REVIEW: Receptor-Mediated Endocytosis and Cytoskeleton
E. S. Kornilova1,2
1Institute of Cytology, Russian Academy of Sciences, Tikhoretsky pr. 4, 194064 St. Petersburg, Russia2Department of Medical Physics, St. Petersburg State Polytechnical University, St. Petersburg, Russia; fax: +7 (812) 297-3169; E-mail: elena.kornilova@gmail.com
Received April 29, 2014
Receptor-mediated endocytosis is the most specific pathway for macromolecules and macromolecular complexes generally designated as ligands to enter cells. Upon binding to their transmembrane receptors, the ligands enter endocytic vesicles that fuse with each other giving rise to the so-called early endosomes. The sorting of ligand–receptor complexes internalized in these endosomes depends on their nature: metabolic receptors are recycled back to the plasma membrane, while signaling receptors and their ligands (e.g. receptor tyrosine kinases or receptors associated with tyrosine kinase) are delivered to internal vesicles of the multivesicular late endosomes and finally are degraded after interaction with lysosomes. During these processes, endosomes undergo translocation from the cell periphery to the juxtanuclear region, which is accompanied by multiple fusion, invagination, tabulation, and membrane fission events. This review considers modern concepts of the sorting mechanisms of ligand–receptor complexes, the crosstalk between endosomes, microtubules, and actin, and the role of this crosstalk in endosome maturation.
KEY WORDS: endosomes, ligand–receptor complexes, sorting complexes, microtubules, microfilaments, domain organization of endosomes, membrane remodelingDOI: 10.1134/S0006297914090041
Abbreviations: EE, early endosomes; EGF, epidermal growth factor; ER, endoplasmic reticulum; JNR, juxtanuclear region; LDL, low density lipoproteins; LE, late endosomes; MT, microtubules; MVBs, multivesicular bodies; PI3P, phosphatidylinositol-3-monophosphate; TGN, trans-Golgi network.
Receptor-mediated endocytosis (RME) is a pathway for highly selective
absorption of macromolecular ligands by a cell, when the ligand bound
to a transmembrane receptor at the cell surface is concentrated in
certain parts of the membrane, giving rise to endocytic vesicles that
are then pinched off inside the cell [1]. The major
groups of macromolecules undergoing clathrin-dependent RME are peptide
signaling growth factors, cytokines, and hormones (e.g. epidermal
growth factor (EGF), platelet-derived growth factor (PDGF), fibroblast
growth factor (FGF), insulin, etc.), macromolecules involved in cell
metabolism (transferrin, low density lipoproteins (LDL), etc.), and a
number of toxins, viruses, and bacteria. This review will consider
endocytosis of only the first two groups of ligands.
In the first case, the signaling function of ligands is performed by their receptor due to the presence of an intrinsic cytoplasmic tyrosine-kinase domain in its sequence or to receptor association with intracellular tyrosine kinases. In both cases, the receptor is activated after the formation of a ligand–receptor complex. The activated receptor is able to stimulate intracellular signals usually organized into cascades of phosphorylation of various targets of tyrosine or serine/threonine residues. Enzymatic activity of the receptor plays a significant role also in its subsequent intracellular fate due to the activation of regulatory transport proteins. In the case of metabolic receptors, their crosstalk with regulatory transport proteins is mediated by small signaling sequences in their cytoplasmic domains [2, 3]. A number of ion channels and transporters also become endosome components [4-6] actively participating in organization of sorting processes.
Both metabolic and signaling receptors are usually internalized through clathrin-coated pits; as a result, endocytosis is designated as clathrin-dependent [7]. This review will consider the involvement of cytoskeleton in the regulation of endocytic pathways initiated by a clathrin-dependent mechanism, with particular attention being given to early and late endosomes and the proteins functioning at these stages. It should be emphasized that the term “endocytosis” in the literature often implies only the initial stage of the process: formation of an endocytic vesicle and its separation from the plasma membrane, though this stage has its own name and is designated as internalization. We will omit this stage and concentrate on the later stages of endocytosis: from early endosomes to lysosomes.
COMPARTMENTS OF ENDOCYTOTIC PATHWAY
Though the process of endocytosis became an object of intensive research as early as in the 1970s, discussions about the major compartments of this pathway (which have been called endosomes) and their biogenesis continue up to now. The primary cause seems to be as follows: in contrast to compartments of the exocytotic pathway (endoplasmic reticulum (ER) and Golgi apparatus) of easily recognizable shape and localization, endosome compartments are represented by vesicles that are usually not combined into large structures. As a consequence, there are some problems with differentiation of endocytic compartments, which result, in particular, in somewhat “nonscientific” character of the accepted terminology: the so-called “early endosomes” (EE) and “late endosomes” (LE) are distinguished. These names come from the initial studies when cargo molecules were the only endosome marker: they were found in tubular–vesicular structures at the cell periphery in the first 5-10 min of endocytosis and, later on, in the vesicles localized in the juxtanuclear region (JNR) also containing lysosomes that are considered the last compartment of the endocytic pathway [8, 9]. It was believed that early and late compartments differ not only in localization but also in their role in endocytosis. It is known that many (especially metabolic) receptors can avoid delivery into lysosomes by entering the recycling pathway: short (peripheral) and long (localized in JNR). Both pathways originate from early endosomes; hence, peripheral and juxtanuclear recycling endosomes are distinguished, while late endosomes are often called a prelysosome compartment [10].
The theory of endosome maturation has become most popular. Its essence is that the de novo formed endocytic vesicle carrying a cargo (e.g. a ligand–receptor complex) grows in size due to homotypic fusion with the same vesicles and is transformed into an early endosome with the main function of cargo sorting for recycling or lysosomal degradative pathways. The receptors recycling back into the plasma membrane are sorted into the tubular part of EE, while the receptors directed for degradation in lysosomes are concentrated in the vesicular part. Endosome membrane composition gradually changes both due to the removal of recycling domains and to fusion with vesicles formed in the trans-Golgi network (TGN) and carrying lysosomal enzymes. In addition, inward invaginations appear at the outer membrane of the endosome. The cargo directed into lysosomes goes away from the outer membrane of the endosome into these invaginations and, after their closing and detachment, becomes inaccessible for the contents of the cytoplasm. The structures being formed are called multivesicular bodies (MVBs) and are considered as late endosomes. Then the mature LE interacts with the lysosome [11].
The mechanism of sorting for the degradation or recycling pathway was originally explained by two factors: first, gradual acidification of the interior space of the endosome due to the work of the vesicular proton pump, resulting in dissociation of ligand–receptor complexes after reaching a certain pH level and, second, to different ratios of membrane surface area to internal volume in the tubular and vesicular regions of EE. As a result, the receptors uniformly distributed over the membrane surface are found mainly in the tubule, while most of the ligand that is solved in the fluid phase is found mainly in the vesicular region [12, 13]. Now it is clear that, although this simple passive mechanism actually contributes to sorting, cargo segregation employs numerous molecular complexes and intracellular structures, making this process active and specific.
One more characteristic of the endocytotic pathway described at early stages of research was its dependence on intact microtubules (MT). In 1979 it was shown that the MT-depolymerizing agent colchicine prevents the appearance of LDL in the juxtanuclear region, suppresses its degradation, and stimulates the recycling of LDL receptor [14]. It is interesting that in the same work the authors found no redistribution of lysosomes from the juxtanuclear region and arrived at a conclusion about independence of lysosome positioning in the cell from MT. As a result of these investigations, the role of MT in a cell was conceived as railways for the movement of endosomes, while the role of actin remained obscure for a long time. In addition, in spite of the well-described phenomenology of the entire process, the basic mechanisms of endocytosis regulation and the proteins performing this regulation are still unknown.
ENDOSOMES: COMPLEX MOLECULAR MACHINES
Research on regulation of endocytosis has demonstrated that endosome, even at the initial stage of its existence when its size is no more than 100-200 nm in diameter, is not a passive “vehicle” but rather a platform with all necessary equipment for performance of numerous functions. First, the ion composition of the endosome lumen is extremely labile and cam be substantially different from the cytoplasmic ion composition. The incorporation of proton pump V0/V1 into endosomal membrane results in gradual acidification of the endosome, which may be essential for “passive sorting” of ligand–receptor complexes depending on the pH optimum of their dissociation [13]. In addition, the endosomal membrane has several types of channels providing maintenance/variation of the concentration of K+, Na+, and Ca2+ ions. Since initially Ca2+ concentration in the endosome lumen corresponds to a high extracellular concentration, the endosome per se is a calcium depot, and the presence of calcium channels of several types (including pH-sensitive) provides local increases in the level of this ion within a limited area at the cytoplasmic side of the endosome membrane. Such local increases in calcium, without affecting overall cell processes, are important for the regulation of fusions, can modulate the properties of cytoskeleton associated with endosomes, and can regulate the activity of numerous proteins in the “near-endosome” region [4, 5].
Second, though it is still unclear whether the cargo packing into endocytic vesicles is receptor type-specific, it has been reliably established that recycling receptors and receptors destined for degradation are found in the same early endosomes [15, 16]. However, these cargoes are not mixed in the plane of the membrane but stay segregated into definite domains, which will be the basis for the formation of recycling tubules [17] or maturing multivesicular endosomes (MVB). MVB formation is associated with invaginations of small membrane regions into the endosome that contain recruited cargo directed for degradation inside them. These invaginations end with the formation of vesicles and their pinching off into the lumen of the endosome [18]. Thus, the cargo becomes incapable of being recycled. Incorporation of the cargoes into the internal vesicles is also important because many signaling receptors with their regulatory domains oriented towards the cytoplasm are able to generate signals inside the cell from the endosomal membrane due to association with components of signaling complexes [19]. Isolation of such receptors from the cytoplasm obviously interrupts the signal.
Thus, endosome sorting and maturation is accompanied by continuous remodeling of the membrane that is associated with its local curving and tubulating as well as multiple membrane fusions and fissions. Both removal of some part of the membranes as a result of recycling and inclusion of new regions as a result of fusion with vesicles carrying lysosomal enzymes from the TGN modify the lipid–protein composition of the endosomal membrane. The mature late endosome with MVB morphology can interact with lysosomes, which results in the degradation of some part of the internal vesicles together with their contents [20]. All these events are regulated by a great number of proteins that are able, in particular, to interact with cytoskeleton.
Finally, the most striking characteristic of the endocytic pathway is long-distance (by cellular standards) endosome movements during endocytosis that are generally maintained by microtubules and motor proteins. The parts recycling by the short pathway after the detachment from an endosome move back to the plasma membrane and must interact with plus-end motors, while the cargoes recycling via the juxtanuclear recycling compartment move toward the minus-end of the MT during a part of the pathway and change for plus-end translocation only after the process of sorting into tubules has been completed. The maturating endosomes moving towards the lysosomes in juxtanuclear localization must use plus-end motors [21]. Thus, it can be expected that the pattern of movements of endosome compartments in the cell will be ordered and easily interpreted. However, the actual pattern of organelle movements, combining long runs (often in opposite directions) with undirected “diffuse” movement within a limited area, causes serious discord in theoretical constructions and active discussions [22-24]. One of the attempts to understand what actually happens here can be the analysis of the available data on the molecular mechanisms of endocytosis and its particular stages, the regulation of which may involve the cytoskeleton.
ROLE OF Rab PROTEINS IN ORGANIZATION OF ENDOCYTOSIS AND
CYTOSKELETAL CROSSTALK
Rab proteins are compartment-specific small (21-25 kDa) GTPases capable of anchoring in the target membrane due to posttranslational attachment of one or two geranyl-geranyl pyrophosphates to the cysteine motif at the protein C-terminus. In spite of their name, the intrinsic GTPase activity of Rab is extremely low, and the protein needs the following effectors for GTP hydrolysis or substitution of GTP by GDP: the GTPase-activating protein (GAP) and the guanine nucleotide exchange factor (GEF). As a rule, one Rab protein has several different GAPs and GEFs, while one effector can work with several small GTPases. Thereby, the coordination of multistage transporting processes is achieved.
As a result, Rab proteins are used by the cell not as enzymes but as timers of regulated processes, because the protein in GTP-bound state is able to interact with a certain set of partners, and this interaction is “switched-off” after hydrolysis of GDP. Active GTP-bound Rab protein is localized on the membrane, while inactive GDP-bound protein may either remain on the membrane or be transferred into the cytoplasm. All these mechanisms are described in detail in some reviews [25, 26].
Originally Rab proteins were supposed to participate in regulation of the first stage of membrane fusion due to their ability to bind with long linear proteins or protein complexes recognizing and tethering the target membrane [27-29]. Further studies have shown that their role in cell life is really fundamental: these small proteins are involved in the maintenance of domain (or mosaic) membrane structure, which makes it possible to form and support various functional domains [29, 30]. The key Rab protein of the endocytotic pathway is Rab5, the actual organizer of the entire pathway, which is localized mainly on early endosomes [31]. Suppressed expression of all three Rab5 isoforms (Rab5a, b, and c) leads to sequential disappearance of all compartments of the endocytotic pathway, including lysosomes [32]. During endosome maturation, Rab5 is replaced by Rab7, which is considered to be a marker of late endosomes (the so-called “Rab5–Rab7 conversion” [33]). Rab4 is typical for the membranes of fast recycling endosomes, while Rab4 and then Rab11 are found on endosomes recycling through the long pathway [30]. In addition, there are more isoforms of Rab proteins localized on endosomes; their functions are still not quite clear [34, 35], but this fact indicates the complexity of sorting processes on endosomes, which is still far from being completely understood. For example, it has been revealed that the proton vesicular pump V0/V1 necessary for endosome acidification is localized on the vesicles formed in the TGN, which do not carry any of the Rab proteins specific for the TGN but the early endosome Rab5a and, at the same time, are negative for the Rab5-dependent endosomal tethering factor EEA1 [36].
The vast majority of the Rab5 population is in the active state and associated with endosomal membranes, though the mechanisms of its stimulation or maintenance in the GTP-bound form are not always clear. In case of EGF receptor, it is known that the stimulation of its tyrosine kinase activity as a result of formation of a ligand–receptor complex leads to activation of the RIN1 protein (the nucleotide exchange factor for Rab5a), which results in transition of this small GTPase into the active state [37]. An event of major importance is the recruitment of phosphoinositol-3-kinase Vps34 and a number of enzymes of metabolism of this lipid by Rab5a-GTP [38]. As a result of Vps34 function, large domains enriched in phosphatidylinositol-3-monophosphate (PI3P) lipid appear on the membrane of the early endosome soon after its formation.
The emergence of PI3P at the surface of the endosome is significant because many proteins involved in membrane fusion, remodeling, and cargo sorting possess domains (FYVE or PX) that recognize exactly this modified lipid and are thereby recruited to the endosome [39]. The major protein of early endosomes tethering, EEA1, recognizes Rab5-GTP but is bound to the membrane with greater affinity via the FYVE domain, which recognizes PI3P and is located near the Rab5-binding domain. The other end of this long dimeric molecule binds also activated Rab5, allowing stabilization of the two similar vesicles opposite each other [40]. The HOPS complex recruited by Rab7 and involved in approaching lysosomes also recognizes the late endosomal membrane enriched in PI3P [41].
SORTING NEXINS (SNX) – THE DOMAIN INSIDE THE
DOMAIN
A large group of proteins that are able to associate with the PI3P-enriched membrane via the PH domain is the SNX (sorting nexins) family. Their active participation in the regulation of transport processes is coupled, first, with the ability to selectively interact with cargo molecules [42] and, second, with the presence in most SNXs of the so-called BAR domain, not only preferring association with highly curved membranes but also involved in membrane deformation that results in tubulation [43, 44]. Members of the SNX family are also revealed in the areas of relatively low curvature, e.g. in the vesicular region of early endosomes, probably stabilized by flat clathrin lattices [45]. Data on their involvement in the life of endosomes are numerous but still highly contradictory. For example, SNX1 and SNX2 are found on endosomes, and some authors report EGF receptor colocalization only with the latter [46], while other authors report also interaction with SNX1, probably indirect [47]. At the same time, SNX1 decorates the entire endocytotic pathway, from early to late endosomes, including the tubular structures associated with recycling [46]. The recycling transferrin receptor interacts with SNX4 [48]. SNX1 and SNX2 are able to form dimers with SNX5 and 6 and, as such, to be components of the retromer complex responsible for reverse transport from late endosomes to the TGN via tubular carriers [49]. However, it can be supposed that diversity of the properties, localization, and interactions of the proteins of this family makes it possible to specifically concentrate various cargoes by forming SNX domains inside PI3P domains, thereby providing “individual” approaches to the sorting of different receptors and coordinating it with rapid dynamic changes in membrane shape.
UBIQUITIN-DEPENDENT SYSTEM FOR RECOGNIZING CARGO AND REGULATORY
PROTEINS ON ENDOSOMES
The second key “organizational” factor of endosome functioning is related to ubiquitin-dependent mechanisms of cargo recognition. In the case of EGF receptor, the internalized receptor TK-dependently recruits ubiquitin ligase c-Cbl, attaching a set of single ubiquitin molecules or short ubiquitin chains to lysine residues exposed at the surface of globular tyrosine kinase domain of the receptor [50, 51]. c-Cbl is permanently associated with the activated receptor on endosome until its deubiquitination and translocation into the internal MVB vesicles [52, 53]. Two deubiquitinating enzymes, UBPY (or UPS8) and AMSH, are also associated with the endocytotic pathway [54]. We have shown that the suppression of EGF receptor tyrosine kinase activity at any moment before the late stage of endocytosis results in rapid deubiquitination and recycling of EGF receptors remaining on the outer endosomal membrane in A431 and HeLa cells [55]; thus, the pattern of receptor ubiquitination at each moment in time is a balance between ubiquitinating and deubiquitinating activities and can be substantially different. The variation of ubiquitination pattern probably orchestrates the order of interaction between the receptor and ubiquitin-recognizing proteins.
ESCRT COMPLEXES DETERMINE THE PATH INTO INTERNAL MVB
VESICLES
In essence, the ubiquitination of membrane cargoes is a signal of their delivery into lysosomes for degradation. The sorting for the lysosomal degradation pathway is implemented by sequential interactions between ubiquitinated cargo and the so-called ESCRT (Endosomal Sorting Complex Required for Transport) complexes concentrating cargo in the PI3P-domains and being involved in the formation of internal vesicles. These four complexes (0-III) are recruited to the membrane, because some of their components possess a phospholipid PI3P-recognizing sequence [56] and interact with the ubiquitinated cargo due to the presence of ubiquitin-recognizing domains [57]. The process is initiated by the ESCRT-0 complex consisting of HRS proteins (Hepatocyte Growth Factor Receptor Substrate), the name of which demonstrates the possibility of its tyrosine phosphorylation by tyrosine kinase receptors, and the STAM protein [56, 58]. STAM contains the SH3 domain, which is believed to participate in recruitment of the deubiquitinating protein UBPY [59]. HRS has the FYVE domain providing for membrane recruitment and the UIM domain recognizing the ubiquitinated cargo [56]. HRS can also binds clathrin, which forms flat lattices on the endosomal membrane [60]. The role of clathrin in this case is not quite clear. However, it is supposed that a spatially restricted lattice makes it possible to concentrate the “HRS–cargo” complex. It is interesting that HRS is initially present in complex with SNX1, exactly with the domain needed for SNX1 binding to EGF receptor, thus preventing formation of the complex HRS–receptor [61]. It has also been reported that HRS undergoes ligand-induced tyrosine phosphorylation at Y329 and Y334 after EGF receptor activation, leading to dissociation of HRS from the membrane and with its subsequent degradation [62]. Combining these data, one can suppose that the activated receptor causes dissociation of HRS from SNX1 by interacting with its UIM domain, and replaces it in the complex. In addition, HRS can undergo UIM-dependent autoubiquitination, which possibly makes the protein incapable of binding ubiquitinated cargoes [63]. All this taken together stops the action of HRS.
Another important role that HRS plays in fusion regulation is as follows: the protein inhibits the formation of endosomal SNARE complex (the syntaxin 13/SNAP-25/VAMP2 complex) by preventing the interaction between VAMP2 and partners [64]. Dissociation of the VAMP2/HRS complex results from the efflux of Ca2+ through the endosomal channel when the intra-endosomal pH decreases to 6.7; as a result, HRS goes away from the endosomal membrane, allowing SNARE to form a functional complex [65]. Thus, the presence of HRS on the membrane (1) allows the concentration of ubiquitinated cargoes in PI3P-enriched domain and (2) impedes untimely homotypic membrane fusion, which is necessary for endosome maturation, thereby preventing endosome enlargement. It can be supposed that HRS provides a certain time window necessary for implementation of the processes, possibly associated with the signaling role of early endosomes.
However, before ESCRT-0 is sidelined, HRS succeeds in recruiting TSG101 to the membrane. It is a component of the ESCTR-I complex consisting of the VPS28, VPS37A, B, and C, as well as MVB12A and B proteins, in addition to the one already mentioned. The TGS101 protein contains an untypical ubiquitin-recognizing domain UEV allowing crosstalk with the cargo transmitted to ESCRT-I by the HRS protein. The ESCRT-I subunit VPS28 also interacts with HRS. ESCRT-I recruitment is followed by the recruitment of ESCRT-II (consisting of EAP30, two copies of EAP20 and EAP45) due to interaction with the same VPS28. In this complex, EAP45 is responsible for binding with ubiquitinated cargo due to the presence of the ubiquitin-recognizing GLUE domain. The largest and most intricately organized ESCRT-III complex is the last one in this “conveyor”. It comprises a number of proteins designated as CHAMP (Charged Membrane Proteins): CHAMP1A and B, CHAMP2A and B, CHAMP3, CHAMP4A, B, and C, CHAMP5, and CHAMP6. It is interesting that the above-mentioned component of the ESCRT-I complex, VPS28, is able to interact also with CHAMP6, which goes beyond the “conveyor” hypothesis, demonstrating the intricate and highly dynamic interrelationships between all of the sorting complexes. All structural aspects are described in detail in a number of reviews [66-69].
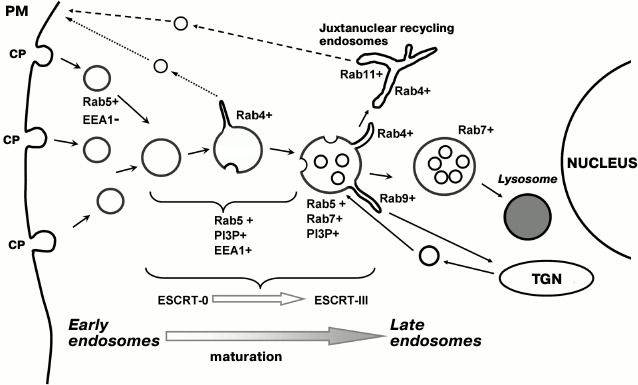
According to the data of mutation analysis, the roles of the complexes are different: the basic mission of ESCRT-0 and -I is to concentrate ubiquitinated cargoes, while ESCRT-II and -III form an internal vesicle and transfer the pre-ubiquitinated cargo into this vesicle [70]. In accordance with modern concepts, invagination occurs due to the fact that CHAMP proteins, being lined up “from head to tail”, are able to form a flat helix as a result of interaction with the membrane and lateral interactions between the coils. The process begins with the widest turn of the helix, which then gradually converges. The “uncoating” AAA-type ATPase VPS4 continuously disassembles the coiled coils; thereby, membrane-bound components gradually constrict the neck of the invagination, transforming it into a cargo-carrying vesicle and finally pinching it off from the outer membrane into the lumen of the endosome. This process is analyzed in several detailed reviews [69, 71]. It should be noted that the formation of vesicles with such direction of invaginations was one of the most enigmatic problems in endosome biogenesis, since the basic mechanism of transport vesicle formation was thought to be the assembly of coats that come from the cytoplasm onto the forming vesicle surface, performed their function, and dissociated back into the cytoplasm for repeated recycling. It is difficult to suppose, however, that a certain coat will “cover” the cavity of the future internal MVB vesicle and undergo degradation each time when moving with it inside and, thus, to be used only once. Indeed, in the mechanism described for ESCRT-III, all regulatory components dissociate into the cytoplasm before the closure of the vesicle and work in many cycles of MVB formation. It is interesting that ESCRT-III or its components proved to be involved in the packaging of viruses on the plasma membrane or membrane abscission in daughter cells after division [67]. Obviously, the exact sequence of all events is yet to be determined; so far we can make a highly simplified representation of the entire process. Figure 1 shows the scheme of endocytosis in linkage to localization of Rab proteins in the recycling and lysosomal degradative pathways and indicates the stages of transformation of early endosomes into late ones associated with the activity of ESCRT complexes. It is obvious that even the sites of beginning and ending of ESCRT-dependent sorting do not yet quite clearly correlate with the morphological changes in endosomes.
Fig. 1. Schematic presentation of the endocytic pathway typical of macromolecules that have entered the cells as a result of receptor-mediated clathrin-dependent endocytosis. Upon detachment from coated pits (CP) on the plasma membrane (PM), endocytic vesicles recruit Rab5 but are incapable of fusion at the very early stage because of the absence of tethering protein (EEA1–) and, probably, the inhibitory role of HRS. Later, EEA1 recruitment leads to endosome fusions, allowing the formation of internal vesicles and giving rise to MVB as a result of the action of ESCRT complexes. Recycling by the short pathway (Rab4-dependent) and long pathway (Rab4- and Rab11-dependent), as well as the influx of proteins from the trans-Golgi network (TGN) (Rab9+) associated with tubule formation, changes the composition of the vesicular part of endosomal membrane, which is reflected in the “Rab5–Rab7 conversion” and makes possible its interaction with the lysosome. The cargo translocated by ESCRT complexes to internal vesicles eventually degrades. The scheme represents the sequence of events related to the maturation of early endosomes into late endosomes. We try to demonstrate three basic points: (1) conditionality of the “interface” between the compartments; (2) domain organization of the processes determined by Rab proteins; and (3) vectorality of the entire process beginning at the cell periphery and ending in the juxtanuclear region.
ENDOCYTIC REGULATORY PROTEINS AND CYTOSKELETON
It has already been mentioned that endocytosis is associated with differently directed long-distance movements and changes in membrane shape as a result of fusions, invaginations, or tubulation. These processes are perfectly well coordinated in space and time and, consequently, susceptible to regulation. It is also obvious that cytoskeletal elements must be involved in most of these events if not in all. Hereinafter, we will try to generalize the data on the crosstalk between proteins involved in endocytic regulation and cytoskeletal components.
In this context, it is highly interesting that Rab proteins are involved in crosstalk with motor proteins. The impression is that just the crosstalk with the motors was the first function of Rab proteins during evolution, so numerous are their interrelationships with the motors both in interphase cells and in mitosis [72]. It has been shown for the Rab proteins maintaining the endocytic pathway that Rab4 and Rab11A interact with kinesin-2 (KIF3B), while Rab11 interacts with kinesin-1 [73]. Another kinesin involved in transferrin receptor recycling by the Rab4/Rab11 pathway, KIF16B, proved to be a member of the nexin family, SNX23. It is interesting that this kinesin is bound directly to endosomal membrane enriched in PI3P due to its PX domain [74]. The recycling pathway in the TGN is regulated by endosomal Rab9 and dynein. Though Rab proteins in principle are able to interact with the motors directly, in most known cases they are connected via adaptor proteins. The pattern of interaction for Rab4 is unknown, while Rab11 and Rab11A are bound with the motors via FIP3 and Rip11 (or FIP5) [73]. As already mentioned, Rab4 and Rab11 work in the recycling pathways to the plasma membrane, and their connection with plus-end directed motors of the kinesin family seem to be natural. However, as early as in 2001 it was found that Rab4A in the GTP-bound form was able to directly bind LIC1, the light intermediate chain of dynein-1 [75]. In addition, it was shown that Rab11A was also able to interact with two subunits of dynein-1, LIC1 and LIC2, via FIP3 [76, 77]. It is interesting that this interaction can result in the simultaneous binding of Rab11 with two motor complexes of dynein-1 [72].
With regard to Rab proteins organizing the delivery of endosomal cargoes into lysosomes, i.e. from the cell periphery to JNR, it would be logical to expect crosstalk with dynein. Indeed, the linker protein RILP (Rab-Interacting Lysosomal Protein) associated with the dynactin subunit p150/Glued and spectrin has been identified for Rab7, which is considered to be a marker of juxtanuclear late endosomes [78].
The situation with the key protein of endocytosis, Rab5, is much more complicated. It is known that this protein becomes incorporated into the membrane of the endocytic vesicle after it has already been separated from the membrane and passed through the layer of cortical actin. Interestingly, this process occurs with the aid of the same actin that can interact with the atypical GTPase dynamin; its molecules form a helix on the neck of a formed vesicle during polymerization. Actin plays a rather controversial role in this process, correlating with the degree of cell adhesion to substrate in the area of endocytic vesicle formation. The closer it is to the adhesion complex, the more effort is needed for membrane curvature, and the force developing due to clathrin “basket” formation becomes insufficient [79]. In the context of movement, small actin filaments polarized on an endosomal membrane may form something like tails, repelling the vesicle from the membrane to the zone where it can be “captured” by the MT plus end. Endosomes are tethered at the dynamic plus end of MT by capping proteins EB1 and EB3 and CLIP-170, which transfer the vesicle to the dynactin–dynein complex [80, 81]. CLIP-170 was one of the first proteins for which association with endosomes was established. Nevertheless, so far it is only known that its C-terminal domain interacts with endosomal membrane [82]. As regards Rab5, the best-studied protein of the Rab family, the sparsity of the data may be surprising. In 1999, the team of M. Zerial showed that the movement of endosomes to JNR depended on activated Rab5 and VPS34 [83]; in addition, Rab5 was shown to coprecipitate dynein [84]. Nevertheless, molecular details of the relationship between Rab5 and dynein are still absent.
As already mentioned, the resultant movement of endosomes is always directed from the membrane to the center of MT convergence, and it would be logical to assume the exceptional involvement of dynein in this process. However, experiments with drosophila have shown that early endosomes can also interact with kinesin-73, a KIF1A analog, due to binding with Rab5 directly via its C-terminus [85]. In addition, using isolated early and late endosomes, kinesin-1 and -2, and dynein in a cell-free system, it was demonstrated that kinesin-1 and dynein participate in the movement of early endosomes, kinesin-2 supports the movement of both late and early endosomes, while dynein does not move late endosomes at all [86]. The authors interpret their results as evidence that dynein is indirectly involved in the mobility of late endosomes if at all, which however contradicts previously obtained data [78]. However, we believe that this contradiction is eliminated if the role of dynein in the juxtanuclear region is considered to be associated not with movement, but rather with positioning and clustering of late endosomes. The reported direct interaction between differently directed cytoplasmic dynein and kinesin-1 [87] may be evidence for the existence of a more complex MT-dependent mechanism for maintenance of endolysosomal localization.
To date, the concept of simultaneous existence of both plus-end and minus-end motors on endosomal membrane, resulting in the free movement of vesicles along the microtubule in both directions, is rather popular. Indeed, it is difficult to make a different conclusion based both on the analysis of proteins associated with the isolated endosomes and on the data of in vitro experiments on the sliding of endosomes along MT. Even the live imaging of endosome movements in cells is evidence in favor of such a concept. The impression is that the rapid runs of endosomes at rates typical of motor-dependent movement can occur in absolutely unpredictable directions, being interrupted by pauses of chaotic movement within a small area. Nevertheless, the characteristic feature of endocytosis, which no one can dispute, is the resulting movement to JNR where the center of MT organization is located. The causes of cessation of movement are quite clear: the endosome exists in an “overcrowded space”; hence, the intersection of microtubules or actin microfilaments, the collision with ER tubules, and other organelles may simply pull the endosome down from MT. The causes and sense of bidirectional movement may be different. First, reverse movements may enhance to a limited extent the probability of encountering endosomes of the same maturity that are ready for tethering and fusion. About 400 endosomes are simultaneously formed, e.g. in HeLa cells and, eventually, about 10 late endosomes are found in the juxtanuclear region [88]. Even assuming the model of pairwise fusion, the number of rounds of such effective contacts resulting in fusion is no more than 5-6 (excluding the fusions of vesicles from the TGN); the number of endosomes is greater on the periphery, where the microtubule network density is lower and, consequently, the probability of contact is also lower. Thus, bidirectional motion can actually simulate the “search-and-capture” logic and be regulated by the current ratio of active kinesin and dynein molecules on the endosomal membrane, which may quickly vary. Contrariwise, even the movement of one endosome in different directions along the same MT can be apparent, associated with the motion not by a single MT but along the bundle of antiparallel MTs, which may be formed by PRC1-type proteins [89]. It is difficult to differentiate such a bundle from a single MT using light microscopy. As a result, an endosome can move forward and backwards by means of dynein but along antiparallel rails. In addition, microtubules may be highly distorted and, as a result, intersect at acute angles; eventually, the jump of endosome in linkage with dynein from one MT to another in the place of their intersection may look like change in direction of motion to the opposite or close to the opposite.
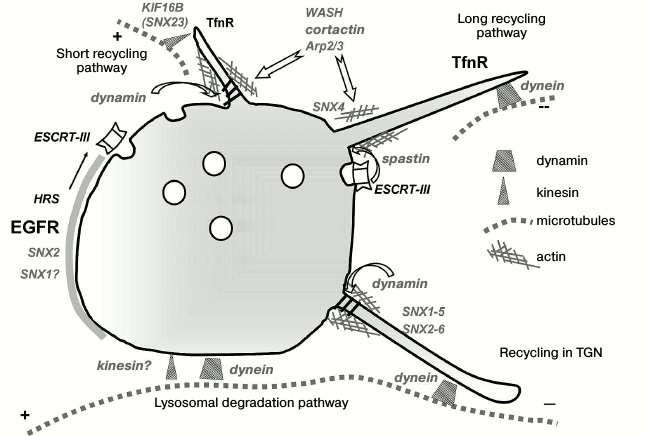
Such reasoning, however, supposes that differently directed motors are localized on endosomes alternately and do not take into account an important aspect such as domain organization of endosomes. Figure 2 generalizes data on the spatial localization of motor proteins and the involvement of MT, actin, and actin-binding proteins in endosome functioning. As mentioned above, Rab proteins can maintain functionally different domains, within which the sorting nexins form still smaller domains to support sorting processes. Sorting is usually associated with the formation of tubules or invaginations, i.e. with higher membrane tension in the region of curvature requiring the application of considerable forces. Most of the data on association with motors indicates precisely their distinct domain localization in the areas of tubule formation. A microtubule in this case is a necessary platform for endosome localization (positioning) due to dynein, on one hand, and formation of a tubule that grows due to the pull developed by the motor (which may be both kinesin and dynein in case of recycling in the TGN), on the other hand. It is not known whether in this case there are any preferences with regard to its own microtubule used as a “support”, or tubulation along the neighboring microtubules is also possible; however, on the face of it, with relatively small dimensions of the organelle, such process of endosome “tearing” may result in bidirectional movement, even in spite of strict compartmentalization of the motors. Dynein may be localized in the Rab5-enriched vesicular domain, while KIF16B (SNX23) will be localized on the Rab4/Rab11 domain of the same endosome, which is responsible for transferrin receptor recycling. It is interesting that bidirectional movements are typical mainly of peripheral early endosomes of no more than 400-500 nm diameter, while the larger perinuclear late endosomes often move unidirectionally or are completely immobile due to spatial restrictions. As already mentioned, Rab7 in linkage with dynein provides not motion but rather positioning of late endosomes and hybrid endolysosomes on stable, highly acetylated MTs, and the endosomes making up small clusters are often localized exactly at the “intersections” of several MTs [90].
Fig. 2. Endosomal domain structure and membrane remodeling are maintained by the tubulin and actin cytoskeleton. The Rab5-positive endosome with the membrane enriched in PI3P contains still smaller functional domains important for cargo receptor sorting. SNX2 and probably SNX1 form a sorting platform on the vesicular part of the endosome. The activated EGF receptor (EGFR) concentrated within this platform is then packed by ESCRT complexes into internal vesicles of MVBs. The recycling transferrin receptor (TfnR) interacts with SNX4, which is involved in membrane curving and tubule formation together with actin-bound proteins. Tubulation, in its turn, is maintained by MTs and MT-bound motors. The “choice” of a motor depends on membrane properties and the supposed direction of tubule movement. Tubule separation is possible with the involvement of both dynamin and spastin interacting with the components of the ESCRT-III complex. The vesicular part of the endosome moves to the MT minus end due to binding with dynein. Thus, endosomal localization of motor proteins is mainly domain-type. The crosstalk between kinesin and dynein on the endosome is discussed in the text.
Participation of actin in long-distance movements is not confirmed in general with the exception of partial cases, though it is difficult to overestimate its local role in transport regulation. Compartmentalization is one of the basic principles of cell organization, and endocytosis makes it possible to coordinate many processes not only in space but also in time. A number of proteins providing crosstalk with both MT and actin interact with the components of ESCRT complexes.
It was believed that the rapid recycling pathway was regulated “by default” as the only alternative to the ubiquitin signal for degradation. However, a work published in 2005 demonstrated that destruction of the complex designated as CART (Cytoskeleton-Associated Recycling or Transport) resulted in disturbance of the short recycling pathway. The components of this complex proved to be myosin-V and BERP bound to HRS via actinin-4 [91]. Then TSG101 (a component of the ESCRT-I complex) proved to be able to recognize the MT-severing protein stathmin/Op18 [92]. We also revealed the association of stathmin with EGF receptor-containing endosomes in the first 15 min of endocytosis, correlating with endosomal localization of activated MAP kinase (unpublished data) that was able to inhibit its activity [93]. Recently, it was shown that another MT-severing protein, spastin, is bound to a component of the ESCRT-III complex, CHAMP1B/IST1, while spastin malfunction results in the appearance of abnormally long recycling tubules [94].
These observations suggest that destruction of microtubules by spastin in this case works as a mechanism of tubule membrane separation from the endosomal body, and this event is coordinated with the formation of internal invaginations of MVB. In principle, as already mentioned, the best known mechanism of membrane separation is associated with the function of the atypical GTPase of dynamin, which is able to form helices on tubules and to break them off during GTP hydrolysis. Indeed, dynamin involvement has been shown for the regulation of recycling tubule separation at earlier stages of EE existence, and its action is supported by Arp2/3-dependent actin polymerization via the cortactin, WASP, and WASH proteins [95, 96]. Actin networks in this case are small in size, and their role probably consists of providing extra effort for the initiation of tubulation and the separation of formed tubules. On another part, it was reported that WASH knockout in MEF cells resulted in the emergence of abnormally large EE, which maintained their domain organization (it also concerns domain enriched in the retromer complex involved in WASH-mediated recycling), though lacking the associated F-actin and Arp2/3, but did not form very long tubules as would be expected. In addition, lysosomes were also shown to undergo dramatic changes, though EGF receptor degradation proceeded normally [97]. These data show a pattern of much finer control of the involvement of actin in regulation and maintenance of integrity in the endolysosomal system.
Many components of the ESCRT-III complex, as well as the disassembling VPS4 ATPase, carry MIT (Microtubule Interacting and Trafficking) domains or their analogs. In fact, the fundamental data set is associated with participation of these domains in the crosstalk of complex components with each other, omitting potential interrelationship with microtubules. Demonstration of how the rapid movement of Rab5-positive endosomes along the MT may switch over to ineffective movement along actin filaments during overexpression of the huntingtin (Htt) and HAP40 protein complex, which is a Rab5 effector [98], confirms the local role of actin and its inability to maintain the “long-distance race” in most cell systems.
CARGO RECEPTORS AND CYTOSKELETON
In conclusion, the interrelationship between endocytosis and cytoskeleton will not be described comprehensively without mentioning the role of cargo receptors in the processes under discussion. Some data show that HDAC6 histone deacetylase involved in maintenance of the dynamic behavior of MT plus ends is associated with endosomes and may be negatively regulated due to tyrosine phosphorylation on Tyr570 by EGF receptor [99]. The authors demonstrated that this event occurs 30 min after stimulation of endocytosis, and this time lag is supposed to be necessary for accumulation of the “threshold level” of phosphorylated inactive form of HDAC6, which results in enhanced acetylation and stabilization of MTs [99]. From our point of view, this interpretation raises strong doubts, because it would be logical to explain this effect by the endosomal localization of HDAC6, as this event occurs after endosomes have reached a certain maturity stage. In addition, Gao et al. [100] have shown that HDAC6 knockout substantially accelerates EGF receptor delivery to lysosomes and its degradation. We have already mentioned previously the HRS-dependent mechanism preventing too rapid maturation of endosomes [64]. It seems that the duration of receptor localization on the outer membrane of the endosome, i.e. the period of time when it remains “signally” active, is extremely important for the coordination of transport and signalization, and this stage is associated with dynamic microtubules. The results obtained in our laboratory confirm that the level of MT acetylation increases 30-60 min after the stimulation of endocytosis of EGF–receptor complexes, mainly due to MT parts located closer to the minus end, i.e. those where endosomes are localized at this moment [90]. In addition, c-Cbl ubiquitin ligase, which maintains ubiquitination of the receptor until it enters the internal MVB vesicles, proved to be able to interact with β-tubulin via its TKB domain (responsible for the binding with ubiquitinated receptors) and to displace HDAC6 from MTs, thereby stabilizing them [101]. Taking into consideration that EGF receptor is continuously connected with c-Cbl right up to moving into the internal vesicles of MVBs, it is obvious that such substitution becomes possible after dissociation of the complex and release of the receptor-binding site. It is interesting that we have also observed that c-Cbl retains its localization on endolysosomal membranes in JNR after the receptor has been degraded [53]; hence, c-Cbl can be related to stabilization of endosome-associated MTs.
This review does not aspire to complete coverage of the subject of crosstalk between the cytoskeleton and the proteins participating in endocytosis. We merely omitted quite a number of crucial points. However, even a very simplified representation allows us to form an idea of the highly dynamic interactions with complicated organization in time and space, which coordinate endocytosis and cytoskeleton. It may be noted that the obvious role of cytoskeleton in directed movements, though best studied, is still a subject of heated discussions and contains many ambiguous points. However, researchers begin to focus their attention on the involvement of cytoskeleton in the regulation of processes such as cargo sorting and membrane remodeling during fusions, invaginations, and tubulation. The already obvious conclusion is strict compartmentalization of membrane-remodeling proteins and lipids, as well as various motors, on the membrane of a single endosome. In addition, the involvement of receptors (especially signaling) and the related regulatory proteins in coordination of these interactions becomes increasingly evident. Development of methods of live cell video imaging, however, has shown an exceptionally high dynamicity of all processes associated with membrane remodeling, the mechanisms of which (e.g. the possibility of exchanging some part of membranes during contacts, etc.) are still far from being understood.
This work was supported by the Russian Foundation for Basic Research (project No. 12-04-00838a).
REFERENCES
1.Stahl, P., and Schwartz, A. L. (1986)
Receptor-mediated endocytosis, J. Clin. Invest., 77,
657-662.
2.Sorkin, A. (2004) Cargo recognition during
clathrin-mediated endocytosis: a team effort, Curr. Opin. Cell
Biol., 16, 392-399.
3.Marks, M. S., Woodruff, L., Ohio, H., and
Bonifacino, J. S. (1996) Protein targeting by tyrosine- and
di-leucine-based signals: evidence for distinct saturable components,
J. Cell Biol., 135, 341-354.
4.Shen, D., Wang, X., and Xu, H. (2011) Pairing
phosphoinositides with calcium ions in endolysosomal dynamics:
phosphoinositides control the direction and specificity of membrane
trafficking by regulating the activity of calcium channels in the
endolysosomes, Bioessays, 33, 448-457.
5.Abe, K., and Puertollano, R. (2011) Role of TRP
channels in the regulation of the endosomal pathway, Physiology
(Bethesda), 26, 14-22.
6.Vina-Vilaseca, A., Bender-Sigel, J., Sorkina, T.,
Closs, E. I., and Sorkin, A. (2011) Protein kinase C-dependent
ubiquitination and clathrin-mediated endocytosis of the cationic amino
acid transporter CAT-1, J. Biol. Chem., 286,
8697-8706.
7.Mousavi, S. A., Malerod, L., Berg, T., and Kjeken,
R. (2004) Clathrin-dependent endocytosis, Biochem. J.,
377, 1-16.
8.Gruenberg, J., Griffiths, G., and Howell, K. E.
(1989) Characterization of the early endosome and putative endocytic
carrier vesicles in vivo and with an assay of vesicle fusion
in vitro, J. Cell Biol., 108, 1301-1316.
9.Ali, N., and Evans, W. H. (1990) Priority targeting
of glycosyl-phosphatidylinositol-anchored proteins to the
bile-canalicular (apical) plasma membrane of hepatocytes. Involvement
of “late” endosomes, Biochem. J., 271,
193-199.
10.Sorkin, A., Krolenko, S., Kudrjavtceva, N.,
Lazebnik, J., Teslenko, L., Soderquist, A. M., and Nikolsky, N. (1991)
Recycling of epidermal growth factor–receptor complexes in A431
cells: identification of dual pathways, J. Cell Biol.,
112, 55-63.
11.Scott, C. C., Vacca, F., and Gruenberg, J. (2014)
Endosome maturation, transport and functions, Semin. Cell Dev.
Biol., pii: S1084-9521(14)00070-6.
12.Linderman, J. J., and Lauffenburger, D. A. (1986)
Analysis of intracellular receptor/ligand sorting. Calculation of mean
surface and bulk diffusion times within a sphere, Biophys. J.,
50, 295-305.
13.Forgac, M., Cantley, L., Wiedenmann, B.,
Altstiel, L., and Branton, D. (1983) Clathrin-coated vesicles contain
an ATP-dependent proton pump, Proc. Natl. Acad. Sci. USA,
80, 1300-1303.
14.Ostlund, R. E., Jr., Pfleger, B., and Schonfeld,
G. (1979) Role of microtubules in low density lipoprotein processing by
cultured cells, J. Clin. Invest., 63, 75-84.
15.Hopkins, C. R., Miller, K., and Beardmore, J. M.
(1985) Receptor-mediated endocytosis of transferrin and epidermal
growth factor receptors: a comparison of constitutive and
ligand-induced uptake, J. Cell. Sci. Suppl., 3,
173-186.
16.Felder, S., Miller, K., Moehren, G., Ullrich, A.,
Schlessinger, J., and Hopkins, C. R. (1990) Kinase activity controls
the sorting of the epidermal growth factor receptor within the
multivesicular body, Cell, 61, 623-634.
17.Van Weering, J. R., and Cullen, P. J. (2014)
Membrane-associated cargo recycling by tubule-based endosomal sorting,
Semin. Cell Dev. Biol., pii: S1084-9521(14)00043-3.
18.Woodman, P. G., and Futter, C. E. (2008)
Multivesicular bodies: coordinated progression to maturity, Curr.
Opin. Cell Biol., 20, 408-414.
19.Gonnord, P., Blouin, C. M., and Lamaze, C. (2012)
Membrane trafficking and signaling: two sides of the same coin,
Semin. Cell Dev. Biol., 23, 154-164.
20.Luzio, J. P., Gray, S. R., and Bright, N. A.
(2010) Endosome-lysosome fusion, Biochem. Soc. Trans.,
38, 1413-1416.
21.Aniento, F., Emans, N., Griffiths, G., and
Gruenberg, J. (1993) Cytoplasmic dynein-dependent vesicular transport
from early to late endosomes, J. Cell Biol., 123,
1373-1387.
22.Herman, B., and Albertini, D. F. (1984) A
time-lapse video image intensification analysis of cytoplasmic
organelle movements during endosome translocation, J. Cell
Biol., 98, 565-576.
23.Wacker, I., Kaether, C., Kromer, A., Migala, A.,
Almers, W., and Gerdes, H. H. (1997) Microtubule-dependent transport of
secretory vesicles visualized in real time with a GFP-tagged secretory
protein, J. Cell Sci., 110, 1453-1463.
24.Bryantseva, S. A., and Zhapparova, O. N. (2012)
Bidirectional transport of organelles: unity and struggle of opposing
motors, Cell Biol. Int., 36, 1-6.
25.Barr, F., and Lambright, D. G. (2010) Rab GEFs
and GAPs, Curr. Opin. Cell Biol., 22, 461-470.
26.Pfeffer, S. R. (2001) Rab GTPases: specifying and
deciphering organelle identity and function, Trends Cell Biol.,
11, 487-491.
27.Cai, H., Reinisch, K., and Ferro-Novick, S.
(2007) Coats, tethers, Rabs, and SNAREs work together to mediate the
intracellular destination of a transport vesicle, Dev. Cell,
12, 671-682.
28.Markgraf, D. F., Peplowska, K., and Ungermann, C.
(2007) Rab cascades and tethering factors in the endomembrane system,
FEBS Lett., 581, 2125-2130.
29.Hutagalung, A. H., and Novick, P. J. (2011) Role
of Rab GTPases in membrane traffic and cell physiology, Physiol.
Rev., 91, 119-149.
30.Sonnichsen, B., De Renzis, S., Nielsen, E.,
Rietdorf, J., and Zerial, M. J. (2000) Distinct membrane domains on
endosomes in the recycling pathway visualized by multicolor imaging of
Rab4, Rab5, and Rab11, Cell Biol., 149, 901-914.
31.Stenmark, H., Parton, R. G., Steele-Mortimer, O.,
Lutcke, A., Gruenberg, J., and Zerial, M. (1994) Inhibition of rab5
GTPase activity stimulates membrane fusion in endocytosis, EMBO
J., 13, 1287-1296.
32.Zeigerer, A., Gilleron, J., Bogorad, R. L.,
Marsico, G., Nonaka, H., Seifert, S., Epstein-Barash, H., Kuchimanchi,
S., Peng, C. G., Ruda, V. M., Del Conte-Zerial, P., Hengstler, J. G.,
Kalaidzidis, Y., Koteliansky, V., and Zerial, M. (2012) Rab5 is
necessary for the biogenesis of the endolysosomal system in
vivo, Nature, 485, 465-470.
33.Rink, J., Ghigo, E., Kalaidzidis, Y., and Zerial,
M. (2005) Rab conversion as a mechanism of progression from early to
late endosomes, Cell, 122, 735-749.
34.Chaineau, M., Ioannou, M. S., and McPherson, P.
S. (2013) Rab35: GEFs, GAPs and effectors, Traffic,
14, 1109-1117.
35.Zhu, H., Liang, Z., and Li, G. (2009) Rabex-5 is
a Rab22 effector and mediates a Rab22-Rab5 signaling cascade in
endocytosis, Mol. Biol. Cell, 20, 4720-4729.
36.Zhang, C., Li, A., Zhang, X., and Xiao, H. (2011)
A novel TIP30 protein complex regulates EGF receptor signaling and
endocytic degradation, J. Biol. Chem., 286,
9373-9381.
37.Barbieri, M. A., Fernandez-Pol, S., Hunker, C.,
Horazdovsky, B. H., and Stahl, P. D. (2004) Role of rab5 in EGF
receptor-mediated signal transduction, Eur. J. Cell Biol.,
83, 305-314.
38.Shin, H. W., Hayashi, M., Christoforidis, S.,
Lacas-Gervais, S., Hoepfner, S., Wenk, M. R., Modregger, J.,
Uttenweiler-Joseph, S., Wilm, M., Nystuen, A., Frankel, W. N.,
Solimena, M., De Camilli, P., and Zerial, M. (2005) An enzymatic
cascade of Rab5 effectors regulates phosphoinositide turnover in the
endocytic pathway, J. Cell Biol., 170, 607-618.
39.Birkeland, H. C., and Stenmark, H. (2004) Protein
targeting to endosomes and phagosomes via FYVE and PX domains, Curr.
Top Microbiol. Immunol., 282, 89-115.
40.Lawe, D. C., Chawla, A., Merithew, E., Dumas, J.,
Carrington, W., Fogarty, K., Lifshitz, L., Tuft, R., Lambright, D., and
Corvera, S. (2002) Sequential roles for
phosphatidylinositol-3-phosphate and Rab5 in tethering and fusion of
early endosomes via their interaction with EEA1, J. Biol. Chem.,
277, 8611-8617.
41.Hickey, C. M., and Wickner, W. (2010) HOPS
initiates vacuole docking by tethering membranes before trans-SNARE
complex assembly, Mol. Biol. Cell, 21, 2297-2305.
42.Haft, C. R., de la Luz Sierra, M., Barr, V. A.,
Haft, D. H., and Taylor, S. I. (1998) Identification of a family of
sorting nexin molecules and characterization of their association with
receptors, Mol. Cell. Biol., 18, 7278-7287.
43.Van Weering, J. R., Verkade, P., and Cullen, P.
J. (2010) SNX-BAR proteins in phosphoinositide-mediated, tubular-based
endosomal sorting, Semin. Cell Dev. Biol., 21,
371-380.
44.Carlton, J., Bujny, M., Peter, B. J., Oorschot,
V. M., Rutherford, A., Mellor, H., Klumperman, J., McMahon, H. T., and
Cullen, P. J. (2004) Sorting nexin-1 mediates tubular endosome-to-TGN
transport through coincidence sensing of high-curvature membranes and
3-phosphoinositides, Curr. Biol., 14, 1791-1800.
45.Skanland, S. S., Walchli, S., Brech, A., and
Sandvig, K. (2009) SNX4 in complex with clathrin and dynein:
Implications for endosome movement, PLoS ONE, 4,
e5935.
46.Gullapalli, A., Garrett, T. A., Paing, M. M.,
Griffin, C. T., Yang, Y., and Trejo, J. (2004) A role for sorting nexin
2 in epidermal growth factor receptor down-regulation: evidence for
distinct functions of sorting nexin 1 and 2 in protein trafficking,
Mol. Biol. Cell, 15, 2143-2155.
47.Chin, L. S., Raynor, M. C., Wei, X., Chen, H. Q.,
and Li, L. (2001) Hrs interacts with sorting nexin 1 and regulates
degradation of epidermal growth factor receptor, J. Biol.
Chem., 276, 7069-7078.
48.Traer, C. J., Rutherford, A. C., Palmer, K. J.,
Wassmer, T., Oakley, J., Attar, N., Carlton, J. G., Kremerskothen, J.,
Stephens, D. J., and Cullen, P. J. (2007) SNX4 coordinates endosomal
sorting of TfnR with dynein-mediated transport into the endocytic
recycling compartment, Nat. Cell Biol., 9, 1370-1380.
49.Wassmer, T., Attar, N., Harterink, M., van
Weering, J. R., Traer, C. J., Oakley, J., Goud, B., Stephens, D. J.,
Verkade, P., Korswagen, H. C., and Cullen, P. J. (2009) The retromer
coat complex coordinates endosomal sorting and dynein-mediated
transport, with carrier recognition by the tans-Golgi network, Dev.
Cell, 17, 110-122.
50.Bowtell, D. D., and Langdon, W. Y. (1995) The
protein product of the c-cbl oncogene rapidly complexes with the EGF
receptor and is tyrosine phosphorylated following EGF stimulation,
Oncogene, 11, 1561-1567.
51.Huang, F., Kirkpatrick, D., Jiang, X., Gygi, S.,
and Sorkin, A. (2006) Differential regulation of EGF receptor
internalization and degradation by multiubiquitination within the
kinase domain, Mol. Cell, 21, 737-748.
52.De Melker, A. A., van der Horst, G., Calafat, J.,
Jansen, H., and Borst, J. (2001) c-Cbl ubiquitinates the EGF receptor
at the plasma membrane and remains receptor associated throughout the
endocytic route, J. Cell Sci., 114, 2167-2178.
53.Melikova, M. S., Kondratov, K. A., and Kornilova,
E. S. (2006) Two different stages of epidermal growth factor (EGF)
receptor endocytosis are sensitive to free ubiquitin depletion produced
by proteasome inhibitor MG132, Cell Biol. Int.,
30, 31-43.
54.Wright, M. H., Berlin, I., and Nash, P. D. (2011)
Regulation of endocytic sorting by ESCRT-DUB-mediated deubiquitination,
Cell Biochem. Biophys., 60, 39-46.
55.Kondratov, K. A., Chernorudsky, A. L., Amosova,
A. P., and Kornilova, E. S. (2009) Analysis of the effect of Tyrphostin
AF1478 on the behavior of internalized EGF receptor at different stages
of endocytosis, Tsitologiya, 51, 520-525.
56.Raiborg, C., Bremnes, B., Mehlum, A., Gillooly,
D. J., D’Arrigo, A., Stang, E., and Stenmark, H. (2001) FYVE and
coiled-coil domains determine the specific localization of Hrs to early
endosomes, J. Cell Sci., 114, 2255-2263.
57.Mageswaran, S. K., Dixon, M. G., Curtiss, M.,
Keener, J. P., and Babst, M. (2014) Binding to any ESCRT can mediate
ubiquitin-independent cargo sorting, Traffic, 15,
212-229.
58.Lim, J., Son, W. S., Park, J. K., Kim, E. E.,
Lee, B. J., and Ahn, H. C. (2011) Solution structure of UIM and
interaction of tandem ubiquitin binding domains in STAM1 with
ubiquitin, Biochem. Biophys. Res. Commun., 405,
24-30.
59.Kato, M., Miyazawa, K., and Kitamura, N. (2000) A
deubiquitinating enzyme UBPY interacts with the Src homology 3 domain
of Hrs-binding protein via a novel binding motif PX(V/I)(D/N)RXXKP,
J. Biol. Chem., 275, 37481-37487.
60.Raiborg, C., Wesche, J., Malerød, L., and
Stenmark, H. (2006) Flat clathrin coats on endosomes mediate
degradative protein sorting by scaffolding Hrs in dynamic
microdomains, J. Cell Sci., 119, 2414-2424.
61.Chin, L. S., Raynor, M. C., Wei, X., Chen, H. Q.,
and Li, L. (2001) Hrs interacts with sorting nexin 1 and regulates
degradation of epidermal growth factor receptor, J. Biol. Chem.,
276, 7069-7078.
62.Stern, K. A., Visser-Smit, G. D., Place, T. L.,
Winistorfer, S., Piper, R. C., and Lill, N. L. (2007) Epidermal growth
factor receptor fate is controlled by Hrs tyrosine phosphorylation
sites that regulate Hrs degradation, Mol. Cell Biol., 27,
888-898.
63.Hoeller, D., Crosetto, N., Blagoev, B., Raiborg,
C., Tikkanen, R., Wagner, S., Kowanetz, K., Breitling, R., Mann, M.,
Stenmark, H., and Dikic, I. (2006) Regulation of ubiquitin-binding
proteins by monoubiquitination, Nat. Cell Biol., 8,
163-169.
64.Sun, W., Yan, Q., Vida, T. A., and Bean, A. J.
(2003) Hrs regulates early endosome fusion by inhibiting formation of
an endosomal SNARE complex, J. Cell Biol., 162,
125-137.
65.Yan, Q., Sun, W., McNew, J. A., Vida, T. A., and
Bean, A. J. (2004) Ca2+ and N-ethylmaleimide-sensitive
factor differentially regulate disassembly of SNARE complexes on early
endosomes, J. Biol. Chem., 279, 18270-18276.
66.Hurley, J. H. (2008) ESCRT complexes and the
biogenesis of multivesicular bodies, Curr. Opin. Cell Biol.,
20, 4-11.
67.Slagsvold, T., Pattni, K., Malerod, L., and
Stenmark, H. (2006) Endosomal and non-endosomal functions of ESCRT
proteins, Trends Cell Biol., 16, 317-326.
68.Hurley, J. H., and Emr, S. D. (2006) The ESCRT
complexes: structure and mechanism of a membrane-trafficking network,
Annu. Rev. Biophys. Biomol. Struct., 35, 277-298.
69.Williams, R. L., and Urbe, S. (2007) The emerging
shape of the ESCRT machinery, Nat. Rev. Mol. Cell Biol.,
8, 355-368.
70.Vaccari, T., Rusten, T. E., Menut, L., Nezis, I.
P., Brech, A., Stenmark, H., and Bilder, D. (2009) Comparative analysis
of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by
efficient isolation of ESCRT mutants, J. Cell Sci., 122,
2413-2423.
71.Adell, M. A. Y., and Teis, D. (2011) Assembly and
disassembly of the ESCRT-III membrane scission complex, FEBS
Lett., 585, 3191-3196.
72.Horgan, C. P., and McCaffrey, M. W. (2011) Rab
GTPases and microtubule motors, Biochem. Soc. Trans., 39,
1202-1206.
73.Schonteich, E., Wilson, G. M., Burden, J.,
Hopkins, C. R., Anderson, K., Goldenring, J. R., and Prekeris, R.
(2008) The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic
protein recycling, J. Cell Sci., 121,
3824-3833.
74.Hoepfner, S., Severin, F., Cabezas, A.,
Habermann, B., Runge, A., Gillooly, D., Stenmark, H., and Zerial, M.
(2005) Modulation of receptor recycling and degradation by the
endosomal kinesin KIF16B, Cell, 121, 437-450.
75.Bielli, A., Thornqvist, P. O., Hendrick, A. G.,
Finn, R., Fitzgerald, K., and McCaffrey, M. W. (2001) The small GTPase
Rab4A interacts with the central region of cytoplasmic dynein light
intermediate chain-1, Biochem. Biophys. Res. Commun.,
281, 1141-1153.
76.Horgan, C. P., Hanscom, S. R., Jolly, R. S.,
Futter, C. E., and McCaffrey, M. W. (2010) Rab11-FIP3 binds dynein
light intermediate chain 2 and its overexpression fragments the Golgi
complex, Biochem. Biophys. Res. Commun., 394,
387-392.
77.Horgan, C. P., Hanscom, S. R., Jolly, R. S.,
Futter, C. E., and McCaffrey, M. W. (2010) Rab11-FIP3 links the Rab11
GTPase and cytoplasmic dynein to mediate transport to the
endosomal-recycling compartment, J. Cell Sci., 123,
181-191.
78.Jordens, I., Fernandez-Borja, M., Marsman, M.,
Dusseljee, S., Janssen, L., Calafat, J., Janssen, H., Wubbolts, R., and
Neefjes, J. (2001) The Rab7 effector protein RILP controls lysosomal
transport by inducing the recruitment of dynein–dynactin motors,
Curr. Biol., 11, 1680-1685.
79.Mooren, O. L., Galletta, B. J., and Cooper, J. A.
(2012) Roles for actin assembly in endocytosis, Annu. Rev.
Biochem., 81, 661-686.
80.Moughamian, A. J., Osborn, G. E., Lazarus, J. E.,
Maday, S., and Holzbaur, E. L. (2013) Ordered recruitment of dynactin
to the microtubule plus-end is required for efficient initiation of
retrograde axonal transport, J. Neurosci., 33,
13190-13203.
81.Lomakin, A. J., Semenova, I., Zaliapin, I.,
Kraikivski, P., Nadezhdina, E., Slepchenko, B. M., Akhmanova, A., and
Rodionov, V. (2009) CLIP-170-dependent capture of membrane organelles
by microtubules initiates minus-end directed transport, Dev.
Cell., 17, 323-333.
82.Pierre, P., Pepperkok, R., and Kreis, T. E.
(1994) Molecular characterization of two functional domains of CLIP-170
in vivo, J. Cell Sci., 107, 1909-1920.
83.Nielsen, E., Severin, F., Backer, J. M., Hyman,
A. A., and Zerial, M. (1999) Rab5 regulates motility of early endosomes
on microtubules, Nat. Cell Biol., 1, 376-382.
84.Zhang, J., Li, S., Fischer, R., and Xiang, X.
(2003) Accumulation of cytoplasmic dynein and dynactin at microtubule
plus ends in Aspergillus nidulans is kinesin dependent, Mol.
Biol. Cell, 14, 1479-1488.
85.Huckaba, T. M., Gennerich, A., Wilhelm, J. E.,
Chishti, A. H., and Vale, R. D. (2011) Kinesin-73 is a processive motor
that localizes to Rab5-containing organelles, J. Biol. Chem.,
286, 7457-7467.
86.Loubery, S., Wilhelm, C., Hurbain, I., Neveu, S.,
Louvard, D., and Coudrier, E. (2008) Different microtubule motors move
early and late endocytic compartments, Traffic, 9,
492-509.
87.Ligon, L. A., Tokito, M., Finklestein, J. M.,
Grossman, F. E., and Holzbaur, E. L. (2004) A direct interaction
between cytoplasmic dynein and kinesin I may coordinate motor activity,
J. Biol. Chem., 279, 19201-19208.
88.Zlobina, M. V., Kharchenko, M. V., and Kornilova,
E. S. (2013) Analysis of the dynamics of EGF receptor endocytosis by
the images of confocal light microscopy on fixed cells,
Tsitologiya, 55, 348-357.
89.Pringle, J., Muthukumar, A., Tan, A., Crankshaw,
L., Conway, L., and Ross, J. L. (2013) Microtubule organization by
kinesin motors and microtubule crosslinking protein MAP65, J. Phys.
Condens. Matter, 25, 374103.
90.Zlobina, M. V., Kharchenko, M. V., Latkin, D. S.,
and Kornilova, E. S. (2010) Acetylation of microtubules during
endocytosis of the epidermal growth factor (c-ErbB1) receptor in
interphasic HeLa cells, Tsitologiya, 52, 466-476.
91.Yan, Q., Sun, W., Kujala, P., Lotfi, Y., Vida, T.
A., and Bean, A. J. (2005) CART: An Hrs/actinin-4/BERP/myosin V
protein complex required for efficient receptor recycling, Mol.
Biol. Cell., 16, 2470-2482.
92.Li, L., and Cohen, S. N. (1996) Tsg101: a novel
tumor susceptibility gene isolated by controlled homozygous functional
knockout of allelic loci in mammalian cells, Cell, 85,
319-329.
93.Lovric, J., Dammeier, S., Kieser, A., Mischak,
H., and Kolch, W. (1998) Activated raf induces the hyperphosphorylation
of stathmin and the reorganization of the microtubule network, J.
Biol. Chem., 273, 22848-22855.
94.Allison, R., Lumb, J. H., Fassier, C., Connell,
J. W., Ten Martin, D., Seaman, M. N., Hazan, J., and Reid, E. (2013) An
ESCRT–spastin interaction promotes fission of recycling tubules
from the endosome, J. Cell Biol., 202, 527-543.
95.Chua, J., Rikhy, R., and Lippincott-Schwartz, J.
(2009) Dynamin 2 orchestrates the global actomyosin cytoskeleton for
epithelial maintenance and apical constriction, Proc. Natl. Acad.
Sci. USA, 106, 20770-20775.
96.Helgeson, L. A., and Nolen, B. J. (2013)
Mechanism of synergistic activation of Arp2/3 complex by cortactin and
N-WASP, Elife, 2, e00884.
97.Gomez, T. S., Gorman, J. A., de Narvajas, A. A.,
Koenig, A. O., and Billadeau, D. D. (2012) Trafficking defects in
WASH-knockout fibroblasts originate from collapsed endosomal and
lysosomal networks, Mol. Biol. Cell., 23, 3215-3228.
98.Pal, A., Severin, F., Hopfner, S., and Zerial, M.
(2008) Regulation of endosome dynamics by Rab5 and
Huntingtin–HAP40 effector complex in physiological versus
pathological conditions, Methods Enzymol., 438,
239-257.
99.Deribe, Y. L., Wild, P., Chandrashaker, A.,
Curak, J., Schmidt, M. H., Kalaidzidis, Y., Milutinovic, N.,
Kratchmarova, I., Buerkle, L., Fetchko, M. J., Schmidt, P., Kittanakom,
S., Brown, K. R., Jurisica, I., Blagoev, B., Zerial, M., Stagljar, I.,
and Dikic, I. (2009) Regulation of epidermal growth factor receptor
trafficking by lysine deacetylase HDAC6, Sci. Signal., 2,
ra84.
100.Gao, Y. S., Hubbert, C. C., and Yao, T. P.
(2010) The microtubule-associated histone deacetylase 6 (HDAC6)
regulates epidermal growth factor receptor (EGFR) endocytic trafficking
and degradation, J. Biol. Chem., 285, 11219-11226.
101.Purev, E., Neff, L., Horne, W. C., and Baron,
R. (2009) c-Cbl and Cbl-b act redundantly to protect osteoclasts from
apoptosis and to displace HDAC6 from β-tubulin, stabilizing
microtubules and podosomes, Mol. Biol. Cell, 20,
4021-4030.