whom correspondence should be addressed.
Received May 13, 2014; Revision received June 2, 2014
Microtubules, actin filaments, and Golgi apparatus are connected both directly and indirectly, but it is manifested differently depending on the cell organization and specialization, and these connections are considered in many original studies and reviews. In this review we would like to discuss what underlies differences in the structural organization of the Golgi apparatus in animal and plant cells: specific features of the microtubule cytoskeleton organization, the use of different cytoskeleton components for Golgi apparatus movement and maintenance of its integrity, or specific features of synthetic and secretory processes. We suppose that a dispersed state of the Golgi apparatus in higher plant cells cannot be explained only by specific features of the microtubule system organization and by the absence of centrosome as an active center of their organization because the Golgi apparatus is organized similarly in the cells of other organisms that possess the centrosome and centrosomal microtubules. One of the key factors determining the Golgi apparatus state in plant cells is the functional uniformity or functional specialization of stacks. The functional specialization does not suggest the joining of the stacks to form a ribbon; therefore, the disperse state of the Golgi apparatus needs to be supported, but it also can exist “by default”. We believe that the dispersed state of the Golgi apparatus in plants is supported, on one hand, by dynamic connections of the Golgi apparatus stacks with the actin filament system and, on the other hand, with the endoplasmic reticulum exit sites distributed throughout the endoplasmic reticulum.
KEY WORDS: cytoskeleton, microtubules, actin filaments, Golgi apparatus, plantsDOI: 10.1134/S0006297914090065
Abbreviations: CESA, cellulose synthase complex; COP, coat protein complex; ER, endoplasmic reticulum; ERES, endoplasmic reticulum exit sites; ERGIC, endoplasmic reticulum–Golgi intermediate compartment; GM130, Golgi matrix protein 130; GRASP, Golgi reassembly and stacking protein; MTOC, microtubule organizing center; tER, transitional endoplasmic reticulum; TGN, trans-Golgi network; γ-TURC, γ-tubulin ring complex.
The localization of organelles in specialized structurally-functional
domains/compartments in the eukaryotic cell cytoplasm depends on
functional loading, growth type, differentiation stages, and on signals
entering the cells [1]. Changes in these parameters
result in translocation of the organelles, reorganization of the
cytoplasm, and formation of new structurally-functional domains. The
organelles are transported intracellularly along cytoskeleton fibrils
represented by microtubules and actin filaments with involvement of
motor proteins linked to them.
In the cells of vertebrates, organelles are transported over long distances due to microtubules and their motor proteins – kinesins and a cytoplasmic dynein – whereas actin filaments are responsible for small local translocations [2, 3]. The actin-mediated trafficking of membrane organelles and particles occurs under the influence of two independent mechanisms: movement along actin filaments with involvement of myosin or pushing away by actin filaments that are gathered, in particular, from the cell surface [4]. Mitochondria, synaptic vesicles, and pigment granules can use for the traffic both microtubules and actin filaments [5].
In the cells of higher plants the trafficking also occurs along cytoskeleton fibrils; however, over long distances organelles are moved along actin filaments with involvement of “rapid” myosins, whereas the microtubules are responsible for “anchoring” or deceleration of the movement of organelles and, possibly, for their transfer over small distances [6]. The cytoskeleton of higher plants is characterized also by the absence of intermediate filaments, which is traditionally thought to be associated with the presence of a cellulose cell wall. Moreover, the microtubules form several systems that successively replace one another during the cell cycle: cortical bundles and/or radial system of microtubules in interphase, the preprophasic ring in the G2 phase, the division spindle during mitosis, and phragmoplast by the end of mitosis and during cytokinesis. The preprophasic ring of microtubules and the phragmoplast are structures typical only for plant cells. The preprophasic ring is involved in determination of the future plate of the cell division and the phragmoplast participates in the targeted delivery of vesicles into the zone of formation of the cell plate between the daughter cells [7]. The cells of higher plants are characterized by the absence of centrioles and basal bodies during all stages of the life cycle and by absence of centrosome and other discrete microtubule organizing centers (MTOC) [8]. The microtubules are assembled with involvement of γ-tubulin ring complexes (γ-TURC), which are fastened to the lateral surface of preexistent microtubules [9-11]. As in many other organisms, the system of actin filaments in plants consists of actin bundles and networks. Interphase cells usually contain networks consisting of short interlaced filaments and cortical or cytoplasmic bundles, whereas mitotic cells include accumulations of filaments around the mitotic spindle and in the phragmoplast zone [12]. In higher plant cells very different in the structure, origin, and functions, the system of actin filaments is directly responsible for movement in the cytoplasm of the endoplasmic reticulum (ER) cisterns, mitochondria, Golgi apparatus, various vacuoles and vesicles, plastids, and chloroplasts [13-20].
Thus, in the cells of vertebrates and higher animals the microtubules and actin filaments have functionally changed their roles as tracks for intracellular transport. This might be associated with the absence in the plant cells of certain families of kinesins, which are involved in the long distance trafficking, but there are “rapid” myosins responsible for effective traffic of endomembranes [6]. Note that differences in the cytoskeleton organization and the transport character have little or no influence on the structural organization of endomembranes except for the Golgi apparatus.
Therefore, in our review we will discuss what underlies the differences in the structural organization of the Golgi apparatus in animals and plants: specific features of the microtubule cytoskeleton organization, the use of different cytoskeleton components for the Golgi apparatus movement and maintenance of its integrity, or specific features of synthetic and secretory processes in plant cells.
THE STACK/DICTYOSOME IS AN ELEMENTARY UNIT OF THE GOLGI
APPARATUS
A stack/dictyosome, which is an accumulation of closed membrane cisterns flattened and piled one above the other and encircled by tubules and vesicles, serves as a structural–functional unit of the Golgi apparatus [21, 22]. Proteins of the Golgi apparatus matrix are responsible for the structural integrity of dictyosomes and their organizing as more complicated aggregations [23]. Within the same stack the Golgi apparatus cisterns are different in morphology, molecular compositions, and functions, and therefore they are subdivided onto: (1) endoplasmic reticulum–Golgi intermediate compartment (ERGIC) or cis-Golgi network; (2) cis-cisterns; (3) medial cisterns; (4) trans-cisterns; and (5) trans-Golgi network (TGN) [22, 24]. Thus, the stacks have a pronounced cis- and trans-polarity that reflects the direction of the secreted product passage across this organelle.
The Golgi apparatus stacks are fully autonomous functionally, and although even one cistern is sufficient for glycosylating and sorting proteins [25, 26], just a stack but not a cistern is considered to be a full-value secretory unit of the Golgi apparatus [22]. Notwithstanding the functional full-value competence of each stack, they often form accumulations, join to one another, and produce large aggregations, as in particular occurs in many cells of vertebrates, especially of mammals.
UNITING OF STACKS AND FORMATION OF A RIBBON-SHAPED GOLGI
APPARATUS IS SPECIFIC FOR MAMMALIAN CELLS
In mammalian cells the Golgi apparatus is an accumulation of stacks/dictyosomes associated into a united system, the so-called Golgi ribbon (Fig. 1, a and c). The ribbon-shaped Golgi apparatus is placed immediately near the centrosome/MTOC localized in the perinuclear region of the cytoplasm [27]. Early studies on mechanisms responsible for the compactness and polar positioning of the Golgi apparatus have shown that such structure and localization of the Golgi apparatus depend on the integrity of centrosomal microtubules [28, 29]. Later it becomes clear that: (1) in addition to the centrosome, the Golgi apparatus itself performs the function of the MTOC; (2) the MTOC-activity of the Golgi apparatus is maintained independently of the centrosome [30-32]; (3) the ribbon-shaped construction of the Golgi apparatus is supported by microtubules that grow from it [33].
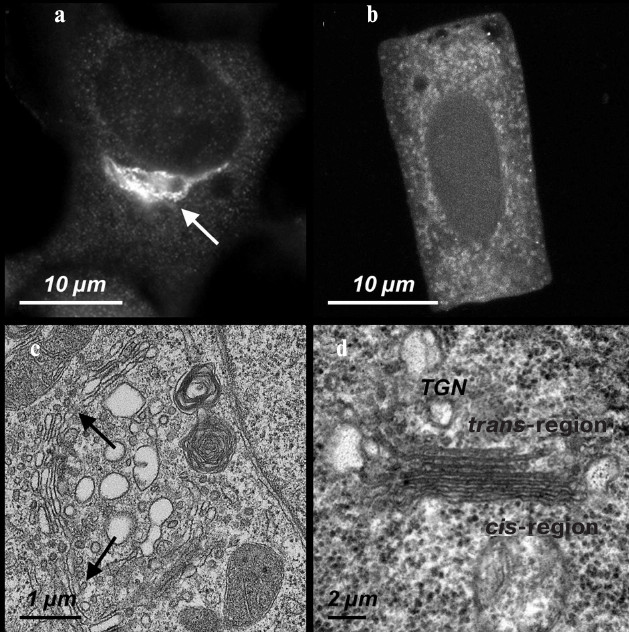
Fig. 1. The Golgi apparatus in mammalian and higher plant cells. a) A ribbon-shaped Golgi apparatus (indicated by the arrow) detected with antibodies against the protein p58K in cultured cells of the human epidermoid carcinoma A431. b) The Golgi apparatus stacks detected with antibodies against the protein p58K in the cells isolated from the wheat T. aestivum seedling root. c) An ultrathin section of a region of the Golgi apparatus ribbon (indicated by two arrow) in the A431 cells. d) The ultrathin section of a separate stack/dictyosome in the meristem cells of the wheat T. aestivum seedling root.
Such a ribbon-shaped configuration is maintained during the functional activity of the Golgi apparatus in interphase, but on entering mitosis the ribbon disintegrates and its functional activity stops [34, 35]. The disintegration during the G2/M begins by disjoining the ribbon into separate stacks, then the stacks are disassembled into separate cisterns, and then the cisterns are disassembled into vesicles. The postmitotic assembly includes the fusion of membrane vesicles and recovery of cisterns, packing the cisterns into stacks, and binding the stacks into a ribbon [36]. These processes occur with the involvement of the Golgi apparatus matrix proteins, various kinases and phosphatases, ubiquitin ligases, and deubiquitinylating enzymes, complexes responsible for outbudding and fusion of vesicles, and, certainly, of cytoskeletal elements [23]. The ribbon-shaped Golgi apparatus is assembled under the influence of both centrosomal microtubules and those derived from the Golgi apparatus [37]. At first, the assembly of microtubules is initiated by recruiting γ-TURC onto the Golgi apparatus stacks. Two cis-golgins, the Golgi matrix protein 130 (GM130) and the Golgi-microtubule associated protein 210 (GMAP210), anchor γ-TURC on the cis-region of separately positioned stacks of the Golgi apparatus [32]. Microtubules derived from the stacks are stabilized under the influence of cytoplasmic linker associated proteins 1/2 (CLASP1/2) [33], which in turn are fastened to membranes of the trans-region of the stacks with the involvement of the trans-golgin GCC185 (GRIP and coiled-coil domain-containing protein 185) [31]. It is supposed that initially mini-stacks of the Golgi apparatus result due to simple fusion of the vesicles and piling the cisterns form clusters on the cell periphery. The clusters are bound to each other through microtubules derived from the stacks. Then the microtubules derived from the clusters interact with centrosomal microtubules, and the clusters are moved along the centrosomal microtubules to the cellular center where they unite into a ribbon. All stages of the Golgi apparatus assembly require involvement of cytoplasmic dynein [38], which is recruited onto the Golgi apparatus membrane under the influence of golgin 160 [39]. Immediately upon the Golgi apparatus localization in the cellular center zone, the microtubules derived from the Golgi apparatus become mainly responsible for its structural integrity. Thus, for assembly of the ribbon-shaped Golgi apparatus a coordinated interaction is needed of the centrosomal and Golgi apparatus microtubules, whereas for maintenance of the produced ribbon and functional activity of the Golgi apparatus the centrosomal microtubules are already not necessary [40].
Thus, the microtubules derived from the Golgi apparatus maintain its ribbon-shaped organization [33] and, as differentiated from the radial centrosomal microtubules, are preferentially targeted to the leading edge of the mobile cells [31]. These microtubules are necessary for polarization and targeted movement of the cells because vesicles are transported to the leading edge of the cell plasma membrane along these microtubules [33]. However, the centrosomal microtubules play an important role in the vesicle traffic from the ER into ERGIC [41] and in the vesicle traffic between the TGN and the endosomal compartment [42].
Microtubules are mainly responsible for maintaining the polarized disposition and integrity of the Golgi apparatus ribbon, whereas actin filaments are mainly involved in the creation of the “architecture” of the Golgi apparatus, formation of its tubulo-vesicular components, and remodeling the membranes during the transfer of vesicles between the ER and Golgi apparatus and the post-Golgi trafficking [43]. Actin filaments are responsible for the specific flattened morphology of cisterns and their structural integrity and stability. Thus, destruction of the actin cytoskeleton is accompanied by swelling and widening of the Golgi apparatus cisterns and the delivery of vesicles to the cell surface is late [44]. Actin was shown to control the generation and outbudding of carrier vesicles in the TGN region [45] and provide for the retrograde transport of the coat protein complex I (COPI) vesicles from the Golgi apparatus into the ER [46, 47].
Certain proteins from the myosin family are also connected with the Golgi apparatus, and myosins are linked to the membranes with involvement of definite Rab proteins. The only known minus-end oriented myosin 6 binds to the Golgi apparatus membrane through the protein optineurin and participates in the maintenance of the Golgi apparatus morphology and transfer of vesicles to the plasma membrane [48]. With the Golgi apparatus membranes non-muscular myosin 2 and myosin 1b are bound, which are also involved in the outbudding of transport vesicles from the cisterns [49, 50]. Myosins are supposed to link to the Golgi apparatus membrane and to generate a force responsible for its remodeling. Thus, myosin 1 regulates cargo transfer in the post-Golgi compartments, creates a force that controls the assembly of F-actin foci, and together with actin filaments promotes the creation of tubules in the TGN [51]. Another protein of this family, myosin 18, controls the normal morphology of the Golgi apparatus ribbon, is responsible for the flattened shape of the cisterns, and also participates in the transfer of vesicles from the Golgi apparatus to the plasma membrane [52].
Various proteins responsible for the actin cytoskeleton state, such as actin-related proteins 2/3 (Arp2/3), formins, nucleation-promoting factors (NPF), small GTPases of the Rho family, etc., are associated with the Golgi apparatus. These molecular regulators of the behavior of actin are supposed to be involved in the outbudding and fusion of membrane carriers moving between the ER and Golgi apparatus, in the traffic from the TGN into the post-Golgi compartments, and in maintaining the Golgi apparatus integrity [43].
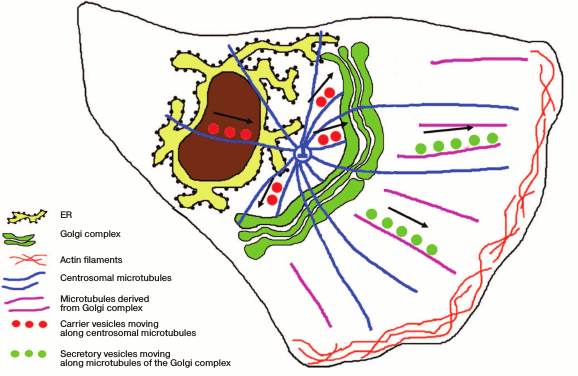
Thus, the ribbon-shaped structure of the Golgi apparatus is formed with involvement of two subpopulations of microtubules – centrosomal and those of the Golgi apparatus itself. Those of the Golgi apparatus are responsible not only for the ribbon integrity but also for the targeted transport of products released from the cell (Fig. 2). Actin filaments are responsible for the finer adjustment of the Golgi apparatus structure and the traffic of secretory vesicles.
Fig. 2. Position of the ribbon-shaped Golgi apparatus with respect to the centrosome in polarized cells. Products of synthesis are delivered from the ER along the centrosomal microtubules to the Golgi apparatus steadily localized near the centrosome. Secretory vesicles are transferred to the plasma membrane along microtubules derived from the Golgi apparatus.
A DISPERSED STATE OF THE GOLGI APPARATUS IS CHARACTERISTIC FOR
PLANT CELLS
Structural characteristics and behavior of the Golgi apparatus. An alternative to the ribbon-shaped configuration is the Golgi apparatus state in higher plant cells consists of a multitude of separate stacks/dictyosomes (Fig. 1, b and d) each of which functions independently of the others. The stacks can be distributed more or less uniformly or be accumulated in certain domains of the cytoplasm [53]. The structure of the stacks and their functional polarity have the same characteristics and features as in animal cells [54]. In plants the Golgi apparatus is involved in the synthesis of complex polysaccharides of the cell wall and of glycolipids of the plasma membrane, and in glycosylation of proteins, which are later targeted to the cell wall, plasma membrane, and vacuoles. The number of stacks varies in different cells depending on the state and functional activity of these cells [55-57]. The Golgi apparatus position depends on the functional specialization of the cells [58-60] and, moreover, it changes during the cell cycle [61, 62]. The Golgi apparatus in plants is characterized by maintaining the structure and functional activity of the stacks during the whole cell cycle, including mitosis. The maintenance of the functional activity of the Golgi apparatus during mitosis is associated with the necessity to provide for the secretion of polysaccharides, which are required in a great amount to form the cell wall during the cytokinesis [57].
The Golgi apparatus in plants also has other features as follows. 1) The absence of the ERGIC compartment. 2) The presence of a clearly pronounced TGN compartment that is localized in the trans-region of stacks and sorts the secreted products directing them to the subsequent post-Golgi compartments. The behavior of TGN varies in different plant cells. In some cases it can separate from the stack, and then the stack and the free TGN begin to move independently [62, 63]. 3) Moreover, TGN in plants concurrently is an early endosomal compartment because components penetrating into the cell by endocytosis come just into TGN. Consequently, TGN of plants is a specific membrane component where the secretory and endocytotic traffics (from the Golgi apparatus and from the plasma membrane, respectively) are combined. This is a difference between TGNs of plant and animal cells, because in animal cells early endosomes and TGN belong to different membrane compartments [60]. A stack with a related TGN compartment is characterized in the literature as a united complex called “Golgi bodies”. 4) The Golgi apparatus of plant cells is mobile, its stacks moving along actin filaments with involvement of myosin.
Thus, the Golgi apparatus in plants is divided into many mobile biosynthetic units each of which is responsible for a controlled import of synthesis products from the ER and the processing, sorting, and targeted delivery of the export products into definite cellular compartments. This is significantly different from the secretory traffic in the cells where the products synthesized in the ER are delivered into the Golgi apparatus constantly localized near the nucleus and then are targeted to the other cellular compartments. To understand the role of the cytoskeleton in the biosynthetic transport flows in plants, it is necessary to consider more in detail specific features of the export and import of synthesis products from the Golgi apparatus and into it.
Export of secretion products from the Golgi apparatus. “Usual” secretion in plants includes the flow of newly synthesized products from the ER to the Golgi apparatus and TGN. TGN is often considered as an element of the post-Golgi compartment, which also contains (1) multivesicular bodies, or a prevacuolar compartment, (2) lytic vacuoles, and (3) protein storage vacuoles [64].
The majority of the secreted proteins together with proteins intended for the lytic and storage vacuoles, plasma membrane, and endosomes, upon being piled and assembled in the ER, are transferred to the Golgi apparatus within COPII vesicles. In the Golgi apparatus the proteins are sorted, and their further fate is determined: whether they will appear in the prevacuolar compartment, central lytic vacuole, protein storage vacuole, plasma membrane, apoplast space, or endosomes [65].
Plant vacuoles are multifunctional organelles which store water, ions, various metabolites, and nutritive components. They accumulate degradation products and excess liquid, they play an important role in programmed cell death, and in many aspects they are similar to lysosomes of animal cells. There are two types of vacuoles in plants – storage and lytic. The storage vacuoles have a higher pH value (close to neutral) and a lower hydrolytic activity than the lytic vacuoles, and they are more specific for the storage tissues of plants. Lytic vacuoles are more often localized in vegetative tissues. Their medium is more acidic and they contain many hydrolytic enzymes. These vacuoles are used for accumulation of unwanted products; they receive extracellular substrates due to endocytosis and phagocytosis and intracellular products – due to autophagy and biosynthetic membrane traffic. The lytic vacuoles are involved in the degradation of various macromolecules and are believed to be key regulators of cell homeostasis [64, 65].
Proteins predestined for storage and lytic vacuoles and proteins (secreted and membranous) targeted to the plasma membrane are sorted in the TGN. The proteins predestined for the storage vacuoles begin to aggregate already in the cis-region of the Golgi apparatus, and this aggregation continues in all compartments of the stacks, then the cargo is detached from the TGN. Proteins of the lytic vacuoles and lysosomal proteins of animals are sorted similarly [64]. Proteins predestined for degradation are moved into the lytic vacuoles through a prevacuolar compartment [59, 64].
Many polysaccharides of the cell wall, including pectins and hemicellulose, are produced under the influence of glycosyl transferases, modified in the Golgi apparatus, and then are delivered through the TGN to the plasma membrane, secreted into the apoplast, and incorporated into the cell wall. On the contrary, cellulose is produced on the plasma membrane under the influence of multienzymatic cellulose synthase complexes (CESA), which, in turn, are assembled in the Golgi apparatus and secreted into the plasma membrane [66].
Thus, the post-Golgi traffic includes the directions to the plasma membrane and into the vacuolar compartment. Actin filaments were shown to participate in the transfer of vesicles from the Golgi apparatus into the lytic vacuoles but not to participate in the transfer from the Golgi apparatus to the plasmalemma [17]. However, up to now there are no data on the involvement of microtubules in any stage of this traffic. It should be especially noted that a possible role of microtubules in the regulation of the endomembrane traffic near the cell wall is now under discussion [6], because in places of the active secretion of polysaccharides a correlation has been found between the localization of microtubules and distribution of the Golgi apparatus stacks [67-69]. It is supposed that the interruptive movement of stacks along actin filaments, so-called “stop-and-go” [70], can be caused by stops of stacks carrying newly synthesized CESA complexes on the adjacent cortical microtubules, and the insertion of CESA into the plasma membrane can be associated with these stops [67].
Import of synthesis products into the Golgi apparatus. Questions about the interaction of ER with separately localized stacks of the Golgi apparatus in the absence of ERGIC are of significant current interest. There are three hypotheses mutually adding to each other that explain how vesicles can be delivered from the ER to the stacks. 1) The vacuum cleaner model supposes that the stacks are continuously moved along the ER gathering the cargo (COPII vesicles). According to this model, the whole surface of the ER can produce endoplasmic reticulum exit sites (ERES) occasionally positioned on the ER [14]. 2) According to the “stop-and-go” model, the stacks are stopped on definitely positioned ERES, gather the vesicles, and then are moved to another similar site [56, 70]. 3) The mobile export sites model supposes that the stacks can form a united secretory system with COPII vesicles derived from the ERES and are moved together as a joined “secretory unit” [71] that allows the cargo to be transferred from the ER to the Golgi apparatus at any time during the movement.
Experimental data have shown that the united mobile systems produced by the Golgi apparatus with the ERES are moved along actin filaments under the influence of myosins [54, 68, 72]. Thus, every stack is an independent mobile unit and is moved along the ER via actin filaments localized near the ER cisterns. Such stacks are called “stacks on track” or “mobile factories” [14, 55, 61, 70]. They gather COPII vesicles separated from the ERES, and the transport flow of vesicles is not linked to the cytoskeletal elements [73]. This is another difference from the cells with a steady polar localization of the Golgi apparatus in which the COPII vesicles are delivered from the ER to the Golgi apparatus along microtubules. Thus, actin filaments and motor proteins linked to them play the leading role in the movement of stacks from one ERES to another.
Motor proteins of higher plants. In the Arabidopsis thaliana genome 17 genes encoding myosins and 61 genes encoding kinesins are found [74]. Myosins are represented by two classes, four members of class VIII and 13 members of class XI [75]. These myosins are found only in plants, and their functions are poorly studied. The family VIII myosin genes includes four proteins: A. thaliana myosin 1 (ATM1), A. thaliana myosin 2 (ATM2), myosin VIIIA, and myosin VIIIB. Myosins of this group are differently expressed in different tissues and can participate in different stages of endocytosis, ER anchoring, and the activity in plasmodesmata [76]. Plasmodesmata are thin cytoplasmic bridges or channels passing across the cell wall and connecting the cytoplasm of neighboring cells. Metabolites can be symplastically transferred through plasmodesmata from one cell into another. Myosin VIII is detected in plasmodesmata of a new cell wall produced between the daughter cells during cytokinesis and is supposed to participate in cell wall maturation and positioning of cytoplasmic actin bands in places of intercellular communications [77]. Class XI myosins are evolutionarily related with myosin V of animals and fungi, where this motor protein participates in the transport of organelles and vesicles, their redistribution during division, localization of the mitotic spindle, and establishment of cell polarity. The myosins XI in plants are responsible for rapid movement of the Golgi apparatus, peroxisomes, mitochondria, and secretory vesicles [78-80], i.e. act as key motors providing motility of organelles in the cytoplasm. Moreover, myosins participate in the control of orientation of the spindle/phragmoplast relative to the cell wall and in the lateral widening of the cellular plate, but they do not significantly contribute to the traffic of vesicles during formation of the cell plate [81].
In addition to myosins, in the A. thaliana genome 61 genes encoding kinesins have been found, and among these are 21 minus-end oriented kinesins. The majority of kinesins are unique for plants [82], and functions of many of them are still poorly studied. Among plant kinesins, AtKinesin-13A and AtKinesin-13B of Arabidopsis and GhKinesin-13A of the cotton Gossypium hirsutum should be noted. These kinesins are members of a superfamily of proteins with the motor domain localized in the central part of the heavy chain. In animals proteins of this superfamily do not perform motor functions and are represented by depolymerases (catastrophins), whereas the plant kinesin-13A is a classic plus-end oriented motor specifically linked to stacks of the Golgi apparatus [83, 84]. In plants mutant in kinesin-13A the Golgi apparatus stacks aggregate and form clusters [83], which suggests a possible involvement of this protein in the maintenance of structure of the Golgi apparatus stacks and in membrane transport associated with post-Golgi traffic. As stated in the section “Export of secretion products from the Golgi apparatus”, at present there are no direct data on the role of microtubules in the delivery of secretory vesicles from the Golgi apparatus to the plasma membrane. However, in one recent review concerning this theme the possible participation of kinesins is discussed in decelerating the movement of the Golgi apparatus and even arresting it on microtubules during the secretion of vesicles carrying CESA complexes to the plasma membrane [6]. In this case, the movement of stacks along actin filaments has to be coordinated with stops on the adjacent microtubules. In turn, CESA complexes synthesize cellulose during their movement along the microtubules [85]. It seems that kinesins can act as motors coordinating the passage from one cytoskeleton track to another.
In the cotton G. hirsutum, a kinesin GhKCH1 has been identified possessing a catalytic domain characteristic for the minus-end of oriented kinesins. This kinesin has also on the N-end a unique CH domain inherent in actin-binding proteins. By now the family of calponin homology domain-containing kinesins (KCH) has been found only in higher plants. The N-terminal domain of GhKCH1 including the CH domain was shown to immediately interact with actin filaments. This kinesin is associated with transverse cortical bundles of actin filaments and interruptedly decorated cortical microtubules [86]. GhKCH1 is supposed to participate in regulation of the dynamic interaction between microtubules and actin filaments. It seems that just such kinesins as GhKCH1 can play an important role in coordination of the movement along actin filaments and cortical microtubules.
A separate group of plant kinesins participate in cytokinesis and provide for trafficking inside the phragmoplast. Thus, a phragmoplast-associated kinesin related protein 2 (AtPAKRP2) is responsible for the translocation of vesicles from the Golgi apparatus along the phragmoplast microtubules into the division plate where the vesicles fuse and form the cell wall between the daughter cells [87].
It can be concluded that the Golgi apparatus stacks in plant cells are transferred along actin filaments under the influence of myosin, although in mitotic cells these functions can be performed by microtubules: during cytokinesis vesicles are moved from the Golgi apparatus along the phragmoplast microtubules, which is considered as a specialized secretion [58]. However, some questions remain, e.g. the involvement of cytoskeleton in post-Golgi traffic.
Why do plant cells need proteins of the Golgi matrix? During the movement by actin filaments, separate and functionally autonomous stacks of the Golgi apparatus maintain not only their own structural integrity and polarization, but also the connection from ERES onto the ER. Certainly, structural support can exist as a scaffold that has to dynamically add a stack to ERES, unite stacks as whole units, and glue cisterns to one another. It seems that this function could be performed by proteins of the Golgi apparatus matrix. A group of the Golgi apparatus matrix proteins has been identified in A. thaliana: similarly to their animal analogs, these proteins are subdivided into cis-golgins (AtCASP, AtGolgin84A, AtGolgin84B, GDAP1, GC4, Atp115) and trans-golgins (AtTMF, AtGRIP). These proteins are responsible for attaching the stacks to ER, attaching “coated” vesicles to target membranes, attaching cisterns to one another, and for the initiation and regulation of biogenesis of the Golgi apparatus stacks [88]. However, the Arabidopsis genome does not contain pronounced homologs of Golgi reassembly and stacking proteins (GRASP) and majority of golgins, such as GM130 and giantin, and this can influence specific features of the Golgi apparatus organization in plants.
WHAT MAINTAINS THE GOLGI APPARATUS IN A DISPERSED STATE?
What is the advantage of the ribbon-shaped organization of the Golgi apparatus? Based on the above-presented data, we shall try to answer the question why in some cells the Golgi apparatus is “subdivided” into structurally and functionally autonomous units (stacks/dictyosomes) capable of the basic processing and sorting the secretion products, whereas in other cells the stacks form a united ribbon-shaped structure localized near the centrosome. It is thought that the polar localization of the Golgi apparatus in mammals promotes secretion targeted to specialized cellular domains that determine the polarized state of the cells. Such organization of the Golgi apparatus is advantageous because it increases the efficiency of glycosylation and facilitates the targeted transfer from the post-Golgi compartment to the plasma membrane, i.e. polarized secretion [21, 22, 89, 90]. Moreover, uniting stacks into a ribbon increases the efficiency of lateral diffusion of glycosylating enzymes between cisterns of neighboring stacks, which leads to a uniform distribution of these enzymes and strengthens the optimal processing of proteins passing across the Golgi apparatus [22, 91]. If there is a steadily and polarly localized ribbon of the Golgi apparatus, COPII vesicles are transferred from the ER along microtubules to the cis-Golgi compartment, and then the vesicles carrying the sorted and modified cargo are moved along the microtubules from the TGN to the plasma membrane or into the endosomal–lysosomal compartment. The cargo is delivered from the ER to the Golgi apparatus mainly along centrosomal microtubules, whereas the secreted material is delivered to the plasma membrane along the centrosomal microtubules derived from the Golgi apparatus (Fig. 2). Nevertheless, although the ribbon-shaped organization of the Golgi apparatus is clearly advantageous, it is not universal.
In what cells are stacks not united into the ribbon-shaped Golgi apparatus? In higher plant cells, even in strongly polarized ones (pollen tube, root hairs, etc.), the ribbon-shaped Golgi apparatus has not been found. The first and most obvious explanation would be an absence in higher plants of the centrosome as a united and dominant MTOC. In fact, if there is no centrosome, there is also no place for delivery of stacks along microtubules to be united into a ribbon. However, plants have microtubules radially derived from the nucleus, but, nevertheless, the stacks are not displaced to the nucleus and united into a ribbon. Thus, there is not a problem of specific features of organization of the microtubule system and of the absence of the centrosome.
This is confirmed by many examples of cells possessing centrosome/MTOC with the Golgi apparatus represented by a multiplicity of separate stacks dispersed in the cytoplasm. Thus, during the differentiation of myoblasts into myotubules, the ribbon-shaped Golgi apparatus disintegrates into fragments, which then are redistributed around the nucleus [92]. Differentiation of urinary bladder epithelium is also associated with fragmentation of the Golgi apparatus pericentrosomal band and with redistribution of stacks in the cytoplasm [93]. In addition to the Golgi apparatus localized perinuclearly near the centrosome/MTOC, neurons have Golgi-like compartments, the so-called “Golgi outposts” localized in dendrites [94]. Some researchers think that the above-listed cells have highly specialized functions, the centrosome/MTOC activity in them can decrease, which leads to reduction of the number of centrosomal microtubules and disturbance in the uniting of stacks into the ribbon [22]. However, in some cases the Golgi apparatus does not form ribbon-shaped structures in cells with an active centrosome/MTOC. Thus, stacks not united in a ribbon are observed in in vitro cultured cells of kangaroo rat kidney (line PtK2) [95], kidney fibroblasts of African grivet (line COS-7) [96], and rat hepatocytes of clone 9 [97]. Finally, the best-known object is drosophila, which has both an active centrosome and Golgi apparatus represented by separate stacks [91]. In drosophila cells the stacks are dispersed in the cytoplasm and are closely associated with transitional endoplasmic reticulum (tER) regions, or ERES, producing structures that are called “tER-Golgi units”. Note that without uniting into a ribbon, the stacks produce in drosophila mini-aggregations, “tER-Golgi units” consisting as a rule of two closely localized stacks. The presence in drosophila of matrix proteins GRASP65, GRASP55, GM130, and other golgins suggests that these aggregations can be primitive precursors of the Golgi ribbon. Authors of a review with the intriguing title “The Golgi apparatus: lessons from Drosophila” [91] think that the “tER-Golgi units” dispersed in the cytoplasm present an archetypical organization of early secretory traffic. Later this organization changed during evolution and began to depend on microtubules and minus-end of the targeted traffic of membranes from the ER to the Golgi apparatus, which resulted in the Golgi apparatus accumulation as a ribbon around the centrosome/MTOC. Nevertheless, the presence of MTOC does not mean that the Golgi apparatus will be inevitably accumulated around it and form a ribbon.
In the above-mentioned review [91] the authors compare the Golgi apparatus of drosophila and mammals, emphasizing the small number of similarities and numerous differences. They indicate some important lessons that can be deduced from studies on the Golgi apparatus of this organism. However, many similar features, which are common for the Golgi apparatus of drosophila and plants, are mentioned only casually. What lessons can be learned by comparing the Golgi apparatus of plants and drosophila?
What is in common in the Golgi apparatus of drosophila and plants? The first feature in common is the division of the Golgi apparatus into separate stacks dispersed in the cytoplasm. The second common feature is the same character of the stack interaction with the ER. Thus, in plants the complex of stack–TGN, or the “Golgi body” is combined through COPII vesicles with the ERES site on the ER into a united secretory system, or a “mobile factory unit” [71]. In drosophila the secretory “tER-Golgi unit” includes ERES, COPII vesicles delivered from it, and a stack of the Golgi apparatus [91]. The third common feature is the necessity for drosophila of actin filaments and proteins regulating the actin cytoskeleton (SCAR/WAVE, dextrin, coronin) for maintaining the structural integrity of the “tER-Golgi units”. In plants, mobile ERES-Golgi are moved along actin filaments under the influence of myosin, whereas regulatory actin proteins are involved in the secretory traffic from the Golgi apparatus [43]. Because the systems of microtubules in drosophila and plants are arranged differently, it is supposed that that the likeness in the Golgi apparatus organization should be caused by relations of the stacks with actin filaments. However, these relations are expressed differently: in drosophila actin controls the structural integrity of “tER-units” and in plants – the movement along the steadily localized ER.
In this connection, consider another feature of drosophila’s Golgi apparatus – the functional independence of the stacks, namely, the “tER-Golgi units” localized in the cytoplasm contain different glycosylating enzymes and therefore are involved in different glycosylation processes and treat different substrates. This means that morphologically similar stacks are different in the functional specialization. The stacks with different functional specializations are localized in definite domains of the cytoplasm, and this promotes the polarity of elimination of the secreted products [98]. Consequently, polar traffic of secreted products can be realized not only by uniting the stacks into the ribbon and its anchoring onto the centrosome. When the Golgi apparatus stacks are dispersed in the cytoplasm, the targeted secretion can be regulated in two variants: (1) each stack is responsible for processing and sorting different products, and in this case the cytoskeleton elements are required for the targeted delivery of the secreted products into the post-Golgi compartments and further into different domains of the cytoplasm; (2) each stack contains a certain set of enzymes and is functionally specialized, and in this case the targeted delivery of the secreted products is realized through regulation of the stack distribution in the cytoplasm. This variant of secretory traffic regulation is observed in drosophila.
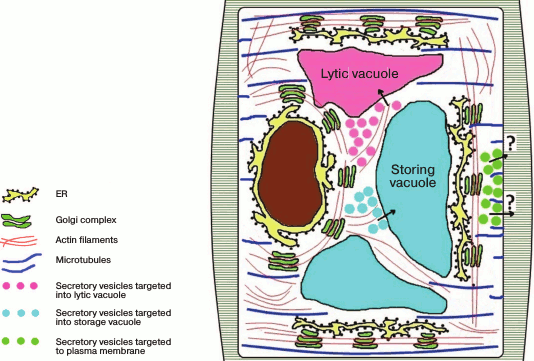
It is still unknown what kind of regulation occurs in plants, but because the stacks are mobile and move with respect to stationary locations of the ER along actin filaments, both variants and even their combination can be realized. Note that it is still unclear what elements of the cytoskeleton are responsible for the traffic of secretory elements from the Golgi apparatus to the plasma membrane, whereas the traffic from the Golgi apparatus into the vacuolar compartment depends on actin filaments [17]. One suggestion is that the stacks in plants, similarly to those in drosophila, are functionally specialized, so the translocation of vesicles from the Golgi apparatus to the plasma membrane does not need cytoskeleton, simply because the stacks that secret products targeted to the plasma membrane are localized within the secretory regions (Fig. 3).
Fig. 3. Golgi apparatus stacks are distributed in the plant cell cytoplasm and are moved along the ER via actin filaments. The secretion products from the Golgi apparatus are transferred into the vacuolar compartment along actin filaments, whereas the traffic of secretory vesicles from the Golgi apparatus to the plasma membrane does not depend on actin but seems to depend on microtubules.
What mechanisms provide and maintain the dispersed state of the Golgi apparatus? The ability of the Golgi apparatus to recruit γ-TURC and nucleate its subpopulation of microtubules is an important factor that determines Golgi apparatus ribbon assembly and maintains this state independently of centrosomal microtubules [33]. Thus, if the stacks are not united into a ribbon in cells possessing a centrosome and centrosomal microtubules, it can be supposed that in these cells the γ-TURC is not recruited onto the Golgi apparatus membrane. However, it was shown recently that the “Golgi outposts” in drosophila neurons recruited γ-TURC and generated new microtubules growing into dendrites [99]. Moreover, separate stacks of the Golgi apparatus and linked to them γ-TURC are involved in the nucleation of microtubules in skeletal muscles [100]. Consequently, in such cells the disjoined stacks of the Golgi apparatus generate their own microtubules but do not unite into a ribbon. But it is still unclear what mechanisms hold such stacks in the dispersed state. This question remains open also for plant cells, but it should be noted that up to now there are no data on the association of γ-TURC with the Golgi apparatus membrane in plants.
In mammalian cells cis-golgins GM130 through linking to the proteins AKAP450 (A-kinase anchoring protein 450) and GMAP210 anchor γ-TURC on the cis-region of the Golgi apparatus stacks dispersed in the cytoplasm, and this triggers the assembly of a ribbon-shaped configuration upon mitosis [32]. Consequently, for recruiting γ-TURC onto the membrane of the stacks, protein mediators are required, and among them proteins of the Golgi apparatus matrix play an important role. A functionally full-valued Golgi apparatus can be assembled de novo in the absence of centrosome and microtubules, but the protein GM130 is necessary [101]. The authors believe that the Golgi apparatus is able to self-organize. The self-organization can occur during nucleation of microtubules from the nuclear envelope and from vesicles, which are precursors of the Golgi apparatus, and this will result in clusterization of precursor vesicles into larger aggregations. In this case the absence in plants of protein GM130 and its analogs seems to explain the non-recruiting of γ-TURC from the cytosol onto the membrane of the Golgi apparatus stacks.
In conclusion, we would like to indicate factors capable of providing and maintaining the Golgi apparatus state characteristic for plant cells. First of all, it should be noted that the dispersed state of the Golgi apparatus in plant cells cannot be explained only by specific features of organization of the system of microtubules and the absence of centrosome as an active center of their organization, because a similar organization of the Golgi apparatus is characteristic also for cells of other organisms that possess the centrosome and centrosomal microtubules. The Golgi apparatus stacks cannot nucleate their microtubules because of absence in the Golgi apparatus of certain proteins responsible for recruiting γ-TURC. However, Golgi apparatus uniting as a ribbon not always occurs in other cells even in the case of microtubule nucleation. The functional uniformity or specialization of the stacks is an important factor influencing the state of the Golgi apparatus. Functional specialization does not suggest uniting of stacks to form a ribbon; therefore, the dispersed state of the Golgi apparatus needs to be supported, certainly, if it does not exist “by default”. The dynamic relations of Golgi apparatus stacks with the system of actin filaments, on one hand, and with the ERES sites distributed in the ER, on the other hand, is a key factor responsible for maintenance of the dispersed state of the Golgi apparatus in plants. Thus, comparison of the Golgi apparatus state in various organisms allows us to suppose that uniting stacks into a ribbon depends mainly on microtubules, whereas the maintenance of separate stacks depends on actin filaments.
REFERENCES
1.Van Zutphen, T., and van der Klei, I. J. (2011)
Quantitative analysis of organelle abundance, morphology and dynamics,
Curr. Opin. Biotechnol., 22, 127-132.
2.Hehnly, H., and Stamnes, M. (2007) Regulating
cytoskeleton-based vesicle motility, FEBS Lett., 581,
2112-2118.
3.Disanza, A., and Scita, G. (2008) Cytoskeletal
regulation: coordinating actin and microtubule dynamics in membrane
trafficking, Curr. Biol., 18, R873-R875.
4.Semenova, I., Burakov, A., Berardone, N., Zaliapin,
I., Slepchenko, B., Svitkina, T., Kashina, A., and Rodionov, V. (2008)
Report actin dynamics is essential for myosin-based transport of
membrane organelles, Curr. Biol., 18, 1581-1586.
5.Soldati, T., and Schliwa, M. (2006) Powering
membrane traffic in endocytosis and recycling, Nat. Rev. Mol. Cell
Biol., 7, 897-908.
6.Brandizzi, F., and Wasteneys, G. O. (2013)
Cytoskeleton-dependent endomembrane organization in plant cells: an
emerging role of microtubules, Plant J., 75, 339-349.
7.Mineyuki, Y. (2007) Plant microtubule studies: past
and present, J. Plant Res., 120, 45-51.
8.Smirnova, E. A., and Bajer, A. S. (1992) Spindle
poles in higher plant mitosis, Cell Motil. Cytoskel., 23,
1-7.
9.Murata, T., Sonobe, S., Baskin, T., Hyodo, S.,
Hasezawa, S., Nagata, T., Horio, T., and Hasebe, M. (2005)
Microtubule-dependent microtubule nucleation based on recruitment of
γ-tubulin in higher plants, Nature Cell Biol., 7,
961-968.
10.Hashimoto, T., and Kato, T. (2006) Cortical
control of plant microtubules, Curr. Opin. Plant Biol.,
9, 5-11.
11.Murata, T., and Hasebe, M. (2007)
Microtubule-dependent microtubule nucleation in plant cells, J.
Plant Res., 120, 73-78.
12.Blancaflor, E. B., Wang, Y.-S., and Motes, C. M.
(2006) Organization and function of the actin cytoskeleton in
developing root cells, Int. Rev. Cytol., 252,
219-264.
13.Liebe, S., and Menzel, D. (1995) Actomyosin-based
motility of endoplasmic reticulum and chloroplasts in
Vallisneria mesophyll cells, Biol. Cell, 85,
207-222.
14.Boevink, P., Oparka, K., Santa Cruz, S., Martin,
B., Betteridge, A., and Hawes, C. (1998) Stacks on tracks: the plant
Golgi apparatus traffics on an actin/ER network, Plant J.,
15, 441-447.
15.Kandasamy, M. K., and Meager, R. B. (1999)
Actin-organelle interaction: association with chloroplast in
Arabidopsis mesophyll cells, Cell Motil. Cytoskel.,
44, 110-118.
16.Van Gestel, K., Kohler, R. H., and Verbelen, J.
P. (2002) Plant mitochondria move on F-actin, but their positioning in
the cortical cytoplasm depends on both F-actin and microtubules, J.
Exp. Bot., 53, 659-667.
17.Kim, H., Park, M., Kim, S. J., and Hwang, I.
(2005) Actin filaments play a critical role in vacuolar
trafficking at the Golgi complex in plant cells, Plant Cell.,
17, 888-902.
18.Kumatani, T., Sakurai-Ozato, N., Miyawaki, N.,
Yokota, E., Shimmen, T., Terashima, I., and Takagi, S. (2006) Possible
association of actin filaments with chloroplasts of spinach mesophyll
cell in vivo and in vitro, Protoplasma,
229, 45-52.
19.Cheung, A. Y., and Wu, H.-M. (2007) Structural
and functional compartmentalization in pollen tubes, J. Exp.
Bot., 58, 75-82.
20.Cai, G., and Cresti, M. (2009) Organelle motility
in the pollen tube: a tale of 20 years, J. Exp. Bot., 60,
495-508.
21.Hua, Z., and Hardham, T. R. (2009) The Golgi
apparatus, in Trafficking Inside Cells: Pathways, Mechanisms
and Regulation (Segev, N., Alfonso, A., Payne, G., and Donaldson,
J., eds.) Landes Bioscience and Springer Science+Business Media, pp.
42-66.
22.Mironov, A., and Beznoussenko, G. (2011)
Molecular mechanisms responsible for formation of Golgi ribbon,
Histol. Histopathol., 26, 117-133.
23.Tang, D., and Wang, Y. (2013) Cell cycle
regulation of Golgi membrane dynamics, Trends Cell Biol.,
23, 296-304.
24.Wilson, C., Venditti, R., Rega, L., Colanzi, A.,
and D’Angelo, G. (2011) The Golgi apparatus: an organelle with
multiple complex functions, Biochem. J., 433, 1-9.
25.Varki, A. (1998) Factors controlling the
glycosylation potential of the Golgi apparatus, Trends Cell
Biol., 8, 34-40.
26.Young, W. W., Jr. (2004) Organization of Golgi
glycosyltransferases in membranes: complexity via complexes, J.
Membr. Biol., 198, 1-13.
27.Sutterlin, C., and Colanzi, A. (2010) The Golgi
and the centrosome: building a functional partnership, J. Cell
Biol., 188, 621-628.
28.Cole, N. B., and Lippincott-Schwartz, J. (1995)
Organization of organelles and membrane traffic by microtubules,
Curr. Opin. Cell Biol., 7, 55-64.
29.Thyberg, J., and Moskalewski, S. (1999) Role of
microtubules in the organization of the Golgi complex, Exp. Cell.
Res., 246, 263-279.
30.Chabin-Brion, K., Marceiller, J., Perez, F.,
Settegrana, C., Drechou, A., Durand, G., and Pous, C. (2001) The Golgi
complex is a microtubule-organizing organelle, Mol. Biol. Cell,
12, 2047-2060.
31.Efimov, A., Kharitonov, A., Efimova, N.,
Loncarek, J., Miller, P. M., Andreyeva, N., Gleeson, P., Galjart, N.,
Maia, A. R., McLeod, I. X., Yates, J. R., 3rd, Maiato, H., Khodjakov,
A., Akhmanova, A., and Kaverina, I. (2007) Asymmetric CLASP-dependent
nucleation of non-centrosomal microtubules at the trans-Golgi
network, Dev. Cell, 12, 917-930.
32.Rivero, S., Cardenas, J., Bornens, M., and Rios,
R. M. (2009) Microtubule nucleation at the cis-side of Golgi
apparatus requires AKAP450 and GM130, EMBO J., 28,
1016-1028.
33.Miller, P. M., Folkmann, A. W., Maia, A. R.,
Efimova, N., Efimov, A., and Kaverina, I. (2009) Golgi-derived
CLASP-dependent microtubules control Golgi organization and polarized
trafficking in motile cells, Nat. Cell Biol., 11,
1069-1080.
34.Sutterlin, C., Hsu, P., Mallabiabarrena, A., and
Malhotra, V. (2002) Fragmentation and dispersal of the pericentriolar
Golgi complex is required for entry into mitosis in mammalian cells,
Cell, 109, 359-369.
35.Colanzi, A., and Corda, D. (2007) Mitosis
controls the Golgi and the Golgi controls mitosis, Curr. Opin. Cell
Biol., 19, 386-393.
36.Rabouille, C., and Kondylis, V. (2007) Golgi
ribbon unlinking an organelle-based G2/M checkpoint cell cycle, Cell
Cycle, 6, 2723-2729.
37.Rios, R. M., Sanchis, A., Tassin, A. M.,
Fedriani, C., and Bornens, M. (2004) GMAP-210 recruits gamma-tubulin
complexes to cis-Golgi membranes and is required for Golgi
ribbon formation, Cell, 118, 323-335.
38.Corthesy-Theulaz, I., Pauloin, A., and Pfeffer,
S. R. (1992) Cytoplasmic dynein participates in the centrosomal
localization of the Golgi apparatus, J. Cell Biol., 118,
1333-1345.
39.Yadav, S., Puthenveedu, M. A., and Linstedt, A.
D. (2012) Golgin 160 recruits the dynein motor to position the Golgi
apparatus, Dev. Cell, 23, 153-165.
40.Vinogradova, T., Raia, P., Grimaldi, A. D.,
Loncarek, J., Miller, P. M., Yampolsky, D., Magidson, V., Khodjakov,
A., Mogliner, A., and Kaverina, I. (2012) Concerted effort of
centrosomal and Golgi-derived microtubules is required for proper Golgi
complex assembly but not for maintenance, Mol. Biol. Cell,
23, 820-833.
41.Zanetti, G., Pahuja, K. B., Studer, S., Shim, S.,
and Schekman, R. (2012) COPII and the regulation of protein sorting in
mammals, Nature Cell Biol., 14, 20-29.
42.Huotari, J., and Helenius, A. (2011) Endosome
maturation, EMBO J., 30, 3481-3500.
43.Egea, G., Serra-Peinado, C., Salcedo-Sicilia, L.,
and Guttieres-Martinez, E. (2013) Actin acting at the Golgi,
Histochem. Cell Biol., 140, 347-360.
44.Valderrama, F., Babia, T., Ayala, I., Kok, J. W.,
Renau-Piqueras, J., and Egea, G. (1998) Actin microfilaments are
essential for the cytological positioning and morphology of the Golgi
complex, Eur. J. Cell Biol., 76, 9-17.
45.Campellone, K. G., Webb, N. J., Znameroski, E.
A., and Welch, M. D. (2008) WHAMM is an Arp2/3 complex activator that
binds microtubules and functions in ER to Golgi transport, Cell,
134, 148-161.
46.Valderrama, F., Luna, A., Babia, T.,
Martinez-Menarguez, J. A., Ballesta, J., Barth, H., Chaponnier, C.,
Renau-Piqueras, J., and Egea, G. (2000) The Golgi-associated
COPI-coated buds and vesicles contain beta/gamma-actin, Proc. Natl.
Acad. Sci. USA, 97, 1560-1565.
47.Valderrama, F., Duran, J. M., Babia, T., Barth,
H., Renau-Piqueras, J., and Egea, G. (2001) Actin microfilaments
facilitate the retrograde transport from the Golgi complex to the
endoplasmic reticulum in mammalian cells, Traffic, 10,
717-726.
48.Sahlender, D. A., Roberts, R. C., Arden, S. D.,
Spudich, G., Taylor, M. J., Luzio, J. P., Kendrick-Jones, J., and Buss,
F. J. (2005) Optineurin links myosin VI to the Golgi complex and is
involved in Golgi organization and exocytosis, Cell Biol.,
169, 285-295.
49.Miserey-Lenkei, S., Chalancon, G., Bardin, S.,
Formstecher, E., Goud, B., and Echard, A. (2010) Rab and
actomyosin-dependent fission of transport vesicles at the Golgi
complex, Nat. Cell Biol., 12, 645-654.
50.Almeida, C. G., Yamada, A., Tenza, D., Louvard,
D., Raposo, G., and Coudrier, E. (2011) Myosin 1b promotes the
formation of post-Golgi carriers by regulating actin assembly and
membrane remodeling at the trans-Golgi network, Nat. Cell
Biol., 13, 779-789.
51.Courdier, E., and Almeida, C. G. (2011) Myosin 1
controls membrane shape by coupling F-actin to membrane,
Bioarchitecture, 1, 230-235.
52.Dippold, H. C., Ng, M. M., Farber-Katz, S. E.,
Lee, S. K., Kerr, M. L., Peterman, M. C., Sim, R., Wiharto, P. A.,
Galbraith, K. A., Madhavarapu, S., Fuchs, G. J., Meerloo, T., Farquhar,
M. G., Zhou, H., and Field, S. J. (2009) GOLPH3 bridges
phosphatidylinositol-4-phosphate and actomyosin to stretch and shape
the Golgi to promote budding, Cell, 139, 337-351.
53.Wang, W., Lazareva, E., Kyreev, I., and Smirnova,
E. (2012) The role of microtubules in the maintenance of regular
localization and arrangement of Golgi apparatus in root cells of
Triticum aestivum L., Process Biochem., 47,
1545-1551.
54.Staehelin, L. A., and Kang, B. H. (2008)
Nanoscale architecture of endoplasmic reticulum export sites and of
Golgi membranes as determined by electron tomography, Plant
Physiol., 147, 1454-1468.
55.Neumann, U., Brandizzi, F., and Hawes, C. (2003)
Protein transport in plant cells: in and out of the Golgi, Annals
Bot., 92, 167-180.
56.Robinson, D. (2003) Vesicle trafficking in
plants, Zellbiologie Aktuell., 2, 29-32.
57.Faso, C., Boulaflous, A., and Brandizzi, F.
(2009) The plant Golgi apparatus: last 10 years of answered and open
questions, FEBS Lett., 583, 3752-3757.
58.Hawes, C. (2005) Cell biology of the plant Golgi
apparatus, New Phytol., 165, 29-44.
59.Matheson, L. A., Hanton, S. L., and Brandizzi, F.
(2006) Traffic between the plant endoplasmic reticulum and Golgi
apparatus: to the Golgi and beyond, Curr. Opin. Plant Biol.,
9, 601-609.
60.Hwang, I. (2008) Sorting and anterograde
trafficking at the Golgi apparatus, Plant Physiol., 148,
673-683.
61.Nebenfuhr, A., Frohlick, J. A., and Staehelin, L.
A. (2000) Redistribution of Golgi stacks and other organelles during
mitosis and cytokinesis in plant cells, Plant Physiol.,
124, 135-151.
62.Seguı-Simarro, J. M., and Staehelin, A. L.
(2006) Cell cycle-dependent changes in Golgi stacks, vacuoles,
clathrin-coated vesicles and multivesicular bodies in meristematic
cells of Arabidopsis thaliana: a quantitative and spatial
analysis, Planta, 223, 223-236.
63.Kang, B.-H. (2011) Shrinkage and fragmentation of
the trans-Golgi network in non-meristematic plant cells,
Plant Signal. Behav., 6, 884-886.
64.Krause, C., Richter, S., Knoll, C., and Jurgens,
G. (2013) Plant secretome — from cellular process to biological
activity, Biochim. Biophys. Acta, 1834, 2429-2441.
65.Jurgens, G. (2004) Membrane trafficking in
plants, Annu. Rev. Cell Dev. Biol., 20,
481-504.
66.Worden, N., Park, E., and Drakakaki, G. (2012)
Trans-Golgi network – an intersection of trafficking
cell wall components, J. Integrat. Plant Biol., 54,
875-886.
67.Crowell, E. F., Bischoff, V., Desprez, T.,
Rolland, A., Stierhof, Y.-D., Schumacher, K., Gonneau, M., Hofte, G.,
and Vernhettesa, S. (2009) Pausing of Golgi bodies on microtubules
regulates secretion of cellulose synthase complexes in
Arabidopsis, The Plant Cell., 21, 1141-1154.
68.Toyooka, K., Goto, Y., Asatsuma, S., Koizumi, M.,
Mitsui, T., and Matsuoka, K. (2009) A mobile secretory vesicle cluster
involved in mass transport from the Golgi to the plant cell exterior,
Plant Cell., 21, 1212-1229.
69.Karahara, L., Staehelin, L. A., and Mineyuki, Y.
(2010) A role of endocytosis in plant cytokinesis, Commun. Integrat.
Biol., 3, 36-38.
70.Nebenfuhr, A., Gallagher, L. A., Dunahay, T. G.,
Frohlick, J. A., Mazurkiewicz, A. M., Meehl, J. B., and Staehelin, L.
A. (1999) Stop-and-go movements of plant Golgi stacks are mediated by
the actomyosin system, Plant Physiol., 121,
1127-1142.
71.Brandizzi, F., Snapp, E. L., Roberts, A. G.,
Lippincott-Schwartz, J., and Hawes, C. (2002) Membrane protein
transport between the endoplasmic reticulum and the Golgi in tobacco
leaves is energy dependent but cytoskeleton independent: evidence from
selective photobleaching, Plant Cell, 14, 1293-1309.
72.DaSilva, L. L. P., Snapp, E. L., Denecke, J.,
Lippincott-Schwartz, J., Hawes, C., and Brandizzi, F. (2004) Plant
Cell, 16, 1753-1771.
73.Saint-Jore, C. M., Evins, J., Batoko, H.,
Brandizzi, F., Moore, I., and Hawes, C. (2002) Redistribution of
membrane proteins between the Golgi apparatus and endoplasmic reticulum
in plants is reversible and not dependent on cytoskeletal networks,
Plant J., 29, 661-678.
74.Lee, Y.-R.-J., and Liu, B. (2004) Cytoskeletal
motors in Arabidopsis. Sixty-one kinesin and seventeen myosins,
Plant Physiol., 136, 3877-3883.
75.Reddy, A. S., and Day, I. S. (2001) Analysis of
the myosins encoded in the recently completed Arabidopsis thaliana
genome sequence, Genome Biol., 2, 0024.1-0024.17.
76.Golomb, L., Abu-Abied, M., Belausov, E., and
Sadot, E. (2008) Different subcellular localizations and functions of
Arabidopsis myosin VIII, BMC Plant Biol., 8,
3.
77.Reichelt, S., Knight, A. E., Hodge, T. P.,
Baluska, F., Samaj, J., Volkmann, D., and Kendrick-Jones, J. (1999)
Characterization of the unconventional myosin VIII in plant cells and
its localization at the post-cytokinetic cell wall, Plant J.,
19, 555-567.
78.Avisar, D., Prokhnevsky, A. I., Makarova, K. S.,
Koonin, E. V., and Dolja, V. V. (2008) Myosin XI-K is required for
rapid trafficking of Golgi stacks, peroxisomes, and mitochondria in
leaf cells of Nicotiana benthamiana, Plant Physiol.,
146, 1098-1108.
79.Avisar, D., Abu-Abied, M., Belausov, E., Sadot,
E., Hawes, C., and Sparkes, I. A. (2009) A comparative study of the
involvement of 17 Arabidopsis myosin family members on the
motility of Golgi and other organelles, Plant Physiol.,
150, 700-709.
80.Peremyslov, V. V., Prokhnevsky, A. I.,
Avisar, D., and Dolja, V. V. (2008) Two class XI myosins function
in organelle trafficking and root hair development in
Arabidopsis, Plant Physiol., 146, 1109-1116.
81.Hepler, P. K., Valster, A., Molchan, T., and Vos,
J. M. (2002) Roles for kinesin and myosin during cytokinesis, Phil.
Trans. R. Soc. Lond., B, 357, 761-766.
82.Lee, Y.-R. J., and Liu, B. (2004) Cytoskeletal
motors in Arabidopsis. Sixty one kinesins and seventeen myosins,
Plant Physiol., 136, 3877-3883.
83.Lu, L., Lee, Y. R., Pan, R., Maloof, J. N., and
Liu, B. (2005) An internal motor kinesin is associated with the Golgi
apparatus and plays a role in trichome morphogenesis in
Arabidopsis, Mol. Biol. Cell, 16, 811-823.
84.Wei, L., Zhang, W., Liu, Z., and Li, Y. (2009)
AtKinesin-13A is located on Golgi-associated vesicle and involved in
vesicle formation/budding in Arabidopsis root-cap peripheral
cells, BMC Plant Biol., 138, doi:
10.1186/1471-2229-9-138.
85.Paredez, A. R., Somerville, C. R., and Ehrhardt,
D. W. (2006) Visualization of cellulose synthase demonstrates
functional association with microtubules, Science, 312,
1491-1495.
86.Preuss, M. L., Kovar, D. R., Lee, Y. R., Staiger,
C. J., Delmer, D. P., and Liu, B. (2004) A plant-specific kinesin binds
to actin microfilaments and interacts with cortical microtubules in
cotton fibers, Plant Physiol., 136, 3945-3955.
87.Lee, Y.-R. J., Giang, H. M., and Liu, B. (2011) A
novel plant kinesin-related protein specifically associated with the
phragmoplast organelles, Plant Cell, 13, 2427-2439.
88.Osterrieder, A. (2012) Tales of tethers and
tentacles: golgins in plants, J. Microsc., 247,
68-77.
89.Glick, B. S., and Nakano, A. (2009) Membrane
traffic within the Golgi apparatus, Annu. Rev. Cell Dev. Biol.,
25, 113-132.
90.Yadav, S., and Linstedt, A. (2011) Golgi
positioning, Cold Spring Harb. Perspect. Biol., 3,
a005322.
91.Kondylis, V., and Rabouille, C. (2009) The Golgi
apparatus: lessons from Drosophila, FEBS Lett.,
583, 3827-3838.
92.Ralston, E. (1993) Changes in architecture of the
Golgi complex and other subcellular organelles during myogenesis, J.
Cell Biol., 120, 399-409.
93.Kreft, M. E., Di Giandomenico, D., Beznoussenko,
G. V., Resnik, N., Mironov, A. A., and Jezernik, K. (2010) Golgi
apparatus fragmentation as a mechanism responsible for uniform delivery
of uroplakins to the apical plasma membrane of uroepithelial cells,
Biol. Cell, 102, 593-607.
94.Sekine, S., Muira, M., and Chihara, T. (2009)
Organelles in developing neurons: essential regulators of neuronal
morphogenesis and function, Int. J. Dev. Biol., 53,
19-27.
95.Keller, P., Toomre, D., Diaz, E., White, J., and
Simons, K. (2001) Multicolor imaging of post-Golgi sorting and
trafficking in live cells, Nature Cell Biol., 3,
140-149.
96.Tawfeek, H. A. W., and Abou-Samra, A. B. (2004)
Important role for the V-type H+-ATPase and the Golgi
apparatus in the recycling of PTH/PTHrP receptor, Am. J.
Physiol., 286, E704-E710.
97.Zuber, C., Fan, J., Guhl B., Parodi, A., Fessler,
J., Parker, C., and Roth, J. (2001) Immunolocalization of
UDP-glucose:glycoprotein glucosyltransferase indicates involvement of
pre-Golgi intermediates in protein quality control, Proc. Natl.
Acad. Sci. USA, 98, 10710-10715.
98.Yano, H., Yamamoto, H., Hirata, R., and Hirano,
A. J. (2005) Distinct functional units of the Golgi complex in
Drosophila cells, Craniofac. Surg., 16,
277-280.
99.Ori-McKenney, K. M., Jan, L. Y., and Jan, Y. N.
(2012) Golgi outposts shape dendrite morphology by functioning as sites
of acentrosomal microtubule nucleation in neurons, Neuron,
76, 921-930.
100.Oddoux, S., Zaal, K. J., Tate, V., Kenea, A.,
Nandkeolyar, S. A., Reid, E., Liu, W., and Ralson, E. (2013)
Microtubules that form the stationary lattice of muscle fibers are
dynamic and nucleated at Golgi elements, J. Cell Biol.,
203, 205-213.
101.Tangemo, C., Ronchi, P., Colombelli, J.,
Haselmann, U., Simpson, J. C., Antony, C., Stelzer, E. H. K.,
Pepperkok, R., and Reynaud, E. G. (2010) A novel laser nanosurgery
approach supports de novo Golgi biogenesis in mammalian cells,
J. Cell Sci., 124, 978-987.