Effect of Sex Hormones on Levels of mRNAs Coding for Proteins Involved in Lipid Metabolism in Macrophages
T. A. Shchelkunova1*, I. A. Morozov2, P. M. Rubtsov2,3, L. M. Samokhodskaya4, I. V. Andrianova5,6, E. G. Rudimov6, I. A. Sobenin7, A. N. Orekhov7, and A. N. Smirnov1#
1Lomonosov Moscow State University, Biological Faculty, Leninskie Gory 1, 119899 Moscow, Russia; fax: (495) 939-4309; E-mail: schelkunova-t@mail.ru2Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, ul. Vavilova 32, 119991 Moscow, Russia; fax: (499) 135-1405; E-mail: rubtsov@eimb.ru
3Moscow Institute of Physics and Technology, Institutskii Pereulok 9, 141700 Dolgoprudny, Moscow Region, Russia; fax: (495) 576-0813
4Lomonosov Moscow State University, Faculty of Fundamental Medicine, Lomonosovsky pr. 31/5, 119899 Moscow, Russia; fax: (495) 932-9904; E-mail: slm@fbm.msu.ru
5Russian Cardiological Scientific Production Complex, ul. 3-ya Cherepkovskaya 15A, 121552 Moscow, Russia; fax: (495) 414-6731; E-mail: irandrianova@yandex.ru
6Institute of Biomedical Problems, Russian Academy of Sciences, Khoroshevskoe Shosse 76a, 123007 Moscow, Russia; fax: (499) 195-1500
7Institute of General Pathology and Pathophysiology, Russian Academy of Medical Sciences, ul. Baltiiskaya 8, 125315 Moscow, Russia; fax: (495) 415-9594; E-mail: office@inat.ru
* To whom correspondence should be addressed.
# Deceased.
Received July 15, 2013; Revision received August 30, 2013
The effects of sex hormones estradiol (E2), testosterone (Te), and 5α-dihydrotestosterone (DT) on cholesterol accumulation induced by modified low density lipoproteins (LDL) in macrophages differentiated from human peripheral blood monocytes and on the levels of mRNAs coding for proteins involved in lipid metabolism have been studied. All three hormones at physiological concentrations (1 nM) are capable of reducing cholesterol accumulation in cells. The treatment of cells with modified and native (not inducing cholesterol accumulation) LDL results in similar alterations in the expression of several mRNAs aimed primarily at homeostatic regulation of lipid metabolism. These alterations depend on the sex of macrophage donors and in some cases are even reversed in cells obtained from male and female donors. The cells not treated with modified LDL have no significant gender differences in the expression of the examined mRNAs. Hormones, either independently or in combination with the modified LDL, influence the levels of some mRNAs, and each hormone shows an individual range of effects. Correlation analysis of changes in mRNA content in the cells showed that the hormones may interfere with coordination of gene expression. Hormone action leads to: (1) reduced coupling of the content of individual mRNAs with their initial levels in the control cells; (2) reduced coupling of different mRNA levels; (3) regrouping of mRNAs between the clusters; and (4) changes in the number of factors that determine the correlation links between mRNAs. The data show that sex hormones may have impact on the level of expression of certain genes and, in particular, on the coordination of gene expression in macrophages.
KEY WORDS: sex hormones, PCR, gene expression, atherogenesis, macrophages, correlation analysisDOI: 10.1134/S0006297913120043
Abbreviations: ABCA1 and ABCG1, ATP-binding cassette transporters A1 and G1; ACAT1, acyl-CoA-cholesterol acyltransferase 1; ApoE, apolipoprotein E; AR, androgen receptor; Arom, aromatase; CCL18, chemokine 18 with a C-C (CCL18) motif; CD36 and CD68, clusters of differentiation 36 and 68; CEH, neutral cholesterol ester hydrolase; DT, 5α-dihydrotestosterone; E2, estradiol; ERα and ERβ, estrogen receptors alpha and beta; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HF, hydroxyflutamide; ICAM1, intercellular adhesion molecule 1; LDLR, low density lipoprotein receptor; LPL, lipoprotein lipase; LXRα and LXRβ, liver X receptors alpha and beta; mLDL and nLDL, modified and native low density lipoproteins, respectively; MP, macrophages; PPARα and PPARγ, peroxisome proliferator-activated receptors alpha and gamma; SR-A, scavenger receptor A; SR-BI, scavenger receptor BI; SREBP1 and SREBP2, sterol regulatory element binding proteins 1 and 2; STS, steroid sulfatase; Te, testosterone; TfR1, transferrin receptor 1; TLR4, Toll-like receptor 4; TNFα, tumor necrosis factor alpha; VCAM1, vascular cell adhesion molecule 1.
Atherosclerosis is one of the diseases characterized by marked gender
differences in the frequency of its development. Women of childbearing
age suffer from this disease much less frequently than male peers.
However, the risk of development of atherosclerosis-related
cardiovascular diseases substantially increases as women approach
menopause. Hence, estrogens in females are considered to be a natural
antiatherogenic factor [1]. The role of androgens
in male gender differences in atherosclerosis development is less
distinct. High doses of anabolic steroids stimulate atherogenesis [2]. However, male hypogonadism is also accompanied by
accelerated development of atherosclerosis, and androgen replacement
therapy in this case reduces the disease risk [3].
Hence, it can be supposed that the normal level of androgens in males
also has an atheroprotective effect, though obviously less efficient
compared to the effect of estrogens in females.
Liver is one of the known sites of the antiatherogenic effect of estrogens. In particular, due to the induction of expression of low density lipoprotein receptor (LDLR) in hepatocytes, estrogens contribute to more effective removal of proatherogenic, cholesterol-rich lipoprotein particles from the bloodstream [4, 5]. It has also been shown that sex hormones can affect the basic cellular elements of the arterial wall: endothelial and smooth muscle cells [6-8]. No less than half of the atheroprotective effect of estrogens is supposed to be realized at the level of the arterial wall [9]. One of the targets of such an effect may be the LDLR, with mRNA level in the intima of female aorta much higher than in male aorta. We believe that more rapid elimination of low density lipoproteins (LDL) from the subendothelial space may prevent LDL modifications making these particles atherogenic [10].
Macrophages are a lesser part of the cell population in intact intima of arteries. However, as atherosclerosis develops, their number very substantially increases [11]. So, the number of hematogenic cells represented mostly by macrophages in atherosclerotic plaques of the coronary arteries may reach 45% [12]. Taking into consideration the important role of macrophages as foam cell precursors, as antigen-presenting cells, and as sources of proinflammatory cytokines, macrophages are often used as a surrogate model of atherogenesis. Macrophages can respond to the action of estrogens and androgens [13] and, thereby, are potentially able to contribute to the formation of gender differences in the risk of atherosclerosis. Although researchers quite often address this subject, data on the regulatory effect of sex hormones on macrophages are still very ambiguous and fragmentary. This study was undertaken to systematically analyze potential targets of the regulatory effects of sex hormones on macrophages at the level of transcription of genes directly or indirectly related to lipid exchange.
MATERIALS AND METHODS
Isolation of monocytes/macrophages. Mononuclear cells were isolated from the venous blood of 17 healthy donors (nine women and eight men, 21- to 57-years-old) on empty stomach, with their written informed consent. The cells were isolated by centrifugation in a Ficoll-Paque Plus density gradient (Amersham Biosciences, Sweden) according to the manufacturer’s protocol.
Macrophages were obtained as follows: DMEM suspension of isolated cells (Gibco BRL Life Technologies, USA) containing 10% fetal calf serum (ICN Biochemicals Inc., USA), 2 mM glutamine, 100 mg/ml kanamycin, and 2.5 mg/ml amphotericin B was poured into 35-mm plastic culture dishes (Corning Costa, USA) at 3·106 cells per dish. The cells were cultivated at 100% humidity and 37ºC in a CO2 incubator (mixture of 5% CO2 and 95% atmospheric air). The next day the unattached cells were removed, and the cells attached to the substrate (monocytes) were cultivated under the same conditions for 7-10 days with the medium changed every two days.
Lipid loading of macrophages and their incubation with hormones. One day before the beginning of the experiment, the cultivation medium was replaced by fresh medium without phenol red but with fetal calf serum treated with activated charcoal to remove endogenous steroid hormones. In a typical experiment, mRNA was measured in eight dishes with cells from the same donor: 1) control (without additional impacts); 2) incubation with mLDL only; 3-5) simultaneous incubation with modified LDL (mLDL) and sex hormones (estradiol (E2), testosterone (Te), and 5α-dihydrotestosterone (DT), respectively); 6-8) incubation with sex hormones only (E2, Te, and DT, respectively). Additional dishes or plates corresponding to these dishes 1-5 were used in experiments with cholesterol measurements. Lipid-loaded macrophages were obtained using freshly isolated mLDL. The mLDL were isolated from the combined blood serum of patients with diagnosed atherosclerosis, with their written informed consent, using a two-stage procedure of ultracentrifugation in a stepwise sodium bromide density gradient. Thereby obtained LDL fraction was dialyzed against phosphate-buffered saline (PBS) for 12 h at 4ºC and sterilized by filtration through a 0.45-µm filter. The mLDL were added to the cells to the final concentration of 100 µg/ml followed by 18-h incubation. In some experiments, native LDL (nLDL) isolated from the serum of healthy donors by the method described above were used simultaneously with mLDL. Hormones (Sigma, USA) were added to the medium at final concentration of 10–9 M from 1 mM ethanol solution after appropriate dilution with the culture medium. The same amount of ethanol was added to the control cells. The competitive androgen receptor hydroxyflutamide (HF) kindly provided by Professor A. G. Reznikov (Kiev, Ukraine) was additionally used in some experiments at final concentration of 1 µM. After 18-h incubation, the cells were washed three times with PBS and, after the addition of Trizol, stored at –70ºC. Some part of the cells was used for cholesterol measurement.
The cells were three times extracted with hexane–isopropyl alcohol mixture (3 : 2 v/v) to measure mLDL-induced cholesterol accumulation in the macrophages and to estimate the effects of hormones on this indicator. The content of cholesterol was measured enzymatically using the Biocon® Diagnostik reagent kit (Marienhagen, Germany) and normalized by the protein using the Lowry assay [14].
Measurement of mRNA content. mRNA content was determined by quantitative PCR in the real time mode. The methods of analysis have been described in detail previously [15]. RNA was isolated from frozen samples using TRIzol Reagent (Invitrogen, USA). cDNA was synthesized on the total RNA using the ImProm-II™ Reverse Transcription System (Promega, USA). The synthesized cDNA was used as a template for PCR in real time mode in a Rotor-Gene 3000 amplifier (Corbett Research, Australia), with a kit of reagents including SYBR Green I intercalating dye (Syntol, Russia) by the technique supplied with the kit. The primers used have been described previously [16]. For verification of the correspondence between the amplification products and the anticipated cDNA fragments, they were sequenced using an ABI PRISM® BigDye™ Terminator v.3.1 in an ABI PRISM 3100-Avant automated DNA sequencer. PCR results were taken into consideration only when the melting temperature and electrophoretic mobility of the amplification products corresponded to the expected values. The data of analysis of the content of individual mRNAs in the studied samples are expressed in percentage of the mRNA content of glyceraldehyde phosphate dehydrogenase (GAPDH) used as an internal reference.
Statistics. Less than half of the data on mRNA content in the samples corresponded to normal distribution. Therefore, the correlations between the values were estimated by the Spearman rank correlation coefficient (Rs), and differences in mRNA content were estimated by the Mann–Whitney criterion. Statistica 8.0 was used for statistical processing of the results. The correlations or differences with p < 0.05 were considered statistically significant. The weight of the correlation graph Gh calculated as a sum of modules of significant correlation coefficients Rs for the analyzed pairs of mRNA, Gh = Σ|Rs|, was used as an integral indicator of the degree of coupling between mRNA contents [17]. Cluster analysis and factor analysis were performed on the basis of Spearman correlation matrices. Cluster analysis was performed using the options of preset number of clusters and assortment of distances with selection of observations at constant intervals. The principal component method was used for the factor analysis.
RESULTS
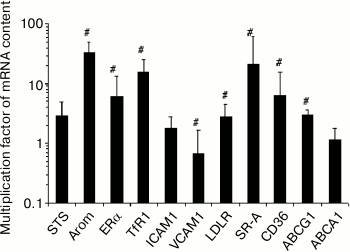
Monocyte-to-macrophage differentiation. The differentiation of monocytes to macrophages during 7-10 days under basal conditions was accompanied by changes in the expression of 8 out of 11 of the studied mRNAs. The content of three of them (Arom, TfR1, SR-A) in the cells was more than an order of magnitude higher (Fig. 1).
Fig. 1. Changes in the content of mRNA during 7-10-day differentiation of monocytes to macrophages. The content of mRNA in monocytes was taken as a unit. Symbol # denotes statistically significant differences between monocytes and macrophages. The cells of blood from 2 males and 4 females were used.
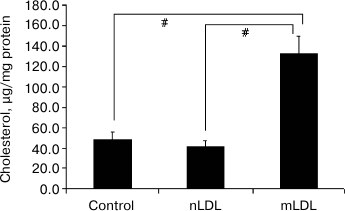
Lipid accumulation in macrophages. The 18-h incubation of macrophages with the modified LDL was accompanied by a 2-3-fold increase in the content of cholesterol. Native LDL isolated from the blood of healthy donors did not stimulate cholesterol accumulation (Fig. 2).
Fig. 2. Variations of cholesterol content in macrophages after 18-h incubation with native or modified low density lipoproteins (nLDL and mLDL) (100 µg/ml). Symbol # denotes statistically significant differences between groups.
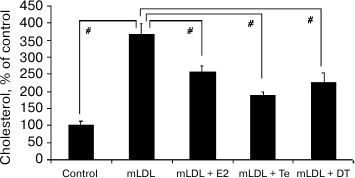
The addition of estradiol (E2), testosterone (Te), or 5α-dihydrotestosterone (DT) at final concentration of 1 nM to the culture medium noticeably decreased cholesterol accumulation induced by the mLDL (Fig. 3).
Fig. 3. Effects of sex hormones (1 nM) on cholesterol accumulation in macrophages induced by mLDL (100 µg/ml). E2, estradiol; Te, testosterone; DT, 5α-dihydrotestosterone. Symbol # denotes statistically significant differences between groups.
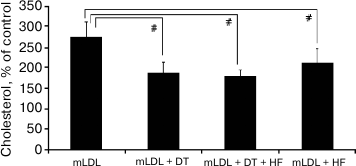
Expression of androgen receptor (AR) mRNA in macrophages is extremely low (~0.04% of GAPDH mRNA), which is more than three orders of magnitude below the content of AR mRNA in the intima of intact aorta [10]. The problem of the involvement of AR in implementation of the inhibitory effect of androgens on cholesterol accumulation was elucidated by studying the effect of the androgen receptor antagonist hydroxyflutamide (HF) at final concentration of 1 µM on the efficiency of 5α-dihydrotestosterone (1 nM). As one can see from Fig. 4, hydroxyflutamide did not block the inhibitory effect of 5α-dihydrotestosterone on cholesterol accumulation. Moreover, it suppressed cholesterol accumulation induced by the mLDL.
Fig. 4. Effect of hydroxyflutamide (HF, 1 µM) on cholesterol accumulation in macrophages induced by mLDL (100 µg/ml) in the presence and absence of 5α-dihydrotestosterone (DT, 1 nM). The content of cholesterol in the control macrophages was taken as 100%. Symbol # denotes statistically significant differences between groups.
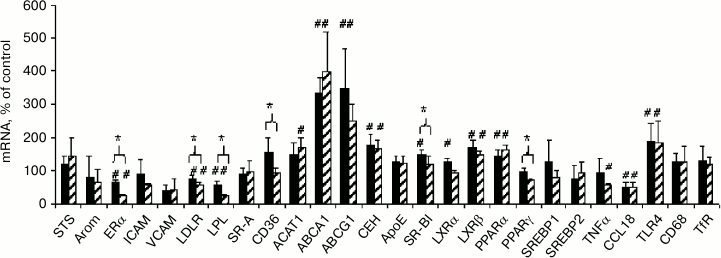
Comparison of effects of native and modified LDL on mRNA expression. The effects of both types of LDL on the content of lipid metabolism-related mRNAs in macrophages were estimated to elucidate the possible cause of the crucial difference between modified and native LDL, which is manifested in the ability of mLDL to induce cholesterol accumulation in cells and the absence of such ability in nLDL (Fig. 2). One can see (Fig. 5) that the alterations of mRNA expression induced by native and modified LDL are very similar in general and are related mostly to homeostatic regulation at the level of lipid metabolism-related mRNAs: inhibition of the expression of LDLR and lipoprotein lipase (LPL) supplying lipids to the cells, stimulation of reverse cholesterol transport due to the induction of cholesterol transporters ABCA1 and ABCG1 and cholesterol ester hydrolase (CEH), and enhancement of the sensitivity of lipid sensors LXRβ and PPARα due to their induction. The two types of LDL also had a unidirectional influence on the expression of mRNAs of estrogen receptor α (ERα) and inflammatory response-related factors CCL18 and TLR4. The degree of influence of the two types of LDL on mRNA content, however, could be different in some cases. The modified LDL inhibited the expression of ERα, LDLR, LPL, and TNFα and induced the expression of ACAT1 more effectively compared to the native LDL, while the native LDL more effectively stimulated the expression of SR-BI and LXRα. Elucidation of probable relationships between these differences and cholesterol accumulation in cells is a task for further research.
Fig. 5. Comparison of effects of native (black columns) and modified (shaded columns) LDL on content of studied mRNAs in macrophages obtained from the male donor used in the experiment in Fig. 2. Symbol # denotes statistically significant differences from control cells incubated without LDL. Symbol * denotes differences in the effects of the two types of LDL.
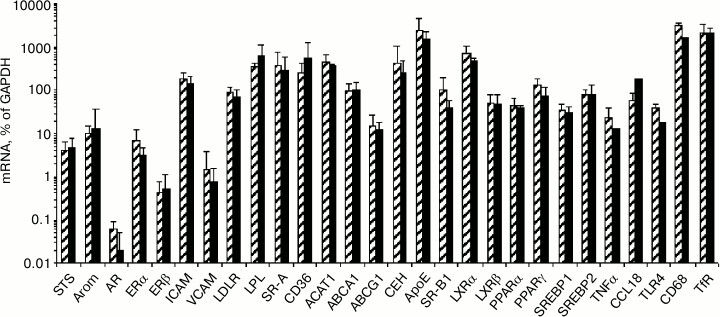
Influence of modified LDL and sex hormones on content of studied mRNAs in macrophages. Among the studied 30 types of mRNA, three types of mRNA (estrogen sulfotransferase, androgen receptor, E-selectin) showed an extremely low level of expression in macrophages and were excluded from further analysis. No gender differences were found in the content of the studied mRNA in the control macrophages, i.e. in cells not exposed to the effect of mLDL and/or sex hormones (Fig. 6).
Fig. 6. Content of studied mRNAs in control macrophages from females (shaded columns) and males (black columns).
The treatment of cells with the modified LDL and hormones resulted in variations of the content of some mRNAs. Statistically reliable variations are given in Table 1.
Table 1. Effects of treatment of macrophages
with mLDL (100 µg/ml) and sex hormones (1 nM) on expression
of studied mRNAs. The arrows on the black and gray background mark the
stimulatory and inhibitory effects, respectively. The frames mark the
p values for gender differences in the response of macrophages
to the treatment. The control of hormonal effects was untreated cells
(“0”) or mLDL-treated cells
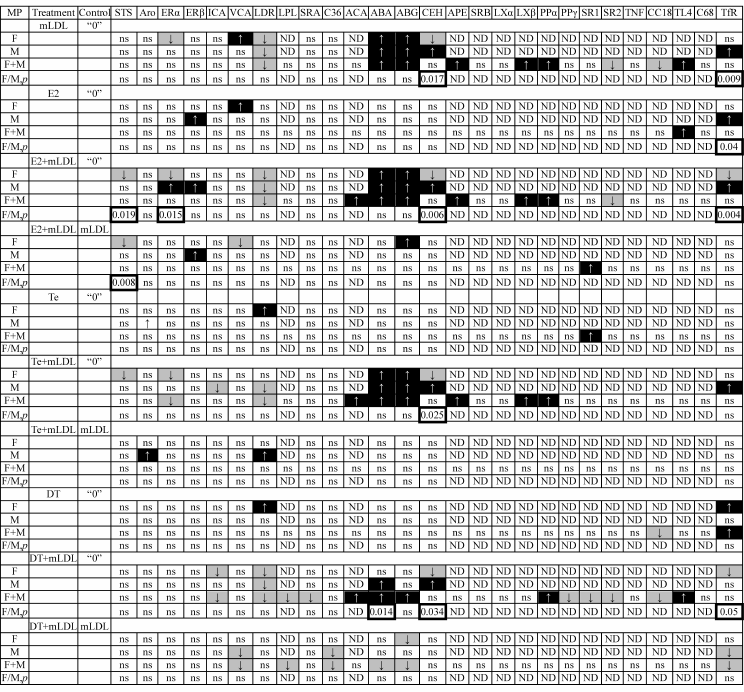
Note: F, females; M, males; ns, not significant; ND, not determined
separately in male and female cells.
One can see that the treatment of cells with mLDL in the absence and presence of hormones induced the expression of mRNAs encoding ACAT1, ABCA1, ABCG1, ApoE, LXRβ, PPARα, and TLR4 and inhibited the expression of LDLR, SREBP2, and CCL18 mRNAs. Some effects of mLDL were observed only in cells isolated from donors of a certain sex (mRNA of STS, ERα, ERβ, ICAM1, VCAM1, CEH, TfR1) and, in some cases, the direction of responses to the treatment of cells isolated from female and male donors was exactly the opposite (ERα, CEH, TfR1).
Correlation in the expression of the studied mRNAs in the presence of mLDL only and during the simultaneous treatment of cells with mLDL and hormone was analyzed to elucidate the potential targets of the “antiatherogenic” effect of sex hormones (Fig. 3). As Table 1 shows, the number of responses to the hormone increased in the order: testosterone < estradiol < 5α-dihydrotestosterone. Against the background of mLDL, estradiol reduced the content of STS and VCAM1 mRNAs in female cells and increased the content of mRNAs of ABCG1 in female cells, ERβ in male cells, and SREBP1 in a mixed population of male and female cells. Testosterone increased the levels of Arom and LDLR mRNAs in male cells. 5α-Dihydrotestosterone against the background of mLDL had an inhibitory effect only. It reduced the expression of mRNAs of VCAM1, LPL, CD36, ABCA1, ABCG1, and TfR1.
The isolated effects of hormones (i.e. in the absence of mLDL) on mRNA expression rarely coincided with the effects of the same hormones in the presence of mLDL, and in some cases could even be opposite. So, the only coincidence of the effects of estradiol during its isolated application and in combination with mLDL was the stimulation of the ERβ mRNA in male macrophages. The isolated application of testosterone and its application together with mLDL had no targets in common. The cross point of isolated action of 5α-dihydrotestosterone and its combination with mLDL was the TfR1 mRNA: its level was stimulated in cells from female donors and in the mixed population during isolated treatment with the hormone and, on the contrary, suppressed in the cells from male donors and in the mixed population during co-treatment with mLDL and the hormone.
Correlations between mRNA contents. As Table 2 shows, the expression of most types of mRNA during incubation with mLDL maintains a relationship with the initial level of expression in the control macrophages (17/23, Gh = 13.63, lower lines of Table 2). The exceptions are ERα, ERβ, LPL, LXRα, PPARγ, and SREBP1 mRNAs. At the same time, Table 1 shows the absence of any changes in the content of the above mRNAs under the action of mLDL. Thus, in addition to the quantitative changes in gene expression, mLDL cause an implicit reorganization of their regulation. The addition of estradiol in the presence of mLDL provides the high-level continuity in mRNA expression (21/23, Gh = 17.80). In the presence of testosterone and especially 5α-dihydrotestosterone, this continuity turns out to be much less (Gh = 14.23 and 12.46, respectively). The same regularity is observed also during the treatment of cells by hormones in the absence of mLDL: in case of estradiol introduction, the coherence in mRNA expression between the control and experimental cells is rather high, while testosterone and 5α-dihydrotestosterone reduce this coherence. The expression of Arom, ERβ, ABCA1, ACAT1, PPARγ, SREBP1, and SREBP2 mRNAs is most sensitive to the disturbing effects of androgens.
Table 2. Coherence of expression of
individual mRNAs in macrophages without treatment (“0”) and
after treatment with mLDL and hormones
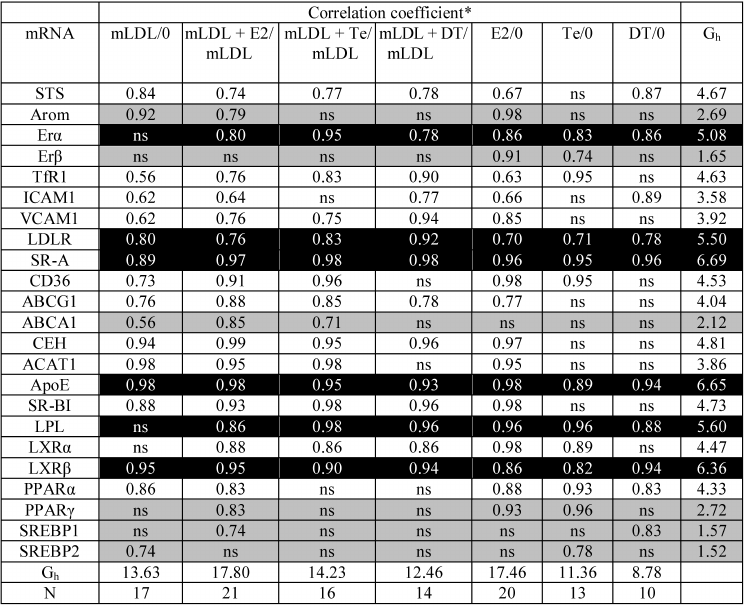
* ns, absence of statistically significant correlation. The
correlations for mRNAs with high (Gh > 5.0, column on
right) and low (Gh < 3.0, column on right) coherence of
expression in untreated cells and after treatment with mLDL and/or
hormones are indicated by black and gray background.
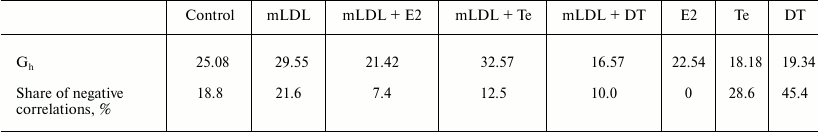
The data from Table 3 show that sex hormones can affect the coherence in expression of various types of mRNA. In the presence of mLDL, estradiol and 5α-dihydrotestosterone reduce and testosterone slightly increases the coherence. During isolated application, all three hormones slightly reduce the coherence between mRNAs. Attention is drawn to the fact that the proportion of negative correlations increases during isolated application of androgens, but they disappear completely in the presence of estradiol. In the presence of mLDL, all three hormones reduce the proportion of negative correlations.
Table 3. General characteristics of
correlations between mRNAs in MP
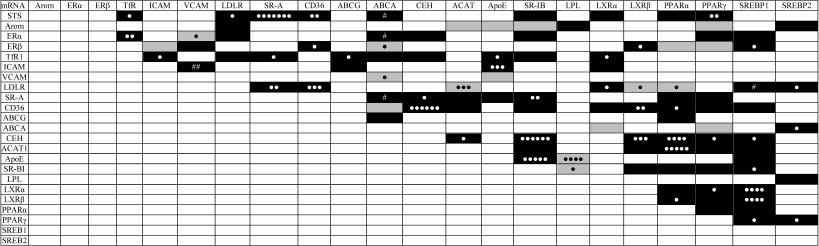
The imposition of correlations between different types of mRNA in undisturbed cells and in cells incubated with mLDL and/or hormones (Table 4) revealed altogether 104 out of 253 potential correlations (41%). Forty-nine of the connections revealed (47%) were conservative, i.e. found in the cells exposed to various impacts. The correlations in the STS/SR-A, ICAM1/ApoE, LDLR/CD36, LDLR/ACAT1, CD36/CEH, CEH/SR-BI, CEH/LXRβ, CEH/PPARα, ACAT1/PPARα, ApoE/SR-BI, ApoE/LPL, LXRα/SREBP1, and LXRβ/SREBP1 mRNA pairs were especially conservative, revealed at least in the four types of cell samples. These connections are obviously most resistant to the action of sex hormones.
Table 4. Imposition of statistically
significant correlations between the contents of different mRNAs in
macrophages without treatment and after treatment with mLDL and/or sex
hormones. Positive and negative correlations are indicated by black and
gray background, respectively. Symbols ●, ●●, etc.
denote the correlations common for two, three, and more types of cell
samples. Symbol # denotes correlations changing their sign in one of
the sample types
Visual estimation of the mosaic of correlations in Table 4 suggests the presence of a certain order of their distribution in the matrix. Cluster analysis of correlations was performed for all types of cell samples. When choosing the optimal number of clusters, we proceeded from the fact that the basis of such clusters may be the above-listed mRNA pairs with highly conservative bonds (13 pairs in all). Divergence of the members of such pairs into different clusters may imply inadequate fractionation of the correlations. It turned out that, with the mRNA population being divided into 3, 4, and 5 clusters, the convergence of members of the pairs of conservative correlations in a single cluster (56%) was greatest in case of 3 clusters. When the number of clusters increases to 4 and 5, this value decreased to 49 and 46%, respectively. It is interesting to note that the members of conservative negative correlations (LDLR/ACAT1 and ApoE/LPL) were actually never found in the same cluster.
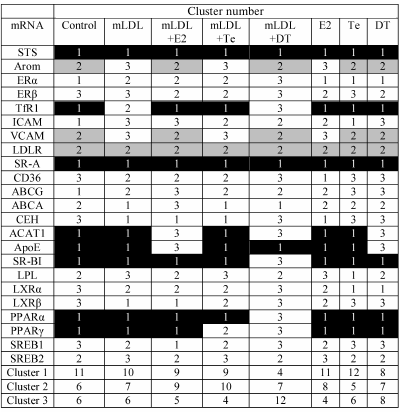
Table 5 shows mRNA distribution between three clusters in the samples of macrophages exposed to various impacts. One can see that most part of the mRNAs can shift from one cluster to another under the influence of mLDL and/or sex hormones. The most marked mRNA redistribution is observed during the treatment of cells with mLDL + DT, when the portion of mRNAs comprising cluster 1 drastically decreases, and the weight of cluster 3 increases. This fact is in agreement with the data of Table 1 on the numerous effects of 5α-dihydrotestosterone in the presence of mLDL. Estradiol and testosterone had no such marked effect on quantitative composition of the clusters.
Table 5. Effects of mLDL and sex hormones on
distribution of studied mRNAs between three clusters. The clusters were
numerated in accordance with the inclusion of STS and LDLR mRNAs
(cluster 1 and cluster 2, respectively). Cluster 3 had no constant
representation of any mRNA. The inclusion of mRNAs in cluster 1 or 2 in
at least 5 out of 8 types of cell samples is denoted by black and gray
background, respectively
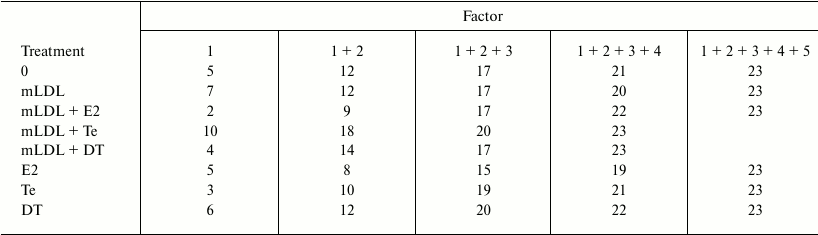
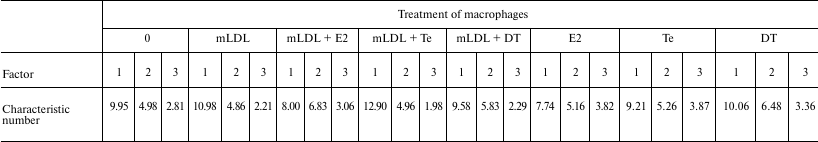
Factor analysis was performed to reveal the effects of sex hormones on the spectrum of regulatory impacts that determine coordination of the expression of the studied genes in macrophages. The data from Table 6 show that testosterone in the presence of mLDL substantially decreases the number of regulatory factors determining most correlations between the mRNAs. Estradiol independently and in the presence of mLDL, on the contrary, broadens the spectrum of such factors. No marked effect of 5α-dihydrotestosterone on this value was revealed.
Table 6. Cumulative contribution of factors
1-6 to correlations between the mRNAs in control macrophages
(“0”) and macrophages treated with mLDL and/or hormones.
The number of mRNA types (out of 23) is presented, where correlations
are determined by combined effects of the factors by more than 70%
The method of principal components (Table 7) leads to the same conclusions.
Table 7. Effect of treatment of macrophages
with mLDL and hormones on the contributions of factors 1-3 in the
correlation between 23 types of mRNAs
DISCUSSION
The addition of all three sex hormones (estradiol, testosterone, and 5α-dihydrotestosterone) at a physiological concentration (1 nM) to the culture medium reduced the modified LDL-induced cholesterol accumulation in macrophages (Fig. 3). Female and male macrophages have similar levels of expression of mRNAs of the two types of estrogen receptors (ERα and ERβ; Fig. 5), which probably mediate the atheroprotective effect of estradiol [8]. At the same time, the level of expression of the androgen receptor (AR) mRNA in both male and female macrophages is extremely low (Fig. 5), being about 1000-fold less than AR expression in the intima of the aorta [10]. We cannot fully exclude the possible effect of androgens on cholesterol accumulation in macrophages via this receptor; however, the probability of this pathway seems to be quite some. The results of experiments with the AR antagonist hydroxyflutamide, that not only does not suppress the effect of 5α-dihydrotestosterone but also inhibits cholesterol accumulation (Fig. 4), confirm the supposition that AR is not involved in the inhibitory effect of androgens on cholesterol accumulation in macrophages. Our results are in agreement with the findings of some other authors, namely, transduction of the signal of BSA-conjugated testosterone inaccessible for intracellular AR [18], induction of fast responses to androgen in a cell line not expressing AR [19], inability of competitive AR antagonist to block the effect of androgen on the cells [18], and maintenance of the ability of androgens to inhibit the proinflammatory activity of macrophages in mice with testicular feminization syndrome lacking functional AR [20]. As follows from the data in Fig. 1, the differentiation of monocytes to macrophages is accompanied by an approximately 30-fold increase in the expression of aromatase mRNA. Hence, it may be assumed that testosterone can have an effect on macrophages in two ways: (1) via transformation into estradiol and then through estrogen receptors, and (2) via the supposed (membrane?) androgen sensor used by the non-aromatized androgen 5α-dihydrotestosterone.
The expression of some lipid metabolism-related mRNAs was analyzed to find the potential targets of the action of sex hormones in macrophages. At the same time, it suddenly turned out that, in spite of the absence of gender differences in the content of all mRNAs under study in the control macrophages (Fig. 6), the response of cells to the disturbing effect of mLDL in the presence or absence of hormones in some cases depended on the sex of the monocyte/macrophage donors. Gender differences in the response were recorded for several types of mRNAs and proved to be particularly marked for the ERα, CEH, and TfR1 mRNAs, the content of which varied in male and female cells in opposite directions (Table 1). The results can be interpreted as the existence of “sex memory” in macrophages differentiated from peripheral blood monocytes. Some other authors also noted gender differences in the properties of macrophages, but these differences were attributed to the levels of the receptors of sex hormones [21], the expression of which is similar in female and male cells according to our data. The phenomenon of hormonal imprinting is well known in relation to some sex-differentiated functions of the brain and liver [22-24]. The imprinting of acyclic type of functioning of the gonads, male sexual behavior, and the male pattern of growth hormone secretion in male rodents occurs in the perinatal period under the influence of testis androgens and includes the induction of aromatase providing high local level of estrogen in the brain. Reproduction of the imprinting by perinatal introduction of high doses of estrogens to females and, on the contrary, the blocking of imprinting by introduction of anti-estrogens and the absence of imprinting with a knocked-out estrogen receptor or upon introduction of the non-aromatized androgen 5α-dihydrotestosterone to the animals demonstrate that the active principles of imprinting are really the estrogens formed locally in the brain [25, 26]. In addition to formation of long-term “memory” for the effect of hormones, the hormonal imprinting of brain functions is characterized by a so-called “critical period” during which the imprinting of hormonal effect is possible. At the same time, the programming of the masculinizing effect of androgens on the expression of some sexually differentiated liver proteins has no marked upper boundary of critical period and may be reproduced by the introduction of the hormone into adult animals. Estrogens do not reproduce such effect of androgens on the liver, suggesting that the active principles in this case (in contrast to the brain) are androgens [22]. The “sexual memory” in the primary hepatocyte cultures of female and male rats during the entire period of cultivation was proved in case of the content of estrogen sulfotransferase [27] and cytochrome P450 CYP2C12 isoform, as well as in the growth hormone signal transduction system (level of Erk1/2 phosphorylation, CBP activity, etc.) [28]. The phenomenon of hormonal imprinting in humans actually has not been studied, and the revealed gender differences in the response of macrophages to the treatment with mLDL could be the starting point for such research. The primary objectives seem to be as follows: (1) elucidation of the level of differentiation of cell precursors of macrophages capable of imprinting; (2) determination of the type of sex hormones (estrogens or androgens) providing hormonal imprinting; and (3) analysis of age-related dependence of manifestation of “sex” program in macrophages.
The effects of sex hormones on the variations in mRNA levels induced by simultaneous introduction of mLDL and a hormone were determined by way of comparison with the isolated action of mLDL. It has been shown that all three hormones can influence the expression of some mRNAs (Table 1). As regards the number of induced responses, the hormones can be arranged in the following series: testosterone < estradiol < 5α-dihydrotestosterone. However, only two objects proved to be a common target for the hormones: ABCG1 mRNA, with its expression stimulated by estradiol and inhibited by 5α-dihydrotestosterone in female cells, and VCAM1 mRNA, the level of which was suppressed by estradiol and 5α-dihydrotestosterone in female and male cells, respectively.
Under the isolated action of the hormones, the number of regulated parameters was less than under their joint action with mLDL. The common target for the two androgens was the LDLR mRNA, the level of which in female cells was stimulated by both testosterone and 5α-dihydrotestosterone. Estradiol and 5α-dihydrotestosterone had a unidirectional effect on TfR1 mRNA, increasing its level in male and female cells, respectively. Comparison of the effects of hormones during their isolated application and in combination with mLDL shows that mLDL very substantially modifies the character of mRNA responses to the hormones. First, the targets for the effect of a given hormone under different conditions overlap only partially. Unidirectional effects of isolated application of the hormones and their combinations with mLDL were observed for the stimulating effect of estradiol on the ERβ mRNA in male cells and the effect of testosterone on the LDLR mRNA in female and male cells, respectively. Second, mLDL can change even the direction of the hormonal effect. Thus, the regulatory effects of estradiol in the absence and presence of mLDL were opposite against VCAM1 in female cells and opposite against the TfR1 mRNA under the influence of 5α-dihydrotestosterone on female and male cells. The influence of lipoproteins on the estrogen effects in macrophages was also noted by other researchers [29].
The used model of effects of sex hormones on mRNA expression in macrophages has revealed considerable new data on the potential targets of regulation that could be related to lipid metabolism in these cells. Steroid sulfotransferase (STS) provides reactivation of many signaling compounds such as estrogens, androgens (e.g. dehydroepiandrosterone), and oxysterols. Oxysterol sulfates, in contrast to the initial oxysterols, act not as agonists but as antagonists of oxysterol sensors LXR [30, 31]. The revealed ability of estradiol to inhibit the expression of STS mRNA can thereby result in reduction of the efficiency of oxysterol signaling in the cell. The revealed stimulatory effect of testosterone in the presence of mLDL on the expression of aromatase mRNA is in agreement with the data obtained previously in the THP-1 myeloid monocyte cell line [32] and primary human osteoblasts [33]. This effect confirms the ability of testosterone to influence macrophages in two ways: independently and via local transformation into estrogens. The revealed stimulation by estradiol of the expression of ERβ mRNA both during isolated action of the hormone and its action in combination with mLDL on male cells is somewhat unique for macrophages, because estradiol inhibited ERβ expression in some other types of cells, such as bone marrow mesenchymal stem cells [34]. The expression of VCAM1 mRNA is one of the few targets unidirectionally regulated by both estrogen and androgen in the presence of mLDL. Inhibition of VCAM1 expression under the influence of estradiol and 5α-dihydrotestosterone was previously reported for endothelial cells [35, 36]. Differently directed effects of estradiol and 5α-dihydrotestosterone against the background of mLDL were shown in case of expression of ABCG1 mRNA. 5α-Dihydrotestosterone in the presence of mLDL decreased mRNA expression also for the second cholesterol transporter, ABCA1. ABCA1 expression was also inhibited by androgen in the prostate cancer cell line LNCaP [37]. However, according to the data of Langer et al. [38], testosterone had no effect on ABCA1 expression in macrophages. This discrepancy between these and our data may be a result of different types of androgens used and different applications of mLDL. Obviously, inhibition of the expression of components of reverse cholesterol transport (ABCA1 and ABCG1) by 5α-dihydrotestosterone should have contributed to cholesterol accumulation in cells, which is not observed in fact (Fig. 3). Similarly, the stimulation of LDLR expression by androgens does not correspond to the final biological effect either. In hepatocytes, the situation is reversed: androgens inhibit the estrogen-induced expression of LDLR [39]. The inhibition of cholesterol accumulation could be facilitated by the revealed decrease in LPL and CD36 expression under the influence of 5α-dihydrotestosterone in the presence of mLDL.
In general, the revealed effects of three sex hormones on the content of studied mRNAs in the cells, which are of certain interest per se, do not explain the commonness of their final biological effect: inhibition of mLDL-induced cholesterol accumulation. It is not improbable that all three hormones have a uniform effect on expression of genes not studied in this work. This assumption is also favored by the data on similar changes in the expression of the studied mRNAs during the treatment of cells by native and modified LDL (Fig. 5) with differentiation of final biological effects (Fig. 2). However, it may be assumed that hormonal effects are based not on selective modulation of expression of separate genes, but rather on rearrangement of connections between the genes. We have analyzed the correlations between the content of the studied mRNAs to test this hypothesis. It has been revealed (Table 2) that the expression of individual types of mRNAs during hormonal treatment maintains the relationship with expression in the cells not exposed to hormones. However, the levels of some mRNAs during hormonal treatment lose the connection with expression in the control cells. Disturbance of continuity in the expression was minimal with estradiol and maximal in the presence of 5α-dihydrotestosterone. The coherence between the expressions of different types of mRNAs also depended on hormones (Table 3): with the exception of the effect of testosterone in the presence of mLDL, the hormones reduced the coherence between different mRNAs. The cluster analysis of correlations between mRNAs has shown (Table 5) that hormones can influence the distribution of relationships between mRNAs, 5α-dihydrotestosterone being the most efficient modulator. As regards the number of factors determining the coherence between mRNAs, testosterone and estradiol acted in different directions, while 5α-dihydrotestosterone was indifferent (Tables 6 and 7). The results show that sex hormones can have profound influence on coordination in gene expression. However, each hormone is characterized by an individual spectrum of effects. The absence of marked commonality of these spectra as yet prevents distinguishing the parameters that determine the antiatherogenic effects of all three hormones on macrophages. The complexity of interpretation of hormonal effects, which often have opposite directions, has also been noted by other researchers [8].
This work was supported by the Russian Foundation for Basic Research (project No. 09-04-00329-a).
Some experiments were performed at the instrumentation facilities of the Genome Center for Collective Use of Equipment at the Institute of Molecular Biology of the Russian Academy of Sciences.
REFERENCES
1.Barton, M. (2013) Curr. Opin. Lipidol.,
24, 214-220.
2.Kanayama, G., Hudson, J. I., and Pope, H. G., Jr.
(2008) Drug Alcohol. Depend., 98, 1-12.
3.Maggio, M., and Basaria, S. (2009) Int. J.
Impot. Res., 21, 261-264.
4.Li, C., Briggs, M. R., Ahlborn, T. E., Kraemer, F.
B., and Liu, J. (2001) Endocrinology, 142, 1546-1553.
5.Croston, G. E., Milan, L. B., Marschke, K. B.,
Reichman, M., and Briggs, M. R. (1997) Endocrinology,
138, 3779-3786.
6.Akishita, M., and Yu, J. (2012) Hypertens.
Res., 35, 363-369.
7.Novella, S., Dantas, A. P., Segarra, G., Medina,
P., and Hermenegildo, C. (2012) Front. Physiol., 3,
165.
8.Nofer, J. R. (2012) J. Mol. Endocrinol.,
48, R13-R29.
9.Rubanyi, G. M., Johns, A., and Kauser, K. (2002)
Vascul. Pharmacol., 38, 89-98.
10.Shchelkunova, T. A., Morozov, I. A., Rubtsov, P.
M., Samokhodskaya, L. M., Andrianova, I. V., Sobenin, I. A., Orekhov,
A. N., and Smirnov, A. N. (2013) Biochemistry (Moscow),
78, 463-470.
11.Andreeva, E. R., Mikhaylova, I. A., Pugach, I.
M., and Orekhov, A. N. (1999) Angiol. Sosud. Khirurg., 5
(Appendix), 6-26.
12.Bobryshev, Yu. V., Karagodin, V. P.,
Kovalevskaya, Zh. I., Myasoyedova, V. A., Shapyrina, E. V., Salyamov,
V. I., Kargapolova, Yu. M., Galaktionova, D. Yu., Melnichenko, A. A.,
and Orekhov, A. N. (2011) Tsitologiya, 53, 815-825.
13.Villablanca, A. C., Jayachandran, M., and Banka,
C. (2010) Clin. Sci. (Lond.), 119, 493-513.
14.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and
Randall, R. J. (1951) J. Biol. Chem., 193, 265-275.
15.Shchelkunova, T. A., Morozov, I. A., Rubtsov, P.
M., Samokhodskaya, L. M., Kireev, R. A., Andrianova, I. V., Orekhov, A.
N., and Smirnov, A. N. (2008) Biochemistry (Moscow), 73,
920-928.
16.Shchelkunova, T. A., Albert, E. A., Morozov, I.
A., Rubtsov, P. M., Samokhodskaya, L. M., Sobenin, I. A., Orekhov, A.
N., and Smirnov, A. N. (2011) Biochemistry (Moscow), 76,
1178-1184.
17.Razzhevaykin, V. N. (2010) Zh. Obshch.
Biol., 71, 75-84.
18.Wunderlich, F., Benten, W. P., Lieberherr, M.,
Guo, Z., Stamm, O., Wrehlke, C., Sekeris, C. E., and Mossmann, H.
(2002) Steroids, 67, 535-538.
19.Guo, Z., Benten, W. P., Krucken, J., and
Wunderlich, F. (2002) J. Biol. Chem., 277,
29600-29607.
20.Kelly, D. M., Sellers, D. J., Woodroofe, M. N.,
Jones, T. H., and Channer, K. S. (2013) Endocr. Res., 38,
125-138.
21.Ng, M. K., Quinn, C. M., McCrohon, J. A., Nakhla,
S., Jessup, W., Handelsman, D. J., Celermajer, D. S., and Death, A. K.
(2003) J. Am. Coll. Cardiol., 42, 1306-1313.
22.Smirnov, A. N. (2009) Ontogenez,
40, 334-354.
23.Scordalakes, E. M., Imwalle, D. B., and Rissman,
E. F. (2002) Reproduction, 124, 331-338.
24.Juntti, S. A., Tollkuhn, J., Wu, M. V., Fraser,
E. J., Soderborg, T., Tan, S., Honda, S., Harada, N., and Shah, N. M.
(2010) Neuron, 66, 260-272.
25.Ogawa, S., Washburn, T. F., Taylor, J., Lubahn,
D. B., Korach, K. S., and Pfaff, D. W. (1998) Endocrinology,
139, 5058-5069.
26.Wu, M. V., Manoli, D. S., Fraser, E. J., Coats,
J. K., Tollkuhn, J., Honda, S., Harada, N., and Shah, N. M. (2009)
Cell, 139, 61-72.
27.Smirnova, O. V., Vishnyakova, T. G., Rozen, V.
B., Shnyra, A. A., Bocharov, A. V., and Spirov, V. G. (1990) J.
Steroid Biochem., 35, 457-463.
28.Thangavel, C., and Shapiro, B. H. (2008) Drug
Metab. Dispos., 36, 1884-1895.
29.Corcoran, M. P., Lichtenstein, A. H., Meydani,
M., Dillard, A., Schaefer, E. J., and Lamon-Fava, S. (2011) J. Mol.
Endocrinol., 47, 109-117.
30.Ma, Y., Xu, L., Rodriguez-Agudo, D., Li, X.,
Heuman, D. M., Hylemon, P. B., and Pandak, W. M. (2008) Am. J.
Physiol. Endocrinol. Metab., 295, E1369-E1379.
31.Cook, I. T., Duniec-Dmuchowski, Z., Kocarek, T.
A., Runge-Morris, M., and Falany, C. N. (2009) Drug. Metab.
Dispos., 37, 2069-2078.
32.Jakob, F., Homann, D., Seufert, J., Schneider,
D., and Kohrle, J. (1995) Mol. Cell. Endocrinol., 110,
27-33.
33.Enjuanes, A., Garcia-Giralt, N., Supervia, A.,
Nogues, X., Mellibovsky, L., Carbonell, J., Grinberg, D., Balcells, S.,
and Diez-Perez, A. (2003) Eur. J. Endocrinol., 148,
519-526.
34.Zhou, S., Zilberman, Y., Wassermann, K., Bain, S.
D., Sadovsky, Y., and Gazit, D. (2001) J. Cell. Biochem.,
36 (Suppl.), 144-155.
35.Mukherjee, T. K., Dinh, H., Chaudhuri, G., and
Nathan, L. (2002) Proc. Natl. Acad. Sci. USA, 99,
4055-4060.
36.Norata, G. D., Tibolla, G., Seccomandi, P. M.,
Poletti, A., and Catapano, A. L. (2006) J. Clin. Endocrin.
Metab., 91, 546-554.
37.Fukuchi, J., Hiipakka, R. A., Kokontis, J. M.,
Hsu, S., Ko, A. L., Fitzgerald, M. L., and Liao, S. (2004) Cancer
Res., 64, 7682-7685.
38.Langer, C., Gansz, B., Goepfert, C., Engel, T.,
Uehara, Y., von Dehn, G., Jansen, H., Assmann, G., and von Eckardstein,
A. (2002) Biochem. Biophys. Res. Commun., 296,
1051-1057.
39.Croston, G. E., Milan, L. B., Marschke, K. B.,
Reichman, M., and Briggs, M. R. (1997) Endocrinology,
138, 3779-3786.