REVIEW: Spectral and Kinetic Parameters of Phosphorescence of Triplet Chlorophyll a in the Photosynthetic Apparatus of Plants
A. A. Krasnovsky, Jr.1,2* and Yu. V. Kovalev2
1Bach Institute of Biochemistry, Russian Academy of Sciences, Leninsky pr. 33, 119071 Moscow, Russia; fax: (495) 954-27322Lomonosov Moscow State University, 119899 Moscow, Russia; E-mail: phoal@mail.ru
* To whom correspondence should be addressed.
Received January 24, 2014
Spectral and kinetic parameters and quantum yield of IR phosphorescence accompanying radiative deactivation of the chlorophyll a (Chl a) triplet state were compared in pigment solutions, greening and mature plant leaves, isolated chloroplasts, and thalluses of macrophytic marine algae. On the early stages of greening just after the Shibata shift, phosphorescence is determined by the bulk Chl a molecules. According to phosphorescence measurement, the quantum yield of triplet state formation is not less than 25%. Further greening leads to a strong decrease in the phosphorescence yield. In mature leaves developing under normal irradiation conditions, the phosphorescence yield declined 1000-fold. This parameter is stable in leaves of different plant species. Three spectral forms of phosphorescence-emitting chlorophyll were revealed in the mature photosynthetic apparatus with the main emission maxima at 955, 975, and 995 nm and lifetimes ~1.9, ~1.5, and 1.1-1.3 ms. In the excitation spectra of chlorophyll phosphorescence measured in thalluses of macrophytic green and red algae, the absorption bands of Chl a and accessory pigments – carotenoids, Chl b, and phycobilins – were observed. These data suggest that phosphorescence is emitted by triplet chlorophyll molecules that are not quenched by carotenoids and correspond to short wavelength forms of Chl a coupled to the normal light harvesting pigment complex. The concentration of the phosphorescence-emitting chlorophyll molecules in chloroplasts and the contribution of these molecules to chlorophyll fluorescence were estimated. Spectral and kinetic parameters of the phosphorescence corresponding to the long wavelength fluorescence band at 737 nm were evaluated. The data indicate that phosphorescence provides unique information on the photophysics of pigment molecules, molecular organization of the photosynthetic apparatus, and mechanisms and efficiency of photodynamic stress in plants.
KEY WORDS: chlorophyll, triplet state, phosphorescence, photodymanic stress, photosynthesisDOI: 10.1134/S000629791404004X
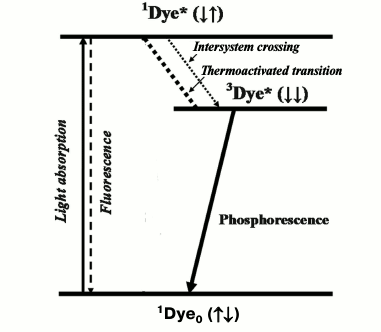
A concept stating that the metastable state of dyes, which is responsible for dye phosphorescence, is a triplet was advanced by A. N. Terenin in 1943 [1] and independently by G. Lewis and M. Kasha in 1944 [2]. Initially this concept met strong resistance from the school of J. Frank. Later it was proved in papers of many authors, and 10 years later it was also accepted by Frank and his followers [3]. Acceptance of this concept caused reconsideration of the well-known Jablonski scheme [4], which after these papers was considered as evidence for the existence of singlet and triplet excited states of dyes. Direct radiative deactivation of the singlet state is accompanied by short-lived fluorescence with a lifetime in the nanosecond time range. Direct radiative deactivation of the triplet state causes millisecond phosphorescence (Fig. 1). Another important consequence of this concept was the acceptance of the fact that emission of fluorescence and phosphorescence is a basic photophysical property of dye molecules.
Fig. 1. Simplified Jablonski diagram. 1Dye0, 1Dye*, and 3Dye* are dye molecules in the ground and excited singlet and triplet states.
It is natural that soon after these papers the question arose whether this scheme could be applied to chlorophyll, whose phosphorescence had not been discovered at that time. This question was clearly formulated for the first time by A. N. Terenin and A. A. Krasnovsky (my father) at the 1st All-Union Conference on Photosynthesis in 1946 (Moscow) [5, 6], who claimed also that knowledge of the roles of the triplet and singlet states in photosynthesis is one of the major problems of the mechanism of this process.
As measurement of phosphorescence was at that time the only way to detect triplet states, attempts to observe the phosphorescence of chlorophylls (Chl) a and b were repeatedly made by scientists from recognized research laboratories during 1948-1969. However, reliable data were not obtained [7-15]. In particular, the first papers that reported the registration of Chl a phosphorescence in frozen (77 K) solutions using a photographic technique were from Becker’s group [12, 14] (Table 1). In the nonpolar solvent 3-methylpentane, they observed luminescence of Chl a with a maximum at 755 nm and quantum yield of about 10%, which was proposed to be n-π phosphorescence of the pigment [12]. In the solvent mixture EPA (diethyl ether–ethanol–pentane 5 : 3 : 5 v/v), which forms a transparent glass at 77 K, they observed phosphorescence of Chl a with maxima at 885 and 925 nm. The luminescence lifetime was estimated to be ≥0.5 ms, and the quantum yield was about 50 times less that the quantum yield of fluorescence [14]. In 1969, Amster repeated these measurements using a mechanical phosphoroscope and photographic plates of a new generation [15]. He could not detect chlorophyll afterglow with the quantum yield indicated in published papers [12, 14]. According to Amster, in both 3-methylpentane and a mixture of ether and 3-methylpentane, the phosphorescence was several orders of magnitude weaker, though an accurate value of the quantum yield was not reported. In both solvents, the maximum of phosphorescence of Chl b was found at 865 nm. The maxima of the Chl a phosphorescence were found at 800 and 840 nm. Using a phosphoroscope, Amster did not observe afterglow in the region 730-760 nm. However, this emission was detected in 3-methylpentane if measurements were performed without the phosphoroscope. Therefore, he attributed it to fluorescence of chlorophyll aggregates of higher order than chlorophyll dimers [15].
Table 1. Results of the first reported
measurement of Chl a phosphorescence
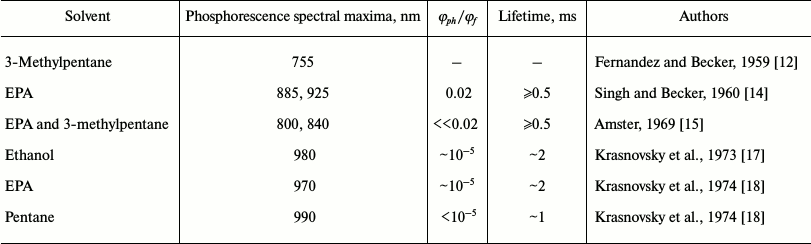
Note: φph/φf is the ratio of the quantum
yields of phosphorescence and fluorescence.
The first reliable measurements of chlorophyll phosphorescence in frozen solutions were carried out by our group in papers of 1971-1975 using setups with mechanical phosphoroscopes [16-20]. In the first paper of this series (1971), IR phosphorescence of 4-vinylprotochlorophyll and 4-vinylprotopheophytin was discovered, which had never been studied before [16]. Photomultipliers with S-11 spectral response were used as detectors, which are sensitive in the infrared up to 900 nm. Ethanol was used as the solvent. In these experiments, phosphorescence was detected that corresponds to the shortest-wavelength form of protochlorophyll present in the ethanol solutions. The emission maximum was observed at 870-880 nm, the lifetime was 4-5 ms, and the quantum yield was about 10–5 [16].
For further measurements, setups were constructed whose sensitivity in the infrared was increased by 2-4 orders of magnitude. Cooled photomultipliers FEU-83 (S-1) sensitive in the 400-1300 nm range, powerful (1 kW) xenon lamps, and high-throughput grating monochromators were used. Technical parameters of the phosphoroscopes were greatly changed. Detailed description of these setups was presented in papers [21-27]. The new instruments allowed measurement of spectra and lifetimes of IR phosphorescence at 700-1300 nm with quantum yield ≥10–9-10–10 and lifetimes ≥0.5 ms.
The use of these setups allowed us to reliably detect phosphorescence of Chls a and b and their magnesium-free analogs. More accurate data on protochlorophyll phosphorescence were also obtained [16-20]. It was found that the parameters of phosphorescence of Chl a are quite different from those described in the papers cited above (Table 1). In both polar and nonpolar solvents, the maxima of Chl a phosphorescence were observed at λ > 920 nm. The emission at 755 nm in the nonpolar solvent pentane was shown to be fluorescence of aggregated chlorophyll, as Amster also claimed [15]. Three spectral forms of chlorophyll phosphorescence – monomers Mg-monosolvates (Mg-L), monomers Mg-disolvates (Mg-L2), and aggregates (dimers and oligomers, Chln) – were also observed. It was also the first experimental observation of the triplets of these forms [18-20].
Apparently, the results of our phosphorescence studies actually disproved the experiments of well-known groups. In 1977-1979, our data were confirmed by other authors (Table 2). As a result of these studies, researchers obtained a new method of spectral analysis that allows precise measurement of energy and lifetimes of triplets and analysis of interaction of the pigments with environment.
Table 2. Parameters of phosphorescence of
chlorophylls and pheophytins reported in papers of different groups
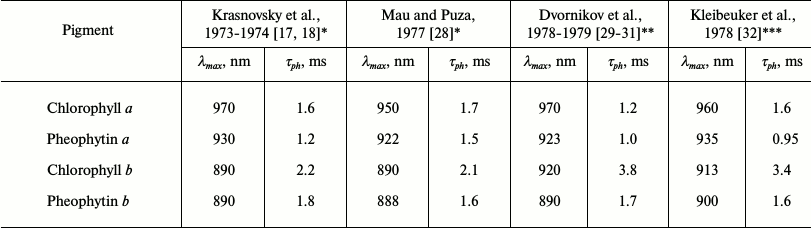
* EPA (mixture of diethyl ether, pentane and ethanol) as solvent.
** Mixture of diethyl ether and petroleum ether (1 : 1) as solvent.
*** Methyltetrahydrofuran as solvent.
In addition in 1975, phosphorescence of chlorophyll and protochlorophyll was observed in etiolated plant leaves. Also, phosphorescence was discovered in solutions of Chlorobium chlorophylls and their magnesium-free analogs [19, 20]. The results of these studies were outlined in papers [33-36].
In 1978-1981, the authors of the present review discovered and investigated phosphorescence of Chl a in mature leaves and chloroplasts of normal and mutant plants, in cells of green, red and brown algae, and in suspensions of cyanobacteria at 77 K [37-41]. Since that time many data have been accumulated (see reviews [27, 42-45]). Nonetheless, this information is poorly known. Moreover, a certain part of experimental materials dealing with mature leaves and chloroplasts was described only in dissertation works of these authors. Objectives of this mini review consist in introduction of certain unpublished data of the authors and in reconsideration of experimental information on the spectral and kinetic parameters and quantum yields of chlorophyll phosphorescence in chloroplasts of green plants and algae.
Chlorophyll a in solutions and model systems. For analysis of the photosynthetic systems one should shortly describe modern views on phosphorescence of chlorophyll in solutions and model systems. Detailed discussion is presented in a recent review [27]. Investigation of phosphorescence in chlorophyll solutions is complicated by the fact that upon freezing to 77 K chlorophyll often forms several spectral species whose concentrations depend on the concentration of the pigments and also on nature and state of solvents. Approximate parameters of these forms obtained by our group and other researchers are summarized in Table 3. It is of interest that the spectral properties of phosphorescence are more sensitive to solvation and aggregation of chlorophyll than the parameters of the absorption and fluorescence spectra. For instance, as Table 3 shows, maxima of the absorption spectra of Mg-L2 and maxima of the excitation spectra of Mg-L2 phosphorescence are shifted by 12-14 nm to longer wavelengths compared to Mg-L, whereas the phosphorescence spectra of Mg-L2 are shifted by 40-50 nm compared to Mg-L.
Table 3. Parameters of phosphorescence of Chl a in different states of solvation and aggregation [22, 27, 33-35, 42-49]
Note: λex denotes the main red maximum of the excitation spectrum; λph denotes the maxima of the phosphorescence spectra. (Mg-0) is a chlorophyll molecule with nonsolvated magnesium atom; τph is the lifetime corresponding to the main phosphorescence maximum.
For comparison with the photosynthetic apparatus, it is convenient to use systems in which only one chlorophyll species dominates. Such systems are diluted (1-3 µM) solutions of chlorophyll in pyridine and diethyl ether frozen as snow, in which monomeric Mg-L2 or Mg-L chlorophyll molecules dominate [27, 46, 47]. Heterogeneous aqueous suspensions containing Chl a solubilized by micelles of the detergent Triton X-100 and chlorophyll-containing suspensions of liposomes are different examples of such systems. In these systems, pentacoordinate (Mg-L) monomeric molecules of chlorophyll are formed whose phytol tail is located in the hydrophobic phase of the micelles and the magnesium atom is connected with the hydrophilic polar groups of detergents or lipids [27, 46, 47].
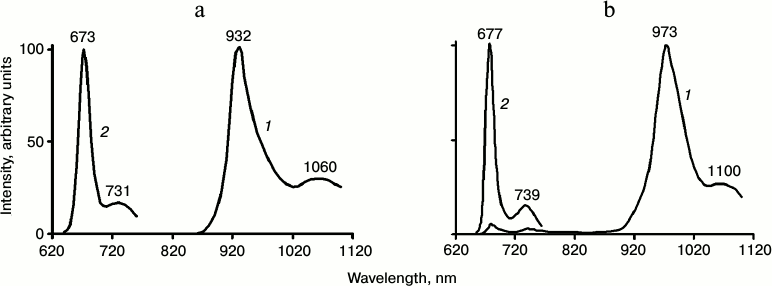
Spectra of phosphorescence and fluorescence of Chl a in pyridine and detergent micelles at 77 K are shown in Fig. 2. Each spectrum has two bands. The energy gap between the maxima of these bands is equal to 1250 ± 50 cm–1 in both systems. Thus, the spectra of radiative deactivation of both the singlet and triplet states have similar vibronic structure.
Fig. 2. Phosphorescence (1) and fluorescence (2) spectra of Chl a solutions (3-5 µM) at 77 K: a) in aqueous micellar solutions of detergent Triton X-100; b) in pyridine. Phosphorescence was measured under excitation by red light λ ≥ 620 nm; the monochromator slits corresponded to 6 nm. Fluorescence was excited by monochromatic light corresponding to the maximum of the Soret band; the monochromator slits corresponded to 2 nm (data of papers [20, 27, 46, 47]). Numbers over the curves show wavelengths of maxima in nanometers.
The bandwidth (FWHM) of the main phosphorescence bands in both pyridine and detergent micelles was 55 ± 2 nm (~620 cm–1). This is much greater than the bandwidth of the main fluorescence band (19 ± 2 nm, ~450 cm–1). The energy of the chlorophyll triplet state in pyridine and detergent (calculated from the maxima of the phosphorescence spectra) corresponds to 1.3 ± 0.03 eV. The energy of singlet–triplet splitting is 0.56 eV in pyridine and 0.51 eV in detergent micelles (~4500 cm–1). The spectra of chlorophyll phosphorescence are rather similar in diethyl ether and detergent micelles (Table 4). In solvent mixtures forming a glass at 77 K (for instance EPA or a mixture of diethyl and petroleum ethers, 1 : 1) the phosphorescence spectra are close to the phosphorescence spectra of the Mg-L2 in pyridine [18, 27, 29-31, 46, 47].
According to the data of several groups, the quantum yield of Chl a phosphorescence (φph) is (2 ± 1)·10–5 [17-20, 28, 30]. It should be noted that φph is determined by the following equation:
φph = φst × kr × τph, (1)
where φst is the quantum yield of the triplet state population, kr is the rate constant of radiative deactivation of the triplet state, and τph is the phosphorescence lifetime. Hence:
φst × kr = φph/τph. (2)
Table 4 summarizes relative quantum yields of chlorophyll phosphorescence in different media. One can see that in pyridine and diethyl ether the quantum yields (φph) are rather similar (the difference does not exceed ±10%). The ratio φph/τph in detergent micelles, pyridine, and diethyl ether are also almost equal within the precision of our measurements.
According to flash photolysis data, φst ~ 0.6 for monomeric chlorophyll molecules. The degree of solvation of the central magnesium atom changed φst by not more than by 10% [50]. A similar result was also obtained upon measurement of the quantum yields of singlet oxygen generation by Mg-L- and Mg-L2-solvates of chlorophyll [51]. Hence using Eq. (2), one can conclude that the rate constant of radiative deactivation of the triplet chlorophyll molecules (kr) almost does not depend on the degree of solvation of the central magnesium atom.
At the same time, as Table 4 shows, solvation of the magnesium atom does not change also the fluorescence quantum yield (φf) and the ratio φph/(τphφf). Similar values of these parameters were obtained in other model systems in which chlorophyll molecules are monomeric, as for instance, chlorophyll-containing liposomes [27, 46, 47].
Thus, parameters of φph, φph/τph and φph/(τphφf) have rather constant values for monomeric chlorophyll molecules solvated only by the central magnesium atom (Mg-L and Mg-L2). Deviation from these values indicates a change in a state of pigment molecules in the systems under investigation.
For instance, these parameters are markedly reduced in solutions of chlorophyll in ethanol (Table 4), which were used for a long time as a reference in our measurements of the quantum yields of chlorophyll phosphorescence in the photosynthetic systems [38]. It is likely that the phosphorescence of Chl a in frozen ethanol solutions mostly correspond to Mg-L2 solvates, whose C=O group of the V-ring is simultaneously hydrogen bonded by ethanol. Detailed discussion of this type of solvation is given in papers [22, 27]. According to our data, strong decrease in all parameters – φph, φph/τph, and φph/(τphφf) – is observed upon aggregation of chlorophyll molecules [18-20, 27].
It should be noted that chlorophyll phosphorescence is always accompanied by low temperature delayed fluorescence (Fig. 3). Under conditions of the experiments illustrated by Fig. 3, delayed fluorescence was 300 times weaker than phosphorescence in detergent solutions and 15 times weaker than phosphorescence in pyridine. In both cases, the intensity of delayed fluorescence is proportional to the square of the intensity of the exciting light. The lifetime of phosphorescence was around 2 ms. Stronger delayed fluorescence was observed in chlorophyll-containing liposomes [27, 42, 46, 47]. The data indicate that low temperature delayed fluorescence accompanies a recombination process whose efficiency strongly depends on solvents and chlorophyll concentration. It seems improbable that triplet–triplet annihilation might occur in rigid solutions with low, micromolar chlorophyll concentrations. An assumption was proposed that afterglow arises as a result of interaction of triplet chlorophyll molecules with a metastable photoproduct that appears as a result of reaction of triplets with solvent molecules [42].
Greening etiolated leaves. The first measurements of phosphorescence of protochlorophyll and chlorophyllide in frozen etiolated and greening leaves were reported by our group in 1975 [19, 20]. To the best of our knowledge, these data were the first experimental evidence of population of pigment triplet states in the developing photosynthetic apparatus of green plants.
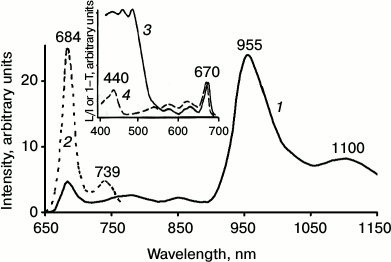
It is known that protochlorophyllide is the major pigment of etiolated leaves ([52, 53] and references therein). Spectral and kinetic parameters of protochlorophyllide phosphorescence were many times reported in papers of our lab [20, 27, 33-35, 54-56]. Upon illumination of etiolated leaves, photoreduction of protochlorophyllide occurred followed by chlorophyll accumulation. After 1 h of greening (just after the Shibata shift) the major pigment is Chl a ([52, 53] and references therein). In our experiments, etiolated seedlings of bean, pea, and wheat were studied. In all these seedlings, after 1 h greening Chl a was formed with absorption and fluorescence maxima at 670-672 and 682-684 nm, respectively.
Simultaneously, at 77 K chlorophyll phosphorescence was observed with the main maximum at 955 ± 5 nm and the vibrational satellite at 1100 nm (Fig. 3). The lifetime in the main maximum was 1.8-2 ms. The bandwidth (FWHM) of the major bands of fluorescence and phosphorescence spectra were about 20 and 55 nm. Thus, the spectral parameters of chlorophyll phosphorescence in greening leaves resemble those in micelles of detergent and pyridine.
Table 4. Quantum yields (φph)
and lifetimes (τph) of phosphorescence of Chl a
in different solvents at 77 K according to the data our group [22, 27, 38,
42, 46, 47, 57]
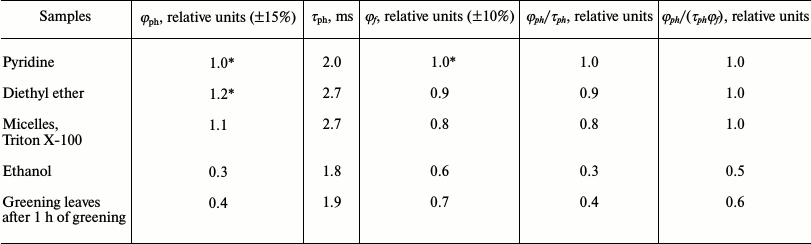
* The absolute quantum yield in pyridine and ether is (2 ±
1)·10–5; the fluorescence yield at 77 K is
55-60% [30].
Fig. 3. Low temperature (77 K) phosphorescence spectra (1) and fluorescence spectra (2) of Chl a in etiolated bean leaves after 1 h of greening; 3) the absorption spectrum of the leaf at 77 K; 4) phosphorescence excitation spectrum upon registration of light emission at λ ≥ 900 nm. L/I is the ratio of the phosphorescence intensity to the intensity of excitation; 1–T is the fractional absorbance of a leaf [20, 27, 33-35, 57].
On the other hand, the maximum of the phosphorescence spectrum in greening leaves is shifted to longer wavelengths compared to the Mg-L solvates in solutions, and to shorter wavelengths compared to the Mg-L2 solvates (compare the data of Table 3 and Figs. 2 and 3). The phosphorescence lifetime of chlorophyll in greening leaves is also markedly less than that of the Mg-L species in solutions. Thus, we can conclude that according to the spectral and temporal parameters, phosphorescence of Chl a in leaves on the early stage of greening does not correspond to phosphorescence Mg-L or Mg-L2 species of monomeric chlorophylls.
Comparison of the data presented in Tables 3 and 4 leads to an assumption that chlorophyll phosphorescence in greening leaves belongs to short wavelength aggregates (probably dimers) of chlorophyll. In agreement with this assumption, φph and the parameters φph/τph and φph/(φfτph) are about two times less in greening leaves than in solutions of monomeric chlorophyll molecules (Table 4). As mentioned above, the quantum yield of the triplets in solutions of monomeric chlorophyll is about ~0.6 [30, 50]. If one takes into account Eq. (2) and proposes that the rate constants of radiative deactivation of chlorophyll triplets (kr) are equal in greening leaves and solutions, one can conclude that the quantum yield of the Chl a triplet state (φst) in greening leaves is about 0.25 [22, 27, 42, 54, 57]. This value can be regarded as the lower limit of φst, because it is not excluded that kr might be smaller in greening leaves than in solutions.
In both chlorophyll solutions and greening leaves, phosphorescence was accompanied by low temperature delayed fluorescence. The relative intensity of delayed fluorescence in leaves was greater than in solutions (Fig. 3). The intensity of delayed fluorescence was proportional to the square of the intensity of excitation.
The maximum of the excitation spectrum of chlorophyll phosphorescence in greening leaves was observed at 670 nm. Hence, it is located closely to the absorption maximum of chlorophyll (Fig. 3). As Fig. 3 shows, the excitation spectrum does not have the bands of carotenoids at 400-500 nm, though the bands of carotenoids dominate in the absorption spectra of leaves.
Thus, the data indicate that in agreement with Butler’s data [58], there is no energy transfer from the singlet states of carotenoids to chlorophyll at this stage of development of the photosynthetic apparatus. Also, as seen from Table 4, the lifetime of phosphorescence is about 2 ms. Hence, there is no energy transfer from the triplet state of Chl a to carotenoids [20, 33-35, 54, 57].
Phosphorescence of chlorophyll in mature plant leaves. Further development of the photosynthetic apparatus led to a sharp decrease of the phosphorescence yield, though the phosphorescence lifetime was almost unchanged [20, 22, 24, 42, 54, 57]. Table 5 illustrates the dependence of the phosphorescence yield on time of greening of etiolated seedlings of wheat. During greening the main maximum of the phosphorescence spectrum was shifted from 953 to 980 nm. After 48 h of greening, the phosphorescence yield reached the minimum value, about 2000 times smaller than the quantum yield of phosphorescence of monomeric chlorophyll in solutions (Table 5). Further “greening” did not change the phosphorescence yield. Similar data were obtained upon greening of etiolated leaves of pea, bean, and barley.
Table 5. Changes in intensity of Chl
a phosphorescence in etiolated wheat leaves during greening*
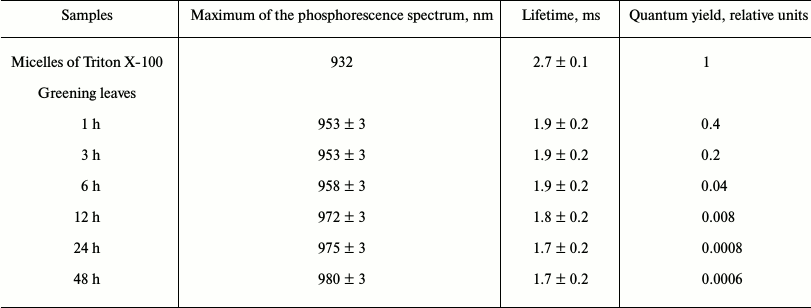
* Phosphorescence was measured at 77 K under excitation by a xenon lamp
through a KS-15 cut-off filter (λ ≥ 640 nm). Greening
was observed under white light of 1000 lx (Kovalev and Krasnovsky,
unpublished data).
Because of the very low yield of chlorophyll phosphorescence in mature leaves, reliable detection of this light emission was a difficult problem whose solution required from us much effort. The first measurement of chlorophyll phosphorescence in mature leaves and suspensions of cells of green, brown, and red algae and cyanobacteria was reported by us in 1978 [37]. Later these measurements were many times repeated by our group using different photosynthetic organisms.
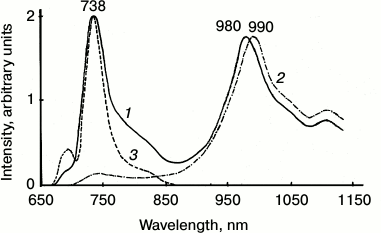
Figure 4 shows the spectrum of chlorophyll phosphorescence in a mature pea leave. One can see that upon excitation by red light through the cut-off red filter transmitting light at λ ≥ 650 nm, the main phosphorescence maximum was observed at ~980 nm. The phosphorescence spectrum has also a long-wavelength satellite at ~1100 nm, whose intensity was about two times less. The phosphorescence lifetime was ~1.7 ms. Upon excitation by dark red light, the main phosphorescence maximum was shifted to 990 nm, and the lifetime decreased to ~1.3 ms. Thus, phosphorescence is emitted by at least two spectral forms of chlorophyll. Judging by the fact that the phosphorescence lifetime is millisecond, the triplet states of these forms are not quenched by carotenoids.
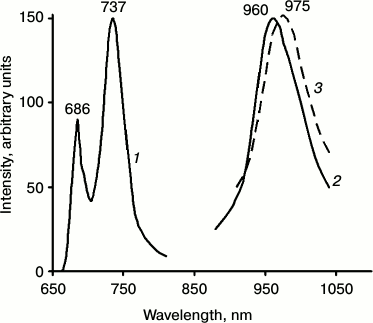
Fig. 4. Spectra of delayed luminescence (1, 2) and fluorescence (3) of chlorophyll in mature pea leaves at 77 K. Spectra 1 and 2 were recorded under excitation by red light λ ≥ 640 nm (1) and far red light λ ≥ 680 nm (2). Monochromator slit width was 20 nm. The spectrum of fluorescence (3) was recorded upon excitation by 440 nm monochromatic light. The width of the monochromator slit was 2 nm. Absorbance of the leaf in the red absorption maximum was ~2.
Figure 5 shows the major band of the phosphorescence spectrum in a suspension of pea chloroplasts. The absorbance in the maximum of the absorption band of this sample was 0.8 at 77 K, being much smaller than in mature leaves. Under excitation by red light transmitted by a KS-17 cut-off filter (λ ≥ 650 nm), the maximum of the phosphorescence spectrum was observed at 960 nm. The lifetime was 2 ms. Under excitation by dark red light (λ ≥ 680 nm), the maximum was shifted to 975 nm. The lifetime decreased to 1.4 ms [38-41]. The band at 990 nm was observed only in dense chloroplast suspensions whose absorbance was close to that of mature leaves.
Fig. 5. Spectra of fluorescence (1) and phosphorescence (3, 4) of chlorophyll in suspensions of pea chloroplasts at 77 K. Fluorescence was excited by 440 nm blue light, phosphorescence was excited through cut-off red filters KS-17 (λ ≥ 650 nm) (1) and KS-18 (λ ≥ 680 nm) (2). Upon fluorescence measurement, the absorbance in the red absorption maximum of suspensions was 0.05, and the slit width was 1 nm. Upon measurement of phosphorescence the absorbance was 1, and the slit width was 20 nm. Data of reference [39] and further unpublished experiments of the authors.
IR phosphorescence of chlorophyll was always accompanied by delayed fluorescence whose major spectral maximum coincided with the maximum of the low temperature chlorophyll fluorescence (735-740 nm) (Fig. 4). In green leaves, the relative intensity of delayed fluorescence was much higher than in chlorophyll solutions and greening leaves. It was shown that the main maximum of the excitation spectrum of delayed fluorescence was observed at 350 nm, i.e. it does not belong to chlorophyll [37, 38, 40-42, 59]. The nature of this phenomenon deserves a special discussion that is out of the scope of this review.
Millisecond phosphorescence and delayed fluorescence of chlorophyll with the same spectral and temporal parameters was detected in mature leaves of all plants we investigated (maize, barley, pea, birch, maple, rowan-tree, and others). The quantum yield of chlorophyll phosphorescence in plant leaves grown under natural conditions, which were taken for analysis in daytime, was always 1000 times less than in greening leaves after 1 h of greening and 2000 times less than in solutions of monomeric chlorophyll. Chlorophyll phosphorescence in suspensions of the class B chloroplasts was usually three times stronger than in mature leaves [39].
Thus, the quantum yield of chlorophyll phosphorescence in mature leaves and chloroplasts of green plants grown under normal conditions is ~10–8 [38-42, 44, 45, 57]. As so strong decrease in the phosphorescence yield is not accompanied by the decrease in the phosphorescence lifetime, we conclude that phosphorescence is emitted by a minor part of the chlorophyll molecules spatially separated from carotenoids of the photosynthetic apparatus. In this case, the quantum yield of chlorophyll phosphorescence in leaves can be described by the following equation:
φph = kr × φst × τph × αchl, (3)
where αchl is a molar fraction of phosphorescence-emitting chlorophyll relative to the entire chlorophyll concentration. It is most likely that the product kr × φst × τph has the same value in both etiolated leaves after 1 h of greening and in mature leaves. This leads to the φph ratio for phosphorescence in mature leaves compared to greening leaves after 1 h of greening ((φph)mature/(φph)etiol) equal to 0.1%. Hence, only 0.1% of the overall chlorophyll is involved in phosphorescence emission in leaves. In isolated chloroplasts this value is about 0.3%, as mentioned above.
It was observed that the intensity of chlorophyll phosphorescence in leaves strongly depended on external factors. For instance, phosphorescence increased 3-fold if plants were grown under low illumination (less than 50 lx). Phosphorescence intensity increased under any actions that damaged the chloroplast structure. For instance, after heating, the action of organic solvents, detergents, and herbicides, and also in mutant plants. Especially strong increase of phosphorescence intensity was observed after inhibition of biosynthesis of carotenoids or change of carotenoid composition [38, 40, 41].
It should be noted that mature leaves and chloroplasts cannot be used for reliable measurement of the excitation spectra of chlorophyll phosphorescence. Such measurements were hampered by the high absorbance of leaves and by strong delayed fluorescence, the long wavelength tail of which overlaps with the phosphorescence bands of chlorophyll. Such overlapping is especially strong under excitation by green or blue light. Rough estimate has shown that the red maximum (~670 nm) of the excitation spectrum of chlorophyll phosphorescence in leaves and chloroplasts is shifted to shorter wavelengths compared to the red absorption maximum (676 nm) [39].
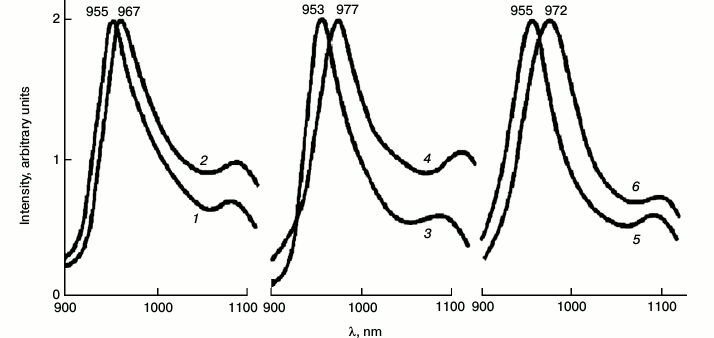
Macrophytic algae. Transparent thalluses of macrophytic algae are much more convenient for spectral measurements. Absorbance of thalluses did not exceed 0.7. The spectra of phosphorescence emission are shown in Fig. 6. Under excitation by red light the emission band at 955 nm dominated, which resembles the band of chlorophyll phosphorescence in etiolated leaves after 1 h of greening. The lifetime corresponding to this band was 2.0 ± 0.2 ms. Under excitation by far red light, the maxima were shifted to 972 ± 4 nm (Fig. 6). The lifetime decreased to 1.3 ± 0.2 ms [60]. Low temperature delayed fluorescence of chlorophyll in these algae was much weaker than in leaves.
Fig. 6. Spectra of chlorophyll phosphorescence in thalluses of macrophytic algae: green Ulva fenestrata (1, 2), brown Sargassum pallidum (3, 4), and red Grateloupia turuturu (5, 6) under excitation via the cut-off light filters KS-15 (λ ≥ 640 nm) (1, 3, 5) and KS-18 (λ ≥ 680 nm) (2, 4, 6) at 77 K. The width of monochromator slits was 15 nm. Data of reference [60].
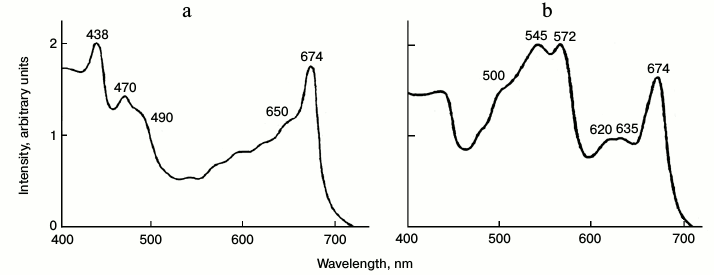
The combination of favorable properties, transparent thalluses with relatively low absorbance of chlorophyll and relatively low intensity of delayed fluorescence, allowed us to measure the excitation spectrum of chlorophyll phosphorescence in the visible region (Fig. 7). Figure 7 shows that in green algae the main maxima of excitation spectra (438 and 674 nm) correspond to Chl a. The excitation spectrum also has the distinct maxima of Chl b (650 and 470 nm) and carotenoids (490-500 nm). In the excitation spectrum of chlorophyll phosphorescence in red algae, the maxima of chlorophyll (674 nm) and maxima of phycobilins are clearly seen (Fig. 7). It is noteworthy that the red (chlorophyll) excitation maximum is slightly shifted to shorter wavelengths compared to the main absorption maximum of chlorophyll (678 nm) [60].
Fig. 7. Excitation spectrum of chlorophyll phosphorescence in thalluses of green algae Ulva fenestrate (a) and red algae Grateloupia turuturu (b) at 77 K. Phosphorescence was measured through an IR cut-off filter transmitting light at λ ≥ 900 nm. The ratio of the fluorescence intensity and the intensity of exciting light is indicated on the Y axis in relative units. The monochromator slit width was 7 nm. Data of reference [60].
These data suggest that phosphorescence is emitted mostly by the short wavelength chlorophyll forms, which are connected with the light harvesting complex containing accessory pigments (Chl b, carotenoids and phycobilins). Therefore, one can assume that the chlorophyll molecules responsible for emission of millisecond phosphorescence are a part of the natural pigment protein complex of chloroplasts. As phosphorescence-emitting chlorophyll molecules can also fluorescence, it was of interest to estimate a contribution of this fluorescence to the overall chlorophyll fluorescence in chloroplasts.
Comparison of phosphorescence and fluorescence of chlorophyll in chloroplasts. For these studies, suspensions of isolated chloroplasts were used. It is known that at 77 K the main maximum of chlorophyll fluorescence in chloroplasts is observed at 737 nm (Fig. 5). At the same time, the fluorescence spectra have also the short wavelength maxima at 686 and 695 nm. Deconvolution of the low temperature spectra reveals two more minor bands at 680 and 703 nm [61-65].
As the red maximum of the excitation spectra is shifted by several nanometers to shorter wavelengths compared to the main absorption maximum of chloroplasts (676 nm), it is logical to ascribe fluorescence of the phosphorescence-emitting chlorophyll to the short wavelength forms 680, 686, and 695 nm. According to Murata et al., the contribution of the short wavelength fluorescence (600-700 nm) to the overall fluorescence spectrum of spinach chloroplasts is 25% [62]. A similar value was obtained by Litvin et al. for bean chloroplasts [64, 65].
The general yield of the low temperature fluorescence of chloroplasts obtained by Murata et al. upon excitation the Chl b band (475 nm) was 75% [62]. We repeated this measurement upon excitation the absorption band of Chl a (440 nm). As a reference, solutions of Chl a in ethanol were used. For the first try we used relatively dense chloroplast suspensions with absorbance equal to 0.4 at 676 nm at 77 K. The yield of overall fluorescence was estimated to be 15% [39]. This value is now corrected by using for fluorescence measurement dilute chloroplast suspensions in which reabsorption of fluorescence is reduced. As references, chlorophyll solutions in diethyl ether and pyridine were employed whose fluorescence yield was shown to be 55-60% [30]. When the paper [39] was published, the paper [30] has not appeared yet and also we did not know that at 77 K the fluorescence yield in ethanol is two times smaller than in pyridine and ether. As a result (Table 4), we obtained that the overall fluorescence yield is ~30%, two times smaller than in Murata’s measurements. For further analyses, we used the average value 55% for overall fluorescence yield and 14% for the short wavelength bands.
It is natural to propose that the ratio φph/φf for molecules of the phosphorescence-emitting chlorophyll is the same in mature leaves and etiolated leaves after 1 h of greening. As shown in Table 4, the yield of chlorophyll phosphorescence in greening leaves (φf)etiol ≈ 40%. Hence using Eq. (3) one can obtain:
(φf)mature ≈ αchl × (φf)etiol = 0.0004-0.0012. (4)
Comparing this value with the quantum yield of the short wavelength fluorescence of chloroplasts (14%), one arrives to the conclusion that the phosphorescence-emitting chlorophyll makes up 0.3-1% of the short wavelength fluorescence of chlorophyll in chloroplasts.
We tried also to reveal the phosphorescence corresponding to the long wavelength fluorescence at 737 nm. It is likely that energy of the singlet–triplet splitting (ΔEst) for this fluorescence band is equal to ΔEst for the main fluorescence band of chlorophyll in the greening leaves. From the maxima of chlorophyll fluorescence and phosphorescence spectra indicated in Fig. 3 one can obtain that ΔEst = 4100 cm–1. Therefore, the phosphorescence maximum of this form should be located at ~1050 nm. From linear dependence between the energy of triplets and logarithm of their lifetimes, which was shown to be valid for chlorophylls [27, 36, 42, 43], one can calculate that the lifetime of this band should be ~0.5 ms. This value corresponds to the technical limit for temporal resolution of our set-ups. In agreement with this estimate, it was observed that aggregated molecules of bacteriochlorophyll c having the absorption maximum at 725 nm, emitted phosphorescence with the maximum at 1060 nm and the lifetime 0.6 ms [19, 20, 27, 33-35].
Comparison of Figs. 3 and 4 supports the assumption that long wavelength phosphorescence might exist in mature leaves. One can see that in mature leaves the maximum at ~1100 nm was two times smaller than the main maximum of the phosphorescence spectrum. In etiolated leaves after 1 h of greening, the phosphorescence maximum at ~1100 nm is three times weaker than the main phosphorescence band. Special experiments made to reveal a long wavelength phosphorescence band showed that upon shift of excitation to longer wavelengths, the relative intensity of phosphorescence at 1100 nm increased compared to the main phosphorescence maximum. However, insufficient sensitivity of our technique to luminescence in this spectral and temporal region does not allow us to get a final solution of this problem.
Thus, this review briefly describes the history of discovery of low temperature (77 K) phosphorescence of chlorophylls and characterizes parameters of phosphorescence of Chl a in solutions, certain model systems, greening leaves just after the Shibata shift, and in mature leaves and thalluses of macrophytic algae. It was shown that the phosphorescence of newly formed chlorophyll in greening leaves resembles best the phosphorescence of the short wavelength aggregates (probably dimers) in chlorophyll solutions. At this stage of development of leaves, the phosphorescence belongs to the bulk chlorophyll of leaves. The quantum yield of the chlorophyll triplet state is ≥25%. Further (after Shibata shift) formation of the photosynthetic apparatus caused strong decrease of the phosphorescence yield, which reached a minimum after 48 h of greening. In mature leaves of normal plants grown under normal illumination, the phosphorescence yield is 1000 less than in etiolated leaves after the Shibata shift. This value is stable in leaves of different plants. As a result of analysis of spectral and kinetic parameters of chlorophyll phosphorescence in leaves, isolated chloroplasts, and thalluses of macrophytic algae, three spectral forms of phosphorescence-emitting chlorophyll were observed with the main maxima at 955, 975, and 995 nm and the lifetimes ~1.9, ~1.5, and 1.1-1.3 ms. Excitation spectra of chlorophyll phosphorescence measured in the transparent thalluses of macrophytic green and red algae showed the maxima of Chl a and accessory pigments – carotenoids, Chl b, and phycobilins. The data indicate that phosphorescence is emitted by the short wavelength forms of Chl a, which are coupled with the light harvesting complex but their triplets are not quenched by carotenoids.
The concentration of the phosphorescence-emitting chlorophyll in mature leaves and its contribution to the overall chlorophyll fluorescence have been estimated. As a reference, phosphorescence of the newly formed chlorophyll in greening leaves was employed. It is shown that in mature leaves and chloroplasts the relative concentration of phosphorescence-emitting chlorophyll is 0.1-0.3%. The contribution of these molecules to the short wavelength (600-700 nm) fluorescence of chlorophyll was estimated to be 0.3-1%.
An attempt was made to reveal the phosphorescence corresponding to the long wavelength fluorescence at 737 nm, which dominates in the fluorescence spectra in leaves and chloroplasts (Figs. 4 and 5). Analysis showed that the spectral maximum of this phosphorescence should be at ~1050 nm. The lifetime should be ~0.5 ms. The experimental data indicate that this phosphorescence might exist in mature leaves and chloroplasts. However, insufficient sensitivity of our technique to luminescence in this spectral and temporal region does not allow us to a get final solution of this problem.
The nature of the phosphorescence-emitting chlorophyll is presently not quite clear. In previous papers, we suggested that phosphorescence belongs to newly synthesized chlorophyll in the centers of pigment biosynthesis [44, 45]. The concentration of the phosphorescence-emitting chlorophyll is consistent with the concentration of young chlorophyll estimated by the Shlyk school [66]. Indeed, it was shown in experiments of our laboratory that phosphorescence of chlorophyll and protochlorophyll strongly increased if leaves were kept in darkness for many hours, especially in the presence of 5-aminolevulinic acid and metal chelators [37, 67]. However, this suggestion is not quite consistent with the fact that several spectral forms of the phosphorescence-emitting chlorophyll were observed in chloroplasts developed under normal illumination conditions. Another assumption states that phosphorescence belongs to chlorophyll of the reaction centers of PS-2 [44, 45]. It is known that chlorophyll molecules in these reaction centers are spatially separated from carotenoids. As a result, carotenoids do not quench chlorophyll triplets, and therefore their lifetime is long, sufficient for efficient singlet oxygen generation [68, 69]. Preliminary data of our laboratory showed that indeed, in isolated reaction centers (D1D2-cyt b559 complexes) strong millisecond chlorophyll phosphorescence appears [70]. However, the samples used for this experiment contained rather much uncoupled chlorophyll molecules. Presently, more detailed studies are in progress. Again, the existence of several spectral forms of the phosphorescence-emitting chlorophyll in normal chloroplasts is not quite consistent with this assumption. It is not excluded that under normal conditions, when reaction centers are inside native (not damaged) structure of chloroplasts, the reaction center triplets are not formed or are quenched. According to the third idea, normal chlorophyll–protein complexes of chloroplasts generate a certain amount of triplet, which are not quenched by carotenoids. Probably, this is a consequence of the complex structure of the pigment apparatus. In this case, the “constant” (0.1-0.3% concentration of phosphorescence-emitting chlorophyll) found in our experiments shows the minimum concentration of carotenoid-detached chlorophyll molecules, which plants can stand. It might be that further reduction of the yield of chlorophyll triplets is not effective. It is simpler to protect native structures from photodynamic activity of this small group of chlorophyll molecules. This assumption correlates with the data of Santabarbara et al. [71], who, using FDMR, also observed triplet states of chlorophyll not connected with photosynthesis reaction centers.
Nevertheless, in aerobic conditions triplet molecules of chlorophyll and its precursors in biosynthesis transfer their energy to O2 with formation of reactive singlet oxygen (1O2), which readily oxidizes biologically important molecules [21, 22, 44, 45, 51, 72]. It is thought that this process is responsible for photoinhibition of photosynthesis and photodestruction of the photosynthetic apparatus ([44, 45, 69, 72] and references therein).
Based on these ideas, we expect that the intensity of the low temperature phosphorescence should correlate with the rate of photodestructive processes under physiological conditions. The first experiments supporting this assumption were carried out by the authors of this review in collaboration with A. Faludi-Daniel (Szeged, Hungary). It was shown that in lycopene and ζ-carotene mutants of maize, in which the rate of photodestruction of chlorophyll is much stronger than in normal plants, the phosphorescence yield is also higher, and what is more, the phosphorescence yield and the photodestruction rate correlate [38]. Later, this problem was studied in detail [44, 45, 57]. Apparent correlation between the phosphorescence yield at 77 K and chloroplast photodestruction at room temperature was revealed. This fact allows for the conclusion that namely the phosphorescence-emitting fraction of chlorophyll molecules is responsible for photodynamic destruction and photoinhibition of the photosynthetic apparatus. Thus, the yield of chlorophyll phosphorescence shows the degree of plant protection against photodynamic action of the native pigment apparatus. In particular, measurement of phosphorescence allows for investigation of mechanisms and efficiency of action of photodynamic herbicides [44, 45, 59, 67].
Thus, the data indicate that phosphorescence provides unique information on the photophysics of pigment molecules, molecular organization of the photosynthetic apparatus, and mechanisms and efficiency of photodynamic stress in plants.
The authors thank the colleagues whose names are cited in the list of joint research papers for collaboration in the experimental work. Support of the Program “Molecular and Cell Biology” of the Russian Academy of Sciences is gratefully acknowledged.
REFERENCES
1.Terenin, A. N. (1943) Acta Phisicochim.
(USSR), 18, 210-241.
2.Lewis, G. N., and Kasha, M. (1944) J. Amer.
Chem. Soc., 66, 2100-2116.
3.Kasha, M. (2005) Spectrum, 18,
5-11.
4.Jablonski, A. (1935) Z. Physik, 94,
38-46.
5.Terenin, A. N. (1947) Bull. USSR Acad. Sci. Ser.
Biol., 3, 369-376.
6.Krasnovsky, A. A. (1947) Bull. USSR Acad. Sci.
Ser. Biol., 3, 377-396.
7.Calvin, M., and Dorough, G. D. (1947)
Science, 105, 433-434.
8.Calvin, M., and Dorough, G. D. (1948) J. Am.
Chem. Soc., 70, 699-706.
9.Livingston, R. (1949) in Photosynthesis in
Plants (Frank, J., and Loomis, W. E., eds.) The Iova State College
Press, pp. 179-196.
10.Livingston, R. (1960) Quart. Rev.,
14, 174-199.
11.Becker, R. S., and Kasha, M. (1955) J. Am.
Chem. Soc., 77, 3669-3670.
12.Fernandez, J., and Becker, R. S. (1959) J.
Chem. Phys., 31, 467-471.
13.Terenin, A. N. (1959) in Problems of
Photosynthesis (Kursanov, A. L., et al., eds.) Publishing House of
the USSR Academy of Sciences, Moscow, pp. 10-21.
14.Singh, J. S., and Becker, R. S. (1960) J.
Amer. Chem. Soc., 82, 2083-2084.
15.Amster, R. L. (1969) Photochem.
Photobiol., 9, 331-338.
16.Krasnovsky, A. A., Jr., Shuvalov, V. A., Litvin,
F. F., and Krasnovsky, A. A. (1971) Dokl. AN SSSR,
199, 1181-1184.
17.Krasnovsky, A. A., Jr., Romaniuk, V. A., and
Litvin, F. F. (1973) Dokl. AN SSSR, 209, 965-968.
18.Krasnovsky, A. A, Jr., Lebedev, N. N., and
Litvin, F. F. (1974) Dokl. AN SSSR, 216, 1406-1409.
19.Krasnovsky, A. A., Jr., and Litvin, F. F. (1975)
Bull. USSR Acad. Sci. Ser. Phys., 39, 1968-1971.
20.Krasnovsky, A. A., Jr., Lebedev, N. N., and
Litvin, F. F. (1975) Dokl. AN SSSR, 225, 207-210.
21.Krasnovsky, A. A., Jr. (1979)
Photochem. Photobiol., 29, 29-36.
22.Krasnovsky, A. A., Jr. (1983) Singlet Oxygen
in Biological Processes: Doctor-of-Science Thesis [in Russian],
Lomonosov Moscow State University, Moscow.
23.Krasnovsky, A. A., Jr. (1993) SPIE Proc.,
1887, 177-186.
24.Krasnovsky, A. A., Jr. (2004) Biofizika,
49, 289-306.
25.Krasnovsky, A. A., Jr. (2007) in Photodynamic
Therapy at the Cellular Level (Uzdensky, A. B., ed.) Research
Signpost, Trivandrum-695 023, Kerala, India, pp. 17-62.
26.Krasnovsky, A. A., Jr. (2008) J.
Photochem. Photobiol. A, 196, 210-218.
27.Krasnovsky, A. A., Jr. (2014) in Handbook of
Porphyrin Science (Kadish, K., et al., eds.) Vol. 33, World
Scientific Publishing, pp. 77-166.
28.Mau, A. W. H., and Puza, M. (1977) Photochem.
Photobiol., 25, 601-603.
29.Dvornikov, S. S., Knyukshto, B. N., Sevchenko, A.
N., Solovyov, K. N., and Tsvirko, M. P. (1978) Dokl. AN SSSR,
240, 1457-1460.
30.Dvornikov, S. S., Knyukshto, B. N., Sevchenko, A.
N., Solovyov, K. N., and Tsvirko, M. P. (1979) Opt. Spektr.,
46, 689-695.
31.Dvornikov, S. S., Knyukshto, B. N., Solovyov, K.
N., and Tsvirko, M. P. (1979) J. Luminescence,
18/19, 491-494.
32.Kleibeuker, J. F., Platenkamp, R. J., and
Schaafsma, T. J. (1978) Chem. Phys., 27, 51-64.
33.Krasnovsky, A. A., Jr., Lebedev, N. N., and
Litvin, F. F. (1977) Stud. Biophys., 65, 81-89.
34.Krasnovsky, A. A., Jr. (1977) Acta Phys.
Chem. (Szeged University, Hungary), 23, 147-154.
35.Krasnovsky, A. A., Jr., Lebedev, N. N., and
Litvin, F. F. (1978) in Proc. 3rd Int. Seminar on Energy Transfer in
Condensed Matter (Fiala, J., ed.) Universita Karlova, Prague, pp.
111-119.
36.Lebedev, N. N., and Krasnovsky, A. A., Jr. (1978)
Biofizika, 23, 1095-1096.
37.Krasnovsky, A. A., Jr., and Kovalev, Yu. V.
(1978) Biofizika, 23, 920-922.
38.Krasnovsky, A. A., Jr., Kovalev, Yu. V., and
Faludi-Daniel, A. (1980) Dokl. AN SSSR (Biophys.), 251,
1264-1267.
39.Krasnovsky, A. A., Jr., Kovalev, Yu. V.,
Kukarskikh, G. P., and Gulayev, B. A. (1980) Biofizika,
25, 821-826.
40.Krasnovsky, A. A., Jr., Kovalev, Yu. V., and
Lehoczki, E. (1981) Dokl. Akad. Nauk SSSR, 256,
726-730.
41.Kovalev, Yu. V., Krasnovsky, A. A., Jr.,
Lehoczki, E., and Maroti, I. (1981) Biofizika, 26,
891-893.
42.Krasnovsky, A. A., Jr. (1982) Photochem.
Photobiol., 36, 733-741.
43.Hoff, A. J. (1986) in Light Emission of Plant
and Bacteria (Govindjee, Ametz, J., and Fork, D. C., eds.) Academic
Press, New York, pp. 225-266.
44.Krasnovsky, A. A., Jr. (1994) Proc.
Roy. Soc. Edinburgh, 102B, 219-235.
45.Krasnovsky, A. A., Jr. (1994)
Biofizika, 39, 236-250.
46.Krasnovsky, A. A., Jr., and Semenova, A. N.
(1981) Photobiochem. Photobiophys., 3,
11-18.
47.Krasnovsky, A. A., Jr., and Semenova, A. N.
(1981) Dokl. Akad. Nauk SSSR, 257, 729-732.
48.Solovyov, K. N., Dvornikov, S. S., Knyukshto, B.
N., and Turkova, A. E. (1983) J. Appl. Spectr. (Minsk),
38, 87-95.
49.Losev, A. P., Nichiporovich, I. N., Sagun, E. I.,
and Vasilenok, G. D. (1987) Dokl. AN BSSR, 31,
131-134.
50.Losev, A. P., Sagun, E. I., and Nichiporovich, I.
N. (1987) Khim. Fizika, 6, 907-914.
51.Egorov, S. Yu., Krasnovsky, A. A., Jr.,
Vychegzanina, I. V., Drozdova, N. N., and Krasnovsky, A. A. (1990)
Dokl. Akad. Nauk SSSR, 310, 471-474.
52.Raskin, V. I. (1981) Photoreduction of
Protochlorophyllide [in Russian], Nauka i Tekhnika, Minsk.
53.Belyaeva, O. B. (2009) Light-Dependent
Synthesis of Chlorophyll [in Russian], Binom, Moscow.
54.Lebedev, N. N., Krasnovsky, A. A., Jr., and
Litvin, F. F. (1991) Photosynth. Res., 30, 7-14.
55.Krasnovsky, A. A., Jr., Belyaeva, O. B., Kovalev,
Yu. V., Ignatov, N. V., and Litvin, F. F. (1999) Biochemistry
(Moscow), 64, 587-591.
56.Ignatov, N. V., Krasnovsky, A. A., Jr., Litvin,
F. F., Belyaeva, O. B., and Walter, G. (1983)
Photosynthetica, 17, 352-360.
57.Egorov, S. Yu., Krasnovsky, A. A., Jr., and
Kulakovskaia, L. I. (1985) Russ. Plant Physiol., 32,
668-673.
58.Butler, W. L. (1961) Arch. Biochem.
Biophys., 92, 287-295.
59.Krasnovsky, A. A., Jr., and Neverov, K. V. (1988)
Dokl. AN SSSR, 302, 252-255.
60.Kovalev, Yu. V., and Krasnovsky, A. A., Jr.
(1986) Biofizika, 31, 444-448.
61.Goedheer, J. C. (1964) Biochim. Biophys.
Acta, 88, 304-317.
62.Murata, N., Nishimura, M., and Takamiya, A.
(1966) Biochim. Biophys. Acta, 126, 234-243.
63.Govindjee and Yang, L. (1966) J. Gen.
Physiol., 49, 763-780.
64.Litvin, F. F., and Sineshchekov, V. A. (1975) in
Bioenergetics of Photosynthesis (Govindjee, ed.) Academic Press,
Inc., San Francisco, pp. 620-661.
65.Litvin, F. F., Shubin, V. V., and Sineshchekov,
V. A. (1976) Biofizika, 21, 669-674.
66.Shlyk, A. A. (1975) in Biosynthesis and the
State of Chlorophyll in Plants [in Russian], Nauka i Tekhnika,
Minsk, pp. 104-160.
67.Neverov, K. V., Shalygo, N. Y., Averina, N. G.,
and Krasnovsky, A. A., Jr. (1996) Russ. Plant Physiol.,
43, 62-73.
68.Takahashi, Y., Hansson, O., Mathis, P., and
Satoh, H. (1987) Biochim. Biophys. Acta, 893, 49-59.
69.Telfer, A., Dhami, S., Bishop, S. M., Phillips,
D., and Barber, J. (1994) Biochemistry, 33,
14469-14474.
70.Neverov, K. V., and Krasnovsky, A. A., Jr. (2004)
Biofizika, 49, 493-498.
71.Santabarbara, S., Bordignon, E., Jeninngs, R. C.,
and Carbonera, D. (2002) Biochemistry, 41, 8184-8194.
72.Krasnovsky, A. A., Jr. (1986) Zh. Vsesouz.
Khim. Obshchestva im. D. I. Mendeleeva, 31, 562-567.