Evidence for Aging Theories from the Study of a Hunter–Gatherer People (Ache of Paraguay)
G. Libertini
Independent Researcher; E-mail: giacinto.libertini@tin.it
Received April 25, 2013
In the late seventies, a small tribal population of Paraguay, the Ache, living under natural conditions, was studied. Data from this population turn out to be useful for considerations about evolutionary hypotheses on the aging phenomenon. 1) Ache show an age-related increasing mortality, which strongly limits the mean duration of life, as observed in other studies on mammal and bird species. 2) According to current theories on aging, in the wild very few or no individual reach old age and, so, aging cannot be directly influenced by natural selection. However, data from our population show that a significant proportion of the population reaches in the wild 60 and 70 years of age. 3) Data from Ache are also in agreement with the observation about an inverse correlation between extrinsic mortality and deaths due to the age-related increasing mortality. 4) For many gerontologists, the age-related decline of vital functions is a consequence of the gradual decline of cell turnover, genetically determined and regulated by the declining duplication capacities of stem cells. The current interpretation is that these restrictions are a general defense against the proliferation of any tumoral mass. However, among wild Ache cancer is virtually unknown in non-elderly subjects, and only among older individuals are there deaths attributable to oncological diseases. Moreover, fitness decline begins long before oncological diseases have fatal effects in significant numbers. This completely disproves the current hypothesis, because a supposed defense against a deadly disease cannot exterminate a population before the disease begins to kill. These data are consistent with similar data from other species studied under natural conditions, and they bring new arguments against the non-adaptive interpretation of aging and in support of the adaptive interpretation.
KEY WORDS: senescence, evolution, telomere, telomerase, cancerDOI: 10.1134/S0006297913090083
For the study of a species, it is fundamental to observe it under natural conditions. For the human species, the closest condition to the natural one is that of the few residual hunter–gatherer populations, which is equivalent to the human condition in the Paleolithic period. These populations live in areas of difficult access, and as such it is very difficult to contact and study them. Moreover, when they come in contact with “civilized” subjects, most of them fall victim of infectious diseases they are not adapted to. It is also worth noting that these people are illiterate and do not use numbers or have specific memories of past events. The precise study of a hunter–gatherer population is therefore a formidable scientific challenge.
One of the most comprehensive studies in the field was conducted in the late seventies on a small tribal population of Paraguay, the Ache, best known with the depreciative name Guayaki used by their “civilized” neighbors. The report of this study was published in 1996 [1]. Many of the data are unique or with few matches in other similar studies [2-4].
In this paper, I want to summarize some of the data from Hill and Hurtado’s study [1] concerning the life table of this hunter–gatherer people in wild conditions and the causes of their mortality. These data will be used for considerations about the evolutionary hypotheses on aging phenomenon.
DATA FROM LIFE TABLE OF ACHE PEOPLE AND THEIR IMPLICATIONS FOR
EVOLUTIONARY HYPOTHESES ABOUT AGING
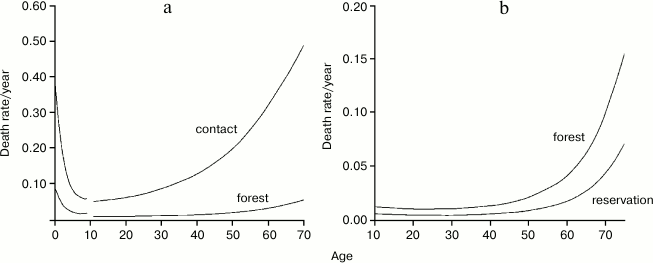
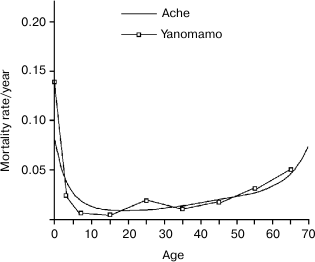
Hill and Hurtado’s very demanding study (“fourteen years of data collection” among the Ache and “nearly five years of writing” [1]) is a very valuable work and an almost unique source of information. Such a study would be unlikely repeatable because hunter–gatherer populations are disappearing. Figures 1-3 summarize the life table of Ache under natural conditions prior to the close and friendly contact with the modern populations (not friendly contacts with “civilized” people were in existence for many years as indiscriminate killings of Ache as soon as they were spotted by Paraguayans or, in other cases, their capture and enslavement). Figure 3 compares the data obtained from the Ache with those obtained from the Yanomamo, one of the few other populations studied under natural conditions.
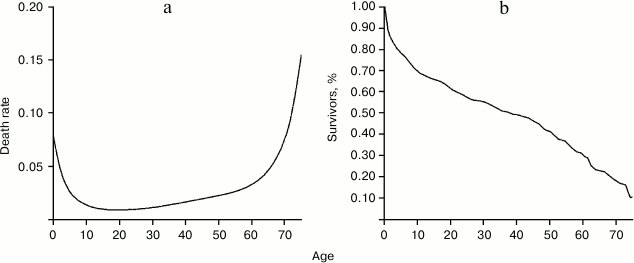
The data document that, in wild conditions, the Ache – despite the ruthless killings and captures by Paraguayans, which caused about one third of the overall mortality (missing persons included) – had a life table similar to that of modern populations, with a high and decreasing mortality in the first part of life, then a phase in which the mortality was low and stable, with a minimum at around 15-20 years (about 0.9%/year), and finally a third phase in which the mortality grew slowly at first and then rapidly.
It is noteworthy that, at ages 60 and 70 years, approximately 30 and 20%, respectively, of Ache survived.
Another important fact is that the increased mortality reported for the Ache in wild conditions in the third phase is not at all indifferent for the overall mean duration of life (ML).
Fig. 1. a) Probability of death as a function of age; b) life table of Ache people in wild conditions (forest period). Data from Hill and Hurtado [1].
Fig. 2. Comparison of age-specific mortality between the period lived in the forest and during the first contact (a) or during life in the reservation (b). Data from Hill and Hurtado [1].
Fig. 3. Comparison between the life tables of Ache and Yanomamo, another hunter–gatherer population. The curves are essentially overlapping. Data from Hill and Hurtado [1] and Early and Peters [4].
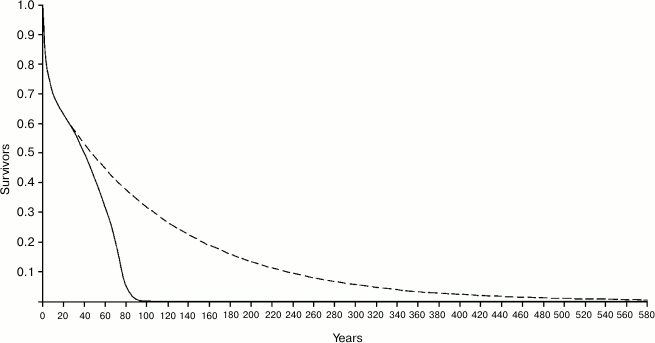
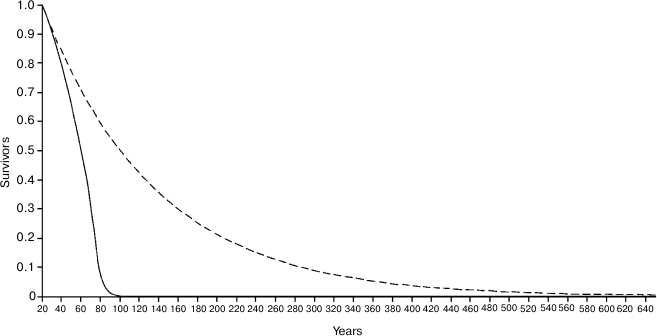
Figure 4 shows the Ache life table in natural conditions and the hypothetical life table that would occur in the case that there was no age-related increase of mortality, i.e. in the case in which individuals would not grow old. In the real life table, the ML is equal to 38.8 years, while in the hypothetical life table ML is equal to 87.75 years, with a ratio (Ratio 1) between the two values equal to 2.260. It is worth noting that the length of the abscissas is extended up to 580 years, since – with a mortality rate of 0.9%/year, which is about the estimated minimum mortality in natural conditions – at that age even 0.5% of the population would survive!
Fig. 4. Continuous line, life table of Ache in natural conditions (forest period) (data from Hill and Hurtado [1]); dashed line, hypothetical life table without age-related increasing mortality.
If we consider only the individuals surviving at the age of 20 years (Fig. 5), the ML of Ache in the wild was 20 + 38.1 = 58.11 years, while for the hypothetical curve the ML is 20 + 116.04 = 136.04 years (!), with a ratio (Ratio 2) between the two values equal to 3.044. In Fig. 5, the abscissas extend up to 640 years, since at the age of 634 years about 0.5% of the population would survive!
The hypothetical curves of Figs. 4 and 5, in the sections where the mortality is constant, is calculated using the simple formula:
Yt = Y0(1 – m0)t, (1)
where Y0 – survivors at time 0; Yt – survivors at time t; m0 – minimum mortality; t – time.
Ratio 1 and Ratio 2 may be visualized, in each figure, as the ratio between the area defined by the hypothetical curve and the area subtended by the real curve.
Fig. 5. Same as Fig. 4 but only individuals surviving at the age of 20 years are considered.
These results, for a human population in wild conditions, are consistent with those reported in a previous work for various species of mammals [5], where the range of Ratio 1 and Ratio 2 were 1.55-3.21 and 2.42-5.09, respectively (Table 1).
Table 1. Ratio 1: ratio between ML in
natural conditions and ML in the hypothetical condition that the
mortality rate remains stable at its lowest value for various mammal
species. Ratio 2: the same as Ratio 1 but excluding the first periods
of life, i.e. before mortality reaches its lowest value. Data from
Libertini [5], sources: (a) Spinage, 1972; (b)
Laws, 1966; (c) Laws, 1968; (d) Spinage, 1970; (e) Deevey, 1947, and
from Hill and Hurtado [1] (in italics). For all:
sexes combined; time unit – year
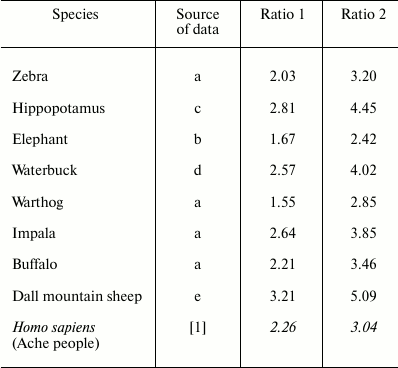
These data show that, for the Ache people in wild conditions: (i) the increase of mortality is evident at ages existing in natural conditions; (ii) such mortality increase strongly reduces the ML; (iii) a substantial part of the population reaches ages commonly considered as senile.
Since the age-related increase of mortality, i.e. the age-related reduction of fitness alias aging, is clearly present in natural conditions, it is therefore certainly subjected to natural selection. This contradicts a basic assumption of the non-adaptive theories of aging (mutation accumulation theory [6-10], antagonistic pleiotropic theory [11, 12], disposable soma theory [13, 14]), according to which aging is not subject to natural selection due to the rarity of elderly individuals under natural conditions: “… there is scant evidence that senescence contributes significantly to mortality in the wild … As a rule, wild animals simply do not live long enough to grow old. Therefore, natural selection has limited opportunity to exert a direct influence over the process of senescence” [15].
It is good to point out the following. Although the percentages of Ache who reached the ages of 60 and 70 years are certainly remarkable and perhaps surprising (see Fig. 1), the key issue is not so much the percentages of individuals who reach those ages but the percentage of individuals who, by age-related fitness reduction, die before. It is important to avoid the confusion between the gradual decline of fitness, or aging, and the result of this process, namely the elderly individuals (see the previous quotation).
The age-related fitness decline is clearly a phenomenon subject to natural selection and in need of an evolutionary explanation, regardless of the percentage – in wild conditions – of individuals called “elders” because they have reached a certain threshold of decline, arbitrarily defined.
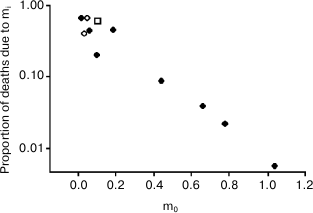
Another important fact is that these results are also in agreement with Ricklefs’ observation [16] about an inverse correlation – documented in some mammal and bird species – between extrinsic mortality (m0) and the deaths due to the age-related increasing mortality (Ps), a relation that disproves non-adaptive aging theories and supports adaptive aging hypotheses [17], which consider vertebrate aging a type of phenoptosis, or programmed death of an individual [18, 19].
With a minimum mortality (m0, approximately 1%/year) and a share of deaths due to senescence (Ps, on the ordinates) equal to about 67%, the position of the human species (Ache people) in the graph (Fig. 6) is highlighted with an open square.
Fig. 6. Inverse relation between extrinsic mortality (m0) and the proportion of deaths (Ps) due to intrinsic mortality (mi), i.e. the deaths due to the age-related increasing mortality. Open rhombs, mammal species; closed rhombs, bird species; open square, Ache people in wild conditions. Data are from Ricklefs [16], Table 2 (p. 30). Ricklefs’ Fig. 7 (p. 34) has been redrawn and the datum from Ache people [1] has been added. Ordinates are in logarithmic scale.
DATA ABOUT DEATH CAUSES IN ACHE PEOPLE AND THEIR IMPLICATIONS FOR
THE HYPOTHESIS OF AGE-RELATED FITNESS DECLINE AS A DEFENSE AGAINST
CANCER
Death causes for Ache people under natural conditions (forest period) are shown in Table 2 [1].
Table 2. Causes of death for Ache people in
natural conditions (forest period) (data from Hill and Hurtado [1])
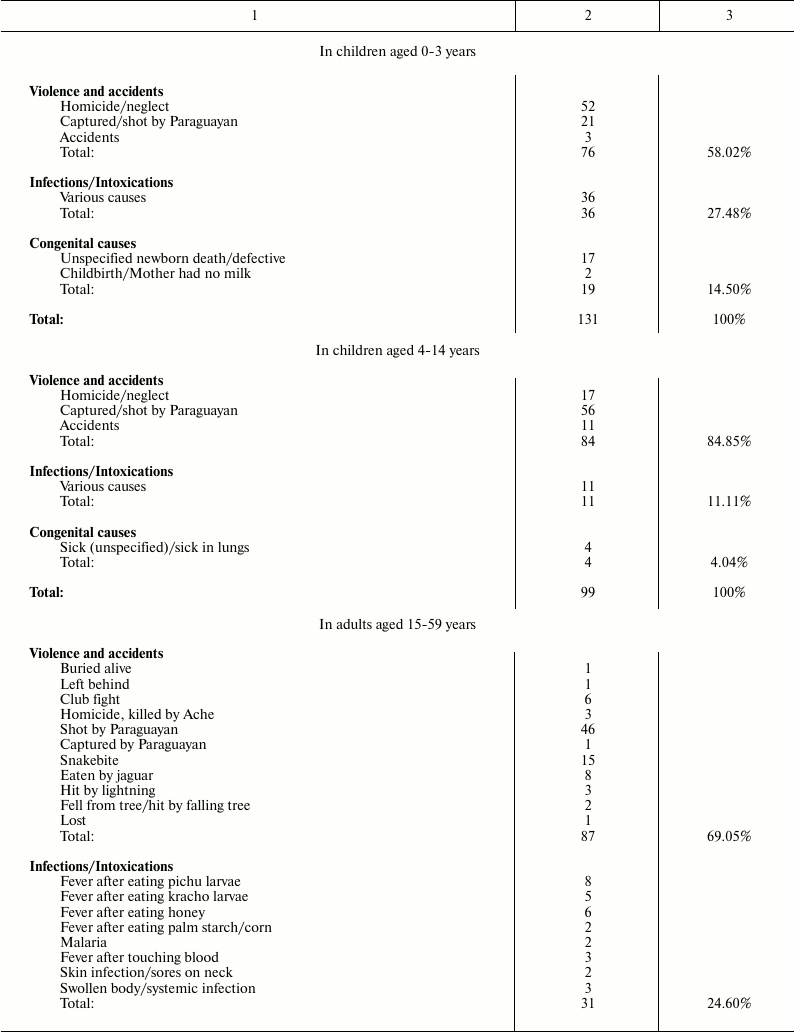
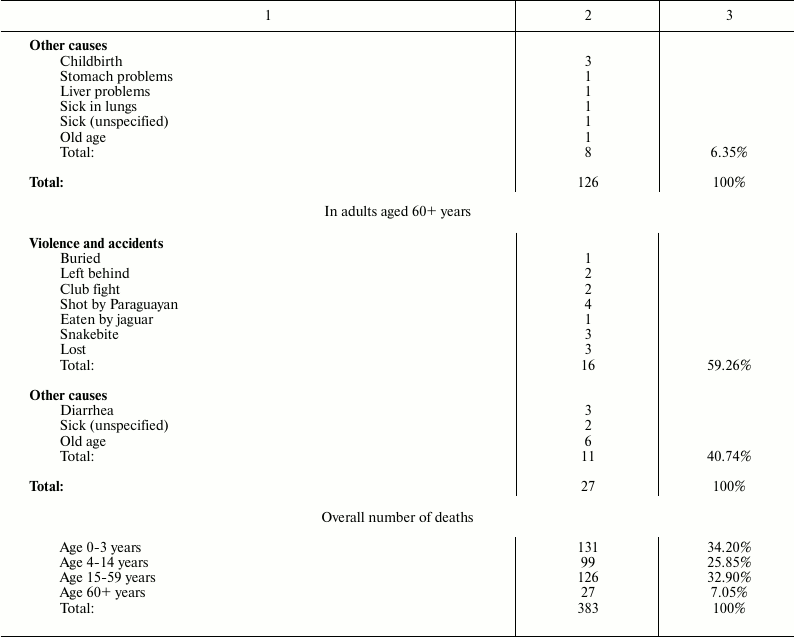
It is worth noting that, in Ache people in wild conditions, the main causes of death for modern western populations (heart attacks, diabetes, hypertension, etc.) are absent. Moreover, cases of death by cancer are not reported, although, in the group “adult aged 60+ years”, some deaths attributed generically to unspecified causes or to “old age” could be the result of neoplastic diseases. In any case, the data indicate that neoplastic diseases were rare events in Ache people in the forest period. This rarity of cancer in primitive conditions is not a new thing and is confirmed elsewhere.
For example, some anecdotal, but authoritative, information about the immunity from cancer of primitive populations are reported by Price [20]: Dr. J. Romig, “a surgeon (of Anchorage) of great skill and with an experience among the Eskimos and the Indians, both the primitives and the modernized … stated that in his thirty-six years of contact with these people he had never seen a case of malignant disease among the truly primitive Eskimos and Indians, although it frequently occurs when they become modernized” (p. 83).
Dr. J. R. Nimmo, the government physician in charge for Torres Strait Islands people told Dr. Price that: “in his thirteen years with them he had not seen a single case of malignancy, and seen only one that he had suspected might be malignancy among the entire four thousand native populations. He stated that during this same period he had operated on several dozen malignancies for the white populations, which numbers about three hundred” (p. 179).
These data can be used to settle the dispute between non-adaptive and adaptive interpretations of aging. Non-adaptive theories do not predict at all the existence of genetically determined and regulated mechanisms that progressively reduce the fitness. The existence of such mechanisms would indeed falsify non-adaptive theories, forcing their total abandonment.
On the contrary, for the plausibility of aging adaptive theories (e.g. [5]), it is indispensable the existence of genetically determined and regulated mechanisms for the age-related mortality increase [21, 22].
The age-related decline of vital functions is well explained as a consequence of the gradual decline of cell turnover, determined by the declining duplication capacities of stem cells by effect of the telomere–telomerase system [22, 23]. This cell turnover decline is in accordance with the adaptive interpretation of aging, while, for the non-adaptive interpretation, it cannot be accepted as a mechanism causing senescence and, therefore, a different rationale is absolutely necessary.
The absence of a valid or at least plausible explanation for these mechanisms would indeed disprove all non-adaptive aging theories, transforming them, once and for all, from potentially valid theories into hypotheses of only historical value. This would change the validity of a huge number of works and experiences based on the assumption of aging as a non-adaptive phenomenon. Some of the best researchers have therefore sought a plausible justification for the above-mentioned mechanisms different from that which explains them purely and simply as determinants of aging.
The current authoritative justification, in fact the only hypothesis widespread and supported in the scientific world, is that these restrictions are a general defense against cancer, because they would limit the pathological proliferation of any tumoral mass [24-26].
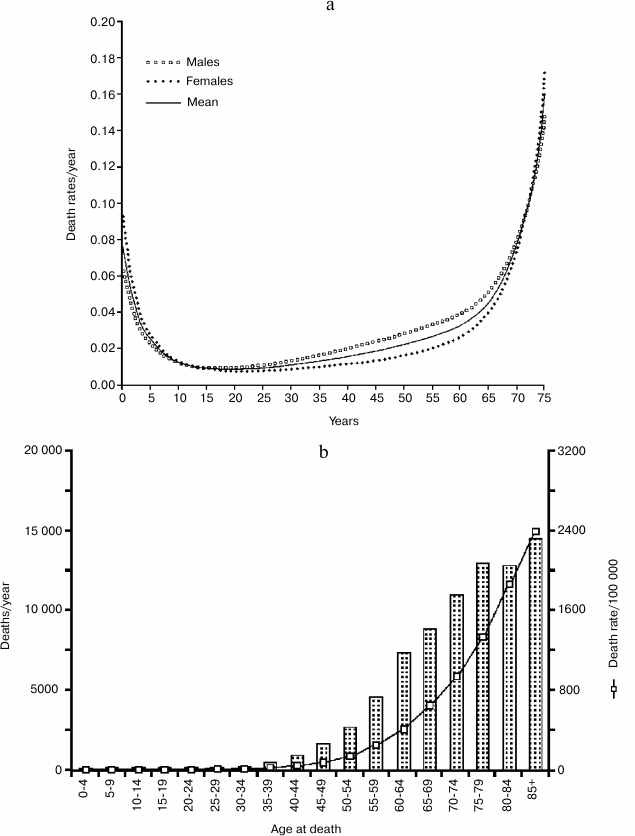
If we compare the age-related increasing mortality in natural conditions (Ache population; Fig. 7a) with the incidence of cancer and the deaths caused by it in a modern population (Great Britain; Fig. 7b), at first sight this explanation could seem plausible.
Fig. 7. a) Life table of Ache people in natural conditions (forest period); data from Hill and Hurtado [1]; b) incidence of cancer and cancer deaths in a modern western population (UK) [27-29].
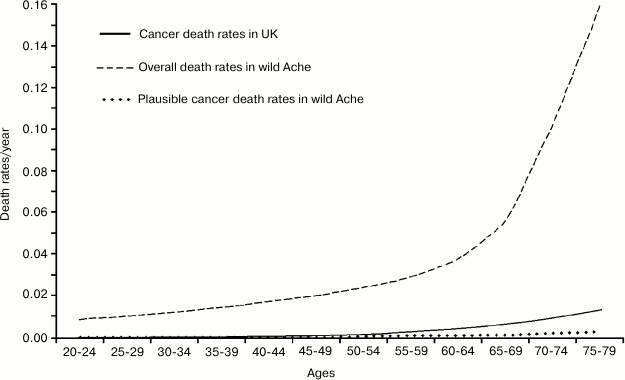
The appearance is misleading and data presented in such a way lead to a false confirmation of current opinion. It is necessary to draw, on a single graph and using a single scale, the rates of: a) total mortality for Ache under natural conditions; b) plausible cancer death rates in wild Aches; and, by comparison: c) cancer death rates in the modern population (Fig. 8). It is evident that, in the wild, the increase in overall mortality (over the minimum value of about 1%) anticipates and is much higher than the mortality from cancer both in the wild and in the modern population. Moreover, under natural conditions, when there are the first possible cases of deaths by cancer, fitness decline has already determined the death of most individuals.
This completely disproves the hypothesis that the reduction of cell duplication capacities would be a defense against cancer: it would be like arguing that a defense against a deadly disease has the effect of mass-killing before the disease begins to kill! On the contrary, according to the adaptive hypothesis of aging, the decline of defense against cancer results also from the decline of cell replication capacities and the cases of cancer in old age are part of aging characteristics [22].
Fig. 8. Total mortality and plausible cancer death rates for Ache under natural conditions and cancer death rates in a modern population (Great Britain).
CONCLUSION
For aging research, the observation of a species under natural conditions is essential because it allows the evaluation of the phenomenon in the real conditions in which natural selection acts. This enables the avoidance of possible misconceptions on which any theoretical construction would be fallacious and the verification of whether, in natural conditions, some theoretical predictions are confirmed or falsified.
The study of the human species under natural conditions should give, and gives, results similar to those obtained from studies of other vertebrate species.
1) According to current non-adaptive interpretation of aging, very few or no individual reach old age and, so, aging cannot be directly influenced by natural selection [15]. However, data from a human population in the wild show that a significant proportion of the population reaches 60 and 70 years of age (about 30 and 20%, respectively). Moreover, if we consider not the ill-defined concepts of “aging” and “old age” but the age-related mortality increase, a perfectly definable parameter, this phenomenon greatly reduces the mean duration of life (67% in Ache people in wild conditions!) and, therefore, it is absurd to consider this increase in mortality as something that is not influenced by natural selection or of no importance for selective process.
These data are consistent with similar data from other vertebrate species studied under natural conditions [16] and invalidate the main theoretical tenet of current evolutionary hypotheses about aging.
2) Non-adaptive aging theories predict a direct relation between the deaths due to the age-related increasing mortality and extrinsic mortality [15] while adaptive aging theories predict an inverse relation [5, 17]. Ricklefs’ data on some mammal and bird species [16] confirm the prediction of the adaptive aging hypothesis and falsify the opposite thesis. The Ache life table is perfectly consistent with Ricklefs’ data.
3) Non-adaptive aging theories offer no valid explanation about the known limitations in cell replication and cell turnover that are the most plausible interpretation of age-related fitness decline.
The supporters of non-adaptive theories of aging, in the attempt to formulate a plausible explanation, hypothesize that these limits are a general defense against cancer. However, data from a population in natural conditions, and even from modern populations where cancer incidence is greatly increased, show that deaths from cancer are chronologically subsequent to the age-related increase in mortality and have a frequency much lower than the deaths caused by this increase in mortality. This shows that from a logical point of view the hypothesis that the aforesaid limits are a general defense against cancer is untenable.
In short, the study of a human population under natural conditions brings new arguments against the non-adaptive interpretation of aging. This hypothesis (actually, a group of ill-defined theories) is commonly presented as the true and only scientific explanation of aging, but, when a hypothesis is widely and repeatedly disproved by empirical data, the scientific method requires that it must be considered as no longer scientifically acceptable and confined within the hypotheses of historical interest.
The blowgun of the humble Ache has a poison that turns out to be fatal to old and widespread beliefs.
REFERENCES
1.Hill, K., and Hurtado, A. M. (1996) Ache Life
History, Aldine De Gruyter, New York.
2.Howell, N. (1979) Demography of the Dobe
!Kung, Academic Press, New York.
3.Melancon, T. (1982) Marriage and Reproduction
among the Yanomamo Indians of Venezuela: Ph. D. Dissertation,
Pennsylvania State University.
4.Early, J., and Peters, J. (1990) The Population
Dynamics of the Mucajai Yanomamo, Academic Press, New York.
5.Libertini, G. (1988) J. Theor. Biol.,
132, 145-162.
6.Medawar, P. B. (1952) An Unsolved Problem in
Biology, H. K. Lewis, London; reprinted in: Medawar, P. B. (1957)
The Uniqueness of the Individual, Methuen, London.
7.Hamilton, W. D. (1966) J. Theor. Biol.,
12, 12-45.
8.Edney, E. B., and Gill, R. W. (1968) Nature,
220, 281-282.
9.Mueller, L. D. (1987) Proc. Natl. Acad. Sci.
USA, 84, 1974-1977.
10.Partridge, L., and Barton, N. H. (1993)
Nature, 362, 305-311.
11.Williams, G. C. (1957) Evolution,
11, 398-411.
12.Rose, M. R. (1991) Evolutionary Biology of
Aging, Oxford University Press, New York.
13.Kirkwood, T. B. L. (1977) Nature,
270, 301-304.
14.Kirkwood, T. B. L., and Holliday, R. (1979)
Proc. R. Soc. Lond. B. Biol. Sci., 205, 531-546.
15.Kirkwood, T. B. L., and Austad, S. N. (2000)
Nature, 408, 233-238.
16.Ricklefs, R. E. (1998) Am. Nat.,
152, 24-44.
17.Libertini, G. (2008)
TheScientificWorldJOURNAL, 8, 182-193.
18.Skulachev, V. P. (1997) Biochemistry
(Moscow), 62, 1191-1195.
19.Skulachev, V. P. (1999) Biochemistry
(Moscow), 64, 1418-1426.
20.Price, W. A. (1939) Nutrition and Physical
Degeneration, Paul B. Hoeber, New York-London.
21.Libertini, G. (2006)
TheScientificWorldJOURNAL, 6, 1086-1108.
22.Libertini, G. (2009) in Telomeres:
Function, Shortening and Lengthening, Nova Science
Publishers Inc., New York.
23.Fossel, M. B. (2004) Cells, Aging and
Human Disease, Oxford University Press, New York.
24.Campisi, J. (1997) Eur. J. Cancer,
33, 703-709.
25.Campisi, J. (2000) In vivo, 14,
183-188.
26.Wright, W. E., and Shay, J. W. (2005) J. Am.
Geriatr. Soc., 53, S292-294.
27.General Register Office for Scotland 2010,
Deaths Time Series Data, Deaths in Scotland in 2009.
28.Northern Ireland Statistics and Research
Agency, Registrar General Annual Report 2010.
29.Office for National Statistics, Mortality
Statistics: Deaths Registered in 2009, England and Wales 2010.